Abstract
In this randomized controlled pilot trial, we explored the feasibility, technology compliance, and preliminary efficacy of the Education for Action (EDU-ACT), a multimodal intervention combining evidence-based strategies of physical activity (PA) education, and coaching, in PA levels over 4 weeks between EDU-ACT and control groups. We also assessed pre-post changes in neurocognitive function, functional mobility and dual-task performance, sleep and quality of life. Thirty-two sedentary older adults with memory complaints (age=66±5.3) completed the study (EDU-ACT=18, control=14). The EDU-ACT adherence rate was 95%, and compliance of daily PA reporting was, on average, 22.7 days (94.6%). The EDU-ACT group demonstrated significantly greater number of steps, processing speed, and dual-task performance when compared with controls (p<0.05). In this study, a multimodal, evidence-based, low-cost intervention was feasible, well-accepted, with high adherence and compliance rates, and effective at promoting clinically meaningful increases in PA, for at least one-month post-intervention, in older adults with memory complaints.
Trial Registration:
The study is registered at the Brazilian Registry of Clinical Trials database (ReBec; http://www.ensaiosclinicos.gov.br, #RBR-73JPWY).
Keywords: health education, coaching, behavior change, exercise, cognition
INTRODUCTION
In projections for 2050, the number of older adults over 60 years of age will increase from approximately 960 million to 2.1 billion (United Nations, 2017). Non-communicable diseases such as diabetes, heart disease and cognitive impairment have devastating effects on cognitive and physical function and societal participation, leading to a worldwide yearly economic burden upward of US$800 billion (CDC, 2011; The Alzheimer’s Association, 2020; Wimo et al., 2017). Developing cognitive impairment causes one’s direct medical costs to double (Greysen et al., 2017; Leibson et al., 2015; Zhu et al., 2013), and the additional cost of a caregiver can further increase this estimate by at least 5 times (Zhu et al., 2013). There is a lack of effective therapies to treat age-related cognitive decline (Cummings et al., 2014). Thus, effective strategies to promote cognitive brain health in aging populations remains a critical and unmet need.
Exercise has been demonstrated to effectively and consistently promote cognitive brain health in aging. There are a least 50 meta-analyses that collectively suggest modest, but significant improvements in cognitive abilities following participation in exercise interventions, such as aerobic and resistance training, and mind-body exercises (Chen et al., 2020; Falck et al., 2019; Northey et al., 2018; Smith et al., 2010; Young et al., 2015). Syntheses of systematic review and meta-analyses have reached the same conclusion (Erickson et al., 2019; Zubala et al., 2017). The limited exercise adherence among older adults is a major challenge for the effective implementation of exercise programs for cognitive brain health in aging. Most clinicians recommend that their patients increase their physical activity (PA) levels (Lobelo et al., 2014; U.S. National Academy of Medicine, 2019; WHO, 2013), but less than a third of adults know and understand PA guidelines (Knox, Musson, & Adams, 2015; Vaara, Vasankari, Koski, & Kyröläinen, 2019). Participation in supervised exercise interventions immediately increases PA levels, but is less effective in helping individuals maintain increased PA levels (Olson, McAuley, & Olson, 2015; Wanigatunga et al., 2017). Thus, recommending that individuals exercise, or even helping them get started does not guarantee a sustained change in PA levels.
Health education interventions have been most successful at helping older adults improve and maintain high PA levels. Several effective strategies have been employed, including health counseling (Morey et al., 2006), self-efficacy and self-regulation strategies (Olson et al., 2015; Stathi et al., 2020), PA workshops (Groessl et al., 2016; Silva-Smith et al., 2013), health technology tools (Hekler et al., 2013; Notthoff & Carstensen, 2014), booklets (Shields et al., 2013), and the use of activity monitors (Mendoza et al., 2015). There is insufficient evidence on the effectiveness of PA education strategies in older adults with memory complaints, whom are reportedly less compliant than their age-matched counterparts with physical activity interventions (Di Lorito et al., 2020; Tak et al., 2012). Furthermore, a combination of successful strategies may be more effective than individual interventions in promoting change in PA especially in this population.
In addition to health education strategies, over the last decades there have been great advances in the science of behavior change, conceptualized by models of health and coaching principles and theories such as the Biopsychosocial (BPS) model of health, the Transtheoretical Model (TTM), and Motivational Interviewing (MI) (Please see Figure 1 for a schematic figure of the conceptual framework). The BPS model of health considers the individuals’ context through an ecological perspective by helping them to understand factors (e. g. social determinants of health) and barriers to behavior change, including their characteristics, social networks, cultural norms, and built environment (Fisher, 2008). The TTM emphasizes behavior change as a dynamic process that is highly dependent on individuals’ readiness to change (Gourlan et al., 2016; Prochaska & Di Clemente, 1982). MI is a supportive behavior change tool that respects and harnesses the individual’s autonomy to change their own behavior by building achievable goals using four main communication elements: open-ended questions, affirmations, reflective listening and summarizing (Miller & Rollnick, 2013; Stewart & Fox, 2011). Rather than only instructing individuals, these theories propose a different approach, where the coach partners with individuals to assist him/her in developing, implementing and eventually achieving their own specific goals. Such coaching principles and theories have shown promise in promoting lifestyle behavior changes as adjunct to pediatric rehab, (Baldwin et al., 2013; Cueto et al., 2019), management of hypertension and diabetes, weight control, tobacco cessation, and alcohol consumption in older adults (Cummings et al., 2009; Sforzo et al., 2018). However, PA education strategies and coaching interventions have not been fully investigated in older adults with subjective memory complaints.
Figure 1 —
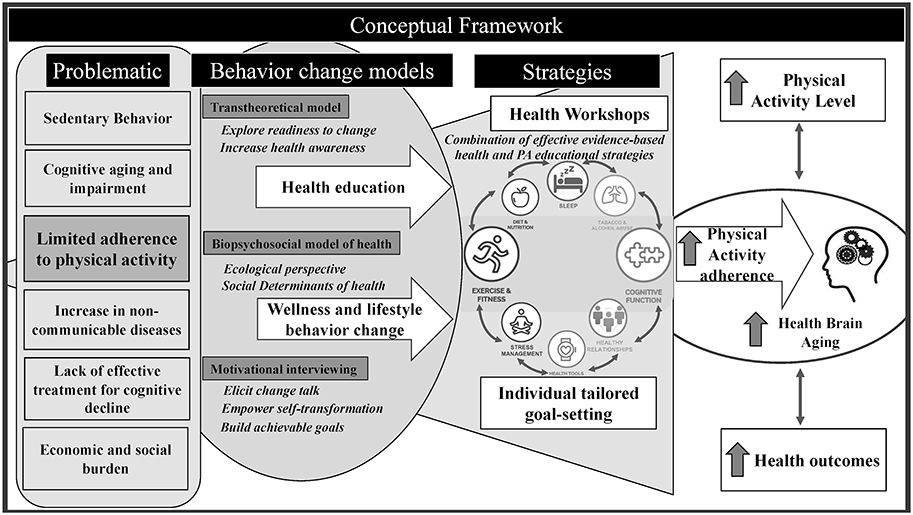
Conceptual framework. PA = physical activity.
Given the limited evidence on both, PA education strategies and coaching interventions for individuals with subjective memory complaints, we developed the Education for Action Program (EDU-ACT), a 3-day multimodal program combining evidence-based strategies of coaching and PA education, tailored to older adults with subjective memory complaints as a starting point in the process of behavior change. Our primary objective was to explore the study feasibility, technology compliance, and preliminary efficacy of the EDU-ACT intervention in older adults with memory complaints. We hypothesized that participants in the EDU-ACT group would adhere to the intervention and demonstrate greater improvements in PA over 4 weeks, when compared to a control group. Our secondary objective was to test the hypothesis that the participants in the EDU-ACT group would demonstrate greater preliminary improvements in cognition, mobility, sleep and quality of life, when compared to a control group.
METHODS
The methods of this study follows the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement (Moher et al., 2012).
Study design
This was a prospective randomized controlled study. All participants did the following: 1) completed a pre-assessment battery, 2) were randomized into the EDU-ACT intervention or a control group, 3) underwent a 4-week PA monitoring period, and 4) then returned for a post-assessment battery. The study was conducted at the Specialized Rehabilitation Center and Kinesiology Laboratory of the Alagoas State University of Health Sciences. The study protocol was approved by the local institutional research ethics committee, and registered in the Brazilian Registry of Clinical Trials database (ReBec; http://www.ensaiosclinicos.gov.br, #RBR-73JPWY). We collected data from September 2017 to March 2020.
Participants
Participants were recruited through brochures and posters placed in common areas of the Alagoas State University of Health Sciences campus and local basic community health centers. Interested individuals participated in an in-person screening visit, and if eligible, signed an informed consent form. The inclusion criteria were: 1) individuals aged ≥ 60 years; 2) subjective memory complaints or global cognitive score compatible with cognitive impairment as per the Mini-Mental State Examination (≥18 and ≤29 corrected by the education level) (Brucki et al., 2003); 3) sedentary behavior defined by International Physical Activity Questionnaire Short Form (IPAQ); and 4) being at the precontemplation, contemplation or preparation stage of change of the TTM model. Individuals were excluded if: 1) illiterate (and thus could not complete all study procedures); 2) unable to obtain exercise clearance (as per the Physical Activity Readiness Questionnaire–PAR-Q) or, 3) presenting with other health related conditions that precluded participation in all study procedures.
A single evaluator was responsible for the simple randomization process (computerized random numbers using the website http://www.randomization.com). Participants were assigned to 1 of 2 groups following a 1:1 allocation. The randomization process was completed using sealed envelopes only opened at the time of the allocation by an investigator (who was not involved in the earlier randomization process). This was a single-blind controlled trial. Participants did not know whether they had been randomized to the EDU-ACT or control group.
EDU-ACT Intervention and Control Group
Four study investigators underwent formal training before delivering the intervention (at least 4 practical and theoretical sessions, each consisting of 4 hours). Each group was led by an instructor and a co-instructor. All instructors followed a uniform script to ensure consistent information delivery. The EDU-ACT intervention consisted of a 12-hour theoretical-practical curriculum delivered over 3 days (Figure 2). In general, didactic material covered presentations and discussions on evidence on exercise, aging and brain health, balanced nutrition, and exercised demonstrations [Health education]. This included exercise benefits, assessment methods, parameters, recommended guidelines, and basic concepts of understanding the process of aging, its effects on cognitive brain health, and how lifestyle and behavior change strategies, such as increasing exercise and PA levels, can effectively increase brain health in older adults. Specific PA recommendations were based on a large systematic review on exercise for cognitive brain health in aging (Gomes-Osman et al., 2018), and global guidelines on physical activity for health (recommendations for adults aged 65 years and above are to engage in at least 150 minutes of moderate-intensity aerobic physical activity throughout the week; and include muscle-strengthening activities involving major muscle groups on 2 or more days a week) (World Health Organization, 2010). In our program, we discussed various modalities of exercise such as leisure time and recreational activities, sports activities, and planned exercise (aerobic, resistance training, and mind-body exercises).
Figure 2 —
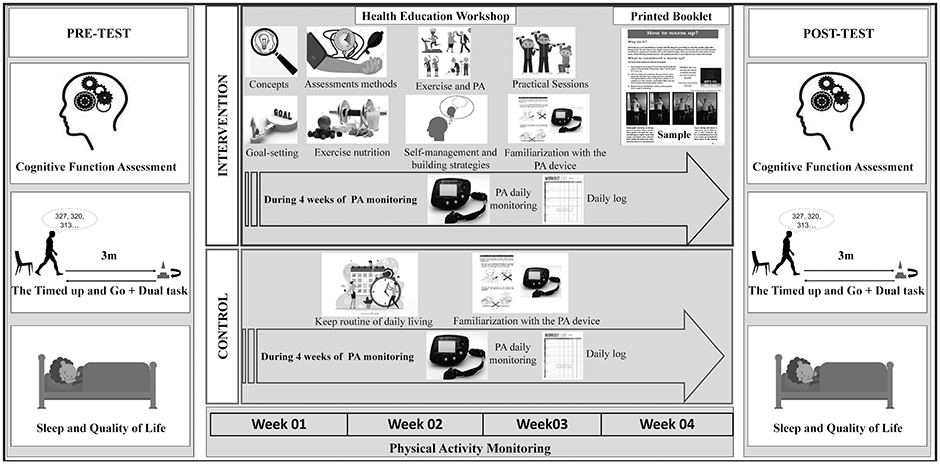
Study design. PA = physical activity
The training was also comprised of individual and small-group wellness coaching sessions focused on identifying supportive influences and barriers/motivators to physical exercise [BPS model], improving confidence toward an active lifestyle, eliciting change talk [MI approach], engaging participants to increase awareness and capacity for change, and to define individual SMART goals (goals that are specific, measurable, attainable, relevant, and time-bound) applied to exercise [TTM and MI]. During group and individual sessions, we applied the five grounded principles of MI: “1) Demonstrate empathy through reflective listening; 2) Develop discrepancy between clients' goals or values and their current behavior; 3) Avoid argument and direct confrontation; 4) Adjust to client resistance rather than opposing it directly; and 5) Support self-efficacy and optimism.” (Miller, 1999, pp. 41) All these strategies were employed to help participants solve ambivalence (the state of having simultaneous and conflicting attitudes or feelings), which prevents them from realizing their personal goals (Miller & Rollnick, 2002). During discussions, participants were encouraged to evoke and to discuss potential barriers and facilitators of individual characteristics, social networks and personal relationships, built environment, and social and cultural norms, that were relevant for their behavior change. Following these discussions, participants were prompted to develop their own progressive program and plan activities considering their actual fitness level, exercise preference, availability, and goals. Plan and goals development were facilitated by one-on-one meetings with the instructors. Aiming to facilitate improved PA and carryover of learned information, we allowed participants to bring a family/caregiver/friend to the session (not mandatory).
Participants also received a user-friendly printed booklet [Health Education] covering all theoretical material, exercise examples, strategies to overcome barriers and increase motivation, and various sources to access complementary information. The booklet contained accessible and easy-to-understand language, and figures to illustrate the content (See supplemental material for a booklet sample). Table 1 shows detailed information about the workshop content. A summary of studies used to conceptualize the EDU-ACT program is available in supplemental material table e-1.
Table 1.
A detailed description of the EDU-ACT program.
| Topic | Content |
|---|---|
| Basic Concepts | 1. Aging 2. Cognition and Cognitive aging 3. Exercise 4. Physical Activity 5. Understanding Lifestyle and Behavior Change |
| Motivational | 6. Empowering people to change 7. Eliciting OARS questions: open-ended questions, affirmations, reflective listening and summarizing |
| Assessments methods | 8. How to assess sedentary lifestyle 9. Self-Exertion measures 10. Cardiovascular assessment |
| Exercise and Physical Activity | 11. Benefits of PA and how to improve health through PA 12. Starting to practice 13. Types and Categories 14. Parameters 15. How to minimize risks and optimize benefits of exercise? 16. Physical activity guidelines and safety |
| Exploring other health outcomes | 17. Sleep matters 18. Stress management 19. Mindfulness and relaxation 20. Building healthy social relationships 21. Be positive |
| Practical Sessions | 22. Examples of home-based exercise and clinic-based exercises (e.g. gym) 23. How could I apply the recommendations in my life or guide a family member or a friend? 24. Tips and clues to maximize daily living physical activities |
| Goal setting | 25. Introduction of physical activity goal setting Introducing SMART goals: goals that are specific, measurable, attainable, relevant, and time-bound. 26. Set specific, manageable physical activity goal for the next week 27. Examine goals and adjust based on experience |
| Exercise Nutrition | 28. Healthy eating in aging adults 29. The Nutrition and health connection 30. Main types and sources of nutrients 31. The importance of nutrition during a physical activity program |
| Identifying barriers and increasing self-regulation and confidence | 32. Identify personal barriers to physical activity 33. Strategies for troubleshooting (anticipate barriers) 34. Confidence building 35. Cognitive reframing 36. Relapse prevention and recovery |
| Tracking Physical Activity | 37. Accelerometer instructions 38. Daily diary |
Individuals in the control group were instructed to follow their routine of daily living. Upon completing the post-assessment battery, participants in the control group were invited to participate in the EDU-ACT intervention. This was a specific request of the local ethics committee.
Outcome measures
Primary Outcome: Feasibility of the Intervention and technology compliance
Feasibility of the Intervention.
The adherence rate was used as a primary outcome measure of feasibility of the EDU-ACT intervention, calculated by 1) the proportion of participants that initiated the intervention out of the total allocated; and 2) the proportion of participants that completed the whole planned intervention out of the participants that initiated the intervention. We also assessed the proportion of participants lost to follow-up, the proportion of participants who withdrew, and the proportion of participants who rescheduled sessions.
Technology compliance.
This was assessed by evaluating the reporting compliance (usage and daily notes) on the Power Walker™ PW-610 accelerometer, used to assess PA levels (physical activity levels described in the following section)”.
Secondary Outcomes
Physical Activity Levels
Participants in both groups received a Power Walker™ PW-610 accelerometer (Yamax Corporation, Tokyo, Japan) along with a user manual, and underwent a familiarization on the use of the device, keeping a daily log, and troubleshooting scenarios. Participants were monitored for the following 4 consecutive weeks and were required to complete a daily log for the accelerometer data. Yamax Corporation accelerometers and pedometers are highly reliable (Cronbach's α =0.98) and moderately to highly valid for use in scientific studies (convergent validity, r = 0.45-0.99; and criterion validity reported accurate step counts at all speeds when compared to actual counted steps) (Bassett & John, 2010; Clemes et al., 2010; Coffman et al., 2016; Crouter et al., 2003; Scheider et al., 2003; Schneider et al., 2004). The outcomes were the average steps per week and the total average number of steps over 4 weeks after the EDU-ACT intervention. Activity data were checked weekly by a study investigator, and were deemed useable if adhering to the following criteria: at least 10h of wear time per day; at least 5 days of valid data per week; and a minimum of 1,000 steps per day.
Neurocognition, Functional Performance, Quality of Life and Sleep
Stroop Color-Word Test (SCWT).
The SCWT was used to evaluate processing speed and executive functions (Trenerry, M. R., Crosson, B., DeBoe, J. & Leber & Trenerry, 1995). The Torga version of the Stroop Test showed a very good internal consistency (Cronbach's α = 0.99), moderate to high test-retest reliability (r =0.48-0.95), and moderate convergent validity (Queiroz Garcia et al., 2016). In this study, we recorded the number of words read, errors, corrections, and the time that was taken to complete each part of the test (SCWT reading, and SCWT naming) (Queiroz Garcia et al., 2016).
Rey Auditory-Verbal Learning Test (RAVLT).
The RAVLT was utilized to assess learning and verbal memory (Rey, 1964), with a version validated to normative standards for the Portuguese language (Malloy-Diniz et al., 2000). Evidence indicates that RAVLT had moderate convergent validity (r = 0.44) and presented good internal consistency (Cronbach's α = 0.83) in neuropsychological assessment in older adults (Magalhães et al., 2012). The test consisted of a 15 words-list ‘List A’, an interference list ‘List B’ and followed by a series of recall tests. We quantified total list learning, retroactive interference (RI), and proactive interference (PI). Total list learning was defined as the sum of words evoked correctly from lists A1 to A5. Retroactive interference (RI) was defined by the degree of influence of the presentation of list B in the learning of list A, and proactive interference (PI) was defined by the degree of influence of the presentation of list A on the learning of list B.
Trail Making Test Parts A and B (TMT-A and TMT-B).
TMT is a widely, valid (convergent validity, r > .73) and reliable measure (test-retest, r > 0.79) used to assess processing speed and attention (Battery, 1994; Ble et al., 2005; Papandonatos et al., 2015; Rabin et al., 2005; Sánchez-Cubillo et al., 2009). We quantified the total time (in seconds) to complete both TMT-A and TMT-B tests.
The Timed up and Go (TUG).
The TUG was used to assess functional mobility and dual-task behavior. Participants were asked to rise from a chair, walk three meters, turn and return to a sitting position in the shortest time possible (Richardson, 1991). The TUG, along with a secondary motor (TUG-motor) or cognitive activity (TUG-cog), was used to assess dual-task performance (Berg et al., 1992; Fatori et al., 2015; J. Magalhães et al., 2008). Evidence indicates that TUG dual-task is a valid and a prognostic measure (Area under the Receiver Operating Characteristics (ROC) Curve > 0.65) to predict falls in older adults with a moderate test-retest reliability (Cronbach's = 0.60) (Hofheinz & Mibs, 2016; Tang et al., 2015; Venema et al., 2019). A dual-task effect (DTE) was computed as the percent change of the TUG dual-task from the TUG single task. The TUG-DTE represents the extent to which the addition of a motor or cognitive task impairs walking speed (Kelly et al., 2010). Values were inverted so that higher scores represent better performance (less cost).
Sleep and Quality of Life.
The Pittsburgh Sleep Quality Index (PSQI) is a valid (discriminative validity, p <0.05) and reliable measure indicating a high internal consistency (Cronbach's α = 0.82) that evaluates sleep quality over the last month, providing an index of severity and nature of the sleep disorder (Bertolazi, 2008). Quality of Life was assessed using the short form of the World Health Organization’s quality of life-brief version assessment instrument (WHOQOL-BREF) (da Silva et al., 2018). The WHOQOL-BREF had demonstrated good reliability (Cronbach's α = 0.81) and adequate convergent validity (Average variance extracted [AVE] ≥ 0.50) (da Silva et al., 2018).
STATISTICAL ANALYSIS
All statistical analyses were performed using JMP Pro (v14.0, The SAS Institute Inc., Cary, North Carolina, USA), using a two-tailed 95% confidence interval (ɑ=.05). Data were tested for normality of distribution using the Shapiro-Wilk test, and homogeneity of variances using the Levene’s test. Additionally, we assumed homoscedasticity of the data.
The adherence rate to the intervention and technology compliance was reported in percentage (%) of the total participants completing the intervention and total of daily reported PA data, respectively. To investigate the impact of EDU-ACT or control on PA level, the average total steps were entered into a mixed-effects linear model with a main effect of time over the 4-week period (week 01, week 02, week 03, week 04). Planned t-tests were used to compute pairwise comparisons of each time point between groups. The Tukey Honestly Significant Difference (HSD) test was used as a post-hoc test of the four means to protect against spurious family-wise Type 1 error. We used pooled t-test (equal variances) or independent samples t-tests (unequal variances) to assess between-group differences in the secondary outcomes.
Given the fact that level of education is likely to be a major confounder regarding the impact of an educational program that includes by design not just coaching but also lectures and the offering of instructional sessions, in an exploratory aim we evaluated the impact of educational level on the results. A mixed-linear regression using a full-factorial model was used to investigate group interactions between baseline education level, and primary and secondary outcome measures (Neurocognition and Functional Performance) (Shaw & Spokane, 2008).
The effect size was analyzed by Cohen's d. Between-group effect size was calculated using the ratio of the difference between group means and the pooled standard deviation (Cohen, 1988). Within-group effect size was calculated using the ratio pre-to-post mean change and mean pre/post standard deviation (Cumming, 2012). Interpretation for Cohen’s d is as follows: non-significant <0.19; small 0.20 to 0.49; moderate 0.50 to 0.79; and large >0.80. Our total sample of 32 participants provided 80% power to detect at least a large between-group effect (Cohen’s d ≥1.03).
RESULTS
Participants
A total of 77 individuals were screened for eligibility. Of these, 46 were eligible and were allocated into the EDU-ACT group (n=24; age = 65.3 ± 3.5; 87.5% females) and control group (n=22; age= 66.9 ± 6.1; 90.9% females). Baseline demographic and clinical characteristics are presented in detail in Table 2. Due to attrition and loss to follow-up (n=11) and incomplete data (n=3), the final analysis included 32 participants (intervention=18, control=14)].
Table 02.
Demographic characteristics.
| Participants' characteristics | Total (n = 46) |
Intervention (n = 24) |
Control (n = 22) |
|---|---|---|---|
| Age, years | 66.1 ± 4.9 | 65.3 ± 3.5 | 66.9 ± 6.1 |
| age, range | 60-86 | 60-75 | 60-86 |
| Gender, n (%) | |||
| male | 5 (10.8) | 3 (12.5) | 2 (9.1) |
| female | 41 (89.2) | 21 (87.5) | 20 (90.9) |
| Marital status, n (%) | |||
| with partner | 21 (45.6) | 10 (41.7) | 11 (50) |
| without partner | 25 (54.4) | 14 (58.3) | 11 (50) |
| Weight, kg, mean± SD | 67.9 ± 13.6 | 68.8 ± 12.1 | 66.9 ± 15.4 |
| Height, cm, mean±SD | 156.8 ± 7.2 | 157.5 ± 7.6 | 156.1 ± 6.8 |
| BMI, n (%) | |||
| normal (18.5 – 24.9) | 15 (32.6) | 7 (29.2) | 8 (36.4) |
| overweigh (25.0 – 29.9) | 15 (32.6) | 9 (37.5) | 6 (27.3) |
| obese (≥ 30.0) | 16 (34.8) | 8 (33.3) | 8 (36.3) |
| BMI, mean± SD, kg/m2 | 27.7 ± 5.2 | 27.8 ± 4.9 | 27.5 ± 5.7 |
| Education level, n (%) | |||
| low education | 7 (15.2) | 2 (8.3) | 5 (22.7) |
| medium | 15 (32.6) | 8 (33.3) | 7 (31.8) |
| high education | 24 (52.2) | 14 (58.4) | 10 (45.5) |
| Education level, years, mean± SD | 13.5 ± 4.4 | 14.12 ± 3.5 | 12.7 ± 5.2 |
| IPAQ total, METs, mean± SD | 525.6 ± 409.3 | 555.9 ± 432.1 | 504.8 ± 389.8 |
| IPAQ weekday sitting, hours, mean± SD | 9.3 ± 3.6 | 8.6 ± 3.1 | 10.1 ± 4.1 |
| MMSE total, mean±SD | 26.3 ± 2.7 | 26.5 ± 2.6 | 26.1 ± 2.8 |
Abbreviations. n = sample; % = percentage; SD = standard deviation; kg = kilogram; kg / m2 = kilograms per square meter; BMI = Body Mass Index; METs = metabolic equivalent; IPAQ = International Physical Activity Questionnaire; MMSE = Mini Mental State Examination.
Feasibility of the EDU-ACT intervention and technology compliance
From the 24 participants allocated in the EDU-ACT group, 21 started the intervention (87.5%), and 19 completed the whole planned intervention, resulting in an adherence rate of 95% when only considering the individuals who initiated the intervention. Two individuals were lost to follow-up. Only two individuals (10%) had to reschedule a session, but successfully completed the intervention. Figure 3 shows the full details of the participants’ CONSORT flowchart.
Figure 3 —
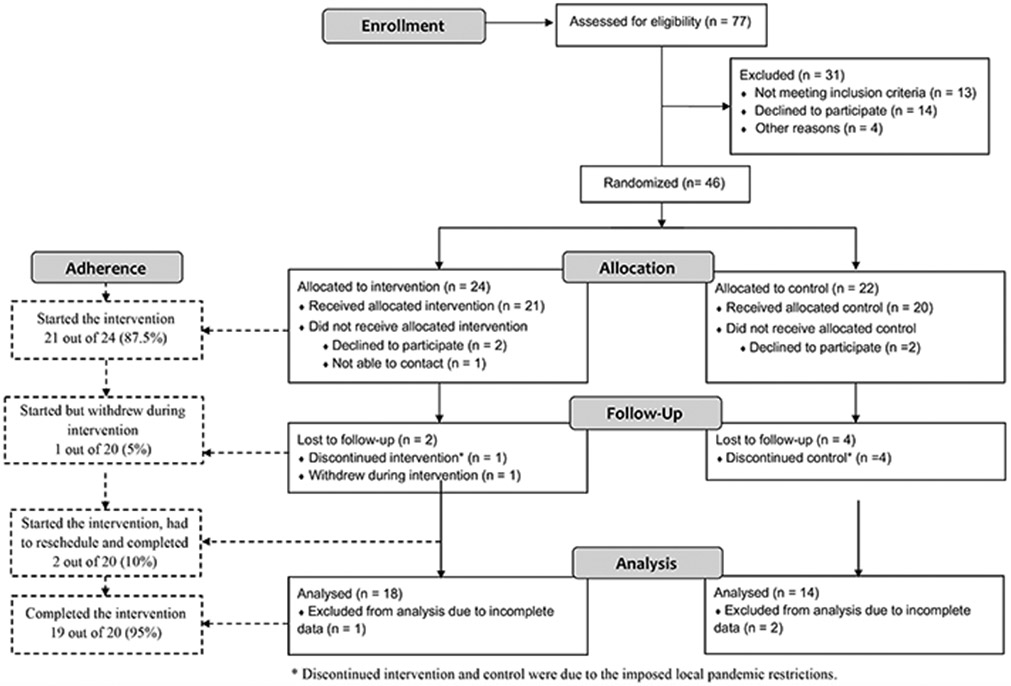
Study flowchart.
Compliance of daily PA reporting data was an average of 22.7 out of 24 days (94.6%).
Physical Activity Results
Table 3 shows detailed data on all pre-post outcome measures. The mixed-effects linear model showed the total average steps over the 4-week varied significantly between the EDU-ACT and control groups. Individuals in the EDU-ACT group demonstrated a greater increase in average steps, when compared to the control group (F1,29=8.9, p=.0057), which was associated with a large effect size (d=.93) (Figure 4). Planned t-test comparisons revealed that the between-group difference was driven by significant changes at all times points: week 01 (t=2.51, p=.01), week 02 (t=2.61, p=.01), week 03 (t=2.9, p=.006), and week 04 (t=2.07, p=.04).
Table 3.
Physical Activity, neuropsychological, mobility, and sleep and quality of life measures.
| Outcomes Mean change ± SD |
Intervention (n = 18) |
Control (n = 14) |
Statistical Analysis |
df | F/t | p- value |
∣d∣ |
|---|---|---|---|---|---|---|---|
| Physical activity, steps | |||||||
| Total mean | 10221 ± 1085 | 8000 ± 2832 | Mixed-effects linear model | 1, 29 | 8.9 | .0057* | .93 |
| week 01 | 10303 ± 1428 | 8086 ± 3006 | Planned t-test | 29 | 2.51 | .01* | .97 |
| week 02 | 10284 ± 1792 | 7973 ± 2686 | Planned t-test | 29 | 2.61 | .01* | 1.03 |
| week 03 | 10456 ± 1972 | 7925 ± 3288 | Planned t-test | 29 | 2.9 | .006* | .96 |
| week 04 | 9842 ± 1441 | 8016 ± 3526 | Planned t-test | 29 | 2.07 | .04* | .70 |
| Cognitive function | |||||||
| TMT-A, seconds | −4.1 ± 9.0 | 2.8 ± 6.3 | Pooled t-test | 30 | 2.46 | .02* | .88 |
| TMT-B, seconds | −18.7 ± 51.3 | −13.7 ± 50.1 | Pooled t-test | 30 | .28 | .78 | .10 |
| Stroop Word reading, score | 7.8 ± 15.5 | −0.3 ± 1.2 | t-test | 17.3 | 2.2 | .04* | .69 |
| Stroop Color naming, score | 5.2 ± 10.8 | 4.6 ± 14.5 | Pooled t-test | 30 | .14 | .88 | 05 |
| RAVLT A1-A5, score | 4.4 ± 12.2 | 5.1 ± 7.1 | t-test | 20.8 | .17 | .87 | .07 |
| RAVLT RI, score | −0.06 ± 0.3 | 0.1 ± 0.5 | Pooled t-test | 26 | 1.12 | .27 | .39 |
| RAVLT PI, score | −0.14 ± 0.5 | −0.13 ± 0.4 | Pooled t-test | 26 | .07 | .94 | .02 |
| TUG, seconds | |||||||
| TUG-regular | .29 ± 1.44 | .38 ± 1.27 | Pooled t-test | 25 | .18 | .85 | .07 |
| TUG-motor | .15 ± 1.08 | .28 ± 1.28 | Pooled t-test | 25 | .28 | .78 | .11 |
| TUG-DTE, motor, % | 1.0 ± 11.0 | .09 ± 8.5 | Pooled t-test | 25 | .03 | .97 | .19 |
| TUG-cog01 | −0.61 ± 1.7 | 0.50 ± 1.54 | Pooled t-test | 25 | 1.78 | .08** | .69 |
| TUG-DTE, cog01, % | 10.0 ± 17.0 | −1.0 ± 14.0 | Pooled t-test | 25 | 1.98 | .05* | .07 |
| TUG-cog02 | 0.10 ± 1.6 | 0.57 ± 1.63 | Pooled t-test | 25 | .75 | .46 | .29 |
| TUG-DTE, cog02, % | 2.3 ± 16.9 | −1.7 ± 14.4 | Pooled t-test | 25 | .65 | .51 | .14 |
| Sleep and Quality of Life | |||||||
| PSQI, score | −0.35 ± 4.8 | −0.50 ± 3.05 | Pooled t-test | 26 | .09 | .92 | .04 |
| WHOQOL, score | |||||||
| Physical | 3.5 ± 13.8 | −3.65 ± 9.75 | Pooled t-test | 26 | 1.58 | .13 | .59 |
| Psychological | 0.29 ± 13.7 | −2.15 ± 10.73 | Pooled t-test | 26 | .52 | .60 | .20 |
| Social | −1.71 ± 18.9 | 11.57 ± 18.22 | Pooled t-test | 26 | 1.89 | .07 | .71 |
| Environmental | −6.21 ± 14.36 | −1.28 ± 14.93 | Pooled t-test | 26 | .89 | .38 | .34 |
Abbreviations. n = sample; SD = standard deviation; df = degree of freedom; ∣d∣ = Cohen’s d effect size; TMT = Trail Making Test; RAVLT = Rey Auditory Visual Learning Test; TUG = Timed up and Go; DTE = Dual task effect; PSQI = Pittsburgh Sleep Quality Index; WHOQOL = World Health Organization’s quality of life.
Note.
= p < .05
= p < .09
Figure 4 —
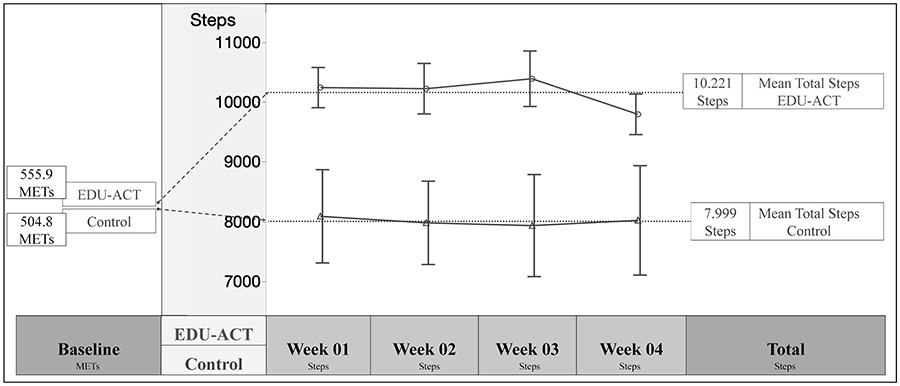
Physical activity over the 4 weeks. This figure shows the PA behavior observed at baseline and over the course of 4 weeks after the EDUACT intervention and control condition. At baseline, both groups were in the same low PA level. The intervention group showed an increase in PA participation and maintained consistency throughout the 4 weeks. METs = metabolic equivalent per week measured by the IPAQ showing sedentary behavior at baseline; EDU-ACT= Education for Action program.
Other Outcome Results
With regards to other secondary outcome measures, we found between-group differences in TMT-A and SCWT reading scores, with the EDU-ACT group demonstrating improved performance when compared with the control group (t=2.46, p=.02, d=.88, and t=2.2, p=.04, d=.69, respectively). No other between-group differences in neurocognitive measures were found. Within-group comparisons revealed that the EDU-ACT group demonstrated significant pre to post differences in the SCWT naming (t=2.05, p=.05, d=.39).
In addition, we found a trend toward between-group differences in TUG-cog dual-task (t=1.78, p=.08 d=.69), with the intervention group demonstrating greater dual-task performance when compared with the control group. Also, we found significant between-group differences in the TUG-cog-DTE (t=2.0, p=.05, d=.76), with the EDU-ACT group demonstrating 10% less cognitive-motor interference when compared with the control group that increased 1%. No other statistically significant between-group or within-group differences were found.
Our exploratory analysis including baseline education level as a covariate of both, the primary and secondary outcome measures revealed that interaction of group*education level was a significant predictor of variability in cognitive outcomes, but not PA levels. Specifically, education level predicted between-group differences in SCWT reading performance (F2,26=4.8, p=.0167, d=.78, Figure 5B) and TUG-cog performance (F2,21=6.27, p=.0073, d=.89, Figure 5C). Planned t-test comparisons showed that differences were driven by the low education subgroup, which showed greater improvements both, in TUG-cog performance and SCWT reading for participants randomized to the EDU-ACT group (p<.001, Figure 5B and 5C). The inclusion of baseline education level (Figure 5A) as a covariate did not significantly affect the between-group difference in physical activity (p>.05, See supplement Table e-2 for detailed information).
Figure 5 —
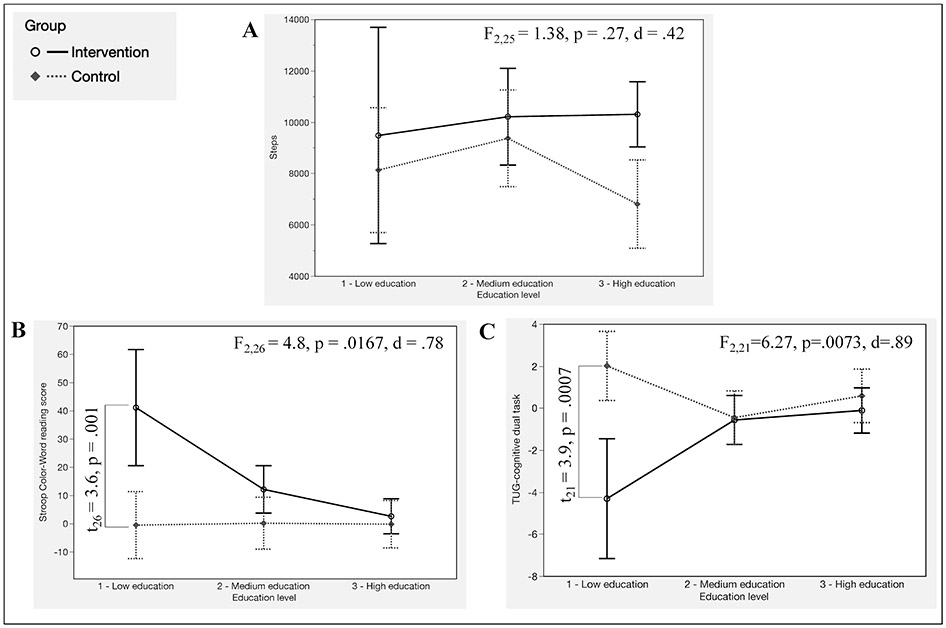
Results of the interaction of Group × Education level. (a) Education level did not significantly affect the between-group difference in PA level (steps). (b) Education level was a significant predictor of increase in performance in Stroop Color–Word Test reading score, and the difference was driven by the low education subgroup. (c) Education level was a significant predictor of increase in performance in TUG-cognitive dual task, and the difference was driven by the low education subgroup.
DISCUSSION
We present the preliminary results of EDU-ACT, a 3-day multimodal program combining evidence-based strategies of coaching and PA educational strategies tailored to older adults with subjective memory complaints as a starting point in the process of behavior change. In this randomized controlled pilot trial, the EDU-ACT intervention was feasible and well-accepted, with high adherence and compliance rates (both over 90%). In addition, preliminary data demonstrated clinically meaningful increases in physical activity levels in the EDU-ACT participants, when compared with the control group over the same period.
The high adherence and compliance rates observed in the EDU-ACT group are promising given the paucity of evidence on engagement in PA education and exercise interventions specifically in individuals with subjective memory complaints. In a large-scale systematic review of exercise studies aimed at improving cognition in older healthy adults and those with mild cognitive impairment and dementia, only 35.7% of trials reported moderate to high adherence (i.e., 80% or greater) (Gomes-Osman et al., 2018). Secondary analyses from these data indicate an expected decrease in exercise adherence in studies of those with cognitive impairment, compared with older healthy adults (Di Lorito et al., 2020; Gomes-Osman et al., 2018). Our preliminary findings serve as a starting point to inform more individually tailored interventions aiming to improve PA adherence rate in this population.
Participants in the EDU-ACT intervention were able to maintain an average of 10,200 steps/day for at least 4 weeks, which was approximately 2,200 steps/day greater than the control group. This exceeds the public recommendations that advise older adults to maintain a minimum of 6,000 steps/day to achieve at least 150 minutes of walking or moderate activity per week (Tudor-Locke et al., 2011). In fact, participants in the EDU-ACT intervention actually met the recommended physical activity levels for the young adult population (approximately 7,000 to 11,000 steps/day) (Tudor-Locke et al., 2011). The overall average of 8,000 steps/day in the control group likely reflects the previously reported finding that physical activity monitoring by itself can lead to increased physical activity (Bravata et al., 2007; Clemes & Parker, 2009; Nishiwaki et al., 2014).
We believe that the high adherence and compliance rates and the clinically meaningful improvements in PA can be attributed to a combination of the coaching and the evidence-based education strategies used in this trial. While evidence on the effect size of coaching strategies for PA in older adults is limited (0.27) (Oliveira et al., 2017), we found an effect size of 0.93, and an improvement of 2,000 steps/day relative to the control group. According to a recent meta-analysis, this is greater than the effect size associated with PA improvement from other reported multimodal PA education interventions in older adults (0.18, and 620 steps/day, respectively) (Chase, 2015).
We did not expect that our 3-day EDU-ACT intervention would lead to direct improvements in neurocognitive function, and this was supported by the minimal relative gains in processing speed and dual-task performance observed in the EDU-ACT group. We were instead, mostly interested in examining whether EDU-ACT would set in motion ‘signs of a lifestyle change’ in our participants, which would then, perhaps accompany preliminary neurocognitive gains. We are cognizant that the study was not designed to fully control for educational level, but we believed that this exploratory analysis would be of value in planning subsequent studies of EDU-ACT. Interestingly, while education levels did not modify between-group change in physical activity, between-group neurocognitive gains differed according to education levels. Individuals with low education reaped the greatest neurocognitive benefits when randomized to the EDU-ACT group, demonstrating significant improvements in executive function and dual-task performance, when compared with individuals with a moderate or high level of education.
High levels of education throughout the lifespan are one of many factors that have been shown to maintain cognitive reserve in aging adults, conferring protection against the risk of developing dementia later in life (Livingston et al., 2020). While individuals with higher education levels tend to demonstrate: 1) greater engagement in PA (Florindo et al., 2001; Shaw & Spokane, 2008), and 2) better cognitive outcomes in later life (Castro-Costa et al., 2019; de Azeredo Passos et al., 2015; Díaz-Venegas et al., 2019), our results suggest that older adults with low level of education might show more robust cognitive benefit from an educational approach targeting improved PA. These preliminary findings begin to shed light on inter-individual differences in responsiveness to behavioral interventions targeting increased physical activity in older adults.
The strengths of this study are that we present a 3-day, multimodal, evidence-based, low-cost intervention (EDU-ACT) that is feasible and effective at promoting clinically meaningful increases in PA, for at least one-month post-intervention in older adults with memory complaints. Another strength is that our EDU-ACT program emphasizes the scientific evidence by employing objective outcomes to assess gains, which are less reliant on subjective constructs, such as satisfaction. There are clinically available commercial programs that aim to also harness evidence-based approaches to engage older adults to increase brain health and well-being, but unfortunately lack objective cognitive assessment metrics of impact (Bendheim, 2009; Green, 2010).
Some limitations should be mentioned in our study. By not measuring baseline PA in steps, we were unable to compute change scores. However, we measured baseline PA using the IPAQ, which is the gold-standard instrument that is used to classify PA behavior, and this data demonstrate that individuals in both groups were sedentary prior to study enrollment. Future studies should account for education, language, and culture that could impact retention/carryover of information and group dynamics. Other limitations include modest sample size, low representation of male participants, and lack of a longer follow-up period, which would have been useful to evaluate potential longer-lasting changes. Future studies should implement this program on an ongoing basis using a larger sample size and/or incorporate at other outpatient/inpatient settings.
CONCLUSIONS
Our findings demonstrate that a multimodal PA education and coaching intervention is feasible and well accepted by older adults with memory complaints, and when compared with a control group, the participation in the EDU-ACT program was associated with: 1) significant increase in physical activity participation for at least 4 weeks and; 2) minimal improvements in cognition (processing speed and dual-task performance) in older, sedentary individuals with memory complaints. Education level did not predict an increase in physical activity participation, but did predict preliminary improvement in neurocognitive performance, with individuals with lower education demonstrating greater improvements.
Supplementary Material
Funding:
Gomes-Osman was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR002737. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Cabral, Santos, and Oliveira were supported by the Alagoas State Research Support Foundation (FAPEAL).
Footnotes
Declaration of Conflict of interest: None.
REFERENCES
- Baldwin P, King G, Evans J, McDougall S, Tucker MA, & Servais M (2013). Solution-Focused Coaching in Pediatric Rehabilitation: An Integrated Model for Practice. Physical & Occupational Therapy In Pediatrics, 33(4), 467–483. 10.3109/01942638.2013.784718 [DOI] [PubMed] [Google Scholar]
- Bassett DR, & John D (2010). Use of pedometers and accelerometers in clinical populations: validity and reliability issues. Physical Therapy Reviews, 15(3), 135–142. 10.1179/1743288X10Y.0000000004 [DOI] [Google Scholar]
- Battery AIT (1994). Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office. [Google Scholar]
- Bendheim PE (2009). The Brain Training Revolution: A Proven Workout for Healthy Brain Aging. United States: Sourcebooks. [Google Scholar]
- Berg KO, Maki BE, Williams JI, Holliday PJ, & Wood-Dauphinee SL (1992). Clinical and laboratory measures of postural balance in an elderly population. Archives of Physical Medicine and Rehabilitation, 73(11), 1073–1080. 10.5555/uri:pii:000399939290174U [DOI] [PubMed] [Google Scholar]
- Bertolazi AN (2008). Tradução, adaptação cultural e validação de dois instrumentos de avaliação do sono: escala de sonolência de Epworth e índice de qualidade de sono de Pittsburgh [Universidade Federal do Rio Grande do Sul]. In Digital Repositoy of the UFRGS. http://hdl.handle.net/10183/14041 [Google Scholar]
- Ble A, Volpato S, Zuliani G, Guralnik JM, Bandinelli S, Lauretani F, Bartali B, Maraldi C, Fellin R, & Ferrucci L (2005). Executive function correlates with walking speed in older persons: the InCHIANTI study. Journal of the American Geriatrics Society, 53(3), 410–415. 10.1111/j.1532-5415.2005.53157.x [DOI] [PubMed] [Google Scholar]
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, & Sirard JR (2007). Using pedometers to increase physical activity and improve health: A systematic review. In Journal of the American Medical Association (Vol. 298, Issue 19, pp. 2296–2304). American Medical Association. 10.1001/jama.298.19.2296 [DOI] [PubMed] [Google Scholar]
- Brucki SMD, Nitrini R, Caramelli P, Bertolucci PHF, & Okamoto IH (2003). Sugestões para o uso do mini-exame do estado mental no Brasil [Suggestions for Utilization of the Mini-Mental State Examination in Brazil]. Arquivos de Neuro-Psiquiatria, 61(3B), 777–781. 10.1590/S0004-282X2003000500014 [DOI] [PubMed] [Google Scholar]
- Castro-Costa E, Lima-Costa MF, Andrade F. B. de, de Souza Junior PRB, & Ferri CP (2019). Cognitive function among older adults. Revista de Saúde Pública, 52(Suppl 2), 4s. 10.11606/s1518-8787.2018052000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2011). Cognitive impairment: A Call for Action, Now! www.cdc.gov/brfss.
- Chase J-AD (2015). Interventions to Increase Physical Activity Among Older Adults: A Meta-Analysis. The Gerontologist, 55(4), 706–718. 10.1093/geront/gnu090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FT, Etnier JL, Chan KH, Chiu PK, Hung TM, & Chang YK (2020). Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. In Sports Medicine (Vol. 50, Issue 8, pp. 1451–1467). Springer. 10.1007/s40279-020-01292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemes SA, Mears R, Brown C, & Varela-Silva I (2010). Accuracy Of The Yamax PW-611 Pedometer For Measuring Steps, Distance Walked And Pace. Medicine & Science in Sports & Exercise, 42, 481. 10.1249/01.mss.0000385075.24927.10 [DOI] [Google Scholar]
- Clemes SA, & Parker RAA (2009). Increasing our understanding of reactivity to pedometers in adults. Medicine and Science in Sports and Exercise, 41(3), 674–680. 10.1249/MSS.0b013e31818cae32 [DOI] [PubMed] [Google Scholar]
- Coffman MJ, Reeve CL, Butler S, Keeling M, & Talbot LA (2016). Accuracy of the Yamax CW-701 Pedometer for measuring steps in controlled and free-living conditions. Digital health, 2, 205520761665252. 10.1177/2055207616652526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (Routledge Academic (ed.); p. 40). New York, NY: Routledge Academic. [Google Scholar]
- Crouter SE, Schneider PL, Karabulut M, & Bassett DR (2003). Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Medicine and Science in Sports and Exercise, 35(8), 1455–1460. 10.1249/01.MSS.0000078932.61440.A2 [DOI] [PubMed] [Google Scholar]
- Cueto V, Wang CJ, & Sanders LM (2019). Impact of a Mobile App–Based Health Coaching and Behavior Change Program on Participant Engagement and Weight Status of Overweight and Obese Children: Retrospective Cohort Study. JMIR MHealth and UHealth, 7(11), e14458. 10.2196/14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G (2012). Understanding the New Statistics: Effect sizes, Confidence Intervals, and Meta-Analysis. New York, NY: Routledge Academic. [Google Scholar]
- Cummings JL, Morstorf T, & Zhong K (2014). Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Research & Therapy, 6(4), 37. 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SM, Cooper RL, & Cassie KM (2009). Motivational Interviewing to Affect Behavioral Change in Older Adults. Research on Social Work Practice, 19(2), 195–204. 10.1177/1049731508320216 [DOI] [Google Scholar]
- da Silva WR, Bonafé FSS, Marôco J, Maloa BFS, & Campos JADB (2018). Psychometric properties of the world health organization quality of life instrument-abbreviated version in portuguese-speaking adults from three different countries. Trends in Psychiatry and Psychotherapy, 40(2), 104–113. 10.1590/2237-6089-2017-0058 [DOI] [PubMed] [Google Scholar]
- de Azeredo Passos VM, Giatti L, Bensenor I, Tiemeier H, Ikram MA, de Figueiredo RC, Chor D, Schmidt MI, & Barreto SM (2015). Education plays a greater role than age in cognitive test performance among participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). BMC Neurology, 15(1), 1–9. 10.1186/s12883-015-0454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorito C, Bosco A, Booth V, Goldberg S, Harwood RH, & Van der Wardt V (2020). Adherence to exercise interventions in older people with mild cognitive impairment and dementia: A systematic review and meta-analysis. In Preventive Medicine Reports, 19, 101–139. Elsevier Inc. 10.1016/j.pmedr.2020.101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Venegas C, Samper-Ternent R, Michaels-Obregón A, & Wong R (2019). The effect of educational attainment on cognition of older adults: results from the Mexican Health and Aging Study 2001 and 2012. Aging and Mental Health, 23(11), 1586–1594. 10.1080/13607863.2018.1501663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, & Powell KE (2019). Physical Activity, Cognition, and Brain Outcomes. Medicine & Science in Sports & Exercise, 51(6), 1242–1251. 10.1249/MSS.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck RS, Davis JC, Best JR, Crockett RA, & Liu-Ambrose T (2019). Impact of exercise training on physical and cognitive function among older adults: a systematic review and meta-analysis. Neurobiology of Aging, 79, 119–130. 10.1016/j.neurobiolaging.2019.03.007 [DOI] [PubMed] [Google Scholar]
- de Fatori CO, Leite CF, de Souza LAPS, & Patrizzi LJ (2015). Dupla tarefa e mobilidade funcional de idosos ativos. Revista Brasileira de Geriatria e Gerontologia, 18(1), 29–37. 10.1590/1809-9823.2015.13180 [DOI] [Google Scholar]
- Fisher EB (2008). The Importance of Context in Understanding Behavior and Promoting Health. Annals of Behavioral Medicine, 35(1), 3–18. 10.1007/s12160-007-9001-z [DOI] [PubMed] [Google Scholar]
- Florindo AA, do de Latorre MRDO, Tanaka T, Jaime PC, & de Zerbini CAF (2001). Fatores associados à prática de exercícios físicos em homens voluntários adultos e idosos residentes na Grande São Paulo, Brasil. Revista Brasileira de Epidemiologia, 4(2), 105–113. 10.1590/S1415-790X2001000200005 [DOI] [Google Scholar]
- Gomes-Osman J, Cabral DF, Morris TP, McInerney K, Cahalin LP, Rundek T, Oliveira A, & Pascual-Leone A (2018). Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurology: Clinical Practice, 8(3). 10.1212/CPJ.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlan M, Bernard P, Bortolon C, Romain AJ, Lareyre O, Carayol M, Ninot G, & Boiché J (2016). Efficacy of theory-based interventions to promote physical activity. A meta-analysis of randomised controlled trials. Health Psychology Review, 10(1), 50–66. 10.1080/17437199.2014.981777 [DOI] [PubMed] [Google Scholar]
- Green CR (2010). Total Brain Health Toolkits. https://totalbrainhealth.com/#toolbox [Google Scholar]
- Greysen SR, Stijacic Cenzer I, Boscardin WJ, & Covinsky KE (2017). Functional Impairment: An Unmeasured Marker of Medicare Costs for Postacute Care of Older Adults. Journal of the American Geriatrics Society, 65(9), 1996–2002. 10.1111/jgs.14955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessl EJ, Kaplan RM, Castro Sweet CM, Church T, Espeland MA, Gill TM, Glynn NW, King AC, Kritchevsky S, Manini T, Rushing J, & Pahor M (2016). Cost-effectiveness of the LIFE Physical Activity Intervention for Older Adults at Increased Risk for Mobility Disability. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 71(5), 656–662. 10.1093/gerona/glw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekler EB, Buman MP, Otten J, Castro CM, Grieco L, Marcus B, Friedman RH, Napolitano MA, & King AC (2013). Determining who responds better to a computer- vs. human-delivered physical activity intervention: Results from the community health advice by telephone (CHAT) trial. International Journal of Behavioral Nutrition and Physical Activity, 10(1), 1. 10.1186/1479-5868-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofheinz M, & Mibs M (2016). The Prognostic Validity of the Timed Up and Go Test With a Dual Task for Predicting the Risk of Falls in the Elderly. Gerontology and Geriatric Medicine, 2, 233372141663779. 10.1177/2333721416637798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly VE, Janke AA, & Shumway-Cook A (2010). Effects of instructed focus and task difficulty on concurrent walking and cognitive task performance in healthy young adults. Experimental Brain Research, 207(1–2), 65–73. 10.1007/s00221-010-2429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox ECL, Musson H, & Adams EJ (2015). Knowledge of physical activity recommendations in adults employed in england: Associations with individual and workplace-related predictors. International Journal of Behavioral Nutrition and Physical Activity, 12(1), 1–8. 10.1186/s12966-015-0231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Long KH, Ransom JE, Roberts RO, Hass SL, Duhig AM, Smith CY, Emerson JA, Pankratz VS, & Petersen RC (2015). Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population-based study. Alzheimer’s & Dementia, 11(8), 917–932. 10.1016/j.jalz.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, … Cooper C (2020). The Lancet Commissions Dementia prevention, intervention, and care: 2020 report of the Lancet Commission The Lancet Commissions. The Lancet, 396, 413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobelo F, Stoutenberg M, & Hutber A (2014). The Exercise is Medicine Global Health Initiative: a 2014 update. British Journal of Sports Medicine, 48(22), 1627–1633. 10.1136/bjsports-2013-093080 [DOI] [PubMed] [Google Scholar]
- Magalhães J, Barbosa M, De Sá B, Prates S, Ferreira Gonçalves C, Aquino AR, Parentoni AN, & Barbosa JMM (2008). Efeito da realização simultânea de tarefas cognitivas e motoras no desempenho funcional de idosos da comunidade Dual task effects on functional performance in community-dwelling elderly. Fisioter Pesq, 15(4), 374–383. [Google Scholar]
- Magalhães SS, Malloy-Diniz LF, & Hamdan AC (2012). Validity convergent and reliability test-retest of the rey auditory verbal learning test. Clinical Neuropsychiatry, 9(3), 129–137. [Google Scholar]
- Malloy-Diniz L, Cruz M, Torres V, & Cosenza R (2000). O teste de aprendizagem auditivo-verbal de Rey: normas para uma populacao brasileira. Revista Brasileira de Neurologia, 36(3), 79–83. [Google Scholar]
- Mendoza L, Horta P, Espinoza J, Aguilera M, Balmaceda N, Castro A, Ruiz M, Díaz O, & Hopkinson NS (2015). Pedometers to enhance physical activity in COPD: a randomised controlled trial ∣. Eur Respir J, 45, 347–354. 10.1183/09031936.00084514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W ., & Rollnick S (2013). Motivational interviewing: Preparing people to change. New York, NY: Guilford Press. [Google Scholar]
- Miller WR (1999). Chapter 3: Motivational Interviewing as a Counseling Style. In Enhancing Motivation For Change in Substance Abuse Treatment (35th ed., p. 41). Substance Abuse and Mental Health Services Administration. https://www.ncbi.nlm.nih.gov/books/NBK64967/ [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational Interviewing: Preparing People For Change (2nd ed.). Guilford Press. [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, & Altman DG (2012). CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. International Journal of Surgery, 10(1), 28–55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Morey MC, Ekelund C, Pearson M, Crowley G, Peterson M, Sloane R, Pieper C, McConnell E, & Bosworth H (2006). Project LIFE: A partnership to increase physical activity in elders with multiple chronic illnesses. Journal of Aging and Physical Activity, 14(3), 324–343. 10.1123/japa.14.3.324 [DOI] [PubMed] [Google Scholar]
- Nishiwaki M, Kuriyama A, Ikegami Y, Nakashima N, & Matsumoto N (2014). A pilot crossover study: Effects of an intervention using an activity monitor with computerized game functions on physical activity and body composition. Journal of Physiological Anthropology, 33(1). 10.1186/1880-6805-33-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JM, Cherbuin N, Pumpa KL, Smee DJ, & Rattray B (2018). Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-Analysis. In British Journal of Sports Medicine. 52(3), 154–160. BMJ Publishing Group. 10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- Notthoff N, & Carstensen LL (2014). Positive messaging promotes walking in older adults. Psychology and Aging, 29(2), 329–341. 10.1037/a0036748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JS, Sherrington C, Amorim AB, Dario AB, & Tiedemann A (2017). What is the effect of health coaching on physical activity participation in people aged 60 years and over? A systematic review of randomised controlled trials. In British Journal of Sports Medicine, 51(19), 1425–1432. BMJ Publishing Group. 10.1136/bjsports-2016-096943 [DOI] [PubMed] [Google Scholar]
- Olson EA, McAuley E, & Olson EA (2015). Impact of a brief intervention on self-regulation, self-efficacy and physical activity in older adults with type 2 diabetes HHS Public Access. J Behav Med, 38(6), 886–898. 10.1007/s10865-015-9660-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandonatos GD, Ott BR, Davis JD, Barco PP, & Carr DB (2015). Clinical utility of the trail-making test as a predictor of driving performance in older adults. Journal of the American Geriatrics Society, 63(11), 2358–2364. 10.1111/jgs.13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, & Di Clemente CC (1982). Transtheoretical therapy: Toward a more integrative model of change. Psychotherapy, 19(3), 276–288. 10.1037/h0088437 [DOI] [Google Scholar]
- Queiroz Garcia I, Amaral Pessoa I, Monteiro B, Daniel F, Lemos L, & Espírito Santo H (2016). Propriedades psicométricas da versão Torga do Teste Stroop ∣ Psychometric properties of the Torga version of the Stroop Test. Revista Portuguesa de Investigação Comportamental e Social, 2(2), 55–64. 10.19234/ismt.rpics.2016.2.2.41 [DOI] [Google Scholar]
- Rabin LA, Barr WB, & Burton LA (2005). Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Archives of Clinical Neuropsychology : The Official Journal of the National Academy of Neuropsychologists, 20(1), 33–65. 10.1016/j.acn.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’Examen Clinique en Psychologie. Paris: Press Universitaire de France. [Google Scholar]
- Richardson S (1991). The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. Journal of the American Geriatrics Society, 39(2), 142–148. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, Rodríguez-Sánchez JM, Ríos-Lago M, Tirapu J, & Barceló F (2009). Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. In Journal of the International Neuropsychological Society (Vol. 15, Issue 3, pp. 438–450). Cambridge University Press. 10.1017/S1355617709090626 [DOI] [PubMed] [Google Scholar]
- Scheider PL, Crouter SE, Lukajic O, & Bassett DR (2003). Accuracy and Reliability of 10 Pedometers for Measuring Steps over a 400-m Walk. Medicine & Science in Sports & Exercise, 35(10), 1779–1784. 10.1249/01.MSS.0000089342.96098.C4 [DOI] [PubMed] [Google Scholar]
- Schneider PL, Crouter SE, & Bassett DR (2004). Pedometer Measures of Free-Living Physical Activity: Comparison of 13 Models. Medicine and Science in Sports and Exercise, 36(2), 331–335. 10.1249/01.MSS.0000113486.60548.E9 [DOI] [PubMed] [Google Scholar]
- Sforzo GA, Kaye MP, Todorova I, Harenberg S, Costello K, Cobus-Kuo L, Faber A, Frates E, & Moore M (2018). Compendium of the Health and Wellness Coaching Literature. American Journal of Lifestyle Medicine, 12(6), 436–447. 10.1177/1559827617708562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BA, & Spokane LS (2008). Examining the association between education level and physical activity changes during early old age. Journal of Aging and Health, 20(7), 767–787. 10.1177/0898264308321081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CA, Fowles JR, Dunbar P, Barron B, McQuaid S, & Dillman CJ (2013). Increasing Diabetes Educators’ Confidence in Physical Activity and Exercise Counselling: The Effectiveness of the “Physical Activity and Exercise Toolkit” Training Intervention. Canadian Journal of Diabetes, 37(6), 381–387. 10.1016/j.jcjd.2013.08.265 [DOI] [PubMed] [Google Scholar]
- Silva-Smith AL, Fleury J, & Belyea M (2013). Effects of a physical activity and healthy eating intervention to reduce stroke risk factors in older adults. Preventive Medicine, 57(5), 708–711. 10.1016/j.ypmed.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, & Sherwood A (2010). Aerobic Exercise and Neurocognitive Performance: a Meta- Analytic Review of Randomized Controlled Trials. Psychosom Med, 72(3), 239–252. 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathi A, Withall J, Thompson JL, Davis MG, Gray S, De Koning J, Parkhurst G, Lloyd L, Greaves C, Laventure R, & Fox KR (2020). Feasibility Trial Evaluation of a Peer Volunteering Active Aging Intervention: ACE (Active, Connected, Engaged). The Gerontologist, 60(3), 571–582. 10.1093/geront/gnz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EE, & Fox C (2011). Encouraging Patients to Change Unhealthy Behaviors with Motivational Interviewing. In Family Practice Management, 18. Retrieved from: https://www.aafp.org/dam/AAFP/documents/patient_care/nrn/motivinterview2.pdf [PubMed] [Google Scholar]
- Tak ECPM, van Uffelen JGZ, Paw MJMCA, van Mechelen W, & Hopman-Rock M (2012). Adherence to Exercise Programs and Determinants of Maintenance in Older Adults With Mild Cognitive Impairment. Journal of Aging and Physical Activity, 20(1), 32–46. 10.1123/japa.20.1.32 [DOI] [PubMed] [Google Scholar]
- Tang P-F, Yang H-J, Peng Y-C, & Chen H-Y (2015). Motor dual-task Timed Up & Go test better identifies prefrailty individuals than single-task Timed Up & Go test. Geriatrics & Gerontology International, 15(2), 204–210. 10.1111/ggi.12258 [DOI] [PubMed] [Google Scholar]
- The Alzheimer’s Association. (2020). 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 16(3), 391–460. 10.1002/alz.12068 [DOI] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR, & Trenerry M (1995). Stroop neuropsychological screening test (Manual). United State: Psychological Assessment Resources. [Google Scholar]
- Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, Hatano Y, Inoue S, Matsudo SM, Mutrie N, Oppert JM, Rowe DA, Schmidt MD, Schofield GM, Spence JC, Teixeira PJ, Tully MA, & Blair SN (2011). How many steps/day are enough? for adults. International Journal of Behavioral Nutrition and Physical Activity, 8(1), 79. 10.1186/1479-5868-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Academy of Medicine. (2019). Healthy Longevity Global Grand Challenge. https://nam.edu/initiatives/grand-challenge-healthy-longevity/
- United Nations, Department of Economic and Social Affairs, Population Division (2017). World Population Ageing 2017 - Highlights (ST/ESA/SER.A/397). New York: United Nations. [Google Scholar]
- Vaara JP, Vasankari T, Koski HJ, & Kyröläinen H (2019). Awareness and Knowledge of Physical Activity Recommendations in Young Adult Men. Frontiers in Public Health, 7, 310. 10.3389/fpubh.2019.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema DM, Hansen H, High R, Goetsch T, & Siu KC (2019). Minimal Detectable Change in Dual-Task Cost for Older Adults With and Without Cognitive Impairment. Journal of Geriatric Physical Therapy, 42(4), E32–E38. 10.1519/JPT.0000000000000194 [DOI] [PubMed] [Google Scholar]
- Wanigatunga AA, Tudor-Locke C, Axtell RS, Glynn NW, King AC, McDermott MM, Fielding RA, Lu X, Pahor M, & Manini TM (2017). Effects of a Long-Term Physical Activity Program on Activity Patterns in Older Adults. Medicine and Science in Sports and Exercise, 49(11), 2167–2175. 10.1249/MSS.0000000000001340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: World Health Organization Press; 2013. [Google Scholar]
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jönsson L, Liu Z, & Prince M (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s and Dementia, 13(1), 1–7. 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2010). Global Recommendations on Physical Activity for Health. In WHO Press. https://www.who.int/dietphysicalactivity/publications/9789241599979/en/ [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J, & Tabet N (2015). Aerobic exercise to improve cognitive function in older people without known cognitive impairment. In Cochrane Database of Systematic Reviews, 4. John Wiley and Sons Ltd. 10.1002/14651858.CD005381.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS, Peters JJ, & Squibb M (2013). Health-Related Resource Use and Costs in Elderly Adults with and without Mild Cognitive Impairment NIH Public Access. J Am Geriatr Soc, 61(3), 396–402. 10.1111/jgs.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubala A, MacGillivray S, Frost H, Kroll T, Skelton DA, Gavine A, Gray NM, Toma M, & Morris J (2017). Promotion of physical activity interventions for community dwelling older adults: A systematic review of reviews. PLOS ONE, 12(7), e0180902. 10.1371/journal.pone.0180902 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


