Abstract
Pleocatenata, a new genus, is introduced with its type species, Pleocatenatachiangraiensis, which was isolated from withered twigs of two medicinal plants, Clerodendrumquadriloculare (Blanco) Merr (Verbenaceae) and Tarennastellulata (Hook.f.) Ridl (Rubiaceae) in northern Thailand. The genus is characterized by mononematous, septate, brown or dark brown conidiophores, monotretic conidiogenous cells and catenate, obclavate, olivaceous to blackish brown conidia. Phylogenetic analysis of combined LSU, SSU, tef1-α, rpb2 and ITS sequence data showed Pleocatenata forms a distinct phylogenetic lineage in Pleosporales, Dothideomycetes. Therefore, we treat Pleocatenata as Pleosporales genera incertae sedis based on morphology and phylogenetic analyses. Descriptions and illustrations of the new taxa are provided, and it is compared with morphologically similar genera.
Keywords: Genera incertae sedis, hyphomycetes, multi-gene phylogeny, taxonomy
Introduction
Medicinal plants are a rich source of natural products with biological and chemical properties. They are used in health care or treatment of human ailments and have been used since prehistoric times worldwide (Rasool-Hassan 2012). Many fungi have been found on medicinal plants and are members of Dothideomycetes and Sordariomycetes (Bhagat et al. 2012; Long et al. 2019; Ma et al. 2019; Hyde et al. 2020; Tennakoon et al. 2021). They form important associations with medicinal plants and as pathogens or saprobes (Long et al. 2019; Tennakoon et al. 2021), sources of medicines (Strobel et al. 1993; Huang et al. 2008; Hyde et al. 2019), involved in nutrient recycling (Bonnardeaux et al. 2007) and some are used in biological control (Hyde et al. 2019).
Pleosporales is the largest order in Dothideomycetes, which accounts for about a quarter of the class (Zhang et al. 2012; Hyde et al. 2013; Hongsanan et al. 2020a). They have a worldwide distribution with diverse lifestyles, including saprobes, pathogens of plants and humans, endophytes, epiphytes and hyperparasites (Ramesh 2003; Kirk et al. 2008; Zhang et al. 2012; Hyde et al. 2013; Sun et al. 2019; Ferdinandez et al. 2021). Many species in Alternaria Nees, Curvularia Boedijn and Corynespora Güssow, can invade medicinal plants and cause leaf spots and other diseases, as economically important plant pathogens (Mathiyazhagan et al. 2004; Abtahi and Nourani 2017; Zhang et al. 2020), and some also pose a threat to human health (Hyde et al. 2018; Iturrieta-González et al. 2020). Endophytes in Pleosporales also show important biocontrol value (Su et al. 2014; De Silva et al. 2019; Hyde et al. 2019), for example, an extract from Cochliobolusspicifer R.R. Nelson has mosquito-larvicidal activity (Abutaha et al. 2015).
The sexual morph of Pleosporales is characterized by uniloculate ascomata typically with papillae, ostioles and pseudoparaphyses, generally fissitunicate asci bearing mostly septate ascospores of different colours and shapes (Ramesh 2003; Kirk et al. 2008; Zhang et al. 2012; Hyde et al. 2013). Coelomycetes and hyphomycetes are the asexual morphs of pleosporalean taxa (Zhang et al. 2012; Hongsanan et al. 2020a). Recent comprehensive studies on Dothideomycetes treated 91 families in Pleosporales (Hongsanan et al. 2020a). More than 40 genera are recognized as genera incertae sedis in Pleosporales (Hongsanan et al. 2020a; Wijayawardene et al. 2020, 2021). This uncertainty in genetic placement occurs for the following reasons: 1) some genera lack sufficient collections even though molecular data is available, they are not included in any families in phylogenetic analyses, eg. Aegeanispora E.B.G. Jones & Abdel-Wahab, Antealophiotrema A. Hashim. & Kaz. Tanaka and Perthomyces Crous (Li et al. 2016; Abdel-Wahab et al. 2017; Crous et al. 2017); 2) due to the diverse morphology of hyphomycetous asexual morphs, it is difficult to determine their familial placement without the sexual morph and molecular data. Examples are Briansuttonia R.F. Castañeda, Minter & Saikawa, Cheiromoniliophora Tzean & J.L. Chen, Dangeardiella Sacc. & P. Syd and Pleosphaerellula Naumov & Czerepan (Obrist 1959; Tóth 1975; Tzean and Chen 1990; Castañeda-Ruiz et al. 2004).
During the examination of collections from medicinal plants in northern Thailand (Sun et al. 2021), two isolates representing a new species were obtained from Clerodendrumquadriloculare and Tarennastellulata. Morphology and phylogenetic analyses confirmed that it was distinct in Pleosporales, but its familial placement was uncertain. Thus, we introduced a new genus, Pleocatenata (Pleosporales, genera incertae sedis) to accommodate the new species, P.chiangraiensis.
Materials and methods
Collection, examination and isolation
The isolates used in this study were collected from decaying twigs of Clerodendrumquadriloculare and Tarennastellulata from Mae Fah Luang University, Chiang Rai, Thailand during June to July 2020 in terrestrial habitat. The samples were packaged in envelopes and returned to the laboratory as described in Senanayake et al. (2020). The fruiting bodies on natural substrates were observed and photographed using a stereo-microscope (SteREO Discovery, V12, Carl Zeiss Microscopy GmBH, Germany). Morphological characters were observed using a Nikon ECLIPSE Ni compound microscope (Nikon, Japan) and photographed with a Nikon DS-Ri2 digital camera (Nikon, Japan). The Adobe Photoshop CS6 Extended v. 13.0 software was used to make photo-plates. Measurements were done with the Tarosoft (R) Image Frame Work software.
Single spore isolations were used to obtain pure cultures following the methods described by Senanayake et al. (2020). Germinated conidia were transferred to new potato dextrose agar (PDA) plates and incubated at 26 °C for four weeks. The pure cultures obtained were deposited in Mae Fah Luang University Culture Collection (MFLUCC), Chiang Rai, Thailand. Herbaria materials were deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand. Facesoffungi (FoF) and Index Fungorum numbers were acquired as described in Jayasiri et al. (2015) and Index Fungorum (2022).
DNA extraction, PCR amplification and sequencing
Fresh fungal mycelia grown on PDA medium for 4 weeks at 26 °C were scraped with a sterile scalpel. Genomic DNA was extracted from scraped mycelia using the BIOMIGA Fungus Genomic DNA Extraction Kit (GD2416, BIOMIGA, San Diego, California, USA) following the manufacture’s protocol. Five genes were selected in this study: the 28S subunit rDNA (LSU), the 18S subunit rDNA (SSU), the internal transcribed spacers (ITS), the translation elongation factor 1 (tef1-α), and the RNA polymerase II subunit 2 (rpb2). Polymerase chain reaction (PCR) was carried out in 20 μL reaction volume which contained 10 μL 2 × PCR Master Mix, 7 μL ddH2O, 1 μL of each primer, and 1 μL template DNA. The PCR thermal cycle program and primers are given (Table 1). Purification and sequencing of PCR products were carried out at SinoGenoMax (Beijing) Co., China.
Table 1.
Primers and PCR procedures used in this study.
| Locus | Primers | PCR procedures | References | |
|---|---|---|---|---|
| Name | Sequence (5’–3’) | |||
| Large subunit (LSU) | LR0R | ACCCGCTGAACTTAAGC | 94 °C 3 min; 35 cycles of 94 °C 30 s, 52 °C 30 s, 72 °C 1 min; 72 °C 8 min; 4 °C on hold | Vilgalys and Hester (1990), Rehner and Samuels (1994) |
| LR5 | TCCTGAGGGAAACTTCG | |||
| Small subunit (SSU) | NS1 | GTAGTCATATGCTTGTCTC | White et al. (1990) | |
| NS4 | CTTCCGTCAATTCCTTTAAG | |||
| Internal transcribed spacer (ITS) | ITS5 | GGAAGTAAAAGTCGTAACAAGG | ||
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| Elongation factor-1 alpha (tef1-α) | EF1-983F | GCYCCYGGHCAYCGTGAYTTYAT | 94 °C 2 min; 36 cycles of 66 °C – 56 °C (touchdown 9 cycles), 94 °C 30 sec, 56 °C 1 min, 72 °C 1 min; 72 °C 10 min; 4 °C on hold | Rehner and Buckley (2005) |
| EF1-2218R | ATGACACCRACRGCRACRGTYTG | |||
| RNA polymerase II subunit (rpb2) | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | 94 °C 3 min; 40 cycles of 94 °C 20 sec, 55 °C 30 sec, 72 °C 1 min; 72 °C 10 min; 4 °C on hold | Liu et al. 1999 |
| fRPB2-7cR | CCCATRGCTTGYTTRCCCAT | |||
Phylogenetic analyses
BLASTn (https://blast.ncbi.nlm.nih.gov//Blast.cgi) was used to evaluate closely related strains to our new taxa. Other sequences used in this study were obtained from GenBank referring to Zhang et al. (2012, 2018) and Hongsanan et al. (2020a, 2021) (Table 2). The single gene sequences were viewed using BioEdit v. 7.0.9.0 (Hall 1999). Alignments for each locus were generated with MAFFT v.7 (https://mafft.cbrc.jp/alignment/server/) and manually improved using AliView (Larsson 2014) for maximum alignment and minimum gaps. The final single gene alignments were combined by SequenceMatrix 1.7.8 (Vaidya et al. 2011).
Table 2.
Taxa of Pleosporales used in the phylogenetic analysis with the corresponding GenBank accession numbers. The newly generated strains are indicated in bold. N/A: Not available.
| Species names | Strain number | LSU | SSU | ITS | tef1-α | rpb2 |
|---|---|---|---|---|---|---|
| Acrocalymmaaquatica | MFLUCC 11-0208 | JX276952 | JX276953 | JX276951 | N/A | N/A |
| Acrocalymmapterocarpi | MFLUCC 17-0926 | MK347949 | MK347840 | MK347732 | MK360040 | N/A |
| Acuminatisporapalmarum | MFLUCC 18-0264 | MH390437 | MH390401 | NR_163327 | MH399248 | N/A |
| MFLUCC 18-0460 | MH390438 | MH390402 | MN749106 | MH399249 | N/A | |
| Aigialusgrandis | BCC 20000 | GU479775 | GU479739 | N/A | GU479839 | N/A |
| Alternariaalternata | AFTOL ID-1610 | DQ678082 | KC584507 | KF465761 | KC584634 | KC584375 |
| Amniculicolaaquatica | MFLUCC 16-1123 | MK106096 | MK106108 | N/A | MK109800 | N/A |
| Amorocoelophomacassia | MFLUCC 17-2283 | MK347956 | NG_065775 | MK347739 | MK360041 | MK434894 |
| Angustimassarinalonicerae | MFLUCC 15-0087 | KY496724 | N/A | KY496759 | N/A | N/A |
| Anteagloniumparvulum | SMH5223 | GQ221909 | N/A | N/A | GQ221918 | N/A |
| Aquasubmersajaponica | HHUF 30469 | NG_057138 | NG_062426 | NR_154739 | LC194384 | LC194421 |
| Aquasubmersamircensis | MFLUCC 11-0401 | NG_042699 | NG_061141 | JX276954 | N/A | N/A |
| Ascocylindricamarina | MD6011 | KT252905 | KT252907 | N/A | N/A | N/A |
| MF416 | MK007123 | MK007124 | N/A | N/A | N/A | |
| Astragalicolavasilyevae | MFLUCC 17-0832 | MG828986 | MG829098 | NR_157504 | MG829193 | MG829248 |
| Astrosphaeriellafusispora | MFLUCC 10-0555 | KT955462 | KT955443 | N/A | KT955425 | KT955413 |
| Atrocalyxacutisporus | KT 2436 | LC194341 | LC194299 | LC194475 | LC194386 | LC194423 |
| Bahusandhikaindica | GUFCC 18001 | KF460274 | N/A | KF460273 | N/A | N/A |
| Bambusicolabambusae | MFLUCC 11-0614 | JX442035 | JX442039 | JX442031 | N/A | KP761718 |
| Berkleasmiumcrunisia | BCC 17023 | DQ280271 | N/A | DQ280265 | N/A | N/A |
| Berkleasmiumtyphae | BCC 12536 | DQ280275 | N/A | DQ280264 | N/A | N/A |
| Brevicollumhyalosporum | MFLUCC 17-0071 | MG602200 | MG602202 | MG602204 | MG739516 | N/A |
| Brevicollumversicolor | HHUF 30591 | NG_058716 | NG_065124 | NR_156335 | LC271246 | LC271250 |
| Camarosporidiellacaraganicola | MFLUCCC 14-0605 | KP711381 | KP711382 | KP711380 | N/A | N/A |
| Camarosporiumquaternatum | CPC 31081 | NG_064442 | KY929123 | NR_159756 | KY929201 | N/A |
| Camarosporomycesflavigenus | CBS 314.80 | GU238076 | NG_061093 | MH861266 | N/A | N/A |
| Coniothyriumpalmarum | CBS 400.71 | JX681084 | EU754054 | MH860184 | N/A | KT389592 |
| Corynesporacassiicola | CBS 100822 | GU301808 | GU296144 | N/A | GU349052 | GU371742 |
| Corynesporatorulosa | CPC 15989 | KF777207 | N/A | NR_145181 | N/A | N/A |
| Crassiperidiumoctosporum | MAFF 246406 | LC373116 | LC373092 | LC373104 | LC373128 | LC373140 |
| Cryptocoryneumjaponicum | HHUF 30482 | NG_059035 | NG_065118 | NR_153938 | LC096144 | LC194438 |
| Cryptocoryneumpseudorilstonei | CBS 113641 | NG_059036 | LC194322 | NR_153941 | LC096152 | LC194446 |
| Cucurbitariaberberidis | MFLUCC 11-0387 | KC506796 | KC506800 | N/A | N/A | N/A |
| Cyclothyriellarubronotata | CBS 141486 | KX650544 | NG_061252 | NR_147651 | KX650519 | KX650574 |
| Cylindroaseptosporaleucaenicola | MFLUCC 17-2424 | MK347966 | MK347856 | NR_163333 | MK360047 | N/A |
| Dacampiaengeliana | Hafellner 72868 | KT383791 | N/A | N/A | N/A | N/A |
| Dacampiahookeri | Hafellner 73897 | KT383792 | N/A | N/A | N/A | N/A |
| Delitschiachaetomioides | SMH 3253.2 | GU390656 | N/A | N/A | GU327753 | N/A |
| Delitschiawinteri | AFTOL ID-1599 | DQ678077 | DQ678026 | N/A | DQ677922 | DQ677975 |
| Dendryphionfluminicola | MFLUCC 17-1689 | MG208141 | N/A | NR_157490 | MG207992 | N/A |
| Dictyocheirosporabannica | KH 332 | AB807513 | AB797223 | LC014543 | AB808489 | N/A |
| Dictyosporiumelegans | NBRC 32502 | DQ018100 | DQ018079 | DQ018087 | N/A | N/A |
| Didymellaexigua | CBS 183.55 | MH868977 | GU296147 | MH857436 | N/A | N/A |
| Didymellarumicicola | CBS 683.79 | MH873007 | N/A | KT389503 | N/A | KT389622 |
| Didymosphaeriarubi-ulmifolii | MFLUCC 14-0023 | KJ436586 | KJ436588 | MK646049 | N/A | N/A |
| Dimorphosporicolatragani | CBS 570.85 | KU728536 | N/A | KU728497 | N/A | N/A |
| Dothidotthiaaceris | MFLUCC 16-1183 | MK751816 | MK751761 | MK751726 | N/A | N/A |
| Fissuromacalami | MFLUCC 13-0836 | MF588993 | NG_062430 | N/A | MF588975 | N/A |
| Flammeascomabambusae | MFLU 11-0143 | NG_059553 | KP753952 | NR_132915 | N/A | N/A |
| Flavomycesfulophazii | CBS 135761 | NG_058131 | NG_061191 | NR_137960 | N/A | N/A |
| Foliophomafallens | CBS 161.78 | GU238074 | GU238215 | KY940772 | N/A | KC584502 |
| CBS 284.70 | GU238078 | GU238218 | MH859609 | N/A | N/A | |
| Fuscostagonosporacytisi | MFLUCC 16-0622 | KY770978 | KY770977 | N/A | KY770979 | N/A |
| Fuscostagonosporasasae | HHUF 29106 | AB807548 | AB797258 | AB809636 | AB808524 | N/A |
| Fusculinaeucalypti | CBS 120083 | DQ923531 | N/A | DQ923531 | N/A | N/A |
| Fusculinaeucalyptorum | CBS 145083 | MK047499 | N/A | NR_161140 | N/A | N/A |
| Halojulellaavicenniae | BCC 20173 | GU371822 | GU371830 | N/A | GU371815 | GU371786 |
| Halotthiaposidoniae | BBH 22481 | GU479786 | GU479752 | N/A | N/A | N/A |
| Hazslinszkyomycesaloes | CBS 136437 | KF777198 | N/A | KF777142 | N/A | N/A |
| Helminthosporiumvelutinum | L131 | KY984352 | KY984432 | KY984352 | KY984463 | KY984413 |
| Hermatomycesiriomotensis | HHUF 30518 | LC194367 | LC194325 | LC194483 | LC194394 | LC194449 |
| Hermatomycestectonae | MFLUCC 14-1140 | KU764695 | KU712465 | KU144917 | KU872757 | KU712486 |
| Hypsostromacaimitalense | GKM1165 | GU385180 | N/A | N/A | N/A | N/A |
| Hypsostromasaxicola | SMH5005 | GU385181 | N/A | N/A | N/A | N/A |
| Hysteriumangustatum | CBS 123334 | FJ161207 | N/A | N/A | N/A | N/A |
| Hysterobreviumsmilacis | CBS 114601 | FJ161174 | FJ161135 | N/A | FJ161091 | FJ161114 |
| Latoruacaligans | CBS 576.65 | NG_058180 | N/A | N/A | N/A | N/A |
| Latoruagrootfonteinensis | CBS 369.72 | NG_058181 | N/A | N/A | N/A | N/A |
| Lentimurisporaurniformis | MFLUCC 18-0497 | MH179144 | MH179160 | N/A | MH188055 | N/A |
| Lentitheciumclioninum | HHUF 28199 | NG_059391 | NG_064845 | NR_154137 | AB808515 | N/A |
| Lentitheciumpseudoclioninum | HHUF 29055 | NG_059392 | NG_064847 | AB809633 | AB808521 | N/A |
| Lepidosphaerianicotiae | AFTOL ID-1576 | DQ678067 | N/A | N/A | DQ677910 | DQ677963 |
| Leptosphaeriacichorium | MFLUCC 14-1063 | KT454712 | KT454728 | KT454720 | N/A | N/A |
| Leucaenicolaphraeana | MFLUCC 18-0472 | MK348003 | NG_065784 | MK347785 | MK360060 | MK434867 |
| Libertasomycesmyopori | CPC 27354 | NG_058241 | N/A | KX228281 | N/A | N/A |
| Ligninsphaeriajonesii | MFLUCC 15-0641 | NG_059642 | N/A | N/A | N/A | N/A |
| Lindgomycescigarospora | G619 | KX655804 | KX655805 | KX655794 | N/A | N/A |
| Lindgomycesingoldianus | ATCC 200398 | AB521736 | NG_016531 | NR_119938 | N/A | N/A |
| Longiostiolumtectonae | MFLUCC 12-0562 | KU764700 | N/A | KU712447 | N/A | N/A |
| Longipedicellataaptrootii | MFLU 10-0297 | KU238894 | KU238895 | KU238893 | KU238892 | KU238891 |
| Lophiostomamacrostomum | KT508 | AB619010 | AB618691 | N/A | LC001751 | N/A |
| Lophiotremaeburnoides | KT 1424.1 | LC001707 | LC001706 | LC001709 | LC194403 | LC194458 |
| Macrodiplodiopsisdesmazieri | CBS 140062 | NG_058182 | N/A | NR_132924 | N/A | N/A |
| Massariaanomia | CBS 59178 | GU301839 | GU296169 | N/A | N/A | GU371769 |
| Massariainquinans | M19 | N/A | HQ599444 | HQ599402 | HQ599342 | HQ599460 |
| Melanommajaponicum | MAFF 239634 | NG_060360 | NG_065122 | NR_154215 | LC203367 | LC203395 |
| Melanommapulvispyrius | CBS 124080 | MH874873 | GU456302 | MH863349 | GU456265 | GU456350 |
| Misturatosphaeriaaurantonotata | GKM 1238 | NG_059927 | N/A | N/A | GU327761 | N/A |
| Morosphaeriamuthupetensis | NFCCI4219 | MF614796 | MF614797 | MF614795 | MF614798 | N/A |
| Morosphaeriavelatispora | KH221 | AB807556 | AB797266 | LC014572 | AB808532 | N/A |
| Multiloculariabambusae | MFLUCC 11-0180 | KU693438 | KU693442 | KU693446 | N/A | N/A |
| Murisporagalii | MFLUCC 13-0819 | KT709175 | KT709182 | KT736081 | KT709189 | N/A |
| Neocamarosporiumgoegapense | CPC 23676 | KJ869220 | N/A | KJ869163 | N/A | N/A |
| Neoconiothyriumpersooniae | CBS 143175 | MG386094 | N/A | MG386041 | N/A | N/A |
| Neomassariafabacearum | MFLUCC 16-1875 | KX524145 | NG_061245 | N/A | KX524149 | N/A |
| Neomassariaformosana | NTUCC 17-007 | MH714756 | MH714759 | N/A | MH714762 | MH714765 |
| Neomassarinathailandica | MFLU 11-0144 | NG_059718 | N/A | NR154244 | N/A | N/A |
| MFLUCC 17-1432 | MT214467 | MT214420 | MT214373 | N/A | N/A | |
| Neopaucisporarosaecae | MFLUCC 17-0807 | MG829033 | NG_061293 | MG828924 | MG829217 | N/A |
| Neophaeosphaeriaagaves | CPC 21264 | KF777227 | N/A | KF777174 | N/A | N/A |
| Neophaeosphaeriafilamentosa | CBS 102202 | GQ387577 | GQ387516 | JF740259 | GU349084 | GU371773 |
| Neophaeosphaeriaphragmiticola | KUMCC 16-0216 | MG837009 | NG_065735 | N/A | MG838020 | N/A |
| Neoplatysporoidesaloes | CPC 36068 | MN567619 | N/A | NR_166316 | N/A | N/A |
| Neopyrenochaetacercidis | MFLUCC 18-2089 | MK347932 | MK347823 | MK347718 | N/A | MK434908 |
| Neopyrenochaetopsishominis | UTHSC DI16 238 | LN907381 | N/A | LT592923 | N/A | LT593061 |
| Neoroussoellabambusae | MFLUCC 11-0124 | KJ474839 | N/A | KJ474827 | KJ474848 | KJ474856 |
| Neotestudinarosatii | CBS 690.82 | DQ384107 | DQ384069 | N/A | N/A | N/A |
| Neoyrenochaetaacicola | CBS 812.95 | GQ387602 | GQ387541 | NR_160055 | N/A | LT623271 |
| Nigrogranafuscidula | CBS 141556 | KX650550 | N/A | NR_147653 | KX650525 | N/A |
| Nigrogranamackinnonii | CBS 674.75 | GQ387613 | NG_061081 | NR_132037 | KF407986 | KF015703 |
| Occultibambusabambusae | MFLUCC 13-0855 | KU863112 | N/A | KU940123 | KU940193 | KU940170 |
| Occultibambusajonesii | GZCC 16-0117 | KY628322 | KY628324 | N/A | KY814756 | KY814758 |
| Parabambusicolabambusina | KH 139 | AB807537 | AB797247 | LC014579 | AB808512 | N/A |
| Paradictyoarthriniumaquatica | MFLUCC 16-1116 | NG_064501 | N/A | NR_158861 | N/A | N/A |
| Paradictyoarthriniumdiffractum | MFLUCC 13-0466 | KP744498 | KP753960 | KP744455 | N/A | KX437764 |
| Paralophiostomahysterioides | PUFNI 17617 | MT912850 | MN582762 | MN582758 | N/A | MT926117 |
| Parapyrenochaetaprotearum | CBS 131315 | JQ044453 | N/A | JQ044434 | N/A | LT717683 |
| Periconiadelonicis | MFLUCC 17-2584 | NG_068611 | NG_065770 | N/A | N/A | MK434901 |
| Periconiapseudodigitata | KT 1395 | AB807564 | AB797274 | LC014591 | N/A | N/A |
| Phaeoseptummali | MFLUCC 17-2108 | MK625197 | N/A | MK659580 | MK647990 | MK647991 |
| Phaeoseptumterricola | MFLUCC 10-0102 | MH105779 | MH105780 | MH105778 | MH105781 | MH105782 |
| Phaeosphaeriaoryzae | CBS 110110 | KF251689 | GQ387530 | KF251186 | N/A | KF252193 |
| Phaeosphaeriopsistriseptata | MFLUCC 13-0271 | KJ522479 | KJ522484 | KJ522475 | MG520919 | KJ522485 |
| Plenodomussalvia | MFLUCC 13-0219 | KT454717 | KT454732 | KT454725 | N/A | N/A |
| Pleocatenatiumchiangraiense | MFLUCC 21-0222 | OL986398 | N/A | OL986396 | OM240638 | OM117709 |
| MFLUCC 21-0223 | OL986399 | N/A | OL986397 | OM240637 | OM117708 | |
| Pleohelicoonrichonis | CBS 282.54 | N/A | AY856952 | MH857332 | N/A | N/A |
| Pleomonodictysdescalsii | FMR 12716 | KY853522 | N/A | KY853461 | N/A | N/A |
| Preussiafuniculate | CBS 659.74 | GU301864 | GU296187 | N/A | GU349032 | GU371799 |
| Pseudoastrosphaeriellalongicolla | MFLUCC 11-0171 | KT955476 | N/A | N/A | KT955438 | KT955420 |
| Pseudoastrosphaeriellathailandensis | MFLUCC 11-0144 | KT955478 | KT955457 | N/A | KT955440 | KT955416 |
| Pseudoberkleasmiumchiangmaiense | MFLUCC 17-1809 | MK131260 | N/A | MK131259 | MK131261 | N/A |
| Pseudoberkleasmiumpandanicola | KUMCC 17-0178 | MH260304 | MH260344 | MH275071 | N/A | N/A |
| Pseudocoleodictyosporatectonae | MFLUCC 12-0385 | KU764709 | NG_061232 | NR_154338 | N/A | KU712491 |
| Pseudocoleodictyosporathailandica | MFLUCC 12-0565 | KU764701 | NG_062417 | NR_154337 | N/A | KU712494 |
| Pseudodidymosphaeriaspartii | MFLUCC 13-0273 | KP325436 | KP325438 | KP325434 | N/A | N/A |
| Pseudopyrenochaetalycopersici | FMR 15746 | EU754205 | NG_062728 | NR_103581 | N/A | LT717680 |
| Pseudopyrenochaetaterretris | FMR 15327 | LT623216 | N/A | LT623228 | N/A | LT623287 |
| Pseudotetraploalongissima | HC 4933 | AB524612 | AB524471 | AB524796 | AB524827 | N/A |
| Pseudoxylomyceselegans | KT 2887 | AB807598 | AB797308 | LC014593 | AB808576 | N/A |
| Pyrenochaetopsisleptospora | CBS 101635 | GQ387627 | NG_063097 | JF740262 | MF795881 | LT623282 |
| Pyrenochaetopsistabarestanensis | IBRC M 30051 | KF803343 | NG_065034 | NR_155636 | N/A | N/A |
| Quadricrurabicornis | yone 153 | AB524613 | AB524472 | AB524797 | AB524828 | N/A |
| Quercicolafusiformis | MFLUCC 18-0479 | MK348009 | MK347898 | MK347790 | MK360085 | MK434864 |
| Quercicolaguttulospora | MFLUCC 18-0481 | MK348010 | MK347899 | MK347791 | MK360086 | N/A |
| Quixadomycescearensis | HUEFS 238438 | MG970695 | N/A | NR_160606 | N/A | N/A |
| Roussoellanitidula | MFLUCC 11-0634 | KJ474842 | N/A | KJ474834 | KJ474851 | KJ474858 |
| Salsugineaphoenicis | MFLU 19-0015 | MK405280 | N/A | N/A | MK404650 | N/A |
| Salsuginearamicola | KT 2597.2 | GU479801 | GU479768 | N/A | GU479862 | GU479834 |
| Seltsamiaulmi | CBS 143002 | MF795794 | MF795794 | MF795794 | MF795882 | MF795836 |
| Shiraiabambusicola | GZAAS2.629 | KC460980 | N/A | GQ845415 | N/A | N/A |
| Splanchnonemaplatani | CBS 222.37 | KR909316 | KR909318 | MH855895 | KR909319 | KR909322 |
| Sporormiafimetaria | UPS Dissing Gr.81.194 | GQ203729 | N/A | GQ203769 | N/A | N/A |
| Sporormiellaisomera | CBS 166.73 | MH872355 | N/A | AY943053 | N/A | N/A |
| Stemphyliumherbarum | CBS 191.86 | GU238160 | GU238232 | NR_111243 | KC584731 | DQ247794 |
| Striatiguttulanypae | MFLUCC 18-0265 | MK035992 | MK035977 | MK035969 | MK034432 | MK034440 |
| Striatiguttulaphoenicis | MFLUCC 18-0266 | MK035995 | MK035980 | MK035972 | MK034435 | MK034442 |
| Sublophiostomathailandica | MFLUCC 11-0185 | KX534216 | KX534222 | MW136275 | KX550080 | MW088718 |
| MFLUCC 11-0207 | KX534212 | KX534218 | MW136257 | KX550077 | MW088714 | |
| Subplenodomusviolicola | CBS 306.68 | MH870849 | GU238231 | MH859138 | N/A | N/A |
| Sulcatisporaacerina | KT 2982 | LC014610 | LC014605 | LC014597 | LC014615 | N/A |
| Sulcatisporaberchemiae | KT 1607 | AB807534 | AB797244 | AB809635 | AB808509 | N/A |
| Sulcosporiumthailandica | MFLUCC 12-0004 | KT426563 | KT426564 | MG520958 | N/A | N/A |
| Teichosporatrabicola | C134 | KU601591 | N/A | KU601591 | KU601601 | KU601600 |
| Tetraplosphaeriasasicola | KT 563 | AB524631 | AB524490 | AB524807 | AB524838 | N/A |
| Thyridariaacaciae | CBS 138873 | NG_058127 | N/A | KP004469 | N/A | N/A |
| Thyridariabroussonetiae | TB1 | KX650568 | KX650515 | KX650568 | KX650539 | KX650586 |
| Torulaaquatica | MFLUCC 16-1115 | MG208146 | N/A | MG208167 | N/A | MG207977 |
| Torulapluriseptata | MFLUCC 14-0437 | KY197855 | KY197862 | MN061338 | KY197875 | KY197869 |
| Tremateiaarundicola | MFLU 16-1275 | KX274248 | KX274254 | KX274241 | KX284706 | N/A |
| Trematosphaeriagrisea | CBS 332.50 | NG_057979 | NG_062930 | NR_132039 | KF015698 | KF015720 |
| Trematosphaeriapertusa | CBS 122368 | NG_057809 | FJ201991 | NR_132040 | KF015701 | FJ795476 |
| Tzeananiataiwanensis | NTUCC 17-006 | MH461121 | MH461127 | MH461124 | MH461131 | N/A |
| Wicklowiaaquatica | CBS 125634 | MH875044 | NG_061099 | N/A | N/A | N/A |
| Wicklowiasubmersa | MFLUCC 18-0373 | MK637644 | MK637643 | N/A | N/A | N/A |
| Xenopyrenochaetopsispratorum | CBS 445.81 | GU238136 | NG_062792 | MH861363 | N/A | KT389671 |
The single locus and combined analyses were carried out for maximum likelihood (ML) and Bayesian posterior probability (BYPP). The ML analyses were carried out using IQ-TREE (Nguyen et al. 2015; Trifinopoulos et al. 2016) on the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at, 30 September 2021) under partitioned models. The best-fit substitution models were determined by WIQ-TREE (Chernomor et al. 2016): SYM+I+G4 for LSU and SSU; TIM+F+I+G4 for tef1-α; GTR+F+I+G4 for rpb2; TIM2+F+I+G4 for ITS. Ultrafast bootstrap analysis was implemented with 1,000 replicates (Minh et al. 2013; Hoang et al. 2018).
The BYPP analyses were performed in CIPRES (Miller et al. 2010) with MrBayes on XSEDE 3.2.7a (Ronquist et al. 2012). The best nucleotide substitution model for each data partition was evaluated by MrModeltest 2.2 (Nylander 2004). The substitution model GTR+I+G was decided for LSU, SSU, ITS, tef1-α and rpb2 sequences. The Markov chain Monte Carlo (MCMC) sampling approach was used to calculate posterior probabilities (PP) (Rannala and Yang 1996). Six simultaneous Markov chains were run for 10 million generations and trees were sampled every 1,000th generation. The first 20% of trees, representing the burn-in phase of the analyses, were discarded and the remaining trees were used for calculating posterior probabilities (PP) in the majority rule consensus tree.
Phylogenetic trees were viewed using FigTree v1.4.0 (Rambaut and Drummond 2008) and modified in Microsoft Office PowerPoint 2010 and converted to jpg file using Adobe Photoshop CS6 Extended 10.0 (Adobe Systems, San Jose, CA, USA). The new sequences derived from this study were deposited in GenBank. The final alignment and tree were deposited in TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S29199).
Results
Phylogenetic analyses
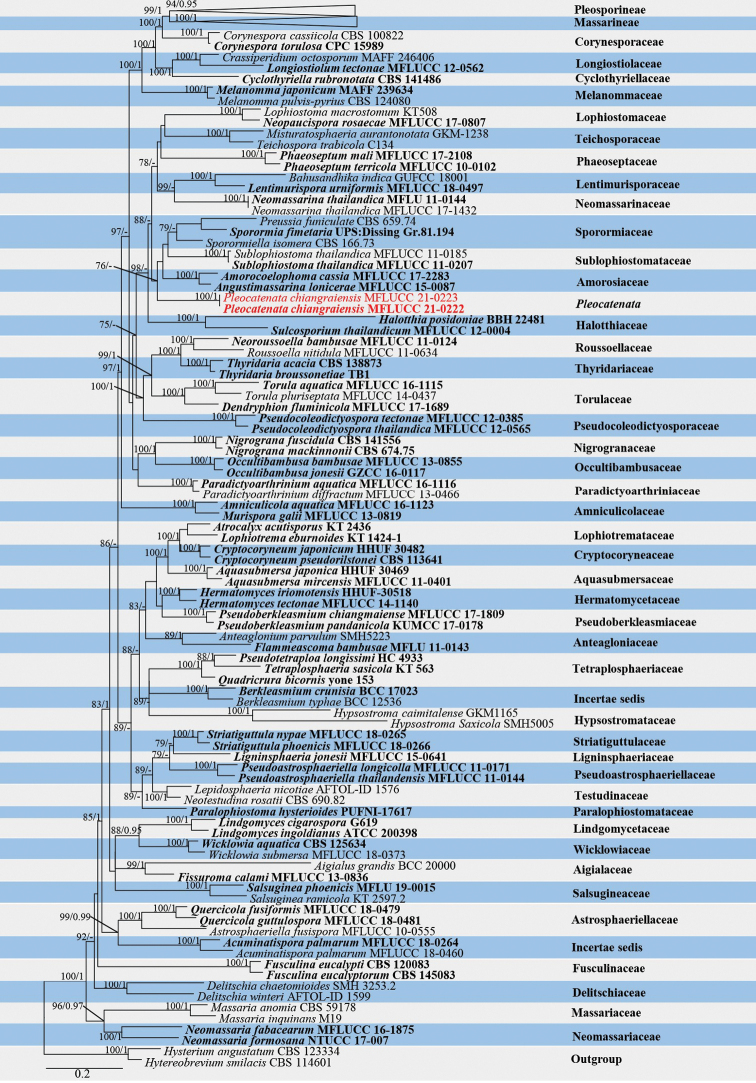
Blast searches of LSU, tef1-α, rpb2 and ITS sequences data in NCBI showed that our sequences were related to Acrocalymmaceae, Amorosiaceae, Sporormiaceae and Sublophiostomataceae. One hundred and seventy-six taxa, representing all families in Pleosporales, with Hysteriumangustatum Alb. & Schwein (CBS 123334) and Hysterobreviumsmilacis (Schwein.) E. Boehm & C.L. Schoch (CBS 114601) as the outgroups, were selected for the analyses. The final combined dataset consisted of 4,953 characters (LSU: 1–850 bp, SSU: 851–1,851 bp, tef1-α: 1,852–2,720 bp, rpb2: 2,721–3,701 bp, ITS: 3,702–4,953 bp), including alignment gaps. Among them, 2,336 characters were constant, 608 variable characters were parsimony-uninformative, and 2,009 characters were parsimony informative. The most likely tree (-ln = 98,965.704) is presented (Figure. 1) to show the phylogenetic placement of the newly introduced genus and its relationship with other members in Pleosporales.
Figure 1.
Maximum likelihood tree generated by IQ-Tree, based on analysis of a combined dataset of LSU, SSU, tef1-α, rpb2 and ITS sequence data. Bootstrap support values for ML greater than 75% and Bayesian posterior probabilities greater than 0.95 are given near nodes, respectively. Ex-type strains are in bold, the new isolates are in red.
Analyses of both ML and BYPP (not shown) yielded almost identical results, and the topology of the trees were similar to previous studies (Zhang et al. 2018; Hongsanan et al. 2020a, 2021). The combined analyses showed that two suborders Massarineae and Pleosporineae were well-supported and formed an upper clade in Pleosporales. Our two newly obtained fungal isolates (MFLUCC 21-0222 and MFLUCC 21-0223) clustered together and formed a distinct clade with maximum support (ML-BS = 100%, BYPP = 1.00) and they grouped with Amorosiaceae, Sporormiaceae and Sublophiostomataceae with weak support.
Taxonomy
. Pleocatenata
Y.R. Sun, Yong Wang bis & K.D. Hyde gen. nov.
402C64E6-8895-5501-81E8-069D0B58EF71
Index Fungorum number:IF559457
Facesoffungi number: FoF 10630
Etymology.
“Pleo-” an abbreviation of Pleosporales, the order in which this fungus is classified; “-catenata” refers to the catenate conidia of this fungus.
Description.
Saprobic on decaying twigs in terrestrial habitats. Asexual morph: Hyphomycetous. Colonies on natural substrate effuse, dark, velvety. Conidiophores macronematous, mononematous, straight or slightly curved, cylindrical, unbranched, septate, brown or dark brown. Conidiogenous cells monotretic, integrated, terminal, cylindrical, brown to dark brown. Conidia catenate, formed in acropetal chains, straight or bent, obclavate, olivaceous to dark brown, multi-euseptate, slightly constricted at septa, distal conidia rounded at apex, truncate at base, intercalary conidia truncate at both ends, with thickened and darkened scars at base or both ends. Sexual morph: Undetermined.
Type species.
Pleocatenatachiangraiensis Y.R. Sun, Yong Wang bis & K.D. Hyde
Notes.
The morphology of Pleocatenata is distinguished from members in other families in Pleosporales by its tretic conidiogenous cells and catenate, euseptate conidia, and phylogenic analyses indicated it does not belong to any existing families. To avoid establishing a new family with only one species, Pleocatenata is introduced as a new genus and assigned to Pleosporales, genera incertae sedis. Pleocatenata is a monotypic genus reported from terrestrial habitats but without a known sexual morph. Further discovery of other species in Pleocatenata or phylogenetic related genera with supported monophyly will determine the familial level of Pleocatenata.
. Pleocatenata chiangraiensis
Y.R. Sun, Yong Wang bis & K.D. Hyde sp. nov.
6151EB30-06BB-56B1-A35A-7ACD4BB5427B
Index Fungorum number:IF559458
Facesoffungi number: FoF 10631
Figure 2.
Pleocatenatachiangraiensis (MFLU 22-0002, holotype) a host (Tarennastellulata) b, c colonies on natural substrate d, e conidiophores with conidia f conidiogenous cells g–k conidia l germinated conidium m, n colonies on PDA (upper view and lower view). Scale bars: 1 mm (b); 100 μm (c); 20 μm (d–l).
Etymology.
The epithet referring to the location in which the fungus was collected.
Holotype.
MFLU: 22-0002
Description.
Saprobic on twigs of Clerodendrumquadriloculare and Tarennastellulata. Asexual morph: Hyphomycetous. Colonies on natural substrate effuse, dark, velvety. Mycelium immersed, composed of septate, branched, hyaline to subhyaline hyphae. Conidiophores macronematous, mononematous, erect, straight or slightly curved, cylindrical, unbranched, robust, 4–6-septate, brown or dark brown, rough, 35–100 µm long, 5.5–8.5 µm wide. Conidiogenous cells monotretic, integrated, terminal, determinate, cylindrical, dark brown. Conidia catenate, formed in acropetal chains of 2–3, straight or curved, obclavate, olivaceous to brown when young, blackish brown when mature, 5–8-euseptate, slightly constricted at septa, distal conidia rounded at apex, truncate at base, intercalary conidia truncate at both ends, with thickened and darkened scars at base or both ends, 34–70 µm long, 6.5–12 µm at the widest. Sexual morph: Unknown.
Culture characteristics.
Conidia germinated on PDA within 12 hours at 26 °C. Germ tubes were produced from both ends. Colony reached 20–25 mm diameter after 4 weeks at room temperature on PDA media. Mycelia superficial, irregularly circular, entire edge, dark brown from above, black from below, pigment produced which turns the media reddish brown.
Material examined.
Thailand, Chiang Rai Province, Mae Fah Luang University, on twigs of Tarennastellulata, 3 July 2020, Y.R. Sun, MFU5 (MFLU 22-0002, holotype, ex-type living culture MFLUCC 21-0222). Thailand, Chiang Rai Province, Medicinal Plants Garden, on twigs of Clerodendrumquadriloculare, 7 June 2020, Y.R. Sun, B45 (MFLU 22-0001, living culture MFLUCC 21-0223).
Notes.
Two isolates collected from different hosts share similar morphology and clustered together in the phylogenic tree. There are no base pair differences in LSU and tef1-α genes between these two isolates. One base pair and two base pair differences (without gaps) are observed in ITS and rpb2, respectively. Therefore, the two isolates MFLUCC 21-0222 and MFLUCC 21-0223 are identified as conspecific.
Discussion
Pleocatenata is phylogenetically related to Amorosiaceae, Sporormiaceae, and Sublophiostomataceae in our multi-gene analyses, but their monophyly was not well-supported, indicating their uncertain phylogenetic affinities. No hyphomycetous asexual morph has been reported in Sporormiaceae or Sublophiostomataceae (Hongsanan et al. 2020a, 2021). However, in Amorosiaceae, only two known hyphomycetous genera, Amorosia and Angustimassarina, are characterized by micronematous to semimacronematous, pale brown conidiophores, monoblastic conidiogenous cells, and single, elongate-clavate conidia (Mantle et al. 2006; Thambugala et al. 2015; Hongsanan et al. 2020a). Pleocatenata can be distinguished from these two genera by having monotretic conidiogenous cells and catenate, obclavate conidia.
A recently introduced species, Corynesporasinensis Jian Ma, X.G. Zhang & R.F. Castañeda, resembles Pleocatenata in its unbranched, cylindrical conidiophores and monotretic, terminal conidiogenous cells that produce catenate, obclavate conidia (Xu et al. 2020). Morphologically, Corynesporasinensis is more similar to P.chiangraiensis than to the type species of Corynespora, C.cassiicola (Berk. & M.A. Curtis) C.T. Wei (Wei 1950). Since Corynespora (Corynesporascaceae, Pleosporales) is a polyphyletic genus (Schoch et al. 2009; Voglmayr and Jaklitsch 2017), and there is no available sequence data for C.sinensis, we presume that C.sinensis may belong to Pleocatenata. However, due to lack of molecular data, and since morphology-based classification is not reliable for many hyphomycetous genera (Shenoy et al. 2006; Su et al. 2016; Yang et al. 2018), we retain the current classification. Sequences of C.sinensis are needed to resolve its phylogenetic placement. Detailed morphological comparison among C.cassiicola, C.sinensis and P.chiangraiensis is provided (Table 3).
Table 3.
Comparison between Corynesporacassiicola, C.sinensis, and Pleocatenatachiangraiensis.
| Species | Conidiophores | Conidiogenous cells | Conidia | References |
|---|---|---|---|---|
| Corynesporacassiicola | Unbranched, cylindrical proliferations, pale to mid brown, up to 9 septate, 110–850 × 4–11 µm | Monotretic, cylindrical, pale to mid brown | Solitary or in chains of 2–6, obclavate to cylindrical, subhyaline to pale olivaceous brown or brown, 4–20 distoseptate, 40–220 × 9–22 µm | Wei 1950 |
| Corynesporasinensis (HJAUP M0156) | Unbranched, cylindrical, brown to dark, 4–8-septate, 53–96.5 × 7–8.5 µm | Monotretic, cylindrical, brown, | In chains of 2, primary conidia obclavate or fusiform, 3(–4)-distoseptate, 31.5–42 × 8–9.5 µm. secondary conidia ellipsoid, 3-distoseptate, 21–28.5 × 8–9.5 µm | Xu et al. 2020 |
| Pleocatenatachiangraiensis (MFLU 21-0222) | Unbranched, cylindrical, brown or dark brown, 4–6-septate, 35–100 × 5.5–8.5 µm | Monotretic, cylindrical, dark brown | In chains of 2–3, obclavate, olivaceous to brown when young, blackish brown when mature, 5–8-euseptate, 34–70 µm × 6.5–12 µm | This study |
Pleocatenata is similar to Sporidesmiumsensu stricto, which is characterized by distinctive, unbranched conidiophores, monoblastic, determinate or proliferating conidiogenous cells, and acrogenous, solitary, transversely septate conidia (Ellis 1958, 1971; Shenoy et al. 2006; Boonmee et al. 2012; Su et al. 2016; Yang et al. 2018). However, Pleocatenata is different from Sporidesmium by having catenate conidia. Additionally, Pleocatenata is phylogenetically distinct from Sporidesmium, supporting the introduction of the new genus.
The catenate, obclavate phragmoconidia of P.chiangraiensis are similar to capnodendron asexual morph of Antennulariella Woron (Antennulariellaceae, Capnodiales) (Hughes 1976, 2000; Seifert et al. 2011). Although sequence data of Antennulariella is not available, morphological characters, such as holoblastic conidiogenous cells and branched conidiophores of Antennulariella, support its separation from P.chiangraiensis (Hughes 1976, 2000; Seifert et al. 2011). Pleocatenata is also similar to Corynesporina Subram (Pezizomycotina, incertae sedis) in having unbranched, robust conidiophores and catenate conidia (Seifert et al. 2011). However, they differ in that the distoseptate conidia form in basipetal chains in Corynesporina and euseptate conidia form in acropetal chains in Pleocatenata.
Supplementary Material
Acknowledgements
We would like to thank Dr. Shaun Pennycook for checking the nomenclature. Ya-Ru Sun thanks Mae Fah Luang University for the award of a fee-less scholarship. Ya-Ru Sun also thanks the director of the Mae Fah Luang University Botanical Garden, the botanist Dr. Jantrararuk Tovaranonte for her support. The study was funded by Guizhou Science Technology Department International Cooperation Basic project ([2018]5806), National Natural Science Foundation of China (No.31972222, 31560489), Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023), and Talent project of Guizhou Science and Technology Cooperation Platform ([2017]57885, [2019]5641 and [2020]5001).
Citation
Sun Y-R, Liu N-G, Hyde KD, Jayawardena RS, Wang Y (2022) Pleocatenata chiangraiensis gen. et. sp. nov. (Pleosporales, Dothideomycetes) from medicinal plants in northern Thailand. MycoKeys 87: 77–98. https://doi.org/10.3897/mycokeys.87.79433
References
- Abtahi F, Nourani SL. (2017) The most important fungal diseases associated with some useful medicinal plants. In: Ghorbanpour M, Varma A. (Eds) Medicinal plants and environmental challenges.Springer International Publishing, Cham, 279–293. 10.1007/978-3-319-68717-9_16 [DOI]
- Abutaha N, Mashaly AM, Al-Mekhlafi FA, Farooq M, Al-shami M, Wadaan MA. (2015) Larvicidal activity of endophytic fungal extract of Cochliobolusspicifer (Pleosporales: Pleosporaceae) on Aedescaspius and Culexpipiens (Diptera: Culicidae). Applied Entomology and Zoology 50: 405–414. 10.1007/s13355-015-0347-6 [DOI] [Google Scholar]
- Barghoorn ES. (1944) Marine fungi: their taxonomy and biology. Farlowia 1: 395–467. 10.5962/p.315987 [DOI] [Google Scholar]
- Barr ME. (1987) Prodromus to class Loculoascomycetes. Amherst. University of Massachusetts, Massachusetts.
- Bhagat J, Kaur A, Sharma M, Saxena AK, Chadha BS. (2012) Molecular and functional characterization of endophytic fungi from traditional medicinal plants. World Journal of Microbiology and Biotechnology 28: 963–971. 10.1007/s11274-011-0894-0 [DOI] [PubMed] [Google Scholar]
- Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K. (2007) Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research 111: 51–61. 10.1016/j.mycres.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Boonmee S, Ko TWK, Chukeatirote E, Hyde KD, Chen H, Cai L, McKenzie EHC, Jones EBG, Kodsueb R, Hassan BA. (2012) Two new Kirschsteiniothelia species with Dendryphiopsis anamorphs cluster in Kirschsteiniotheliaceae fam. nov. Mycologia 104: 698–714. 10.3852/11-089 [DOI] [PubMed] [Google Scholar]
- Castañeda-Ruiz RF, Heredia GP, Arias RM, Saikawa M, Minter DW, Stadler M, Guarro J, Decock C. (2004) Two new hyphomycetes from rainforests of México, and Briansuttonia, a new genus to accommodate Corynesporaalternarioides. Mycotaxon 89: 297–305. [Google Scholar]
- Chernomor O, Von Haeseler A, Minh BQ. (2016) Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65: 997–1008. 10.1093/sysbio/syw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu JC, Liu XZ, Stadler M, Hyde KD. (2014) The sooty moulds. Fungal Diversity 66: 1–36. 10.1007/s13225-014-0278-5 [DOI] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, Hardy GESJ, Barber PA, Alvarado P, Barnes CW, Buchanan PK, Heykoop M, Moreno G, Thangavel R, van der Spuy S, Barili A, Barrett S, Cacciola SO, Cano-Lira JF, Crane C, Decock C, Gibertoni TB, Guarro J, Guevara-Suarez M, Hubka V, Kolařík M, Lira CRS, Ordoñez ME, Padamsee M, Ryvarden L, Soares AM, Stchigel AM, Sutton DA, Vizzini A, Weir BS, Acharya K, Aloi F, Baseia IG, Blanchette RA, Bordallo JJ, Bratek Z, Butler T, Cano-Canals J, Carlavilla JR, Chander J, Cheewangkoon R, Cruz RHSF, da Silva M, Dutta AK, Ercole E, Escobio V, Esteve-Raventós F, Flores JA, Gené J, Góis JS, Haines L, Held BW, Jung MH, Hosaka K, Jung T, Jurjević Ž, Kautman V, Kautmanova I, Kiyashko AA, Kozanek M, Kubátová A, Lafourcade M, La Spada F, Latha KPD, Madrid H, Malysheva EF, Manimohan P, Manjón JL, Martín MP, Mata M, Merényi Z, Morte A, Nagy I, Normand AC, Paloi S, Pattison N, Pawłowska J, Pereira OL, Petterson ME, Picillo B, Raj KNA, Roberts A, Rodríguez A, Rodríguez-Campo FJ, Romański M, Ruszkiewicz-Michalska M, Scanu B, Schena L, Semelbauer M, Sharma R, Shouche YS, Silva V, Staniaszek-Kik M, Stielow JB, Tapia C, Taylor PWJ, Toome-Heller M, Vabeikhokhei JMC, van Diepeningen AD, Van Hoa N, M VT, Wiederhold NP, Wrzosek M, Zothanzama J, Groenewald JZ. (2017) Fungal Planet description sheets: 558–624. Persoonia 38: 240–384. 10.3767/003158517X698941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MB. (1958) Clasterosporium and some allied Dematiaceae Phragmosporae: I. Mycological Papers 7: 1–89. [Google Scholar]
- Ellis MB. (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew.
- Ferdinandez HS, Manamgoda DS, Udayanga D, Deshappriya N, Munasinghe MS, Castlebury LA. (2021) Molecular phylogeny and morphology reveal three novel species of Curvularia (Pleosporales, Pleosporaceae) associated withcereal crops and weedy grass hosts. Mycological Progress 20: 431–451. 10.1007/s11557-021-01681-0 [DOI] [Google Scholar]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In, 95–98.
- Hashimoto A, Matsumura M, Hirayama K, Tanaka K. (2017) Revision of Lophiotremataceae (Pleosporales, Dothideomycetes): Aquasubmersaceae, Cryptocoryneaceae, and Hermatomycetaceae fam. nov. Persoonia 39: 51–73. 10.3767/persoonia.2017.39.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Bhat DJ, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali DS, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana KWT, Samarakoon MC, Ertz D, Bao DF, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Aluthmuhandiram JVS, Abeywickrama PD, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N. (2020a) Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11: 1553–2107. 10.5943/mycosphere/11/1/13 [DOI] [Google Scholar]
- Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Lücking R, Boonmee S, Bhat JD, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones GEB, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Phukhamsakda C, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Thambugala KM, Dai D, Samarakoon MC, Chethana KWT, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang J-F, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Abeywickrama PD, Bao D-F, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Bhunjun CS, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Xu JC, Zheng J, Liu G, Feng Y, Xie N. (2020b) Refined families of Dothideomycetes: orders and families incertae sedis in Dothideomycetes. Fungal Diversity 105: 17–318. 10.1007/s13225-020-00462-6 [DOI] [Google Scholar]
- Hongsanan S, Phookamsak R, Goonasekara ID, Thambugala KM, Hyde KD, Bhat JD, Suwannarach N, Cheewangkoon R. (2021) Introducing a new pleosporalean family Sublophiostomataceae fam. nov. to accommodate Sublophiostoma gen. nov. Scientific Reports 11: e9496. 10.1038/s41598-021-88772-w [DOI] [PMC free article] [PubMed]
- Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Diversity 33: 61–75. [Google Scholar]
- Hughes SJ. (1976) Sooty moulds. Mycologia 68: 693–820. 10.2307/3758799 [DOI] [Google Scholar]
- Hughes SJ. (2000) Antennulariellabatistae n. sp. and its Capnodendron and Antennariella synanamorphs, with notes on Capnodiumcapsuliferum. Canadian Journal of Botany 78: 1215–1226. 10.1139/b00-098 [DOI] [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, De Hoog S, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M. (2013) Families of Dothideomycetes. Fungal Diversity 63: 1–313. 10.1007/s13225-013-0263-4 [DOI] [Google Scholar]
- Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchimbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang QJ, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat DJ, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, McKenzie EHC, Wen TC, Yan JY, Zhao Q. (2018) Mycosphere notes 169–224. Mycosphere 9: 271–430. 10.5943/mycosphere/9/2/8 [DOI] [Google Scholar]
- Hyde KD, Xu JC, Rapior S, Jeewon R, Lumyong S, Niego AGT, Abeywickrama PD, Aluthmuhandiram JVS, Brahamanage RS, Brooks S, Chaiyasen A, Chethana KWT, Chomnunti P, Chepkirui C, Chuankid B, de Silva NI, Doilom M, Faulds C, Gentekaki E, Gopalan V, Kakumyan P, Harishchandra D, Hemachandran H, Hongsanan S, Karunarathna A, Karunarathna SC, Khan S, Kumla J, Jayawardena RS, Liu JK, Liu NG, Luangharn T, Macabeo APG, Marasinghe DS, Meeks D, Mortimer PE, Mueller P, Nadir S, Nataraja KN, Nontachaiyapoom S, O’Brien M, Penkhrue W, Phukhamsakda C, Ramanan US, Rathnayaka AR, Sadaba RB, Sandargo B, Samarakoon BC, Tennakoon DS, Siva R, Sriprom W, Suryanarayanan TS, Sujarit K, Suwannarach N, Suwunwong T, Thongbai B, Thongklang N, Wei D, Wijesinghe SN, Winiski J, Yan J, Yasanthika E, Stadler M. (2019) The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diversity 97: 1–136. 10.1007/s13225-019-00430-9 [DOI] [Google Scholar]
- Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ, Jones EBG, Liu NG, Abeywickrama PD, Mapook A, Wei DP, Perera RH, Manawasinghe IS, Pem D, Bundhun D, Karunarathna A, Ekanayaka AH, Bao DF, Li JF, Samarakoon MC, Chaiwan N, Lin CG, Phutthacharoen K, Zhang SN, Senanayake IC, Goonasekara ID, Thambugala KM, Phukhamsakda C, Tennakoon DS, Jiang HB, Yang J, Zeng M, Huanraluek N, Liu JK, Wijesinghe SN, Tian Q, Tibpromma S, Brahmanage RS, Boonmee S, Huang SK, Thiyagaraja V, Lu YZ, Jayawardena RS, Dong W, Yang EF, Singh SK, Singh SM, Rana S, Lad SS, Anand G, Devadatha B, Niranjan M, Sarma VV, Liimatainen K, Aguirre-Hudson B, Niskanen T, Overall A, Alvarenga RLM, Gibertoni TB, Pfliegler WP, Horváth E, Imre A, Alves AL, da Silva Santos AC, Tiago PV, Bulgakov TS, Wanasinghe DN, Bahkali AH, Doilom M, Elgorban AM, Maharachchikumbura SSN, Rajeshkumar KC, Haelewaters D, Mortimer PE, Zhao Q, Lumyong S, Xu J, Sheng J. (2020) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 100: 5–277. 10.1007/s13225-020-00439-5 [DOI] [Google Scholar]
- Iturrieta‐González I, Pujol I, Iftimie S, García D, Morente V, Queralt R, Guevara-Suarez M, Alastruey‐Izquierdo A, Ballester F, Hernández-Restrepo M. (2020) Polyphasic identification of three new species in AlternariasectionInfectoriae causing human cutaneous infection. Mycoses 63: 212–224. 10.1111/myc.13026 [DOI] [PubMed] [Google Scholar]
- Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, Ghobad-Nejhad M, Nilsson H, Pang KL, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao RL, Zhao Q, Kang JC, Promputtha I. (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Diversity 74: 3–18. 10.1007/s13225-015-0351-8 [DOI] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Staplers JA. (2008) Dictionary of the Fungi 10th edn. CABI Bioscience, UK.
- Larsson A. (2014) AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30: 3276–3278. 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GJ, Hyde KD, Zhao RL, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, De Crop E, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Góes-Neto A. (2016) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 78: 1–237. 10.1007/s13225-016-0366-9 [DOI] [Google Scholar]
- Li H, Sun G, Batzer JC, Crous PW, Groenewald JZ, Karakaya A, Gleason ML. (2011) Scleroramularia gen. nov. associated with sooty blotch and flyspeck of apple and pawpaw from the Northern Hemisphere. Fungal Diversity 46: 53–66. 10.1007/s13225-010-0074-9 [DOI] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among Ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Long H, Zhang Q, Hao YY, Shao XQ, Wei XX, Hyde KD, Wang Y, Zhao DG. (2019) Diaporthe species in south-western China. MycoKeys 57: 113–127. 10.3897/mycokeys.57.35448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell ES. (1955) The ascostromatci Ascomycetes. Mycologia 47: 511–532. 10.2307/3755666 [DOI] [Google Scholar]
- Ma XY, Maharachchikumbura SSN, Chen BW, Hyde KD, McKenzie EHC, Chomnunti P, Kang JC. (2019) Endophytic pestalotiod taxa in Dendrobiumorchids. Phytotaxa 419: 268–286. 10.11646/phytotaxa.419.3.2 [DOI] [Google Scholar]
- Mantle PG, Hawksworth DL, Pazoutova S, Collinson LM, Rassing BR. (2006) Amorosialittoralis gen. sp. nov., a new genus and species name for the scorpinone and caffeine-producing hyphomycete from the littoral zone in The Bahamas. Mycological Research 110: 1371–1378. 10.1016/j.mycres.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Mathiyazhagan S, Kavitha K, Nakkeeran S, Chandrasekar G, Manian K, Renukadevi P, Krishnamoorthy AS, Fernando WGD. (2004) PGPR mediated management of stem blight of Phyllanthusamarus (Schum and Thonn) caused by Corynesporacassiicola (Berk and Curt) Wei. Archives of Phytopathology and Plant Protection 37: 183–199. 10.1080/03235400410001730658 [DOI] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) “Creating the CIPRES Science Gateway for inference of large phylogenetic trees” in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA, 1–8. 10.1109/GCE.2010.5676129 [DOI]
- Minh BQ, Nguyen MAT, von Haeseler A. (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30: 1188–1195. 10.1093/molbev/mst024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JAA. (2004) MrModeltest v2.2. Program distributed by the author: 2. Evolutionary Biology Centre, Uppsala University, 1–2.
- Obrist W. (1959) Untersuchungen über einige” dothideale” Gattungen. Phytopathologische Zeitschrift 35: 357–388. 10.1111/j.1439-0434.1959.tb01833.x [DOI] [Google Scholar]
- Ramesh C. (2003) Loculoascomycetes from India. Rao GP, Manoharachari C, Bhat DJ (Eds) Frontiers of Fungal Diversity in India, International Book Distributing Company, Lucknow, India, 457–479.
- Rambaut A, Drummond A. (2008) FigTree: Tree figure drawing tool, version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh.
- Rannala B, Yang ZH. (1996) Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311. 10.1007/BF02338839 [DOI] [PubMed] [Google Scholar]
- Rasool-Hassan BA. (2012) Medicinal plants (importance and uses). Pharmaceut Anal Acta 3: 2153–2435. 10.4172/2153-2435.1000e139 [DOI] [Google Scholar]
- Rehner SA, Samuels GJ. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. 10.1016/S0953-7562(09)80409-7 [DOI] [Google Scholar]
- Rehner SA, Buckley E. (2005) A beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordycepsteleomorphs. Mycologia 97(1): 84–98. 10.1080/15572536.2006.11832842 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C, Crous PW, Groenewald JZ, Boehm E, Burgess TI, De Gruyter J, De Hoog GS, Dixon L, Grube M, Gueidan C. (2009) A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. 10.3114/sim.2008.61.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, Kendrick B. (2011) The genera of hyphomycetes. CBS–KNAW Fungal Biodiversity Centre, Utrecht.
- Senanayake IC, Rathnayake AR, Marasinghe DS, Calabon MS, Gentekaki E, Lee HB, Hurdeal VG, Pem D, Dissanayake LS, Wijesinghe SN, Bundhun D, Nguyen TT, Goonasekara ID, Abeywickrama PD, Bhunjun CS, Jayawardena RS, Wanasinghe DN, Jeewon R, Bhat DJ, Xiang MM. (2020) Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere 11: 2678–2754. 10.5943/mycosphere/11/1/20 [DOI] [Google Scholar]
- Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD. (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycological Research 110: 916–928. 10.1016/j.mycres.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Strobel G, Stierle A, Stierle D, Hess WM. (1993) Taxomycesandreanae, a proposed new taxon for a bulbilliferous hyphomycete associated with Pacific Yew (Taxusbrevifolia). Mycotaxon. 47: 71–80. [Google Scholar]
- Su H, Kang JC, Cao JJ, Mo L, Hyde KD. (2014) Medicinal plant endophytes produce analogous bioactive compounds. Chiang Mai Journal Science 41: 1–13. [Google Scholar]
- Su HY, Hyde KD, Maharachchikumbura SSN, Ariyawansa HA, Luo ZL, Promputtha I, Tian Q, Lin CG, Shang QJ, Zhao YC, Chai HM, Liu XY, Bahkali AH, Bhat JD, McKenzie EHC, Zhou DQ. (2016) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Diversity 80: 375–409. 10.1007/s13225-016-0362-0 [DOI] [Google Scholar]
- Sun JZ, Liu XZ, McKenzie EHC, Jeewon R, Liu JK, Zhang XL, Zhao Q, Hyde KD. (2019) Fungicolous fungi: terminology, diversity, distribution, evolution, and species checklist. Fungal Diversity 95: 337–430. 10.1007/s13225-019-00422-9 [DOI] [Google Scholar]
- Sun YR, Jayawardena RS, Hyde KD, Wang Y. (2021) Kirschsteiniotheliathailandica sp. nov. (Kirschsteiniotheliaceae) from Thailand. Phytotaxa 490(2): 172–182. 10.11646/phytotaxa.490.2.3 [DOI] [Google Scholar]
- Tan YP, Crous PW, Shivas RG. (2016) Eight novel Bipolaris species identified from John L. Alcorn’s collections at the Queensland Plant Pathology Herbarium (BRIP). Mycological Progress 15: 1203–1214. 10.1007/s11557-016-1240-6 [DOI] [Google Scholar]
- Tennakoon DS, Kuo CH, Maharachchikumbura SSN, Thambugala KM, Gentekaki E, Phillips AJL, Bhat DJ, Wanasinghe DN, de Silva NI, Promputtha I, Hyde KD. (2021) Taxonomic and phylogenetic contributions to Celtisformosana, Ficusampelas, F.septica, Macarangatanarius and Morusaustralis leaf litter inhabiting microfungi. Fungal Diversity 108: 1–215. 10.1007/s13225-021-00474-w [DOI] [Google Scholar]
- Thambugala KM, Hyde KD, Tanaka K, Tian Q, Wanasinghe DN, Ariyawansa HA, Jayasiri SC, Boonmee S, Camporesi E, Hashimoto A, Hirayama K, Schumacher RK, Promputtha I, Liu ZY. (2015) Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Diversity 74: 199–266. 10.1007/s13225-015-0348-3 [DOI] [Google Scholar]
- Tóth S. (1975) Some new microscopic fungi, III. Annales Historico-naturales Musel nationalis Hungarici 67: 31–35. [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44: W232–W235. 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed]
- Tzean SS, Chen JL. (1990) Cheiromoniliophoraelegans gen. et sp. nov. (Hyphomycetes). Mycological Research 94: 424–427. 10.1016/S0953-7562(09)80373-0 [DOI] [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. (2011) SequenceMatrix: concatenation softwarefor the fast assembly of multi‐gene datasets with character set and codon information. Cladistics 27: 171–180. 10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. (2017) Corynespora, Exosporium and Helminthosporium revisited – New species and generic reclassification. Studies in Mycology 87: 43–76. 10.1016/j.simyco.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CT. (1950) Notes on Corynespora. Mycological Papers 34, 10 pp.
- White TJ, Bruns T, Lee SJWT, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Shinsky J, White T. (Eds) PCR protocols: a guide to methods and applications.Academic Press, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wanasinghe DN, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat DJ, Gueidan C, Chomnunti P, De Hoog GS, Knudsen K, Li WJ, McKenzie EHC, Miller AN, Phillips AJL, Piątek M, Raja HA, Shivas RS, Slippers B, Taylor JE, Tian Q, Wang Y, Woudenberg JHC, Cai L, Jaklitsch WM, Hyde KD. (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Diversity 69: 1–55. 10.1007/s13225-014-0309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardene NN, Hyde KD, Al-Ani LKT, Tedersoo L, Haelewaters D, Rajeshkumar KC, Zhao RL, Aptroot A, Leontyev D, Saxena RK, Tokarev YS, Dai DQ, Letcher PM, Stephenson SL, Ertz D, Lumbsch HT, Kukwa M, Issi IV, Madrid H, Phillips AJL, Selbmann L, Pfliegler WP, Horváth E, Bensch K, Kirk PM, Kolaříková K, Raja HA, Radek R, Papp V, Dima V, Ma J, Malosso E, Takamatsu S, Rambold G, Gannibal PB, Triebel D, Gautam AK, Avasthi S, Suetrong S, Timdal E, Fryar SC, Delgado G, Réblová M, Doilom M, Dolatabadi S, Pawłowska JZ, Humber RA, Kodsueb R, Sánchez-Castro I, Goto BT, Silva DKA, de Souza FA, Oehl F, da Silva GA, Silva IR, Błaszkowski J, Jobim K, Maia LC, Barbosa FR, Fiuza PO, Divakar PK, Shenoy BD, Castañeda-Ruiz RF, Somrithipol S, Lateef AA, Karunarathna SC, Tibpromma S, Mortimer PE, Wanasinghe DN, Phookamsak R, Xu J, Wang Y, Tian F, Alvarado P, Li DW, Kušan I, Matočec N, Mešić A, Tkalčec Z, Maharachchikumbura SSN, Papizadeh M, Heredia G, Wartchow F, Bakhshi M, Boehm E, Youssef N, Hustad VP, Lawrey JD, Santiago ALCMA, Bezerra JDP, Souza-Motta CM, Firmino AL, Tian Q, Houbraken J, Hongsanan S, Tanaka K, Dissanayake AJ, Monteiro JS, Grossart HP, Suija A, Weerakoon G, Etayo J, Tsurykau A, Vázquez V, Mungai P, Damm U, Li QR, Zhang H, Boonmee S, Lu YZ, Becerra AG, Kendrick B, Brearley FQ, Motiejūnaitė J, Sharma B, Khare R, Gaikwad S, Wijesundara DSA, Tang LZ, He MQ, Flakus A, Rodriguez-Flakus P, Zhurbenko MP, McKenzie EHC, Stadler M, Bhat DJ, Liu JK, Raza M, Jeewon R, Nassonova ES, Prieto M, Jayalal RGU, Erdoğdu M, Yurkov A, Schnittler M, Shchepin ON, Novozhilov YK, Silva-Filho AGS, Gentekaki E, Liu P, Cavender JC, Kang Y, Mohammad S, Zhang LF, Xu RF, Li YM, Dayarathne MC, Ekanayaka AH, Wen TC, Deng CY, Pereira OL, Navathe S, Hawksworth DL, Fan XL, Dissanayake LS, Kuhnert E, Grossart HP, Thines M. (2020) Outline of Fungi and fungus-like taxa. Mycosphere 11: 1060–1456. 10.5943/mycosphere/11/1/8 [DOI] [Google Scholar]
- Wijayawardene NN, Hyde KD, Anand G, Dissanayake LS, Tang LZ, Dai DQ. (2021) Towards incorporating asexually reproducing fungi in the natural classification and notes for pleomorphic genera. Mycosphere 12: 238–405. 10.5943/mycosphere/12/1/4 [DOI] [Google Scholar]
- Xu ZH, Kuang WG, Qiu L, Zhang XG, Castañeda-Ruíz RF, Ma J. (2020) Corynesporasinensis sp. nov. from Jiangxi, China. Mycotaxon 135: 803–809. 10.5248/135.803 [DOI] [Google Scholar]
- Yang J, Maharachchikumbura SSN, Liu JK, Hyde KD, Jones EBG, Al-Sadi AM, Liu ZY. (2018) Pseudostanjehughesiaaquitropica gen. et sp. nov. and Sporidesmiumsensu lato species from freshwater habitats. Mycological Progress 17: 591–616. 10.1007/s11557-017-1339-4 [DOI] [Google Scholar]
- Zhang Q, Yang ZF, Cheng W, Wijayawardene NN, Hyde KD, Chen Z, Wang Y. (2020) Diseases of Cymbopogoncitratus (Poaceae) in China: Curvulariananningensis sp. nov. MycoKeys 63: 49–67. 10.3897/mycokeys.63.49264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SN, Hyde KD, Gareth Jones EB, Cheewangkoon R, Liu JK. (2018) Acuminatisporapalmarum gen. et sp. nov. from mangrove habitats. Mycological Progress 17: 1173–1188. 10.1007/s11557-018-1433-2 [DOI] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012) Pleosporales. Fungal Diversity 53: 1–221. 10.1007/s13225-011-0117-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.