Abstract
Characterization of enterotoxigenic Escherichia coli (ETEC) has been based almost exclusively on the detection of phenotypic traits such as serotypes and virulence-associated factors: heat-labile (LT) and heat-stable (ST) toxins and colonization factors (CFs). In the present work we show that the analysis of band patterns generated by randomly amplified polymorphic DNA (RAPD) analysis and pulsed-field gel electrophoresis (PFGE) of digested chromosomal DNA can be used to detect genetic diversity among ETEC strains expressing identical phenotypic traits. The study included 29 ETEC isolates from Latin America and Spain expressing the phenotype O153:H45 CFA/I ST plus 1 rough derivative, 2 nonmotile derivatives, and 1 O78:H12 CFA/I ST isolate, and a representative of a genetically distinct ETEC group. The results showed that the O153:H45 CFA/I ST ETEC isolates belong to a single clonal cluster whose isolates share on average, 84% of the RAPD bands and 77% of the PFGE restriction fragments, while the O78:H12 isolate shared only 44 and 4% of the RAPD bands and PFGE fragments, respectively, with the isolates of the O153:H45 group. More relevantly, RAPD and PFGE fingerprints disclosed the presence of different clonal lineages among the isolates of the O153:H45 cluster. Some of the genetic variants were isolated from defined geographic areas, while places like São Paulo City in Brazil and the middle-eastern part of Argentina were populated by several genetic variants of related, but not identical, ETEC strains. These results show that molecular biology-based typing methods can disclose strain diversity, which is usually missed in studies restricted to phenotypic typing of ETEC.
Enterotoxigenic Escherichia coli (ETEC) represents one of the main etiologic agents of diarrhea in infants and travelers in developing countries (5). ETEC strains are identified by the ability to produce enterotoxins, either heat-labile toxin or heat-stable toxin (ST), or both, and surface adhesins known as colonization factors (CFs). Also, characterization of ETEC strains has relied on the serological determination of a number of different combinations of O (lypopolyssacharide) and H (flagellar) serogroups (4, 7, 11, 13, 28). Antigen heterogeneity is a striking feature among ETEC strains, as demonstrated in a survey that evaluated the diversity, distribution, and association of ETEC phenotypes in epidemiological studies carried out in different parts of the world (31).
The application of DNA-based typing methods to investigate the genetic relationship among ETEC strains isolated from humans has been rare (16, 19). Previous studies, based on the use of randomly amplified polymorphic DNA (RAPD) analyses, indicated that, in contrast to other pathogenic E. coli groups (8, 30), ETEC strains that share an O:H serotype but not necessarily the same virulence-associated factors belong to clonal clusters (21, 22, 23). Moreover, RAPD analysis can reveal variant genotypes among ETEC strains that share the same O:H serotype and that belong to the same clonal cluster (22, 23).
The O153:H45 CFA/I ST ETEC phenotype seems to be one of the most frequently found phenotypes in Latin America and Spain, where it represents a leading cause of infantile diarrhea (1, 4, 6, 13, 28). It has been suggested that its prevalence reflects the past and present extensive cultural and economic interchanges by the populations in these regions (12). In the present study we used RAPD analysis and pulsed-field gel electrophoresis (PFGE) as DNA-based typing methods to investigate the genetic relationship among O153:H45 CFA/I ST ETEC isolates collected from diarrheic children in Latin America and Spain. The results show that the isolates with this phenotype are clonally related; in addition, the analysis of DNA profiles unveiled significant intraserotype diversity which would otherwise pass unnoticed.
MATERIALS AND METHODS
Bacterial isolates.
A total of 29 ETEC isolates that belonged to serotype O153:H45 and that expressed the CFA/I ST phenotype were included in the work described here (Table 1). Two nonflagellated isolates and one rough isolate were putatively considered derivatives of a parenteral O153:H45 strain and were included in the present analysis. All isolates were unable to ferment rhamnose and, therefore, belonged to the same biotype. Twelve isolates were collected in Brazil: 11 from São Paulo City (provided by B. E. Guth) and 1 from Recife, in the northeastern region of Brazil (provided by M. Magalhães). Four isolates from Mexico were kindly provided by A. Cravioto, while two isolates from Spain were supplied by the International Escherichia coli and Klebsiella Center, Copenhagen, Denmark. Fourteen isolates were collected from different regions in Argentina: 7 from the middle-eastern region (1 from Buenos Aires, 2 from La Plata, and 4 from Rosario) and 7 from the northeastern region (3 from Las Dolores, 2 from Zaimán, and 2 from Posadas). The serotype, toxin, and CF profiles of these isolates were determined by standard methods (4, 27). One ETEC isolate, isolated in Argentina, that expressed the O78:H12 CFA/I ST phenotype was included in the present work as an outgroup control sample for comparative purposes.
TABLE 1.
Characteristics of the isolates studied
| Isolate | Geographic origina | Date of isolation (mo and yr or yr) | Phenotypic trait
|
RAPD profiles
|
RAPD type | PFGE type | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serotype | Toxin | CF | Rhamb | Primer 1254 | Primer 1290 | |||||
| AR1 | Las Dolores (NE Argentina) | Aug 1988 | O153:H45 | ST | CFA/I | − | A1 | B1 | 1 | 13 |
| AR2 | Las Dolores (NE Argentina) | Dec 1988 | O153:H45 | ST | CFA/I | − | A1 | B1 | 1 | 13 |
| AR3 | Las Dolores (NE Argentina) | Dec 1988 | Rough:H45 | ST | CFA/I | − | A1 | B1 | 1 | 13 |
| AR4 | Posadas (NE Argentina) | Jan 1988 | O153:H− | ST | CFA/I | − | A2 | B1 | 2 | 1 |
| AR5 | Posadas (NE Argentina) | Jan 1988 | O153:H− | ST | CFA/I | − | A2 | B1 | 2 | 1 |
| AR6 | Zaimán (NE Argentina) | Nov 1989 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 2 |
| AR7 | Zaimán (NE Argentina) | Dec 1989 | O153:H45 | ST | CFA/I | − | A2 | B2 | 3 | 4 |
| AR8 | Rosario (ME Argentina) | May 1987 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 2 |
| AR9 | Rosario (ME Argentina) | Apr 1989 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 1 |
| AR10 | Rosario (ME Argentina) | Dec 1987 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 9 |
| AR11 | Rosario (ME Argentina) | Mar 1988 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 9 |
| AR12 | La Plata (ME Argentina) | Jun 1989 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 1 |
| AR13 | La Plata (ME Argentina) | Apr 1988 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 7 |
| AR14 | Buenos Aires (ME Argentina) | Dec 1987 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 7 |
| BR1 | São Paulo (SE Brazil) | 1989 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 8 |
| BR2 | São Paulo (SE Brazil) | 1989 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 8 |
| BR3 | São Paulo (SE Brazil) | 1989 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 10 |
| BR4 | São Paulo (SE Brazil) | 1983 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 7 |
| BR5 | São Paulo (SE Brazil) | 1983 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 3 |
| BR6 | São Paulo (SE Brazil) | 1984 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 12 |
| BR7 | São Paulo (SE Brazil) | 1984 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 3 |
| BR8 | São Paulo (SE Brazil) | 1984 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | ND |
| BR9 | São Paulo (SE Brazil) | 1985 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 3 |
| BR10 | São Paulo (SE Brazil) | 1985 | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 11 |
| BR11 | São Paulo (SE Brazil) | 1985 | O153:H45 | ST | CFA/I | − | A2 | B2 | 3 | 7 |
| BR12 | Recife (NE Brazil) | 1990 | O153:H45 | ST | CFA/I | − | A3 | B3 | 4 | 5 |
| MX1 | Mexico | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 6 | |
| MX2 | Mexico | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 6 | |
| MX3 | Mexico | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 6 | |
| MX4 | Mexico | O153:H45 | ST | CFA/I | − | A2 | B1 | 2 | 6 | |
| SP1 | Spain | O153:H45 | ST | CFA/I | − | A2 | B4 | 5 | 14 | |
| SP2 | Spain | O153:H45 | ST | CFA/I | − | A2 | B4 | 5 | 14 | |
| AR15 | Cordoba (Argentina) | O78:H12 | ST | CFA/I | − | A4 | B5 | 6 | 15 | |
ME, middle-eastern; NE, northeastern; SE, southeastern.
Rham, ability to ferment rhamnose.
RAPD typing.
Bacterial growth, template DNA preparation, amplification reactions, and electrophoretic analysis of products were performed as described previously (22). Each isolate was independently tested with two primers: primer 1254 (5′-CCGCAGCCAA-3′) and primer 1290 (5′-GTGGATGCGA-3′). The whole procedure was repeated at least three times for each isolate, and only those bands consistently detected in different experiments were considered for the RAPD profile definition. RAPD profiles were defined by a capital letter, which indicates a similar band pattern, followed by a number, which indicates the detection of at least one polymorphic band.
PFGE typing.
Bacterial samples grown overnight at 37°C were harvested, and the cell concentration was adjusted to 109 CFU/ml in buffer (75 mM NaCl, 25 mM EDTA [pH 7.4]). DNA was prepared in solid agarose plugs by mixing 500 μl of a bacterial cell suspension with an equal volume of 1.6% Pulse Field Certified Agarose (Bio-Rad), followed by incubation overnight at 50°C in lysis buffer (50 mM Tris-HCl [pH 8.0], 50 mM EDTA, 1% lauryl-sarcosine, 0.5 mg of proteinase K per ml). The plugs were washed three times for 40 min each time at 37°C in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) and were then washed with distilled water for 15 min at 37°C and finally washed with restriction enzyme reaction buffer for 15 min. The samples were treated with fresh reaction buffer containing 20 U of XbaI per plug at 37°C overnight. PFGE was performed in a CHEF DRIII electrophoresis chamber (Bio-Rad) for 21 h at 6 V/cm and 14°C in a 1% agarose gel by using a linear pulse ramp of 5 to 50 s in 0.5× Tris-borate-EDTA electrophoresis buffer. The PFGE patterns were designated by different numbers.
Data analysis.
The relatedness among RAPD or PFGE patterns was estimated by the proportion of shared bands by applying the Jaccard coefficient (15). Data recording and calculations were performed with RAPDistance programs, version 1.03 (3), and phenograms were constructed on the basis of the unweighted pair group method with arithmetic means (UPGMA method) included in Molecular Evolutionary Genetics Analysis software, version 1.02 (17).
RESULTS
RAPD typing.
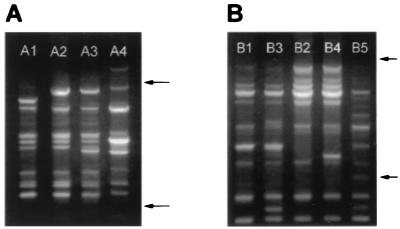
Amplifications performed with primer 1254 resulted in the identification of three RAPD profiles among the O153:H45 isolates (Table 1 and Fig. 1A). These RAPD patterns were distinguished by the presence of three polymorphic bands among a total of 13 amplified bands (23%). For comparative purposes, an O78:H12 CFA/I ST ETEC isolate was used as an outgroup control sample; it showed an RAPD profile similar to those of other O78:H12 CFA/I ST isolates from Brazil and Argentina (data not shown) and was therefore considered representative of a clonal cluster distinct from the O153:H45 group. The band pattern of the O78:H12 isolate showed 53% polymorphic bands compared to the band pattern of the isolates in the O153:H45 group (Fig. 1A). The A2 band profile was shared by 28 of 32 O153:H45 isolates. Intraserotype diversity was detected in the profiles of three isolates from Las Dolores (northeastern Argentina), isolates AR1, AR2, and AR3 (profile A1), and one isolate from Recife (northeastern Brazil), isolate BR12 (profile A3) (Table 1 and Fig. 1A).
FIG. 1.
RAPD profiles of O153:H45 CFA/I ST ETEC strains from Latin America and Spain obtained with primers 1254 (A) and 1290 (B). Profiles are designated as indicated in Table 1 and are indicated at the top. Arrows indicate the positions of 500 and 2,000 bp (bottom and top arrows, respectively). The RAPD profile of a strain that expressed the O78:H12 CFA/I ST phenotype was included for comparison (lane A4 in panel A and lane B5 in panel B).
Amplifications with primer 1290 revealed four RAPD profiles (Table 1 and Fig. 1B) and seven polymorphic bands among a total of 18 amplified bands (39%) among the O153:H45 isolates. When these profiles of O153:H45 isolates were compared with that of the O78:H12 isolate, 57% polymorphic bands were identified (Fig. 1B). Twenty-seven of 30 isolates from Latin America had the B1 RAPD profile (Table 1). Intraserotype diversity was apparent from the variant profiles obtained with primer 1290: only the isolate from Recife (isolate BR12) had the B3 profile, one isolate from São Paulo (isolate BR11) and another isolate from Zaimán (isolate AR7) had the B2 profile, and the B4 profile was exclusively identified among isolates derived from Spain (Table 1).
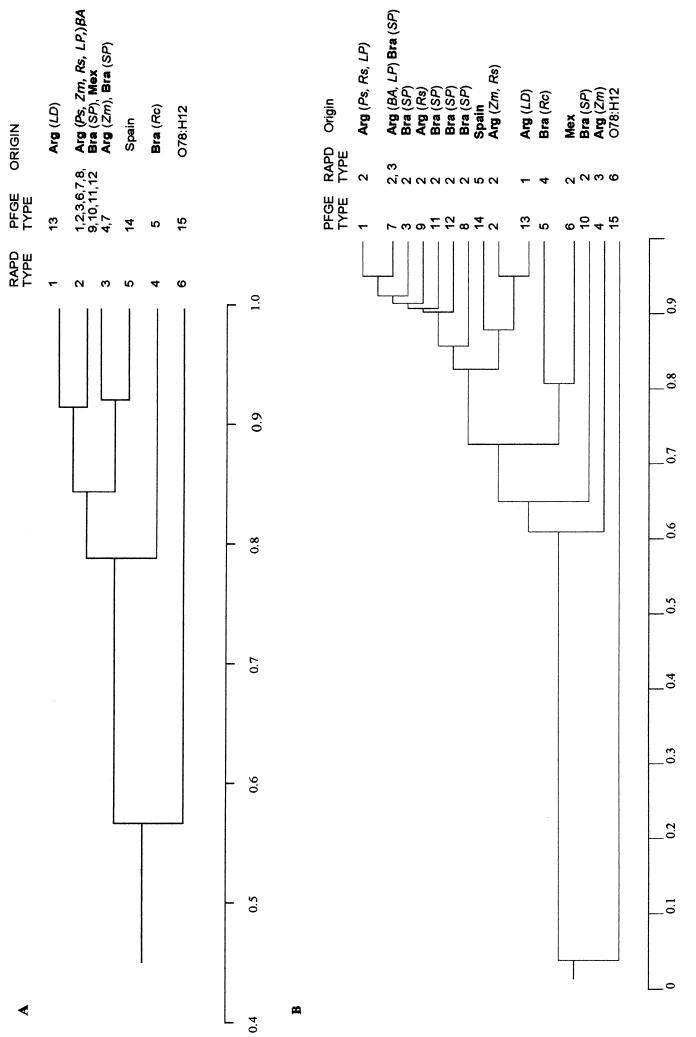
By combining the data obtained with both primers, a total of 31 bands (32% polymorphic) were amplified and five different RAPD types were defined for the O153:H45 isolates (Fig. 1 and Table 1). When the profiles for the isolates in the O153:H45 group were compared to that for the O78:H12 isolate, 44% of the bands were polymorphic. Among the five different RAPD types, type 2 was the most widespread in Latin America and was found among isolates from Brazil, Mexico, and Argentina. RAPD type 1 was found only among ETEC isolates from the community of Las Dolores (isolates AR1, AR2, and AR3). The isolate from Recife (isolate BR12) was the only representative of RAPD type 4. The two isolates from Spain (isolates SP1 and SP2) defined RAPD type 5, which was not shared by any Latin American isolate (Table 1). The degree of similarity among the various RAPD types was estimated from the electrophoretic patterns and was analyzed according to the proportion of shared bands (Fig. 2A). The O153:H45 isolates shared, on average, 84% of the amplified bands (minimum value, 76%; maximum value, 92%). In contrast, the unrelated O78:H12 isolate shared, on average, 56% of the amplified bands (maximum value, 66%) with the O153:H45 group (Fig. 2A). These results indicate that ETEC isolates that express the O153:H45 CFA/I ST phenotype belong to a cluster of genetically related strains. Nevertheless, a significant degree of genetic diversity among the phenotypically indistinguishable O153:H45 CFA/I ST isolates could be detected by RAPD analysis.
FIG. 2.
Phenograms representing the relatedness of O153:H45 ETEC strains on the basis of the results obtained by RAPD typing with two primers (A) or PFGE typing with XbaI-restricted DNA (B). The comparison of samples was based on the proportion of shared bands (indicated on the scale at the bottom of each panel). Groups of similarity were established by the UPGMA method. The RAPD and PFGE types are indicated at the right (designated as indicated in Table 1), and the geographical origins of the strains [countries are indicated in boldface and localities are indicated in italics] are as follows: Arg, Argentina; LD, Las Dolores; Ps, Posadas; Zm, Zaiman; Rs, Rosario; LP, La Plata; BA, Buenos Aires; Bra, Brazil; SP, São Paulo; Rc, Recife; Mex, Mexico). The RAPD and PFGE profiles of an ETEC strain that expressed the O78:H12 CFA/I ST phenotype were included in the analysis for comparative purposes.
PFGE typing.
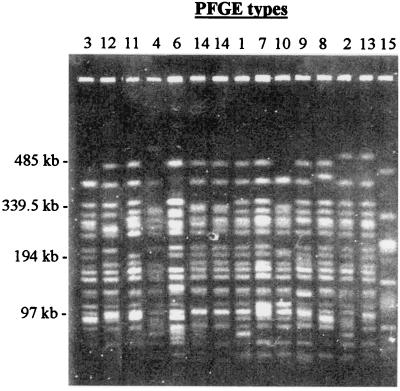
The extent of genetic variability among the O153:H45 isolates was also analyzed by PFGE analysis of XbaI-cleaved chromosomal DNA (Fig. 3 and Table 1). Fourteen different electrophoretic patterns (about 20 bands each) were distinguished. A total of 33 DNA bands were identified; 70% were polymorphic. The PFGE pattern of the representative O78:H12 CFA/I ST isolate, which showed 98% polymorphic bands, was clearly distinct from those detected among the O153:H45 isolates. The discriminatory power of PFGE was higher than that of RAPD analysis with two primers. A phenogram derived from the PFGE data showed a significant degree of similarity among the O153:H45 isolates, which shared, on average, 77% of the amplified bands (minimum value, 56%; maximum valve, 95%), whereas the representative O78:H12 isolate shared, on average, only 4% of the amplified bands with the isolates of the O153:H45 group (Fig. 2B).
FIG. 3.
PFGE profiles of XbaI-restricted DNA of O153:H45 CFA/I ST ETEC strains from Latin America and Spain. Profiles are designated as indicated in Table 1 and are indicated at the top. DNA markers are indicated on the left. The PFGE profile of a strain that expressed the O78:H12 CFA/I ST phenotype was included for comparison (type 15).
As observed by RAPD typing, intraserotype variation was also detected by PFGE analysis, with some of the types being characteristic of certain geographic areas: the 3 isolates from Las Dolores (isolates AR1, AR2, and AR3; type 13), the 4 isolates from Mexico (isolates MX11, MX12, MX13, and MX14; type 6), the two isolates from Spain (isolates SP1 and SP2; type 14) and the isolate from Recife (isolate BR12; type 5) were identified as distinct clones within the O153:H45 CFA/I ST cluster. PFGE analysis also showed that at least six different O153:H45 clones exist in São Paulo City and that at least one of them (type 7) was also isolated from Argentina (La Plata and Buenos Aires). The four isolates from Rosario (isolates AR8, AR9, AR10, and AR11), which were considered identical by RAPD analysis (type 2), proved to represent three different clones (types 2, 1, 9, and 9, respectively). These results confirm the common clonal nature of the O153:H45 CFA/I ST group and disclose a significantly higher degree of diversity among the isolates compared with that detected by the RAPD approach.
The two nonmotile isolates from Posadas (isolates AR4 and AR5) displayed the same RAPD and PFGE types as O153:H45 isolates from Rosario and La Plata (isolates AR9 and AR12, respectively). Similarly, isolate AR3 from Las Dolores, whose O antigen was not typeable, was identical (by both RAPD and PFGE analyses) to the other two isolates from the same region (isolates AR1 and AR2) (Table 1). Although not typeable by certain phenotypic traits, those isolates were shown to belong to the O153:H45 clonal cluster.
DISCUSSION
In this work we have shown that O153:H45 CFA/I ST ETEC isolates from Latin America and Spain share a common genetic origin. The analysis, based on RAPD and PFGE typing, showed that all isolates tested shared a major fraction of their fingerprints (an average of 84% of RAPD bands and 77% of PFGE bands). In contrast, such a high degree of similarity was not observed when this group was compared to an ETEC isolate that expressed the same virulence-associated traits (CFA/I ST) but that belonged to a different serotype, O78:H12 (with which an average of 56% of RAPD bands and 4% of PFGE bands were shared). Our results showed that ETEC isolates of phenotype O153:H45 CFA/I ST derived from different geographic areas represent a cluster of genetically related strains. More interestingly, despite the identical phenotype and common genetic origin of the O153:H45 CFA/I ST ETEC isolates, both RAPD- and PFGE-based typing methods were able to detect variant genotypes among the O153:H45 CFA/I ST ETEC isolates. In addition, using DNA-based typing methods, we were able to define clones among isolates derived from different geographic areas, which suggests that relevant strain diversity is missed in studies based only on phenotypic typing methods.
The observation that a few ETEC O:H serotypes frequently isolated from diarrheic patients expressed fixed combinations of phenotypic traits as biotypes and virulence-associated factors led to the formulation of the clonal concept to describe the structure of bacterial populations (20). Subsequent studies supported the notion that E. coli strains that share a combination of antigenic determinants are probably clonally related (9, 25, 29). A remarkable feature of ETEC strains isolated from humans is the widespread distribution of some combination of phenotypes. (O153:H45 CFA/I ST is one of the most widely distributed and most frequently found ETEC phenotypes). A possible explanation for this fact is the dissemination of one or a few ancestral clones by means of human migration or travel. Another possibility is the horizontal transfer of genes encoding positively selected traits as virulence-associated factors among genetically distinct strains. Our observations, based on RAPD and PFGE analyses, suggest that ETEC strains that express the same O:H serotype but not those that share only O serogroup or virulence-associated factors are clonally related (21, 22, 23; this study). The fact that virulence-associated factors do not represent reliable clonal markers of ETEC strains is not surprising since genes that code for toxins and CFs are usually located in plasmids and are frequently flanked by insertion sequences (10, 14, 32). The previous indication that CFs such as CS17 and CFA/I could represent better clonal markers than serotypes might be attributed to the restricted number of samples analyzed (25).
The definition of clonality should be flexible enough to take into account the variability accumulated in the genomes as a result of recent evolutionary divergence (2). Accordingly, the criteria used for the definition of a clonal group by DNA-based typing methods should be empirically adapted to the discrimination power of the analysis and to the time frame and the population under study. On the basis of the results of previous studies of RAPD, typing the use of a borderline value of approximately 80% shared amplified bands was appropriate to define a clonal group when primers 1254 and 1290 were used (21, 22, 23). For PFGE analysis, which has a higher degree of discriminatory power than RAPD analysis, the minimum number of shared bands needed to identify clonally related ETEC isolates decreases substantially. In the present investigation we considered that isolates that shared more than 50% of their fragments are likely to be genetically related. The definition of such a limit was in accordance with suggested criteria for the interpretation of PFGE patterns (26).
In the present study, the application of DNA fingerprinting methods revealed intraclonal variations among phenotypically identical isolates, and some of the genetic types identified could be correlated with the geographic distribution of the isolates. For example, ETEC isolates from Mexico and Spain could be readily distinguished from each other and from any other isolate from Latin American countries. Moreover, the analysis of DNA profiles revealed that diverse (yet phenotypically identical) ETEC clones may circulate in the same area, as is the case with São Paulo City (at least seven different PFGE types) and the middle-eastern part of Argentina (four PFGE types). In a recent study, ribotyping and plasmid patterns were used, along with phenotypic traits, to characterize ETEC strains isolated in a single area of endemicity in Mexico during a 6-year period (16). Cluster analysis revealed a diversity of clones, and their distribution was related to the time of isolation. Similar to our results, it was observed that ETEC diarrhea was caused by heterogeneous strains and that many strains can be responsible for the disease that occurs in an area of endemicity.
PFGE analysis represents a “gold standard” in epidemiological studies of diverse bacterial pathogens including other E. coli groups (2, 18, 19, 26). In the present study, XbaI-generated DNA fingerprints proved to be more sensitive than RAPD analysis (performed with two primers) for the detection of genetic diversity among phenotypically identical strains. Advances in automation of PFGE analysis have simplified the large-scale use of the technique, and the application of standardized protocols has allowed comparison of patterns generated at different laboratories. Thus, the establishment of a PFGE database of ETEC strains, at least in reference laboratories, could improve the analysis of data generated at different locations. Implementation of DNA-based typing methods for the characterization of ETEC isolates could have a significant impact on epidemiological studies and could also contribute to a better understanding of the genetic structure of natural ETEC populations.
ACKNOWLEDGMENTS
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científíco e Tecnológico (CNPq), PADCT-III, Centro Brasileiro Argentino de Biotecnologia (CBABIO), Financiadora de Estudos e Projetos (FINEP), and Fundación “Alberto Roemmers.”
We thank B. E. Guth, M. Magalhães, and A. Cravioto for providing bacterial strains and Alejandra Manni, Ana Garbini, Celso Pereira, Eduardo Camacho, Griselda Lafuente Devier, and Kelen Soares for technical assistance.
REFERENCES
- 1.Agüero M E, Reyes L, Prado V, O/rskov I, O/rskov F, Cabello F C. Enterotoxigenic Escherichia coli in a population of infants with diarrhea in Chile. J Clin Microbiol. 1985;22:576–581. doi: 10.1128/jcm.22.4.576-581.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbeit R D, Arthur M, Dunn R, Kim C, Selander R K, Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed-field gel electrophoresis to molecular epidemiology. J Infect Dis. 1990;161:230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong J, Gibbs A, Peakall R, Weiller G. RAPDistance programs, version 1.04 for the analysis of patterns of RAPD fragments. Canberra, Australia: Australian National University; 1994. [Google Scholar]
- 4.Binsztein N, Jouve M J, Viboud G I, Moral L L, Rivas M, Orskov I, Ahrén C, Svennerholm A M. Colonization factors of enterotoxigenic Escherichia coli isolated from children with diarrhea in Argentina. J Clin Microbiol. 1991;29:1893–1898. doi: 10.1128/jcm.29.9.1893-1898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black R E. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 6.Blanco J, Gonzalez E A, Blanco M, Garabal J I, Alonso M P, Fernandéz S, Villanueva R, Aguillera A, Garcia M A, Torres J, Rey A, Jansen W H, Guinée P A M. Enterotoxigenic Escherichia coli associated with infant diarrhoea in Galicia, north-western Spain. J Med Microbiol. 1991;35:162–167. doi: 10.1099/00222615-35-3-162. [DOI] [PubMed] [Google Scholar]
- 7.Blanco J, Gonzalez E A, Blanco M, Garabal J I, Alonso M P, Jansen W H, Guinee P A. Prevalence of enterotoxigenic Escherichia coli strains in outbreaks and sporadic cases of diarrhoea in Spain. Eur J Clin Microbiol Infect Dis. 1989;8:396–400. doi: 10.1007/BF01964054. [DOI] [PubMed] [Google Scholar]
- 8.Campos L, Whittam T S, Gomes T, Andrade A, Trabulsi L. Escherichia coli serogroup O111 includes several clones of diarrheagenic strains with different virulence properties. Infect Immun. 1994;62:3282–3288. doi: 10.1128/iai.62.8.3282-3288.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caugant D A, Levin B R, O/rskov I, O/rskov F, Eden C S, Selander R K. Genetic diversity in relation to serotype Escherichia coli. Infect Immun. 1985;49:407–413. doi: 10.1128/iai.49.2.407-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Graaf F K, Gaastra W. Fimbriae of enterotoxigenic Escherichia coli. In: Klemm P, editor. Fimbriae: aspects of adhesion genetics, biogenesis and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 53–83. [Google Scholar]
- 11.Echeverria P, O/rskov F, O/rskov I, Plianbangchang D. Serotypes of enterotoxigenic Escherichia coli in Thailand and the Philippines. Infect Immun. 1982;36:851–856. doi: 10.1128/iai.36.3.851-856.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez E A, Blanco J, Garabal I, Blanco M. Biotypes, antibiotic resistance and plasmids coding for CFA/I and STa in enterotoxigenic Escherichia coli strains of serotype O153:H45 isolated in Spain. J Med Microbiol. 1991;34:89–95. doi: 10.1099/00222615-34-2-89. [DOI] [PubMed] [Google Scholar]
- 13.Guth B E C, Aguiar E G, Griffin P M, Ramos S R T D S, Gomes T A T. Prevalence of colonization factor antigens (CFA) and adherence to HeLa cells in enterotoxigenic Escherichia coli isolated from feces of children in São Paulo. Microbiol Immunol. 1994;38:695–701. doi: 10.1111/j.1348-0421.1994.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamers A M, Pel H J, Willshaw G A, Kusters J G, van der Zeijst B A, Gaastra W. The nucleotide sequence of the first two genes of the CFA/I fimbrial operon of human enterotoxigenic Escherichia coli. Microb Pathog. 1989;6:297–309. doi: 10.1016/0882-4010(89)90103-4. [DOI] [PubMed] [Google Scholar]
- 15.Jaccard P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull Soc Vaudoise Sci Nat. 1901;37:547–579. [Google Scholar]
- 16.Jiang Z, Mathewson J J, Ericsson C D, Svennerholm A M, Pulido C, DuPont H L. Characterization of enterotoxigenic Escherichia coli strains in patients with traveler's diarrhea acquired in Guadalajara, Mexico, 1992–1997. J Infect Dis. 2000;181:779–782. doi: 10.1086/315272. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis, version 1.02. University Park, Pa: The Pennsylvania State University; 1993. [Google Scholar]
- 18.Meng J, Zhao S, Zhao T, Doyle M P. Molecular characterisation of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis and plasmid DNA analysis. J Med Microbiol. 1995;42:258–263. doi: 10.1099/00222615-42-4-258. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuda T, Muto T, Yamada M, Kobayashi N, Toba M, Aihara Y, Ito A, Yokota S. Epidemiological study of a food-borne outbreak of enterotoxigenic Escherichia coli O25:NM by pulsed-field gel electrophoresis and randomly amplified polymorphic DNA analysis. J Clin Microbiol. 1998;36:652–656. doi: 10.1128/jcm.36.3.652-656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O/rskov L, O/rskov F. Special O:K:H serotypes among enterotoxigenic E. coli strains from diarrhea in adults and children. Occurrence of the CF (colonization factor) antigen and of hemagglutinating abilities. Med Microbiol Immunol. 1977;163:99–110. doi: 10.1007/BF02121825. [DOI] [PubMed] [Google Scholar]
- 21.Pacheco A B F, Guth B E C, Soares K C C, de-Almeida D F, Ferreira L C S. Clonal relationships among Escherichia coli serogroup O6 isolates based on RAPD. FEMS Microbiol Lett. 1997;148:255–260. doi: 10.1111/j.1574-6968.1997.tb10297.x. [DOI] [PubMed] [Google Scholar]
- 22.Pacheco A B F, Guth B E C, Soares K C C, Nishimura L, de-Almeida D F, Ferreira L C S. Random amplification of polymorphic DNA reveals serotype-specific clonal clusters among enterotoxigenic Escherichia coli strains isolated from humans. J Clin Microbiol. 1997;35:1521–1525. doi: 10.1128/jcm.35.6.1521-1525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacheco A B F, Soares K C C, de-Almeida D F, Viboud G I, Binsztein N, Ferreira L C S. Clonal nature of enterotoxigenic Escherichia coli serotype O6:H16 revealed by randomly amplified polymorphic DNA analysis. J Clin Microbiol. 1998;36:2099–2102. doi: 10.1128/jcm.36.7.2099-2102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peruski L F, Jr, Kay B A, El-Yazeed R A, El-Etr S H, Cravioto A, Wierzba T F, Rao M, El-Ghorab N, Shaheen H, Khalil S B, Kamal K, Wasfy M O, Svennerholm A M, Clemens J D, Savarino S J. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J Clin Microbiol. 1999;37:2974–2978. doi: 10.1128/jcm.37.9.2974-2978.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommerfelt H, Steinsland H, Grewal H M S, Viboud G I, Bhandari N, Gaastra W, Svennerholm A M, Bahn M K. Colonization factors of enterotoxigenic Escherichia coli isolated from children in north India. J Infect Dis. 1996;174:768–776. doi: 10.1093/infdis/174.4.768. [DOI] [PubMed] [Google Scholar]
- 26.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viboud G I, Binsztein N, Svennerholm A M. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J Clin Microbiol. 1993;31:558–564. doi: 10.1128/jcm.31.3.558-564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viboud G I, Jouve M J, Binsztein N, Vergara M, Rivas M, Quiroga M, Svennerholm A M. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J Clin Microbiol. 1999;37:2829–2833. doi: 10.1128/jcm.37.9.2829-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittam T S. Genetic variation and evolutionary processes in natural populations of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 2708–2722. [Google Scholar]
- 30.Whittam T S, Wolfe M L, Wachsmuth K, Orskov F, Orskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf M. Occurrence, distribution, and association of O and H serogroups, colonization factor antigen, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol. 1997;10:569–584. doi: 10.1128/cmr.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto T, Yokota T. Plasmids of enterotoxigenic Escherichia coli H10407: evidence for two heat-stable enterotoxin genes and a conjugal transfer system. J Bacteriol. 1983;153:1352–1360. doi: 10.1128/jb.153.3.1352-1360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]