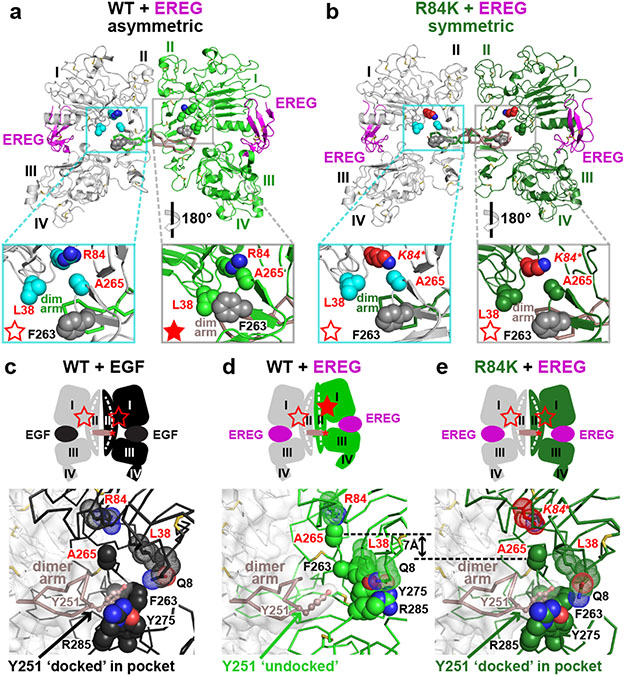
FIGURE 3. R84K GBM mutation symmetrises EREG-induced EGFR dimers.
a. EREG-induced asymmetric sEGFRWT dimer (PDB: 5WB7), with key GBM mutation sites shown as cyan (left) or green (right) spheres. The right-hand insert is rotated 180° about a vertical axis to compare L38/R84/A265 (and F263) positions between molecules. GBM-mutated residues are dispersed in the left-hand molecule (open red star), but clustered in the right (filled star).
b. Symmetric EREG-induced dimer of R84K-mutated sEGFR, with broken autoinhibitory interactions shown in zoomed regions (open red stars).
c.-e. Upper panels: Cartoons of sEGFR dimers, showing symmetry (c and e) or asymmetry (d) and status of autoinhibitory interactions (open or filled red stars). Dashed white curve/line in domain II denotes whether domain II is bent or straight.
Lower panels: Close-up of the Y251 side-chain in the dimer arm of the left-hand molecule docking into its binding site formed by F263, Y275, and R285 from the right-hand molecule in c and e, but remaining undocked in d. The 7 Å shift of A265 (and residues C-terminal to it) between EREG-bound WT (d) and R84K (e) is marked.