Abstract
Context and purpose:
There is an urgent need to develop vitamin D dietary recommendations for dark-skinned populations resident at high latitude. Using data from randomised controlled trials (RCTs) with vitamin D3-supplements/fortified foods, we undertook an individual participant data-level meta-regression (IPD) analysis of the response of wintertime serum 25-hydroxyvitamin (25(OH)D) to total vitamin D intake among dark-skinned children and adults residing at ≥40°N and derived dietary requirement values for vitamin D.
Methods:
IPD analysis using data from 677 dark-skinned participants (of Black or South Asian descent; ages 5–86 years) in 10 RCTs with vitamin D supplements/fortified foods identified via a systematic review and predefined eligibility criteria. Outcome measures were vitamin D estimates across a range of 25(OH)D thresholds.
Results:
To maintain serum 25(OH)D concentrations ≥25 and 30 nmol/L in 97.5% of individuals, 23.9 and 27.3 μg/d of vitamin D, respectively, were required among South Asian and 24.1 and 33.2 μg/d, respectively, among Black participants. Overall, our age-stratified intake estimates did not exceed age-specific Tolerable Upper Intake Levels for vitamin D. The vitamin D intake required by dark-skinned individuals to maintain 97.5% of winter 25(OH)D concentrations ≥50 nmol/L was 66.8 μg/d. This intake predicted that the upper 2.5% of individuals could potentially achieve serum 25(OH)D concentrations ≥158 nmol/L, which has been linked to potential adverse effects in older adults in supplementation studies.
Conclusions:
Our IPD-derived vitamin D intakes required to maintain 97.5% of winter 25(OH)D concentrations ≥25, 30 and 50 nmol/L are substantially higher than the equivalent estimates for White individuals. These requirement estimates are also higher than those currently recommended internationally by several agencies, which are based predominantly on data from Whites and derived from standard meta-regression based on aggregate data. Much more work is needed in dark-skinned populations both in the dose-response relationship and risk characterisation for health outcomes.
Keywords: Dark-skinned, Vitamin D recommendations, Dietary Reference Values, Recommended Dietary Allowance, Individual Participant Data-level meta-regression analyses
Introduction
Vitamin D deficiency has significant implications for human health throughout life [1]. Most worldwide expert bodies agree that serum 25-hydroxyvitamin D [25(OH)D] concentrations below 25/30 nmol/L indicate an increased risk of clinical vitamin D deficiency implicated in the development of childhood nutritional rickets and adult osteomalacia [2–6]. In North America and Europe, dark-skinned racial/ethnic subgroups are at a much higher risk of low vitamin D status in comparison to their White counterparts. For example, across racial/ethnic groups in the National Health and Nutrition Examination Survey 2011–2014 in the US, the prevalence of serum 25(OH)D <30 nmol/L (vitamin D deficiency) in Non-Hispanic white, Hispanic, Non-Hispanic Asian and Non-Hispanic black participants have been reported as 2.1%, 5.9%, 7.6% and 17.5%, respectively [7]. Within the Canadian Health Measures Surveys Cycles 1–3 (covering 2007–2013), the prevalence of vitamin D deficiency was 6% in White versus 20% among participants of ethnic minority [8]. Dark-skinned ethnic groups within Europe are also worryingly at much increased risk of vitamin D deficiency compared to their White counterparts [9,10]. Recent data from the UK Biobank shows that the prevalence of serum 25(OH)D <25 nmol/L among adult participants of South Asian and Black ethnicity was much higher than in Caucasians (57.3% and 36.3% v. 11.7%, respectively) [11]. Within the UK-dwelling South Asian population, those of Pakistani ethnicity had the highest risk of serum 25(OH)D <25 nmol/L (66% v. 54% and 44% for Indian and Bangladeshi, respectively) [12]. Recent data from a population-based health survey among immigrant adults in Finland shows that the prevalence of vitamin D deficiency in Somali and Kurdish participants was much higher than that of the general Finnish population (24.1% and 48.8% v. 0.9%, respectively) [13]. In Sweden, there was also a much higher prevalence of vitamin D deficiency after summer in dark-skinned compared to fair-skinned 5–7 year-old children (17% and 3.7%, respectively) [14].
Dietary Reference Values (DRVs), as estimates of the vitamin D dietary requirements, provide a framework for prevention of vitamin D deficiency among almost all individuals in the population [15]. Despite the fact that DRVs for vitamin D have been re-evaluated by several authorities in the past decade [see 16–18, for reviews], recommendations have been, by and large, based predominantly on an assumption that the dietary requirements between racial groups do not differ. Not from neglect or a lack of consideration of the issues, this assumption is largely due to an absence of data on which to base decisions for vitamin D requirements and the dietary recommendations to achieve them - a key knowledge gap identified by a number of authorities [2–4]. A small number of suitable vitamin D randomized controlled trials (RCTs) of dark-skinned children and adults existed around the time of the recent DRV reviews, ranging from one to 5 RCTs depending on the review year [19–23] and since then, additional RCTs have been published [24–29]. Of particular note, a number of trials compared White versus Black or fair versus dark skinned, and several, but not all [19], reported higher dietary vitamin D requirements for Black and dark-skinned participants compared with their White counterparts [20,24,30].
Increasingly, the use of individual participant data (IPD)-level meta-regression analysis is recognized as best practice [31], as it avoids some of the limitations intrinsic to standard meta-regression based on aggregate data [18,32]. Thus, pooling of the individual data from suitable RCTs using an IPD approach offers a novel opportunity to investigate DRV for dark-skinned individuals residing at higher latitudes, while also ensuring greater representation, by considering data from several studies in different contexts, instead of relying on specific data from one specific study undertaken in a particular context [4].
The aims of the present work were firstly, to identify RCTs with vitamin D3 supplements or fortified foods in dark-skinned participants residing at >40°N using a systematic review process and to undertake an IPD meta-regression analyses of the response of winter serum 25(OH)D to total vitamin D3 intake in both children and adults. Secondly, to compare these IPD-derived vitamin D dietary requirement estimates (i.e., estimates of the daily dietary vitamin D needed in achieving 25(OH)D concentrations above recommended thresholds) for dark-skinned individuals with international DRV, which were largely based on vitamin D3-supplement RCTs of White individuals; as well as comparing these estimates with those from our previous IPDs of White children and adults (based on vitamin D3-supplement and/or -fortified food RCTs [32,33]). Lastly, to inform safety considerations, each of the vitamin D intake requirement estimates generated were compared to the current age-appropriate Tolerable Upper Intake Levels (ULs) for vitamin D [2,34] and the estimates were also used to predict the upper 97.5th percentiles of serum 25(OH)D concentration achieved. These predicted concentrations were bench-marked against those suggested as high serum 25(OH)D concentrations by the Institute of Medicine (IOM) [2] and the European Food Safety Authority (EFSA) [35] as well as in two high-dose vitamin D supplementation trials in older adults with evidence of increased risk of falls and/or factures [36,37].
Materials and methods
The description of the scientific approach and methodology used in the present work has been outlined in a detailed study protocol [38], and aligns very closely with our recent similar IPD of White children and adults [33]. Therefore, only an overview is presented here to provide context and to update on any amendments since the publication of the study protocol.
Adherence to guidelines, registration and ethics approval
The present IPD meta-regression analysis of data from vitamin D RCTs follows the guidance provided as part of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)-IPD statement [39]. The IPD meta-regression analysis was registered with the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42018092343; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=92343). Approval by a research ethics committee to conduct this meta-analysis was not required because the aim of this secondary analysis was consistent with the ethical approval received for the individual studies. The current IPD analysis was conducted on anonymized data.
Systematic review to identify eligible papers
Within the Population Intervention Comparison Outcome (PICO) framework for systematic reviews [40], the populations of interest in this study were specified as dark-skinned male and female children and adults, excluding studies in infants (0–12 months) and young children (12–23.9 months), pregnant or lactating women [32]. While dark-skinned individuals were defined as those with a Fitzpatrick skin type of V or VI [38], we were aware that it is not always measured or reported within studies. Should Fitzpatrick skin type not be reported in the identified studies of dark-skinned individuals in the present work, particular emphasis was placed on studies performed on individuals who identify as of Black or South Asian descent, as these are the population groups most studied to-date in terms of vitamin D dietary requirements. The full details of inclusion and exclusion criteria for the vitamin D RCTs considered for eligibility are outlined in the study protocol and elsewhere [32,33,38]. In addition, participants with missing vitamin D supplement adherence data or with a vitamin D supplement adherence of <70% were excluded from the primary analysis as total vitamin D intake estimates could be considered insecure.
Identification of studies: Information sources and search strategy
During May-July 2018, electronic searches were performed in PubMed, Ovid Medline and Embase online databases as well as three trial registries (ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), and the International Standard Randomized Controlled Trials Number (ISRCTN) registry) from inception to July 31st 2018 (date of the final screen) using structured electronic search strategies, based closely on those used by us previously [33,38,41,42] and which accounted for the inclusion/exclusion criteria outlined above. An exemplar search strategy specifically adapted for PubMed is shown in Supplemental Table 1 in the ‘Online Resource‘. The methods used in the present systematic review per se follow the PRISMA statement [43].
Study selection and inclusion
Study selection was independently conducted by two pre-specified investigators (KDC and MEK), first by a screen of the titles and abstracts, followed by a review of the full text of potentially relevant studies. The same two investigators separately determined which RCTs met the eligibility criteria for inclusion. In addition, the searches were supplemented by searches of review/systematic review articles and reference lists of trial publications as well as from the key international vitamin D DRV reports over the last decade [2–5]. Information on the combined number of records identified, abstracts and full-text articles screened, and articles excluded and included in the review are shown in Figure 1.
Figure 1.
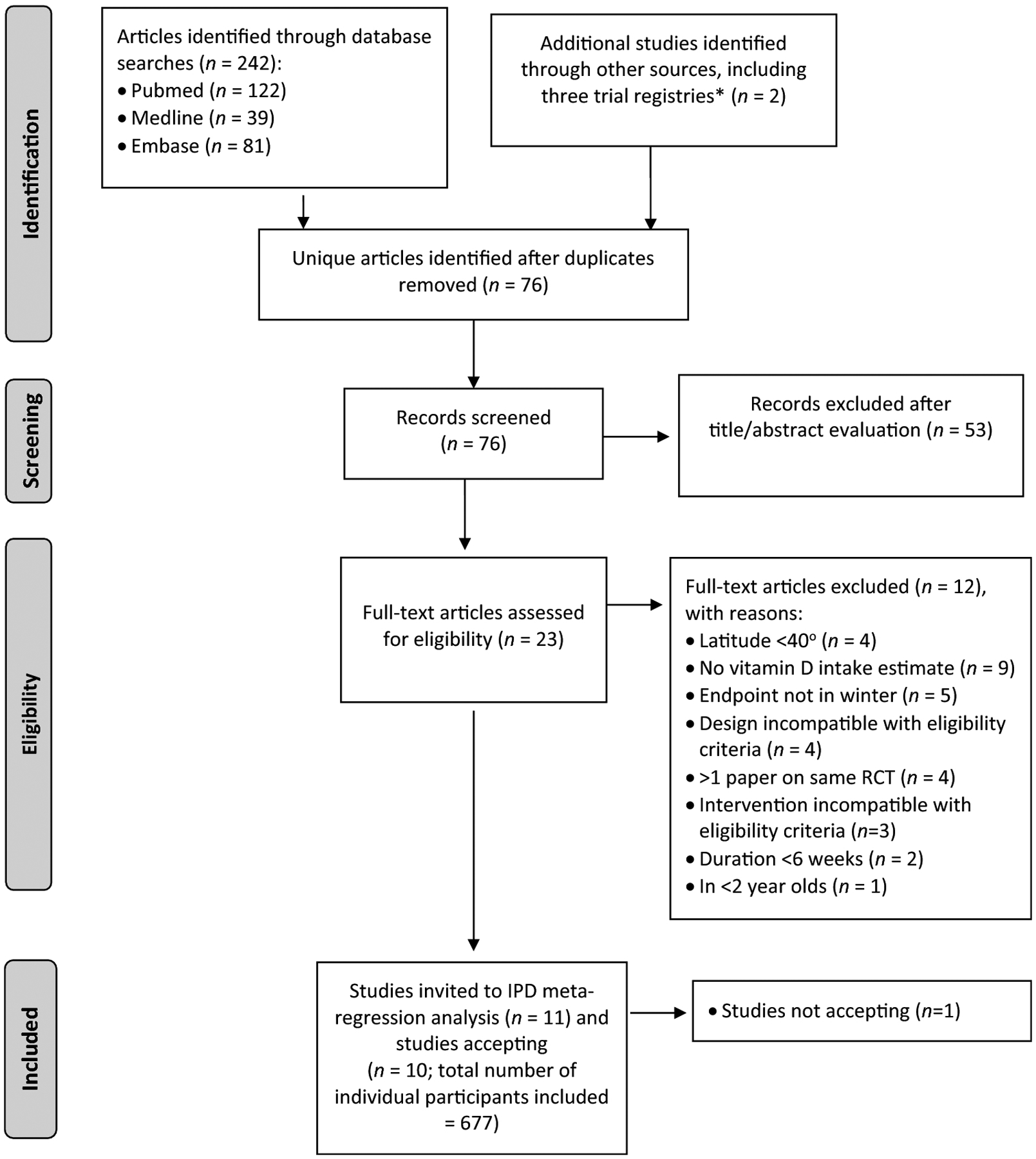
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for study selection procedure.
*ClinicalTrials.gov, Cochrane Central Register of Controlled Trials, and the International Standard Randomized Controlled Trials Number registries.
Data collection processes, data items, IPD integrity, and data protection
Full details of the data collection processes, including data transfer agreement, as well as IPD integrity checks and data protection are presented in the study protocol [38]. In brief, for each eligible RCT, collaboration was requested and negotiated with the principal investigator [44]. Data were initially de-identified at source before encryption and transfer by e-mail. On receipt, a pre-specified investigator (KDC) assessed the data integrity, as described previously [33,38]. In line with recently published principles and recommendations in relation to the sharing and reuse of IPD [45], data within the individual datafiles were used to establish an overall anonymized data file, as follows: only data on the prioritized IPD variables within the transferred files were included, there were no personal identifiers included, the identity of included RCTs was also de-identified by use of a random assignment number. This was based on pseudo-random numbers generated via R version 3.6.2 (R Core Team, Vienna, Austria), using a pre-specified seed, by a researcher with no involvement in the IPD process or analysis. The anonymized data file was held in Excel® V15.30 (Microsoft Corporation, USA). The originally transferred data files from participating RCT groups were fully deleted from the lead PI’s (KDC) files in advance of the synthesis and statistical analyses by the project biostatistician (CR).
Specification of outcomes and effect measures
Serum 25(OH)D concentration (in nmol/L [2.5 nmol/L = 1 ng/mL]) was the sole outcome considered in the IPD meta-regression analysis. Likewise, total vitamin D intake (μg [1 μg = 40 international units (IU)]) was the only predictor considered. Total vitamin D intake was defined as that from the diet (including personal vitamin D supplements, where permitted within an RCT) as well as that from any supplemental vitamin D dose provided in the RCT after accounting for adherence [46–48].
Quality assessment and risk of bias assessment for individual studies
The Jadad scale was used to assess the quality of the included RCTs [49], and an assessment of the risk of bias in these RCTs was performed using the Cochrane Collaboration’s tool for assessing risk of bias [50]. Two pre-specified investigators (KDC and LT) independently assessed study quality and risk of bias in the RCTs.
Statistical analysis
Choice of model
A one-stage IPD meta-analysis [51,52] was carried out using a model which approximated the curvilinear intake-serum concentration relationship over a wide vitamin D intake distribution [2,4]. Specifically, a linear mixed regression model with vitamin D intake as the independent variable (a fixed effect) and square root-transformed serum 25(OH)D concentration as the dependent variable, corresponding to a quadratic regression model on the original scales. Study-specific random intercepts were also included to accommodate between-study variation typically exhibited in IPD analyses. Both unadjusted models and adjusted models including fixed-effect adjustments for baseline serum 25(OH)D concentrations, age and BMI were fitted. These adjustments, which are commonly used [4,32,33], were pre-specified [38]. Models with additional adjustments for methods of vitamin D intake estimation or serum 25(OH)D measurement were also fitted. To inform safety considerations around the various vitamin D intake estimates generated (see below), the present unadjusted and adjusted models were also used to predict the upper 97.5th percentiles of serum 25(OH)D concentration achieved.
Derivation of vitamin D DRV estimates
Lower boundaries of the prediction intervals of the fitted (mean) regression line, corresponding to vitamin D intakes needed to maintain 50, 90, 95, and 97.5% of the participants above serum 25(OH)D thresholds of 25, 30, and 50 nmol/L (where appropriate and feasible) were estimated by means of inverse regression [32]. Corresponding 95% confidence intervals on these estimated lower boundaries were obtained using a parametric bootstrap procedure with 1000 replications, as described previously [32,33].
Leave-one-out sensitivity analysis
To assess if there were any overly influential RCTs, the derivation of vitamin D intake estimates at the 97.5th percentile using the 50 nmol/L threshold based on an unadjusted model (as described above) was repeated leaving out one RCT at a time [33]. The impact of omission of each RCT individually is reported as change, both as μg/d and %, from the vitamin D intake estimate derived using all studies.
Subgroup analyses
A number of specific subgroups had been considered previously [2,4,32,33]. We fitted separate models for subgroups of children (<18 years) and adults (≥18 years) and for subgroups of participants of South Asian and African ethnicity. We also fitted separate models for a subgroup consisting of adult participants with BMI < and ≥30 kg/m2. The present work had individual total vitamin D intake estimates which accounted for adherence to vitamin D supplements/fortified foods by participants (i.e., for all those with vitamin D supplement adherence of ≥70%, as those below this threshold were excluded from the analysis), whereas our previous IPD of White individuals did not build adherence into the estimation of total vitamin D intakes but rather applied a minimum fortified food compliance threshold of 80% for inclusion of participants in the analyses [33]. Therefore, to better facilitate a comparison of the vitamin D DRV estimates from both IPDs, we calculated the total vitamin D intake estimates for Black individuals (children and adults) without accounting for adherence to vitamin D supplements/fortified foods, and fitted separate adjusted models after applying a minimum compliance threshold of 80%, as per our previous IPD of White individuals [33]. The present work allowed for up to 100 μg/d of supplemental vitamin D which led to a maximum individual total vitamin D intake estimate of 124 μg/d, whereas our previous IPD of White individuals had a 50 μg/d total vitamin D intake threshold [32]. Therefore, as an additional subgroup analysis, only those individuals with total vitamin D intake estimates <50 μg/d (applying a minimum compliance threshold of 80%) in the current population were included. The subgroup analyses were pre-specified rendering testing for interaction unnecessary.
Safety considerations of the IPD-derived vitamin D intake requirement estimates
To inform safety considerations, each of the various vitamin D intake requirement estimates generated for children and adults, both separately and pooled, were compared to the current ULs for vitamin D for children (50–75 μg/d, depending on age group and agency) and adults (100 μg/d), respectively [2,34]. Furthermore, and in line with our recent IPD analyses of vitamin D requirements in White individuals [33], we benchmarked the upper 97.5th percentile of serum 25(OH)D concentrations arising from the various vitamin D intake estimates from the present work against serum 25(OH)D concentrations of 150 and 200 nmol/L, as per IOM [2] and EFSA [35], respectively. In addition, the following serum 25(OH)D concentrations achieved in two high-dose vitamin D supplementation trials in older adults with evidence of increased risk of falls and/or factures [36,37] were used as a guide. Following an annual bolus dose of 12,500 μg vitamin D3 [36], median serum 25(OH)D concentrations of older women were 120 and 90 nmol/L at 1 and 3 months, respectively, which is the time frame in which most falls occurred in the trial. At 1 month, 82% of older women were at serum 25(OH)D concentrations of 100 nmol/L, and 24% at 150 nmol/L or higher [36]. Observational analyses within a study of older men and women provided with monthly bolus doses of vitamin D3 and/or 25(OH)D3 showed that those in the highest quartile of serum 25(OH)D at the 12-month follow-up (range: 112–247 nmol/L) had the greatest odds of falling and the most falls compared with those who reached the lowest quartile (range: 53–76 nmol/L) [37].
RESULTS
Study selection and IPD obtained
Our search identified 242 unique articles. Following screening of titles and abstracts, 23 full-text articles were assessed for eligibility, of which 11 studies [19–25, 27–29, 53] fulfilled the eligibility criteria (Figure 1). IPD were sought and obtained for 10 studies, with a total of 677 randomized participants that fulfilled the eligibility criteria. In the case of one RCT for which IPD was sought [53], the data could not be shared due to a lack of agreeable jurisdiction that both institutions could work with in terms of interpretation of the data transfer agreement. A number of the studies had additional participants that did not meet the eligibility criteria (e.g., were White-skinned (in 6 RCTs), sampled outside the specified winter period (in 4 RCT), were given vitamin D2-containing fortified foods (1 RCT), were given vitamin D3 supplements at a dose above our threshold of 4000 IU/d (1 RCT), or had missing data on either adherence or total vitamin D intake (in 4 RCTs)), and the data on these additional participants were not included in the present analysis.
Study and participant characteristics
Table 1 shows the characteristics of the 10 accepted studies and their participants. The RCTs were conducted in the USA and in 4 countries within Europe. Six studies were conducted in Blacks, 3 in South Asians and 1 in a mixed group of dark-skinned individuals. Six studies were conducted in adults, 3 in children, and 1 in both age-groups. Five studies were conducted in adult females only, the rest were conducted in studies with a mixture of males and females.
Table 1.
Selected design parameters of the 10 vitamin D3 randomized controlled trials, conducted in winter and ≥40°N, as well as baseline and vitamin D-related outcome characteristics of dark-skinned subjects who completed the intervention studies and were included in data analyses*.
| Study | Andersen et al. 2008 | Gallagher et al. 2013 | Gallagher et al. 2014 | Ng et al. 2014 |
|---|---|---|---|---|
| (Reference Number) | [23] | [19] | [20] | [21] |
| Trial registry ID | NR | NCT00472823 | NCT00472823 | NCT00585637 |
| Design parameters: | ||||
| Location (°N) | Denmark (56°N) | USA (41°N) | USA (41°N) | USA (42°N) |
| Year of study | 2007 | 2008–2010 | 2010–2011 | 2007–2010 |
| Duration (and Months) | 12 months | 12 months | 12 months | 3 months (within Oct-March)** |
| Vitamin D3 intervention(s) [supplemental dose] | Vit D3 supplement [10 or 20 μg/d] v. placebo | Vit D3 supplement [10, 20, 40, 60, 80 or 100 μg/d] v. placebo | Vit D3 supplement [10, 20, 40 or 60 μg/d] v. placebo | Vit D3 supplement [25, 50 or 100 μg/d] v. placebo |
| Subject characteristics: | ||||
| Baseline | ||||
| N | 140 | 19 | 27 | 190 |
| Racial/ethnic group | South Asian | Black/African American | Black/African American | Black/African American |
| Sex (Male:Female) | 63:77 | 0:19 | 0:27 | 40:150 |
| Age (year) | 35.3 ± 13.21 | 64.9 ± 6.6 | 35.9 ± 6.9 | 52.6 ± 11.7 |
| Weight (kg) | 70.1 ± 15.5 | 88.2 ± 16.3 | 89.0 ± 21.4 | 90.1 ± 21.2 |
| BMI (kg/m2) | 26.0 ± 4.7 | 34.2 ± 5.3 | 31.2 ± 5.9 | 32.4 ± 7.7 |
| Dietary vitamin D (μg/day)2 | 2.5 ± 2.0a | 3.2 ± 2.4b | 2.1 ± 1.1b | 5.1 ± 4.6a |
| Serum/plasma 25(OH)D (nmol/L)3 | 17.9 ± 18.9a | 33.4 ± 10.9b | 36.3 ± 18.9b | 42.8 ± 21.6b |
| Endpoint | ||||
| Total vitamin D intake (μg/day)4 | 12.4 (11.5, 0.5–29.0) | 51.2 (42.8, 0.9–112.3) | 22.8 (12.0, 0.5–63.2) | 49.2, (47.9, 0.2–124.0) |
| Serum/plasma 25(OH)D (nmol/L)5 | 38.5 (3.8–76.4) | 85.0 (22.5–140.0) | 67.5 (15.0–135.0) | 78.6 (8.5–245.6) |
| % (n) <25 nmol/L | 36.4 (51) | 5.3 (1) | 11.1 (3) | 12.6 (24) |
| % (n) <30 nmol/L | 40.7 (57) | 5.3 (1) | 14.8 (4) | 14.2 (27) |
| % (n) <50 nmol/L | 74.3 (104) | 5.3 (1) | 33.3 (9) | 24.7 (47) |
| Study | Adebayo et al. 2018 | Tripkovic et al. 2017 | Grønborg et al. 2019 |
|---|---|---|---|
| (Reference Number) | [25] | [28] | [29] |
| Trial registry ID | NCT02212223 | ISRCTN23421591 | NCT02631629 |
| Design parameters: | |||
| Location (°N) | Finland (60°N) | UK (51.3°N) | Denmark (56°N) |
| Year of study | 2014–2015 | 2011–2012 and 2012–2013 | 2016 |
| Duration (and Months) | 5 months (within Dec-May) | 12 weeks (within Oct-March) | 3 months (Jan-March) |
| Vitamin D3 intervention(s) [supplemental dose] | Vit D3 supplement [10 or 20 μg/d] v. placebo | Vit D3-fortified Orange Juice [15 μg/d] v. Vit D3-fortified Biscuit [15 μg/d] v. Placebo | Vitamin D-fortified low-fat cheese, yoghurt, eggs and crisp bread (supplying 30 μg/d in total) v. non-fortified equivalents |
| Subject characteristics: | |||
| Baseline | |||
| N | 47 | 26 | 50 |
| Racial/ethnic group | Black/East African | South Asian | South Asian |
| Sex (Male:Female) | 0:47 | 0:26 | 0:50 |
| Age (year) | 41.2 ± 8.0 | 40.3 ± 9.8 | 36.8 ± 8.7 |
| Weight (kg) | 78.2 ± 13.3 | 63.6 ± 9.9 | 69.3 ± 12.9 |
| BMI (kg/m2) | 29.4 ± 4.8 | 24.9 ± 4.1 | 27.6 ± 4.8 |
| Dietary vitamin D (μg/day)2 | 11.3 ± 5.1a | 1.5 ± 1.1c | 1.3 ± 0.8a |
| Serum 25(OH)D (nmol/L)3 | 52.2 ± 14.0c | 25.7 ± 18.6c | 48.6 ± 25.0c |
| Endpoint | |||
| Total vitamin D intake (μg/day)4 | 30.0 (27.5, 9.1–94.2) | 12.1 (15.1, 0.2–19.3) | 11.4 (1.4, 0.3–33.6) |
| Serum/plasma 25(OH)D (nmol/L)5 | 55.5 (26.3–127.1) | 56.1 (10.0–89.6) | 41.6 (13.5–137.0) |
| % (n) <25 nmol/L | 0 (0) | 23.1 (6) | 20.0 (10) |
| % (n) <30 nmol/L | 2.1 (1) | 23.1 (6) | 24.0 (12) |
| % (n) <50 nmol/L | 31.9 (15) | 42.3 (11) | 64.0 (32) |
| Study | Rajakumar et al. 2015 | Sacheck et al. 2017 | Öhlund et al. 2017 |
|---|---|---|---|
| (Reference Number) | [22] | [27] | [24] |
| Trial registry ID | NCT00732758 | NCT01537809 | NCT01741324 |
| Design parameters: | |||
| Location (°N) | USA (40.4 and 40.8°N) | USA (42°N) | Sweden (55 and 63°N) |
| Year of study | 2008–2011 | 2011–2013 | 2012–2013 |
| Duration (and Months) | 6 months (enrolled Oct-March) | 6 months (baseline Oct to Dec) | 3 months (within Nov-March) |
| Vitamin D3 intervention(s) [supplemental dose] | Vit D3 supplement [25 μg/d] v. placebo | Vit D3 supplement [25 or 50 μg/d v. recommended 15 μg/d***] | Vit D-fortified lactose-free, UHT milk [12 or 22 μg/d] v. non-fortified lactose-free, UHT milk |
| Subject characteristics: | |||
| Baseline | |||
| N | 44 | 53 | 81 |
| Racial/ethnic group | Black | Black/African American | Dark-skinned (Multiple) |
| Sex (Male:Female) | 25:19 | 26:27 | 38:43 |
| Age (year) | 12.0 ± 1.9 | 11.6 ± 1.4 | 6.3 ± 0.7 |
| Weight (kg) | 53.9 ± 20.8 | 51.6 ± 16.7 | 23.2 ± 4.8 |
| BMI (kg/m2) | 23.6 ± 6.8 | 22.2 ± 5.2 | 15.8 ± 2.1 |
| Dietary vitamin D (μg/day)2 | 6.3 ± 3.9a | 3.1 ± 2.8a | 5.5 ± 2.5a |
| Serum 25(OH)D (nmol/L)3 | 42.4 ± 18.5c | 44.6 ± 18.1c | 47.4 ± 17.6c |
| Endpoint | |||
| Total vitamin D intake (μg/day)4 | 17.2, (14.9, 1.5–38.0) | 34.7 (28.7, 13.2–60.4) | 19.6 (19.9, 3.3, 33.9) |
| Serum/plasma 25(OH)D (nmol/L)5 | 46.3 (13.9–104.9) | 69.3 (20.3–152.0) | 69.0 (24.0–130.0) |
| % (n) <25 nmol/L | 11.4 (5) | 3.8 (2) | 1.2 (1) |
| % (n) <30 nmol/L | 20.5 (9) | 5.7 (3) | 2.5 (2) |
| % (n) <50 nmol/L | 61.4 (27) | 15.1 (8) | 19.8 (16) |
In some cases, additional dark-skinned participants in the study were not included in the analyses as they did not meet with the inclusion criteria (total n = 753).
Not study endpoint but sampling point which fit with sampling months as specified by inclusion criteria.
Study did not include a placebo group for ethical reasons, instead they used a standard care dose of 15 μg/d.
Mean ± SD (all such values).
Habitual dietary vitamin D intake assessed via semi-quantitative FFQa, 7-day food diariesb, or 4-day food/diet diariesb.
Serum 25(OH)D measured by HPLCa, RIAb, or LC-MS/MSc.
Mean (median, min-max) (all such values)
Median (min-max) (all such values)
BMI, body mass index; 25(OH)D, 25-hydroxyvitamin D; NR, not registered.
Mean baseline serum/plasma 25(OH)D concentrations among the 10 RCTs ranged from 17.9 to 52.2 nmol/L, and were between 17–30 nmol/L in 2 RCTs, 30–39.9 nmol/L in 2 RCTs, 40–49.9 nmol/L in 5 RCTs, and 50–59.9 nmol/L in 1 RCT, respectively (Table 1). The 10 RCTs were conducted in latitudes of 40° N or higher, locations of 5 sites were within 40 to 49.9°N and another 5 sites were within 50 to 63°N, respectively. Assays for measuring serum 25(OH)D varied widely amongst the studies and included radioimmunoassay in 3 RCTs, liquid chromatography tandem mass spectrophotometry in 6 RCTs, and high-performance liquid chromatography in 1 RCT, respectively (Table 1).
The daily vitamin D interventions were either vitamin D3 supplementation in 7 RCTs or vitamin D3-fortified food(s) in 3 RCTs. Of the three studies that used vitamin D-fortified food(s), 1 RCT each used milk; orange juice or biscuits; or a combination of 4 foods (vitamin D-fortified low-fat cheese, yoghurt, eggs and crisp bread) (Table 1). The daily dose of vitamin D provided by consumption of the assigned study vitamin D supplement or amount/serving size of the vitamin D-fortified food(s) ranged from 10 to 100 μg/d: 4 studies used ~10–20 μg/d, 1 study each used 25 or 30 μg/d, and 1 study each used 10–60, 15–50, 10–60, 10–100 μg/d (Table 1); one RCT [19] had an additional group which received 120 μg/d, but these subjects were excluded as this dose was above our upper threshold. Study duration ranged from 2 to 12 months. A range of dietary instruments was used to assess vitamin D intake, including 4-day diet diary (1 study), 7-day food diaries (2 studies) and semi-quantitative FFQ (7 studies; with 5 reporting their FFQ as validated for habitual vitamin D intake).
Study quality of included RCTs
All 10 studies achieved a Jadad score of ≥3 (10% and 80% with scores of 4 and 5, respectively). In terms of contributing to these scores, method of randomization was reported in nine studies. One study was reported as blinded, but the method for blinding was unclear based on the information presented within the paper. All 10 studies reported data on dropouts. Participant dropout ranged from 0–45.2% within a study arm and 12 study arms out of a total of 39 had a dropout rate of >20%. It should be noted that the Jadad scale does not assess compliance, which is an important factor in vitamin D intervention studies. Compliance rates were reported in all 10 studies (range of means: 73–96%, with 7 RCTs >85%).
Risk of bias within studies
The summary assessments of risk of bias across domains and across the 10 RCTs are shown in Supplemental Table 2 in ‘Online Resource‘. All 10 RCTs had either a low or unclear risk of selection bias (low risk of random sequence generation and allocation concealment for 9 and 8 RCTs, respectively, and the remainder had unclear risk). In relation to performance and detection bias, a majority of RCTs (n=6–10) had low risk of bias for blinding of participants, personnel and of outcome assessment, with 2–4 RCTs having unclear risk in these domains. In relation to attrition bias, risk of bias in relation to incomplete outcome data was low for 6 RCTs and unclear for 4 RCTs. Risk of bias for selective reporting was low in all 10 RCTs. Overall, most of the information used in the present meta-regression analysis is from studies at low, or to a lesser extent, unclear risk of bias.
The IPD meta-analysis model and vitamin D DRV estimates based on the 1-stage IPD meta-analyses, including sensitivity and subgroup analyses
Vitamin D intake estimates using the 50 nmol/L serum 25(OH)D threshold
The vitamin D intake estimate allowing 90%, 95% and 97.5% of dark-skinned individuals (adults and children) at latitudes ≥40°N to maintain serum 25(OH)D ≥50 nmol/L (the threshold of adequacy as selected by IOM [2], Nordic Council of Ministers’ Nordic Nutrition Recommendations (NNR) [5] and EFSA [4]), assuming minimal UVB exposure, was 51.6, 61.0 and 69.1 μg/d, respectively, using the unadjusted model (n=677) (Table 2, and see Figure 2A). Adjusting for baseline 25(OH)D, age and BMI (n=675), the vitamin D intake estimate allowing 90%, 95% and 97.5% of dark-skinned individuals to maintain serum 25(OH)D ≥50 nmol/L was 50.2, 59.1 and 66.8 μg/d, respectively (Table 2 and see Figure 2B).
Table 2.
Individual Participant Data (IPD) meta-analysis-derived vitamin D intake estimates (μg/day) to maintain stated percentage of the population (percentile) at a serum 25(OH)D level at or above selected concentrations in dark-skinned participants residing between 40–63°N during winter1
| Serum 25(OH)D | 50th Percentile2 | 90th Percentile | 95th Percentile | 97.5th Percentile3 |
|---|---|---|---|---|
| No adjustments | ||||
| All participants (n=677) | ||||
| ≥25 nmol/L | - | 17.5 (10.2, 23.8) | 26.8 (19.3, 33.8) | 34.9 (27.2, 42.1) |
| ≥30 nmol/L | - | 25.4 (18.1, 31.7) | 34.7 (26.1, 41.6) | 42.8 (35.2, 49.7) |
| ≥50 nmol/L | 18.8 (12.6, 25.1) | 51.6 (44.5, 58.6) | 61.0 (53.1, 68.2) | 69.1 (60.2, 77.1) |
| Adjusted model | ||||
| All participants (n=675) | ||||
| ≥25 nmol/L | - | 14.3 (7.8, 20.0) | 23.1 (16.1, 29.0) | 30.8 (23.5, 37.1) |
| ≥30 nmol/L | - | 22.5 (16.1, 28.8) | 31.4 (24.1, 37.5) | 39.0 (31.4, 45.5) |
| ≥50 nmol/L | 19.0 (13.4, 24.6) | 50.2 (43.7, 56.4) | 59.1 (52.1, 65.7) | 66.8 (58.6, 73.8) |
Results based on a 1-stage IPD quadratic model which related serum 25(OH)D concentration as a function of vitamin D3 intake both unadjusted and adjusted for baseline serum 25(OH)D (mean), age (mean) and BMI (mean). 95% CIs for the lower prediction limits were obtained using bias-corrected bootstrap based on 1000 replications.
The vitamin D intake that will maintain serum 25(OH)D concentrations in 50% of individuals above 50 nmol/L during winter, representing an EAR at that threshold; this is not appropriate to do at the 25 and 30 nmol/L thresholds.
The vitamin D3 intake that will maintain serum 25(OH)D concentrations in 97.5% of individuals above the indicated cut-off concentration during winter, representing a Recommended dietary allowance (RDA).
Figure 2.
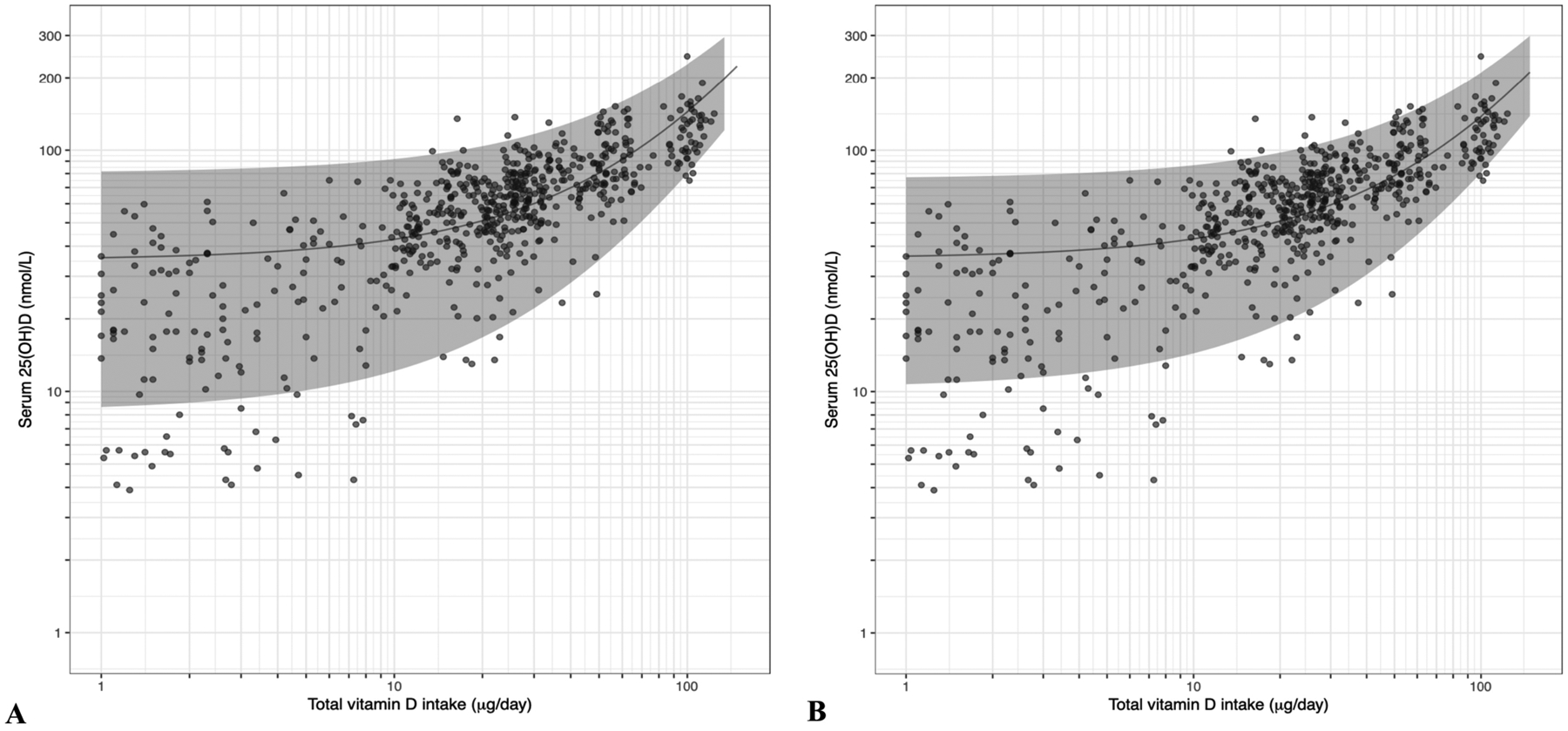
The relation between serum 25-hydroxyvitamin D (25(OH)D) concentrations (in extended winter) and total vitamin D intake in healthy dark-skinned individuals aged 5–86 years living between 40°N and 63°N based on individual participant data (IPD). The solid central diagonal lines correspond to the fitted regression lines based on one-stage IPD meta-analysis (unadjusted model (n=677 individuals) (Panel A) and model adjusted for age, BMI and baseline 25(OH)D (n=675 individuals) (Panel B)) and the corresponding 95% prediction bands are shown in gray. Note: the fitted curve and the 95% confidence band are displayed using logarithmic axes. Overlapping dots make some appear more darkly coloured.
Vitamin D intake estimates using the 25 and 30 nmol/L serum 25(OH)D thresholds
Using the UK’s Scientific Advisory Committee on Nutrition (SACN) 25(OH)D cut-off of ≥25 nmol/L [3], we estimated the vitamin D intake needed to maintain 90%, 95% and 97.5% of dark-skinned individuals (adults and children) above this threshold to be 17.5, 26.8 and 34.9 μg/d, respectively, based on the unadjusted model (n=677); this decreased to 14.3, 23.1 and 30.8 μg/d, respectively, with adjustment for covariates (n=675) (Table 2). The IOM, NNR and EFSA used serum 25(OH)D <30 nmol/L to indicate an increased risk of vitamin D deficiency [2,4,5], but they did not derive a vitamin D intake for this threshold. We estimated a vitamin D intake of 25.4, 34.7 and 42.8 μg/d to maintain 90%, 95% and 97.5% of individuals ≥30 nmol/L, respectively, using the unadjusted model (n=677), and 22.5, 31.4, and 39.0 μg/d, respectively, using the adjusted model (n=675). As the increases in vitamin D intake estimates moving from the 90th to the 97.5th percentile of requirements were large at these lower thresholds, it is important to also emphasize intake estimates at the 90th and 95th percentiles.
Subgroup analysis based on ethnic grouping
The estimated vitamin D intakes needed to maintain 90%, 95% and 97.5% of South Asian and Black individuals at the 25 and 30 nmol/L serum 25(OH)D thresholds are shown in Table 3. We estimated a vitamin D intake of 25.7 and 23.9 μg/d to maintain 97.5% of South Asians ≥25 nmol/L, using the unadjusted (n=216) and adjusted models (n=215), respectively. At the 30 nmol/L serum 25(OH)D threshold, the equivalent vitamin D intake estimates were 29.1 and 27.3 μg/d, respectively. In a subgroup analysis in which only Black individuals (children and adults) with a total vitamin D intake <50 μg/d (n=265) were included, we estimated a vitamin D intake of 21.3 and 27.2 μg/d was needed to maintain 97.5% of individuals ≥25 and 30 nmol/L, respectively, using the adjusted model (see Supplemental Table 3 in ‘Online Resource‘). While presented for Black individuals (Table 3), the estimated vitamin D intakes needed to maintain the higher percentiles of South Asian individuals with the serum 25(OH)D ≥50 nmol/L were not reported as their total vitamin D intakes were below such estimates (maximum intake 33.6 μg/d), and thus, the estimates would be extrapolations.
Table 3.
Individual Participant Data (IPD) meta-analysis-derived vitamin D intake estimates (μg/day) to maintain stated percentage of the population (percentile) at a serum 25(OH)D level at or above selected concentrations in South Asian (A) and Black participants (B) during winter1
| A. South Asian | ||||
|---|---|---|---|---|
| Serum 25(OH)D | 50th Percentile2 | 90th Percentile | 95th Percentile | 97.5th Percentile3 |
| No adjustments | ||||
| All participants (n=216) | ||||
| ≥25 nmol/L | - | 17.9 (10.4, 25.4) | 22.1 (14.6, 31.0) | 25.7 (18.4, 35.7) |
| ≥30 nmol/L | - | 21.2 (14.0, 29.3) | 25.5 (17.6, 34.8) | 29.1 (21.3, 39.1) |
| Adjusted model | ||||
| All participants (n=215) | ||||
| ≥25 nmol/L | - | 17.0 (12.0, 22.7) | 20.7 (15.3, 27.0) | 23.9 (18.4, 31.2) |
| ≥30 nmol/L | - | 20.4 (15.3, 26.8) | 24.1 (18.8, 30.0) | 27.3 (21.8, 34.7) |
| B. Black | ||||
| Serum 25(OH)D | 50th Percentile2 | 90th Percentile | 95th Percentile | 97.5th Percentile3 |
| No adjustments | ||||
| All participants (n=430) | ||||
| ≥25 nmol/L | - | 10.7 (3.3, 18.5) | 20.3 (12.0, 28.8) | 28.7 (20.6, 37.3) |
| ≥30 nmol/L | - | 19.4 (12.4, 26.8) | 29.0 (21.5, 37.8) | 37.3 (29.4, 46.3) |
| ≥50 nmol/L | 14.4 (7.4, 21.3) | 48.3 (41.1, 56.3) | 58.0 (50.0, 66.4) | 66.4 (58.2, 76.5) |
| Adjusted model | ||||
| All participants (n=429) | ||||
| ≥25 nmol/L | - | 6.4 (0.5, 16.0) | 15.9 (6.0, 25.4) | 24.1 (13.6, 35.6) |
| ≥30 nmol/L | - | 15.5 (5.8, 25.5) | 25.0 (15.1, 35.4) | 33.2 (23.7, 44.2) |
| ≥50 nmol/L | 12.6 (3.9, 20.6) | 46.0 (36.6, 55.5) | 55.5 (45.7, 65.6) | 63.8 (53.2, 75.0) |
Results based on a 1-stage IPD quadratic model which related serum 25(OH)D concentration as a function of vitamin D3 intake both unadjusted and adjusted for baseline serum 25(OH)D (mean), age (mean) and BMI (mean). 95% CIs for the lower prediction limits were obtained using bias-corrected bootstrap based on 1000 replications.
The vitamin D intake that will maintain serum 25(OH)D concentrations in 50% of individuals above 50 nmol/L during winter, representing an EAR at that threshold; this is not appropriate to do at the 25 and 30 nmol/L thresholds.
The vitamin D3 intake that will maintain serum 25(OH)D concentrations in 97.5% of individuals above the indicated cut-off concentration during winter, representing a Recommended dietary allowance (RDA).
Additional subgroup and sensitivity analyses
The outcomes of additional subgroup and sensitivity analyses, set at each of the three serum 25(OH)D thresholds, are provided in the Supplemental Material in ‘Online Resource‘. In brief, leave-one-out sensitivity analysis showed that there were no overly influential RCTs (Supplemental Table 4 in ‘Online Resource‘). Using adult RCT data only, limiting the unadjusted analysis to those with a BMI <30 kg/m2 (n=265) reduced the 97.5th percentile vitamin D estimates by 4.3 to 8.0 μg/d, dependent on serum 25(OH)D threshold, compared to those with BMI ≥30 kg/m2 (n=213) (Supplemental Table 5 in ‘Online Resource‘). Using data from Black individuals only, vitamin D intake estimates at the 97.5th percentile were very similar irrespective of whether based on subjects whose total vitamin D intake estimates accounting for adherence to vitamin D supplements/fortified foods or where a minimum of 80% compliance was applied for subject inclusion in the analysis per se rather than applying it in the calculation of total intake estimates (Supplemental Table 3 in ‘Online Resource‘). Additional adjustment for methods of serum 25(OH)D and vitamin D intake assessment yielded estimates which were marginally to modestly higher, respectively, than that from analysis of all individuals irrespective of method (see Supplemental Material in ‘Online Resource‘).
Safety considerations of the IPD-derived dietary vitamin requirement estimates
Using the age-stratified dietary vitamin D requirement estimates from the unadjusted and adjusted models (Table 4), none of the estimates for children or adults at the 25 or 30 nmol/L serum 25(OH)D threshold exceeded the minimum age-specific UL for vitamin D (50 and 100 μg/d for children or adults, respectively [34]). At the 50 nmol/L serum 25(OH)D threshold, none of the estimates for adults or those covering 90 or 95% in children exceeded the UL. The intake estimates covering 97.5% of children at the 50 nmol/L serum 25(OH)D threshold were at or marginally exceeded the UL (50 and 56 μg/d from the adjusted and unadjusted models, respectively).
Table 4.
Estimates of dietary requirement for vitamin D (μg/day) for all and by age grouping, using different serum 25(OH)D thresholds and percentiles, and projected upper 97.5th percentile serum 25(OH)D concentrations achieved at these intake estimates for all participants
| Requirement percentile (at specified threshold) | Vitamin D intake requirement (μg/d) | Projected upper 97.5th percentile serum 25(OH)D concentrations (nmol/L)1 | ||
|---|---|---|---|---|
| Unadjusted model | Children | Adults | All | |
| ≥25 nmol/L: | ||||
| 90th Percentile | 18.4 | 21.8 | 17.5 | 100.8 |
| 95th Percentile | 25.0 | 31.8 | 26.8 | 112.5 |
| 97.5th Percentile | 30.8 | 40.5 | 34.9 | 123.2 |
| ≥30 nmol/L: | ||||
| 90th Percentile | 24.1 | 29.9 | 25.4 | 110.7 |
| 95th Percentile | 30.7 | 39.9 | 34.7 | 122.9 |
| 97.5th Percentile | 36.5 | 48.6 | 42.8 | 134.1 |
| ≥50 nmol/L: | ||||
| 90th Percentile | 43.0 | 56.9 | 51.6 | 146.8 |
| 95th Percentile | 49.8 | 67.0 | 61.0 | 161.0 |
| 97.5th Percentile | 55.7 | 75.7 | 69.1 | 173.9 |
| Adjusted model 2 | ||||
| ≥25 nmol/L: | ||||
| 90th Percentile | 10.2 | 19.0 | 14.3 | 91.2 |
| 95th Percentile | 16.2 | 28.7 | 23.1 | 101.1 |
| 97.5th Percentile | 21.5 | 37.1 | 30.8 | 110.3 |
| ≥30 nmol/L: | ||||
| 90th Percentile | 16.7 | 27.4 | 22.5 | 100.4 |
| 95th Percentile | 22.7 | 37.1 | 31.4 | 111.0 |
| 97.5th Percentile | 28.0 | 45.6 | 39.0 | 120.4 |
| ≥50 nmol/L: | ||||
| 90th Percentile | 38.4 | 55.7 | 50.2 | 135.0 |
| 95th Percentile | 44.6 | 65.5 | 59.1 | 147.3 |
| 97.5th Percentile | 50.1 | 74.0 | 66.8 | 158.3 |
Projected upper 97.5th percentile serum 25(OH)D results based on the 1-stage IPD quadratic unadjusted and adjusted models for ‘All’ participants.
Adjusted for baseline serum 25(OH)D (mean), age (mean) and BMI (mean).
The predicted upper 97.5th percentile serum 25(OH)D concentrations achieved at vitamin D intake estimates for all participants, derived from the unadjusted and adjusted models, are shown in Table 4. None of the vitamin D intake estimates at the 25 or 30 nmol/L serum 25(OH)D threshold, from either the unadjusted or adjusted model, and whether covering 90, 95 or 97.5% of individuals, led to predicted upper 97.5th percentiles of serum 25(OH)D concentrations exceeding 150 or 200 nmol/L. At the 50 nmol/L threshold, while the vitamin D intake estimates covering 90% (either model) or 95% (adjusted model) of individuals predicted upper 97.5th percentiles of serum 25(OH)D concentrations less than 150 nmol/L, those intakes covering 95% (unadjusted) and 97.5% (both models) of individuals yielded serum 25(OH)D concentrations in the range (158–174 nmol/L). The vitamin D intakes corresponding to the EAR (50th percentile) at the 50 nmol/L threshold yielded a predicted upper 97.5th percentile serum 25(OH)D concentration of 102.3 and 96.4 nmol/L, based on the unadjusted and adjusted models, respectively (data not shown).
Discussion
Recent data highlighting the increased risk of vitamin D deficiency and low vitamin D status among dark-skinned populations resident at high latitudes [7–12, 54], which has been referred to as a latitude-skin colour mismatch [55], has emphasized the need to develop targeted dietary recommendations for vitamin D for people of colour resident at high latitude. The present IPD analyses, based on pooled individual data from 10 winter-based RCTs using vitamin D supplements or fortified foods, provides estimates for vitamin D3 intakes needed to maintain serum 25(OH)D concentrations of dark-skinned individuals above commonly used thresholds for defining risk of vitamin D deficiency (25 and 30 nmol/L) and adequacy (50 nmol/L) [2–5]. We estimated that the vitamin D intake required to maintain 97.5% of serum 25(OH)D concentrations ≥50 nmol/L in dark-skinned children and adults residing at >40°N during extended winter to be 67 μg/d, using the adjusted model.
These estimates are much higher than current recommendations and our previous IPD estimates among White-skinned groups. For example, using data from our recent IPD of White individuals [33] and applying the same model as used in the present work to best capture the curvature in the intake-status relationship, the vitamin D intake required to maintain 97.5% of serum 25(OH)D concentrations ≥50 nmol/L was 1.3- to 1.7-times greater for dark-skinned individuals than that for White individuals (depending on unadjusted or adjusted model estimates and method for accounting for participants’ adherence). While BMI and baseline 25(OH)D were on average higher and lower, respectively, in the dark-skinned compared to the White IPD datasets, the adjusted model included these covariates. Our first IPD of White individuals applied an upper total vitamin D intake threshold of 50 μg/day [32], compared to 100 μg/day used in the present IPD. Limiting the present dataset to include Black individuals with total vitamin D intakes <50 μg/day only, showed that the 97.5th percentile vitamin D intake requirement estimate at the 50 nmol/L serum 25(OH)D threshold, based on an adjusted model, was 46.9 μg/day compared to 26.1 μg/day for White individuals [32].
The present analyses also highlights striking differences between the estimates at the 97.5th (as well as the 90th and 95th) percentiles for dark-skinned individuals at the 50 nmol/L threshold and the recommended 10 μg/d by NNR [5] and 15 μg/d by IOM (for those aged 1–70 y) [2] and EFSA [4]. These relate not only to the fact that these Agencies predominantly based their analyses on data from White participants [2,4,5] available at that time, but also, importantly, to the fact that the standard meta-analysis, as applied by these Agencies, is not able to add the two required standard deviations to the median serum 25(OH)D response to cover the 97.5th percentile of individuals, as information on the between-individual variability is not accessible [18]. The IPD approach is highly relevant and applicable in this regard as between-participant variability is crucial for estimating individual-based DRV, such as the Recommended Dietary Allowance (RDA) and its European equivalents [18]. From a safety perspective, the present IPD suggested that the vitamin D intake required to meet or exceed the 50 nmol/L serum 25(OH)D threshold (i.e., 66.8 μg/d from the adjusted model) yielded an upper 97.5th percentile serum 25(OH)D of 158 nmol/L, below EFSA’s 200 nmol/L threshold [35] but marginally higher than the 125–150 nmol/L, as suggested by IOM [2]. However, older individuals at the very top of the serum 25(OH)D distribution arising from an intake of 66.8 μg/d would be attaining concentrations associated with increased risk of falls and factures in monthly/yearly high-dose vitamin D supplementation trials [36,37]. The intake estimates for adults in the present work did not exceed the age-specific UL for vitamin D intake of 100 μg/day, established in 2011 for the US [2] and 2012 for Europe [34]. A 2016 systematic review and meta-analysis of hypercalcaemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation in adults (n=17,801), showed an increased risk of hypercalcemia in vitamin D supplementation groups within 37 studies [56]. Subgroup analyses showed that the risk was not modified by vitamin D dose (≤ 20 [n=3 studies] or >20 μg/day [n=33 studies]) and meta-regression showed no association between vitamin D dose and risk of hypercalcemia [56]. The EFSA will update their ULs for vitamin D in the next year or so, which will be important in terms of latest guidance on safety of vitamin D intakes. In relation to children, using the current age-specific UL of 50 μg/day [34], the intake estimates at the 90th and 95th percentiles were below, whereas the estimate at the 97.5th percentile (based on the adjusted model) just reached the UL.
We estimated that the vitamin D intakes required to maintain 97.5% of serum 25(OH)D concentrations ≥25 nmol/L [3] and ≥30 nmol/L in dark-skinned children and adults residing at >40°N during extended winter were 31 μg/d and 39 μg/d, respectively, based on the adjusted model. These estimates contain large variation, much of which was attributable to less than 10% of the population (i.e., moving from the 90th to 97.5th percentile) and hence this needs to be considered with caution. These estimates will be impacted to some extent by the relatively high total vitamin D intakes (between 50 and 124 μg/day) in some Black individuals, particularly adults, which can influence the slope of intake-status relationship at the lower serum 25(OH)D thresholds, which has generally been considered to be a more linear fit [42]. Using data from the South Asian participants only, which had total vitamin D intakes in the range ~1–34 μg/d, showed that the vitamin D intakes required to maintain 97.5% of individuals with serum 25(OH)D concentrations ≥25 and ≥30 nmol/L were 24–26 μg/d and 27–29 μg/d, respectively, depending on the model. The variation in estimates for both serum 25(OH)D thresholds on moving from the 90th to 97.5th percentiles in the South Asian participants was considerably less than that observed in the Black participants. Furthermore, when the analysis was restricted to only those Black participants who had total vitamin D intakes <50 μg/d, so as to compare with our initial IPD of White individuals [32], the vitamin D intakes required to maintain 97.5% of serum 25(OH)D concentrations ≥25 and ≥30 nmol/L were 21 μg/d and 27 μg/d, respectively, using the adjusted model. These various estimates were about two-times higher than the equivalent for White participants from our previous IPD [32]. In addition, the estimates at the 25 nmol/L threshold, are higher, and strikingly so, than the recommended 10 μg/d by SACN, which based their analyses on data from White participants [3]. All of the various estimates at the two lower serum 25(OH)D thresholds in the present work were well below the age-specific ULs for vitamin D intake and the upper 97.5th percentile serum 25(OH)D concentration of either 150 or 200 nmol/L.
The present IPD analysis had some limitations. The collection of RCTs was such that the baseline vitamin D status, total vitamin D intake and achieved 25(OH)D concentrations in the South Asian versus Black participants were quite heterogeneous which likely increased the variability in estimates at the lower serum 25(OH)D thresholds. This increased variability would explain the higher estimates for vitamin D intake requirement compared to those reported in some of the individual constituent studies [24,25]. It may be that IPD analyses in each ethnic subgroup separately would yield more reflective estimates of vitamin D intake requirements, although the ethnic group-specific datasets can be limited. There were also likely other potential sources of variability within the analyses. For example, there can be substantial variability associated with laboratory measurement of serum 25(OH)D [57]. The use of standardized serum 25(OH)D data has many merits in overcoming some of this method-related differences in estimates [58], but this is not always feasible, particularly for RCTs. However, sensitivity analyses within the present work only showed a marginal effect of assay on the estimates. The estimates of total vitamin D intake used in the analyses are also subject to measurement errors arising from the variety of different dietary assessment techniques used by the various RCTs, as discussed by us previously [33]. Furthermore, differences in the coverage of vitamin D in foods, especially ethnic foods, within different food compositional databases [17,59] used to estimate vitamin D intake, as well as the vitamin D compounds included, may also have introduced variability into our analysis. Sensitivity analyses showed a moderate effect of dietary assessment technique on the requirement estimates.
There are also a number of considerations in relation to the requirement estimates generated in the present work. DRI are intended for a general healthy population [2]. While reflective of the situation within the general population in many countries, it should be noted that a significant portion of the present sample were either overweight or obese, particularly within the adult subset. However, RCTs used by various agencies in establishing the DRI for vitamin D [2–5] would likewise have included participants who were overweight or obese. The present work provided vitamin D requirement estimates which adjusted for BMI and also presented estimates stratified by BMI < and ≥30 kg/m2. It should also be emphasised again that the estimates from the present IPD of dark-skinned individuals were compared with equivalent IPD estimates from White individuals generated in separate analyses, and thus, no direct comparisons between dark-skinned and White individuals have been made in the present work. Furthermore, the analyses did not have the ability to explore reasons for racial differences in requirements, as beyond baseline 25(OH)D and BMI (which were adjusted for), it did not have data on other parameters. For example, it is possible that beyond differences in baseline 25(OH)D, vitamin D stores within the body differed between White and dark-skinned participants, which could impact requirement estimates. Exploration of this and other possible reasons warrants further investigation. The estimates of vitamin D requirement were linked to serum 25(OH)D thresholds identified by a number of expert agencies internationally [2–5]. These thresholds relate to bone health outcomes and are based predominantly on an assumption that physiological vitamin D requirements between racial groups do not differ. This is not from neglect or a lack of consideration of the issues, but largely due to an absence of data. For example, the IOM acknowledged that available, emerging evidence would suggest that there is perhaps a lower requirement for vitamin D among African Americans in relation to ensuring bone health, at least compared with Whites, however, the notable lack of high quality and convincing evidence limited their ability to act on this possibility [2]. Thus, while the 50 nmol/L threshold to ensure adequacy from a bone health perspective may possibly be higher than needed for dark-skinned individuals, the 25 and 30 nmol/L serum 25(OH)D thresholds are protective against rickets and osteomalacia in all ethnic groups [6]. Finally, the use of serum 25(OH)D targets to establish vitamin D intake recommendations in DRI exercises stems from the fact that available data have not sufficiently explored the relationship between total intake of vitamin D per se and health outcomes and dose-response relationship between vitamin D intake and bone health (or other health outcomes) is lacking [2]. In addition, the use of serum 25(OH)D concentrations to simulate a dose-response relationship for bone health, was under conditions of minimal sun exposure, thereby reducing the confounding introduced by the effect of sun exposure on serum 25(OH)D concentrations. In the present work, while only studies conducted during, or at least incorporating, an extended winter (October through April [4]) were included to try and achieve minimal impact of UVB on the vitamin D intake-25(OH)D dose-response relationship, some production of pre-vitamin D3 in the skin of participants, particularly those at lower latitudes, could not be discounted. However, if this were the case, it would likely lower the dietary requirement estimates.
The strengths of the present work include the application of the IPD approach to the data from 10 vitamin D-supplement-/fortified food-based RCTs in both children and adults which met or exceed the eligibility criteria of IOM and/or EFSA, and which were identified through a systematic review thus increasing the external validity of our findings. Study quality was generally high and the majority of data are recent, with 9 of 10 of trials published in the last 8 years. The majority of studies had a low risk of bias across the 7 categories.
In conclusion, this IPD analyses of vitamin D RCTs, several of which had been published since the various DRV review exercises during 2011–2016, has provided new dark-skinned population-specific vitamin D intake estimates required to maintain serum 25(OH)D above commonly used thresholds. These vitamin D intake estimates are radically different from the equivalent estimates for White individuals, and highlight the urgent need to re-configure the approach for setting RDAs for vitamin D. The present IPD-derived RDA estimate of 67 μg/d in dark-skinned individuals, capturing between-participant variability, at the 50 nmol/L 25(OH)D threshold is much higher than the recommendations from NNR, IOM and EFSA (10–15 μg/d), which used standard meta-regression based on aggregate data from vitamin D supplement RCTs, and of predominantly White individuals [2,4,5]. Likewise, the higher estimated vitamin D intakes needed to maintain 97.5% of individuals with serum 25(OH)D concentrations above 25 nmol/L (as recommended by SACN) and 30 nmol/L is of particular note as these thresholds are of critical importance for musculo-skeletal health in all ethnic groups.
Supplementary Material
Funding:
This work was made possible by funding in part from the following sources in the USA and Europe: NCI R01CA205406 and P50CA127003, Project P Fund, Pharmavite, Grant AG28168 from the National Institute on Aging, the Office of Dietary Supplements, the Department of Defense (W81XWH-07-1-201), National Institutes of Health Grants K23HD052550, R01HL112985, and UL1 RR024153, National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL106160 as well as the European Commission (QLK1-CT-2000-00 623 and grant agreement 613977) and the United Kingdom-based Biotechnology and Biological Sciences Research Council (BBSRC) as part of a BBSRC Diet and Health Research Industry Club grant (BB/I006192/1).
Conflicts of Interest:
The following authors had no conflicts of interest: Kevin D. Cashman, Mairead E. Kiely, Rikke Andersen, Ida M. Grønborg, Laura Tripkovic, Christel Lamberg-Allardt, Folasade A. Adebayo, J. Christopher Gallagher, Lynette M. Smith, Jennifer M. Sacheck, Qiushi Huang, Chen Yuan, Edward L Giovannucci, Kumaravel Rajakumar, Charity G. Patterson, Inger Öhlund, Torbjörn Lind, Pia Karlsland Åkeson14, Christian Ritz3
Inge Tetens received free unconditional vitamin D supplements for research purpose from Verman, Finland.
Susan A. Lanham-New is Research Director of D3Tex Ltd which holds the UK and Gulf Corporation Council (GCC) Patents for the use of UVB material in preventing vitamin D deficiency in women who dress for cultural style. SLN also received a small speaker’s honoraria for presentations at two conferences on Vitamin D organised by Solaris.
Kimmie Ng received non-financial research support from Pharmavite.
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- AI
Adequate Intake
- DRI
Dietary Reference Intake
- DRV
Dietary Reference Values
- EAR
Estimated Average Requirement
- EFSA
the European Food Safety Authority
- FFQ
Food Frequency Questionnaire
- IOM
Institute of Medicine
- IPD
Individual Participant Data
- NNR
the Nordic Council of Ministers’ Nordic Nutrition Recommendations
- RCT
Randomized Controlled Trial
- RDA
Recommended Dietary Allowance
- SACN
the Scientific Advisory Committee on Nutrition
- UL
Tolerable Upper Intake Level
- UVB
Ultraviolet B
- WHO-FAO
World Health Organisation–Food and Agriculture Organization
- SR-MA
Systematic Reviews and Meta-Analyses
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Research Registration: PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42018097260)
Code availability: The R code for fitting linear and nonlinear models is presented in Cashman KD, Ritz C (2019). Individual participant data (IPD)-level meta-analysis of randomised controlled trials among dark-skinned populations to estimate the dietary requirement for vitamin D. Syst Rev 8:128. doi: 10.1186/s13643-019-1032-6.
Ethics standards
Approval by a research ethics committee to conduct this meta-analysis was not required because the aim of this secondary analysis was consistent with the ethical approval received for the individual studies. The current analysis was conducted on anonymized data.
References
- 1.Kiely M, Cashman KD (2015) The ODIN project: Development of food-based approaches for prevention of vitamin D deficiency throughout life. Nutr. Bull 40:235–246. [Google Scholar]
- 2.Institute of Medicine (2011) Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- 3.Scientific Advisory Committee on Nutrition (2016) Report on Vitamin D and Health Published online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf (accessed July 21st, 2016)
- 4.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) (2016) Scientific EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) (2016). Scientific opinion on Dietary Reference Values for vitamin D. EFSA Journal, 14:4547, 145 pp. 10.2903/j.efsa.2016.4547 [DOI] [Google Scholar]
- 5.Nordic Council of Ministers (2014) Nordic Nutrition Recommendations 2012, 5th Edition (NNR5). Vitamin D. 10.6027/Nord2014-002 (accessed April 2020). [DOI] [Google Scholar]
- 6.Munns CF, Shaw N, Kiely M, et al. (2016) Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab 101:394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, Potischman N (2019). Vitamin D status in the United States, 2011–2014. Am J Clin Nutr. 110:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks SPJ, Greene-Finestone L, Whiting S, Fioletov VE, Laffey P, Petronella N (2017). An analysis of factors associated with 25-Hydroxyvitamin D levels in white and non-white Canadians. J AOAC Int 100:1345–1354. [DOI] [PubMed] [Google Scholar]
- 9.Cashman KD, Dowling KG, Škrabáková Z, et al. (2016) Vitamin D deficiency in Europe – Pandemic? Am J Clin Nutr Am J Clin Nutr 103:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cashman KD, Dowling KG, Škrabáková Z, Kiely M, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Koskinen S, Lundqvist A, Sundvall J, Linneberg A, Thuesen B, Husemoen LL, Meyer HE, Holvik K, Grønborg IM, Tetens I, Andersen R (2015) Standardizing serum 25-hydroxyvitamin D data from four Nordic population samples using the Vitamin D Standardization Program protocols: Shedding new light on vitamin D status in Nordic individuals. Scand J Clin Lab Invest. 75:549–61. [DOI] [PubMed] [Google Scholar]
- 11.Hastie CE, Mackay DF, Ho F, et al. (2020) Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 14:561–565. doi: 10.1016/j.dsx.2020.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darling AL, Blackbourn DJ, Ahmadi KR, Lanham-New SA (2020) Very High Prevalence of 25-hydroxyvitamin D Deficiency in n 6433 UK South Asian adults: analysis of the UK Biobank Cohort [published online ahead of print, 2020 Jul 22]. Br J Nutr. 1–34. doi: 10.1017/S0007114520002779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adebayo FA, Itkonen ST, Lilja E, Jääskeläinen T, Lundqvist A, Laatikainen T, Koponen P, Cashman KD, Erkkola M, Lamberg-Allardt C (2020) Prevalence and determinants of vitamin D deficiency and insufficiency among three immigrant groups in Finland: evidence from a population-based study using standardised 25-hydroxyvitamin D data. Public Health Nutr. 23:1254–1265. doi: 10.1017/S1368980019004312. Epub 2020 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Åkeson PK, Lind T, Hernell O, Silfverdal SA, Öhlund I (2016) Serum vitamin D depends less on latitude than on skin color and dietary intake during early winter in Northern Europe. J Pediatr Gastroenterol Nutr. 62:643–9. doi: 10.1097/MPG.0000000000001028. [DOI] [PubMed] [Google Scholar]
- 15.Cashman KD (2015) Vitamin D: dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J Steroid Biochem Mol Biol. 148:19–26. [DOI] [PubMed] [Google Scholar]
- 16.Cashman KD, Kiely M (2014) Recommended dietary intakes for vitamin D: Where do they come from, what do they achieve and how can we meet them? J Hum Nutr Diet 27:434–42. [DOI] [PubMed] [Google Scholar]
- 17.Hayes A, Cashman KD (2017) Food-based solutions for vitamin D deficiency: putting policy into practice and the key role for research. Proc Nutr Soc 76:54–63. [DOI] [PubMed] [Google Scholar]
- 18.Cashman KD (2018) Vitamin D requirements for the future - lessons learned and charting a path forward. Nutrients 10. pii: E533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher JC, Peacock M, Yalamanchili V, Smith LM (2013) Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab 98:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher JC, Jindal PS, Smith LM (2014) Vitamin D supplementation in young white and African American women. J Bone Miner Res 29:173–81. [DOI] [PubMed] [Google Scholar]
- 21.Ng K, Scott JB, Drake BF, Chan AT, Hollis BW, Chandler PD, Bennett GG, Giovannucci EL, Gonzalez-Suarez E, Meyerhardt JA, Emmons KM, Fuchs CS (2014) Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr 99:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajakumar K, Moore CG,Yabes J, Olabopo F, Haralam MA, Comer D, Bogusz J, Nucci A, Sereika S, Dunbar-Jacob J, Holick MF, Greenspan SL (2015) Effect of vitamin D3 supplementation in black and in white children: a randomized, placebo-controlled trial. J Clin Endocrinol Metab 100:3183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen R, Mølgaard C, Skovgaard LT, et al. (2008) Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark: a randomised double-blinded placebo-controlled intervention study. Br J Nutr. 100:197–207. [DOI] [PubMed] [Google Scholar]
- 24.Öhlund I, Lind T, Hernell O, Silfverdal SA, Karlsland Åkeson P (2017) Increased vitamin D intake differentiated according to skin color is needed to meet requirements in young Swedish children during winter: a double-blind randomized clinical trial. Am J Clin Nutr 106:105–112. [DOI] [PubMed] [Google Scholar]
- 25.Adebayo FA, Itkonen ST, Öhman T, et al. (2018) Vitamin D intake, serum 25-hydroxyvitamin D status and response to moderate vitamin D3 supplementation: a randomised controlled trial in East African and Finnish women. Br J Nutr. 119:431–441. doi: 10.1017/S000711451700397X [DOI] [PubMed] [Google Scholar]
- 26.Alzaman NS, Dawson-Hughes B, Nelson J, D’Alessio D, Pittas AG (2016) Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutr 104:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacheck JM, Van Rompay MI, Chomitz VR, Economos CD, Eliasziw M, Goodman E, Gordon CM, Holick MF (2017) Impact of three doses of vitamin D3 on serum 25(OH)D deficiency and insufficiency in at-risk schoolchildren. J Clin Endocrinol Metab. 102:4496–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripkovic L, Wilson LR, Hart K, et al. (2017) Daily supplementation with 15 μg vitamin D2compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: a 12-wk randomized, placebo-controlled food-fortification trial. Am J Clin Nutr. 106:481–490. doi: 10.3945/ajcn.116.138693 [DOI] [PubMed] [Google Scholar]
- 29.Grønborg IM, Tetens I, Andersen EW, et al. (2019) Effect of vitamin D fortified foods on bone markers and muscle strength in women of Pakistani and Danish origin living in Denmark: a randomised controlled trial. Nutr J. 18(1):82. doi: 10.1186/s12937-019-0504-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cashman KD, Ritz C, Adebayo FA, et al. (2019) Differences in the dietary requirement for vitamin D among Caucasian and East African women at Northern latitude. Eur J Nutr. 58:2281–2291. doi: 10.1007/s00394-018-1775-1 [DOI] [PubMed] [Google Scholar]
- 31.Vale CL, Rydzewska LH, Rovers MM, Emberson JR, Gueyffier F, Stewart LA, Cochrane IPD Meta-Analysis Methods Group (2015) Uptake of systematic reviews and meta-analyses based on individual participant data in clinical practice guidelines: Descriptive study. BMJ 350:h1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cashman KD, Ritz C, Kiely M, Odin Collaborators (2017) Improved dietary guidelines for vitamin D: Application of Individual Participant Data (IPD)-level meta-regression analyses. Nutrients 9. pii: E469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cashman KD, Kiely ME, Andersen R, et al. (2021) Individual participant data (IPD)-level meta-analysis of randomised controlled trials with vitamin D-fortified foods to estimate Dietary Reference Values for vitamin D [published online ahead of print, 2020 Jun 15]. Eur J Nutr. 60:939–959 [DOI] [PubMed] [Google Scholar]
- 34.EFSA (European Food Safety Authority) (2012) Scientific opinion on the tolerable upper intake level of vitamin D. EFSA Journal 10:2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) (2018) Scientific opinion on the update of the tolerable upper intake level for vitamin D for infants. EFSA Journal;16:5365, 118 pp. 10.2903/j.efsa.2018.5365 [DOI] [Google Scholar]
- 36.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC (2010). Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 303:1815–22. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A (2016) Monthly high-dose vitamin D treatment for the prevention of functional decline: A randomized clinical trial. JAMA Intern Med. 176:175–83. [DOI] [PubMed] [Google Scholar]
- 38.Cashman KD, Ritz C (2019) Individual participant data (IPD)-level meta-analysis of randomised controlled trials among dark-skinned populations to estimate the dietary requirement for vitamin D. Syst Rev. 8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF, PRISMA-IPD Development Group (2015). Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD Statement. JAMA 313:1657–65. [DOI] [PubMed] [Google Scholar]
- 40.Brouwer-Brolsma EM, Berendsen AAM, Vaes AMM, Dullemeijer C, de Groot LCPGM EJM (2016) Collection and analysis of published scientific information as preparatory work for the setting of Dietary Reference Values for Vitamin D. EFSA supporting publication, EN-766, 171 pp. [Google Scholar]
- 41.Seamans KM, Cashman KD (2009) Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr 89:1997S–2008S. [DOI] [PubMed] [Google Scholar]
- 42.Cashman KD, Fitzgerald AP, Kiely M, Seamans KM (2011) A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br J Nutr 106:1638–48. [DOI] [PubMed] [Google Scholar]
- 43.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:2535. [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart LA, Tierney JF, Clarke M, on behalf of the Cochrane Individual Patient Data Meta-analysis Methods Group (2011) Chapter 18: Reviews of individual patient data. In: Higgins JPT, Green S, editors. Part 3: Special topics. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook-5-1.cochrane.org/chapter_18/18_2_the_collaborative_nature_of_ipd_meta_analyses.htm [Google Scholar]
- 45.Ohmann C, Banzi R, Canham S, et al. (2017) Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open 7:e018647. doi: 10.1136/bmjopen-2017-018647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cashman KD, Hill TR, Lucey AJ, et al. (2008) Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr 88:1535–42. [DOI] [PubMed] [Google Scholar]
- 47.Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, Bonham MP, Taylor N, Duffy EM, Seamans K, et al. (2009) Estimation of the dietary requirement for vitamin D in free-living adults ≥64 y of age. Am J Clin Nutr 89:1366–74. [DOI] [PubMed] [Google Scholar]
- 48.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. (2012) A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 367:481. [DOI] [PubMed] [Google Scholar]
- 49.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12. [DOI] [PubMed] [Google Scholar]
- 50.Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (2011) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Part 2: general methods for Cochrane reviews. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration [Internet]. 2011 [updated 2011 Mar; cited 2015 Dec 18]. Available from: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_-bias_in_included_studies.htm. [Google Scholar]
- 51.Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA (2012) Statistical analysis of individual participant data meta-analyses: A comparison of methods and recommendations for practice. PLoS One 7:e46042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris TP, Fisher DJ, Kenward MG, Carpenter JR (2018) Meta-analysis of Gaussian individual patient data: Two-stage or not two-stage? Stat Med 37:1419–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis RD, Laing EM, Hill Gallant KM, et al. (2013) A randomized trial of vitamin D₃ supplementation in children: dose-response effects on vitamin D metabolites and calcium absorption. J Clin Endocrinol Metab. 98:4816–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Öhlund I, Silfverdal SA, Hernell O, Lind T (2013) Serum 25-hydroxyvitamin D levels in preschool-age children in northern Sweden are inadequate after summer and diminish further during winter. J Pediatr Gastroenterol Nutr. 56:551–5. [DOI] [PubMed] [Google Scholar]
- 55.Ames BN, Grant WB, Willett WC (2021) Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities?. Nutrients. 13(2):499. 499. doi: 10.3390/nu13020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R (2016) Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 104:1039–1051. [DOI] [PubMed] [Google Scholar]
- 57.Carter GD (2011) Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets 12:19–28. [DOI] [PubMed] [Google Scholar]
- 58.Brooks SPJ, Sempos CT (2017) The importance of 25-Hydroxyvitamin D assay standardization and the vitamin D standardization program. J AOAC Int 100:1223–1224. [DOI] [PubMed] [Google Scholar]
- 59.Cashman KD, Kiely M (2011) Towards prevention of vitamin D deficiency and beyond: knowledge gaps and research needs in vitamin D nutrition and public health. Br J Nutr 106:1617–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


