Abstract
Simultaneous co-ingestion of prescription medication (e.g., opioid, tranquilizer/sedative, stimulant) and alcohol is associated with overdose and elevated substance use, but no studies have examined prescription drug misuse (PDM) and alcohol co-ingestion in U.S. young adults (18-25 years), despite the high rates of PDM in this age group. We used the 2015-19 National Survey on Drug Use and Health (young adult N= 69,916) to examine prevalence of past-month PDM-alcohol co-ingestion, PDM characteristics, and sociodemographic, physical health, mental health, and substance use correlates. Logistic regression examined correlates, comparing those without past-year PDM, those with past-year but not past-month PDM, those with past-month PDM without alcohol co-ingestion, and those with past-month PDM and alcohol co-ingestion. An estimated 585,000 young adults engaged in any past-month PDM-alcohol co-ingestion, or between 32.7% (opioids) and 44.6% (tranquilizer/sedatives) of those who were engaged in past-month PDM. Co-ingestion varied by educational status and was more common in males and white or multiracial young adults. All PDM-involved groups had elevated odds of suicidal ideation and other psychopathology, but substance use and substance use disorder (SUD) odds were significantly higher in young adults with co-ingestion, versus all other groups. To illustrate, 41.1% with opioid-alcohol co-ingestion had multiple past-year SUDs, versus 2.0% in those without past-year PDM. Young adults with co-ingestion are particularly likely to have problematic alcohol use and higher rates of SUD. Counseling about the risks of PDM-alcohol co-ingestion and screening for co-ingestion among those at risk are warranted to limit poor outcomes.
Keywords: Opioid, tranquilizer, stimulant, alcohol, young adult, co-ingestion
INTRODUCTION
Among examined age groups, young adults (18-25 years) have the highest rates of past-year prescription drug misuse (PDM) in the United States at 6.5%, with much higher rates than adolescents (12-17 years) at 1.5%, or adults older than 26 years, at 1.2% (Substance Abuse and Mental Health Services Administration [SAMHSA], 2019). Notably higher rates of past-year PDM in young adults versus other age groups are also found when examining by medication class, with 6.5% engaged in stimulant PDM, 5.5% engaged in opioid PDM, and 4.9% engaged in tranquilizer/sedative PDM; all of these are at least 2% higher than other age groups (SAMHSA, 2019). Medication examples include methylphenidate and mixed amphetamine-dextroamphetamine for stimulants, oxycodone, codeine, and hydrocodone for opioids, and alprazolam, clonazepam, and zolpidem for tranquilizer/sedatives.
PDM is use of another’s prescribed medication or use of one’s own medication in ways not intended by the prescriber, and it is associated with many concerning correlates in young adults. These include higher rates of psychological distress, depressive symptoms, suicidal ideation, binge alcohol use, cannabis use, other illicit drug use, substance use disorders (SUDs), and other risk behaviors (Arria et al., 2013; Bouvier et al., 2019; Weyandt et al., 2020). Young adult PDM also varies by educational attainment, sex, and race/ethnicity (Hudgins, Porter, Monuteaux, & Bourgeois, 2019; Kelly et al., 2013; Martins et al., 2015), with White non-Hispanic or multiracial young adults and males generally having higher PDM prevalence rates. Also, PDM interacts with education such that stimulant PDM is more common in young adults in college or who have graduated, while opioid or tranquilizer/sedative PDM are more common in young adults with lower educational attainment (Martins et al., 2015; Schepis, Teter, & McCabe, 2018).
In addition to correlates and sociodemographic differences, medication sources for PDM (Ford, Pomykacz, Szalewski, McCabe, & Schepis, 2020) and PDM motives (Blevins, Stephens, & Abrantes, 2017; Drazdowski, Kelly, & Kliewer, 2020) have been examined in young adults. As noted in a review by Hulme and colleagues (2018), the primary medication source for young adult college students is from peers or family. A wider variety of sources was endorsed among young adults not in college, though peers or family were the modal source for opioid and tranquilizer/sedative PDM (McCabe, Teter, Boyd, Wilens, & Schepis, 2018). Young adults engage in prescription stimulant misuse primarily for cognitive enhancement, or to improve concentration or focus and facilitate studying (Schepis, Ford, Wilens, Teter, & McCabe, 2020b). Young adults engaged in opioid or tranquilizer/sedative misuse endorse a greater variety of motives, though the modal motives (pain relief and relaxation, respectively) are consistent with the self-treatment of conditions the medication is indicated for (Schepis, Wastila, Ammerman, McCabe, & McCabe, 2020; Schepis, Wastila, & McCabe, 2020). Recreational motives, or those that are not consistent with the medication’s indication (e.g., to get high) or non-physician sources are generally associated with greater other substance use and psychopathology (Schepis, Wastila, Ammerman, et al., 2020; Schepis, Wastila, & McCabe, 2020; Votaw, Geyer, Rieselbach, & McHugh, 2019).
One important and underexamined area is young adult co-ingestion (or simultaneous co-use) of prescription opioid, tranquilizer/sedative, or stimulant medication with alcohol. Co-ingestion of opioid or tranquilizer/sedative medication with alcohol heightens the risk for severe consequences, including risk for SUD symptoms at age 35 among those with co-ingestion at 17 or 18 years of age (McCabe et al., 2019), motor vehicle accidents in adults 18 and older (Dassanayake, Michie, Carter, & Jones, 2011) and fatal overdose in adults 18 and older (Dai et al., 2020; Fox, Hoffman, Vlahov, & Manini, 2018).Also, co-ingestion of stimulant or tranquilizer/sedative medication with alcohol can impair memory functions in rodent models (Sloan, McGovern, & Buffalari, 2016; Takiguchi, Masuoka, Yamamoto, Mikami, & Kamei, 2006).
The most thorough research on simultaneous PDM-alcohol co-ingestion has occurred in adolescents, where three studies (McCabe, West, Schepis, & Teter, 2015; McCabe, West, Teter, & Boyd, 2012; Schepis, West, Teter, & McCabe, 2016) examined PDM-involved co-ingestion separately by medication class. These found that between 31.3% (stimulants) and 41.6% (tranquilizers) of adolescents with PDM engaged in past-year alcohol co-ingestion. In total, roughly 2% of all adolescents engaged in PDM-alcohol co-ingestion (McCabe et al., 2015; McCabe et al., 2012; Schepis et al., 2016). Adolescent PDM-alcohol co-ingestion was also linked with consumption of at least 10 drinks “in a row” (McCabe, Veliz, & Patrick, 2017). Few studies have investigated PDM-alcohol co-ingestion in young adults, but one found PDM-alcohol simultaneous co-ingestion rates were higher in a college student sample (mean age of 20 years), at 6.9% (McCabe, Cranford, Morales, & Young, 2006). Also, a study of Swiss young men suggested that PDM-alcohol co-ingestion was associated with poorer mental health, physical functioning, and greater substance use, but this study aggregated PDM across medication classes, included non-addictive prescription medications (e.g., beta-blockers) and had a relatively small sample (Baggio et al., 2014).
PDM-alcohol co-ingestion in young adults warrants further investigation. Research is needed that uses nationally representative U.S. data to quantify the extent of co-ingestion in young adults. Such research should also examine potential sex, race/ethnicity, and educational differences by co-ingestion status, PDM characteristics (e.g., motives, sources, and frequency of PDM) of those engaged in co-ingestion, and the correlates of co-ingestion. Such knowledge could direct prevention, screening, and treatment programs to the young adults at greatest likelihood of co-ingestion. To address these outstanding questions about co-ingestion in young adults, we used data from the 2015-19 National Survey on Drug Use and Health (NSDUH).
METHOD
The NSDUH is a yearly survey of substance use and related behaviors (e.g., psychopathology) in U.S. residents 12 and older. It uses an independent, multistage area probability sampling design and provides person-level weights for unbiased, nationally representative U.S. estimates of behavior. Audio computer-assisted self-interviewing (ACASI) is used to assess all sensitive topics, and it is combined with skip-outs and consistency checks using past responses to maximize honest reporting, data completeness, and data accuracy. The weighted screening response rate ranged from 70.5% to 79.7% over the 2015-19 study period, and the weighted interview rate ranged from 64.9% to 69.7%, similar to other recognized nationally representative studies (Grant et al., 2014) The Research Triangle International IRB provided oversight of the NSDUH (Center for Behavioral Health Statistics and Quality [CBHSQ], 2017), and the Texas State University IRB exempted this work from further oversight. Please see (CBHSQ, 2017) for more on the NSDUH.
Participants
The 2015-19 NSDUH public use files contained data on 69,916 U.S. young adults (18-25 years of age). By weighted percentage, the sample was slightly more male (50.2%), with 37.7% 20 years or younger. The majority of participants were white, non-Hispanic/Latino (54.2%), followed by Hispanic/Latino (22.0%), and black, non-Hispanic/Latino (14.1%) young adults. In terms of education, 37.1% were in college/university, 37.9% completed secondary education but were not in college/university, and 11.5% had graduated four-year college.
Measures: Prescription Drug Misuse (PDM)
Past-Year and Past-Month PDM:
PDM is defined as opioid, stimulant, or tranquilizer/sedative use: “in any way a doctor did not direct…including: without a prescription of your own; in greater amounts, more often, or longer than you were told to take it; in any other way a doctor did not direct”. The PDM assessment occurs by medication class, tranquilizers and sedatives were combined due to low sedative PDM prevalence rates, and timing of most recent PDM episode is assessed in those with lifetime PDM. Test-retest reliability (kappa) for past-year PDM exceeds 0.7 for opioids and tranquilizers, with similar agreement for stimulant and sedative medication (SAMHSA, 2010). Both past-year and past-month PDM are imputed by NSDUH staff, using predictive mean neighborhood methodology, which is described in detail in Center for Behavioral Health Statistics and Quality (2017).
Past-Month PDM-Alcohol Co-Ingestion:
Among those with past-month PDM, simultaneous PDM-alcohol co-ingestion is assessed: “During the past 30 days, did you use [medication class] in any way a doctor did not direct you to use [medication class] while you were drinking alcohol or within a couple of hours of drinking?” Test-retest reliability was not assessed for co-ingestion.
PDM Characteristics:
Among those with past-month PDM within a medication class, past-month days of PDM, non-physician PDM sources, and any recreational PDM motives were assessed, separately by class. Sources and motives were queried for the last PDM episode. Sources were dichotomized as physician versus non-physician sources (e.g., purchases from family/friends), and motives were classified as self-treatment or recreational based on the FDA indications for medications in that class. Self-treatment motives are pain relief for opioids, promotion of concentration, studying, alertness, and/or weight loss for stimulants, and promotion of sleep and/or relaxation for tranquilizer/sedatives. These were examined given evidence that non-physician sources and recreational motives are linked to substance use and psychopathology in young adults (McCabe et al., 2018; Schepis, Ford, Wilens, Teter, & McCabe, 2020a). Test-retest reliability was not assessed for the PDM characteristics described in this subsection, and only past-month days of PDM was imputed.
Measures: Correlates
Sociodemographic variables:
These captured sex, race/ethnicity, age, household income, educational attainment, and population density in area of residence. Educational attainment was: [1] in high school (HS); [2] in college; [3] 4-year college graduate; [4] HS graduate, not in school; [5] dropped out of HS, not in school. Sociodemographic test-retest kappa values are 0.9 or greater for all assessed variables (SAMHSA, 2010), and all were imputed except for educational attainment.
Physical health correlates:
These included self-reports of poor/fair health, current insurance status, past-year emergency department use, past-year inpatient hospitalization, and past-year sexually transmitted disease (STD). STD was included because of evidence of greater sexual risk-taking behaviors in young adults engaged in PDM (Bonar et al., 2014; Wells, Kelly, Rendina, & Parsons, 2015). Self-reported health, emergency department use, and inpatient hospitalization had kappa values ranging from 0.65 to 0.76, and it was 0.9 for uninsured status (SAMHSA, 2010). Only insurance status was imputed.
Mental health correlates:
These were (all past-year) mental health treatment, major depression, serious psychological distress (SPD), and suicidal ideation. Major depression was assessed based on the DSM-IV, with good psychometrics (Zanarini & Frankenburg, 2001). SPD was from the K6 assessment of non-specific psychological distress;(Kessler et al., 2003) past-year suicidal ideation was “seriously think[ing] about trying to kill[ing] yourself” in the past 12 months. Test-retest values ranged from 0.52 (past year major depression) to above 0.8 for the assessment of past-year mental health treatment. No mental health variables were imputed.
Substance use correlates:
These were past-month binge alcohol use, past-month marijuana use, past-month nicotine dependence, past-year non-marijuana illicit drug use, past-year any SUD, and past-year multiple SUD diagnoses. Heroin, cocaine, hallucinogen, methamphetamine, and inhalant use are included in non-marijuana illicit drug use. SUD diagnosis comes from the DSM-IV substance use disorder assessment and included both abuse and dependence diagnoses (American Psychiatric Association, 2000) from alcohol, marijuana, cocaine, heroin, hallucinogen, inhalant, methamphetamine, prescription opioid, tranquilizer, sedative, and stimulant use/misuse. Past-month binge alcohol is four or five alcoholic drinks (for females and males, respectively) during one occasion (National Institute of Alcohol Abuse and Alcoholism, 2004).
Past-month reliability values for substance use were not available, but past-year kappa values all exceeded 0.7, and the kappa value for any past-year SUD was 0.67. Validation of NSDUH self-reported substance use data found high rates of agreement (e.g., 90% for past-month cannabis use) between urine drug testing and self-report in young adults, though both reporting of use that was not supported by urine testing and positive urine tests with self-report of no use were found (Harrison, Martin, Enev, & Harrington, 2007). All substance use variables used predictive mean neighborhood imputation (Center for Behavioral Health Statistics and Quality, 2017).
Data Analyses
Analyses were conducted in Stata 16.1 (College Station, TX), accounting for the complex survey design of the NSDUH, and using adjusted-person level weights (weight/5) were used for the five years of pooled data. The Taylor series approximation created robust variance estimates, with adjusted degrees of freedom. Initially, prevalence rates of any past-month PDM and past-month opioid, tranquilizer/sedative, or stimulant PDM were estimated, with 95% confidence intervals (95% CIs). Further analyses estimated prevalence rates and 95% CIs of past-month PDM and past-month co-ingestion by sex, race/ethnicity, and educational attainment. For race/ethnicity, only white, black, Hispanic/Latino, and multiracial young adults were included in estimates, with other racial/ethnic groups excluded due to low cell sizes and unstable prevalence estimates.
Second, analyses examined prevalence and 95% CIs for non-physician sources and any recreational motives by medication class. Estimates of days of PDM and alcohol use in the past-month days were calculated, with 95% CIs, by medication class. These analyses occurred among those with past-month PDM only, and comparisons between those with and without co-ingestion controlled for age, race/ethnicity, sex, educational attainment, population density, and household income. Comparisons for sources and motives used logistic regression, and negative binomial regressions were used for days of PDM or alcohol use. In all cases, we found a significant likelihood ratio test for the alpha parameter in the negative binomial regression analyses, which signaled overdispersion of the data and a better model fit for a negative binomial regression, versus Poisson regression. Finally, bivariable logistic regression examined relationships in young adults between the physical health, mental health, and substance use variables and a four-level PDM variable: [1] no past-year PDM, the reference group, [2] past-year but not past-month PDM, [3] past-month PDM without co-ingestion, and [4] past-month PDM with co-ingestion. All analyses with multiple comparisons used Bonferroni corrections for the p-values to limit the false discovery rate; specific p-values resulting from these corrections are noted in the table footnotes.
RESULTS
Rates of past-month PDM were 1.8% for opioids, 1.5% for tranquilizer/sedatives, and 2.0% for stimulants; past-month simultaneous PDM-alcohol co-ingestion was present in 0.6% for opioids, 0.7% for tranquilizer/sedatives, and 0.8% for stimulants. In all, an estimated 585,000 U.S. young adults engaged in past-month PDM-alcohol co-ingestion across medication classes. One-third or more of young adults engaged in past-month PDM also engaged in co-ingestion, ranging from a low of 32.7% (opioids) to 44.6% (tranquilizer/sedatives), with stimulant co-ingestion (41.7%) intermediate. Past month alcohol use rates ranged from 100% in those with co-ingestion to the lowest rates (range: 53.8-54.9%) in those with no past-year PDM; those with past-year but not past-month PDM (range: 73.0-87.7%) and past-month PDM without co-ingestion (range: 65.2-85.4%) were intermediate.
Per Supplemental Table A (online-only), males had higher rates of past-month stimulant PDM and higher rates of tranquilizer/sedative-alcohol co-ingestion than females. For race/ethnicity-based differences, white young adults generally had significantly higher rates of past-month PDM and co-ingestion from each medication class than black or Hispanic/Latino individuals. To illustrate, 2.9% of white young adults engaged in past-month stimulant PDM, while 0.6% of black and 0.9% of Hispanic/Latino young adults did so. For stimulant-alcohol co-ingestion, rates were 1.2%, 0.1%, and 0.4% in white, black, and Hispanic/Latino young adults, respectively. Rates of PDM and PDM-alcohol co-ingestion were similar in white and multiracial young adults, but multiracial young adults had fewer significant differences from black or Hispanic/Latino young adults. Finally, within sociodemographics, those who dropped out of HS generally had the highest rates of opioid and tranquilizer/sedative PDM and opioid co-ingestion with alcohol, while college students or graduates had the highest rates for stimulant PDM and co-ingestion (see Supplemental Table B, online-only).
Tables 1 (opioids), 2 (tranquilizer/sedatives), and 3 (stimulants) capture correlates of PDM and PDM-involved co-ingestion. The physical health correlates usually were similar across all groups engaged in PDM, though presence of a past-year STD was more likely in those with opioid-alcohol co-ingestion than those with past-year PDM only or no PDM. Similarly, past-year STD rates were higher in young adults with tranquilizer/sedative-alcohol co-ingestion than all other groups. Also, currently being uninsured was associated with lower odds of any stimulant PDM. Similarly, all examined mental health correlates had higher prevalence rates for young adults with any PDM, regardless of co-ingestion status and medication class, versus the reference group of no past-year PDM. With that said, odds of the examined mental health correlates were generally not significantly higher in those with co-ingestion than other groups engaged in PDM, except for past-year mental health treatment for opioid-alcohol co-ingestion. Notably, odds of past-year suicidal ideation in those with co-ingestion were at least 2.35 or greater, versus no past-year PDM. Other mental health correlates had similar, if lower, odds.
Table 1:
Correlates of Opioid PDM and Co-Ingestion Status in Young Adults (18-25 Years of Age)
| No Past-Year Opioid PDM |
Past-Year but not Past-Month Opioid PDM |
Past-Month PDM without Alcohol Co- Ingestion |
Past-Month PDM with Alcohol Co- Ingestion |
|
|---|---|---|---|---|
| Sample Size | 65,176 | 3,472 | 839 | 410 |
| Physical Health Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Self-reported Fair or Poor Health | 1.00 (reference) | 1.55 (1.35-1.78)*** | 2.14 (1.66-2.75)*** | 2.13 (1.46-3.12)*** |
| Currently Uninsured | 1.00 (reference) | 1.02 (0.90-1.17) | 1.08 (0.87-1.34) | 1.40 (0.97-2.03) |
| Past-year Emergency Department Use | 1.00 (reference) | 1.80 (1.64-1.98)*** | 1.63 (1.33-2.01)*** | 2.21 (1.77-2.76)*** |
| Past-year Hospitalization | 1.00 (reference) | 1.76 (1.53-2.03)*** | 1.88 (1.42-2. 49)*** | 2.41 (1.75-3.31)*** |
| Past-year Sexually Transmitted Disease | 1.00 (reference) | 2.48 (2.09-2.93)*** | 2.51 (1.93-3.26)*** | 4.84 (3.23-7.26)***+ |
| Mental Health Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Past-year Mental Health Treatment | 1.00 (reference) | 2.13 (1.90-2.37)*** | 2.19 (1.67-2.87)*** | 3.36 (2.55-4.45)***+ |
| Past-year Major Depression | 1.00 (reference) | 2.54 (2.26-2.87)*** | 2.22 (1.66-2.95)*** | 2.57 (1.87-3.52)*** |
| Past-year Serious Psychological Distress | 1.00 (reference) | 2.61 (2.39-2.84)*** | 2.41 (1.99-2.92)*** | 2.98 (2.31-3.85)*** |
| Past-year Suicidal Ideation | 1.00 (reference) | 2.95 (2.64-3.28)*** | 3.05 (2.44-3.81)*** | 3.54 (2.75-4.55)*** |
| Substance Use Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Past-month Binge Alcohol Use | 1.00 (reference) | 2.68 (2.44-2.96)*** | 1.56 (1.28-1.90)*** | 21.91 (12.82-37.44)***++ |
| Past-month Marijuana Use | 1.00 (reference) | 4.30 (3.95-4.67)*** | 5.57 (4.59-6.77)*** | 12.53 (9.33-16.83)***++ |
| Past-month Nicotine Dependence | 1.00 (reference) | 3.90 (3.47-4.59)*** | 4.68 (3.91-5.60)*** | 8.58 (6.81-10.81)***++ |
| Past-month Non-Marijuana Illicit Drug Use | 1.00 (reference) | 6.65 (5.80-7.64)*** | 7.88 (6.11-10.15)*** | 30.23 (21.24-43.03)***++ |
| Any past-year DSM-IV SUD | 1.00 (reference) | 6.58 (5.91-7.32)*** | 7.63 (6.25-9.30)*** | 18.33 (14.50-23.19)***++ |
| Multiple past-year SUDs | 1.00 (reference) | 10.51 (9.01-12.26)*** | 12.39 (9.94-15.46)*** | 33.79 (27.00-42.29)***++ |
Data Source: 2015-19 National Survey on Drug Use and Health (NSDUH)
PDM = Prescription Drug Misuse; OR = Odds Ratio; 95% CI = 95% confidence interval of the point prevalence estimate; SUD = Substance Use Disorder
Bonferroni-corrected significant differences from the reference group denoted as
p ≤ 0.008
p ≤ 0.0017
p ≤ 0.00017 (adjusted for six comparisons).
denotes significantly different from past-year but not Past-Month PDM
denotes significantly difference from all other categories (both p < 0.008).
Table 2:
Correlates of Tranquilizer/Sedative PDM and Co-Ingestion Status in Young Adults (18-25 Years of Age)
| No Past-Year Tranquilizer/Sedative PDM |
Past-Year but not Past-Month PDM |
Past-Month PDM without Alcohol Co- Ingestion |
Past-Month PDM with Alcohol Co- Ingestion |
|
|---|---|---|---|---|
| Sample Size | 66,398 | 2,550 | 529 | 427 |
| Physical Health Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Self-reported Fair or Poor Health | 1.00 (reference) | 1.36 (1.13-1.63)* | 1.63 (1.14-2.32)* | 2.03 (1.52-2.72)*** |
| Currently Uninsured | 1.00 (reference) | 1.03 (0.91-1.18) | 1.19 (0.85-1.66) | 1.30 (0.87-1.94) |
| Past-year Emergency Department Use | 1.00 (reference) | 1.55 (1.41-1.71)*** | 1.77 (1.46-2.15)*** | 2.07 (1.50-2.85)*** |
| Past-year Hospitalization | 1.00 (reference) | 1.47 (1.22-1.77)*** | 1.59 (1.08-2.35) | 1.79 (1.18-2.71)* |
| Past-year Sexually Transmitted Disease | 1.00 (reference) | 2.85 (2.36-3.44)*** | 2.18 (1.52-3.13)*** | 5.31 (3.84-7.34)***++ |
| Mental Health Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Past-year Mental Health Treatment | 1.00 (reference) | 2.71 (2.40-3.06)*** | 3.16 (2.44-4.10)*** | 3.31 (2.46-4.43)*** |
| Past-year Major Depression | 1.00 (reference) | 2.70 (2.40-3.03)*** | 2.92 (2.27-3.76)*** | 2.71 (1.98-3.72)*** |
| Past-year Serious Psychological Distress | 1.00 (reference) | 2.96 (2.64-3.32)*** | 3.33 (2.68-4.14)*** | 2.94 (2.22-3.90)*** |
| Past-year Suicidal Ideation | 1.00 (reference) | 3.11 (2.78-3.48)*** | 3.37 (2.62-4.34)*** | 3.40 (2.59-4.46)*** |
| Substance Use Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Past-month Binge Alcohol Use | 1.00 (reference) | 3.17 (2.82-3.56)*** | 1.74 (1.44-2.09)*** | 29.42 (16.77-51.62)***++ |
| Past-month Marijuana Use | 1.00 (reference) | 6.15 (5.40-7.00)*** | 9.40 (7.86-11.24)*** | 13.44 (9.56-18.89)***+ |
| Past-month Nicotine Dependence | 1.00 (reference) | 3.72 (3.22-4.30)*** | 5.23 (4.01-6.82)*** | 6.03 (4.36-8.34)***+ |
| Past-month Non-Marijuana Illicit Drug Use | 1.00 (reference) | 7.98 (6.64-9.60)*** | 14.24 (11.28-17.98)*** | 31.49 (23.15-42.84)***++ |
| Any past-year DSM-IV SUD | 1.00 (reference) | 8.20 (7.26-9.27)*** | 8.19 (6.72-9.98)*** | 20.98 (15.42-28.55)***++ |
| Multiple past-year SUDs | 1.00 (reference) | 14.13 (12.36-16.15)*** | 16.75 (13.47-20.84)*** | 40.38 (29.86-54.59)***++ |
Data Source: 2015-19 National Survey on Drug Use and Health (NSDUH)
PDM = Prescription Drug Misuse; OR = Odds Ratio; 95% CI = 95% confidence interval of the point prevalence estimate; SUD = Substance Use Disorder
Bonferroni-corrected significant differences from the reference group denoted as
p ≤ 0.008
p ≤ 0.0017
p ≤ 0.00017 (adjusted for six comparisons).
denotes significantly different from past-year but not Past-Month PDM
denotes significantly difference from all other categories (both p < 0.008).
Table 3:
Correlates of Stimulant PDM and Co-Ingestion Status in Young Adults (18-25 Years of Age)
| No Past-Year Stimulant PDM |
Past-Year but not Past-Month Stimulant PDM |
Past-Month PDM without Alcohol Co- Ingestion |
Past-Month PDM with Alcohol Co- Ingestion |
|
|---|---|---|---|---|
| Sample Size | 65,291 | 3,304 | 755 | 549 |
| Physical Health Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Self-reported Fair or Poor Health | 1.00 (reference) | 0.85 (0.72-1.01)*** | 1.35 (0.92-1.97) | 1.14 (0.77-1.69) |
| Currently Uninsured | 1.00 (reference) | 0.67 (0.57-0.78)*** | 0.60 (0.43-0.83)* | 0.57 (0.40-0.81)* |
| Past-year Emergency Department Use | 1.00 (reference) | 1.08 (0.98-1.19) | 1.32 (1.11-1.57)* | 1.36 (1.09-1.70)* |
| Past-year Hospitalization | 1.00 (reference) | 0.89 (0.77-1.04)** | 1.19 (0.81-1.73) | 0.98 (0.67-1.42) |
| Past-year Sexually Transmitted Disease | 1.00 (reference) | 2.11 (1.77-2.52)*** | 3.35 (2.54-4.42)*** | 3.11 (2.22-4.36)*** |
| Mental Health Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Past-year Mental Health Treatment | 1.00 (reference) | 1.79 (1.59-2.01)*** | 1.66 (1.35-2.04)*** | 2.15 (1.63-2.85)*** |
| Past-year Major Depression | 1.00 (reference) | 1.99 (1.74-2.26)*** | 2.07 (1.62-2.65)*** | 2.07 (1.66-2.58)*** |
| Past-year Serious Psychological Distress | 1.00 (reference) | 1.89 (1.72-2.06)*** | 2.14 (1.75-2.61)*** | 1.85 (1.47-2.32)*** |
| Past-year Suicidal Ideation | 1.00 (reference) | 2.13 (1.87-2.42)*** | 1.85 (1.47-2.32)*** | 2.35 (1.81-3.05)*** |
| Substance Use Correlates | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Past-month Binge Alcohol Use | 1.00 (reference) | 5.23 (4.73-5.79)*** | 5.23 (4.10-6.67)*** | 58.42 (34.51-98.92)***++ |
| Past-month Marijuana Use | 1.00 (reference) | 4.92 (4.48-5.40)*** | 8.26 (6.78-10.05)*** | 12.00 (9.06-15.89)***+ |
| Past-month Nicotine Dependence | 1.00 (reference) | 2.71 (2.34-3.13)*** | 3.07 (2.30-4.11)*** | 3.48 (2.56-4.73)*** |
| Past-month Non-Marijuana Illicit Drug Use | 1.00 (reference) | 7.35 (6.40-8.44)*** | 12.81 (10.77-15.24)*** | 24.06 (19.00-30.46)***++ |
| Any past-year DSM-IV SUD | 1.00 (reference) | 5.87 (5.32-6.47)*** | 8.33 (7.06-9.81)*** | 13.66 (11.11-16.79)***++ |
| Multiple past-year SUDs | 1.00 (reference) | 7.90 (6.94-9.00)*** | 14.55 (11.45-18.50)*** | 23.10 (18.08-29.50)***++ |
Data Source: 2015-19 National Survey on Drug Use and Health (NSDUH)
PDM = Prescription Drug Misuse; OR = Odds Ratio; 95% CI = 95% confidence interval of the point prevalence estimate; SUD = Substance Use Disorder
Bonferroni-corrected significant differences from the reference group denoted as
p ≤ 0.008
p ≤ 0.0017
p ≤ 0.00017 (adjusted for six comparisons).
denotes significantly different from past-year but not Past-Month PDM
denotes significantly difference from all other categories (both p < 0.008).
In contrast, odds of the substance use correlates were almost always significantly higher for those engaged in past-month co-ingestion than the other examined groups. In particular, odds of past-year any SUD ranged from 13.97 (stimulant PDM) to 20.81 (tranquilizer/sedative PDM) versus no past-year PDM, and per Figure 1, odds of having multiple SUDs ranged from 13.66 (stimulant PDM) to 20.98 (tranquilizer/sedative PDM). In terms of prevalence rates, between 66.1% and 75.2% of young adults with co-ingestion had a past-year SUD, while 12.6% or fewer of young adults without PDM did; similarly, between 32.3% and 45.5% of young adults with co-ingestion had multiple SUDs, while 2.1% or fewer of young adults without PDM did.
Figure 1:
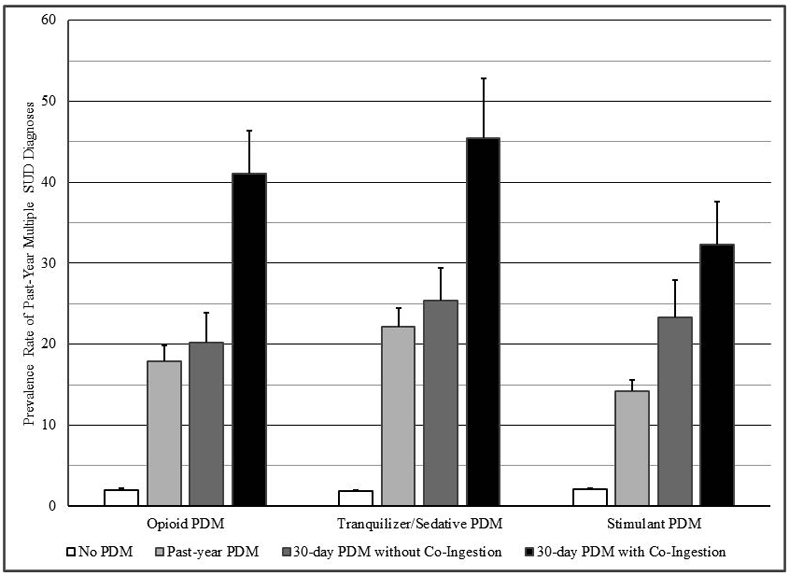
Data Source: 2015-19 National Survey on Drug Use and Health (NSDUH)
Individuals engaged in co-ingestion had a significantly greater number of past-month PDM days versus young adults with past-month PDM but no co-ingestion. For those with co-ingestion, mean days of PDM were 6.56, 5.84, and 4.55 for opioids, tranquilizer/sedatives, and stimulants. Without co-ingestion, mean days were 5.03, 4.26, and 3.47, respectively. Co-ingestion was also significantly associated with higher prevalence rates of recreational motives across PDM classes (all ps≤ 0.001) and with non-physician source use for opioid (p= 0.008) and stimulant PDM (p= 0.02). These results are not in the tables or figures.
Strikingly (see Figure 2), individuals with co-ingestion had a significantly greater number of past-month days of alcohol use, versus all other groups. Days of alcohol use were very similar across co-ingestion groups, ranging from 12.2 (stimulant) to 12.61 (opioid), in contrast to 3.5 days or fewer for those without PDM. Similarly, and per Tables 2 through 4, odds of past-month binge alcohol use were significantly higher than in all other groups, with minimum odds of 21.9 or greater versus no past-year PDM.
Figure 2:
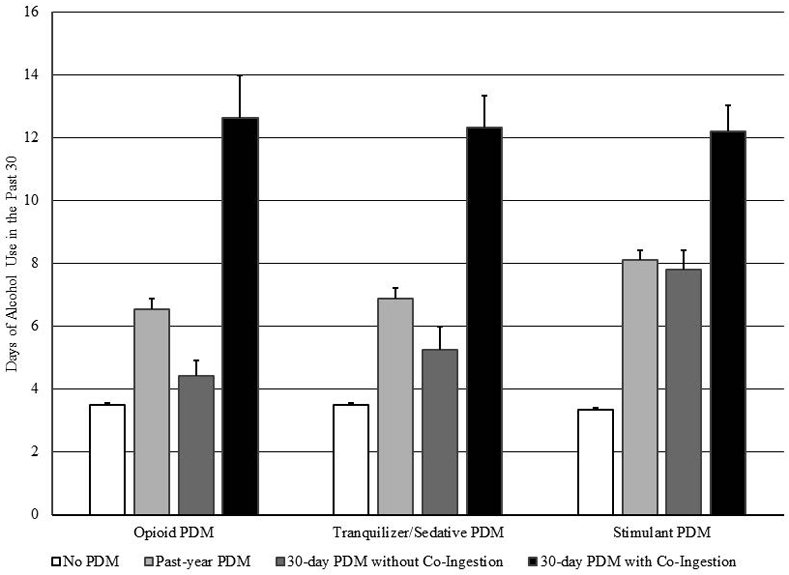
Data Source: 2015-19 National Survey on Drug Use and Health (NSDUH)
DISCUSSION
Using data from the 2015-19 NSDUH, an estimated 585,000 young adults engaged in past-month simultaneous PDM-alcohol co-ingestion, and the findings of this paper indicate they are a particularly at-risk group. Among young adults engaged in past-month PDM, the prevalence of co-ingestion ranged from 32.7% for prescription opioids to 44.6% for prescription tranquilizer/sedatives. Young adults engaged in alcohol-PDM co-ingestion have higher past-year prevalence of SUD diagnoses (except for stimulant-alcohol co-ingestion), higher past-month prevalence of marijuana use and past-year non-marijuana illicit drug use, and higher past-year prevalence of any SUD and multiple SUDs than those without PDM, with past-year but not past-month PDM, or with past-month PDM without co-ingestion. While young adults engaged in PDM are an at-risk group (Arria et al., 2013; Votaw et al., 2019; Weyandt et al., 2020), young adults engaged in alcohol-PDM co-ingestion are even more vulnerable. The elevated risk among those with co-ingestion is further reinforced by their significantly higher rates of recreational motives, non-physician sources (except for tranquilizer/sedative PDM), and greater number of days engaged in PDM. Moreover, the alcohol use patterns of young adults engaged in co-ingestion are reason for concern; they have significantly higher odds of past-month binge alcohol use and engage in alcohol use on at least 40% of the past 30 days.
Young adults engaged in PDM-alcohol co-ingestion have similar prevalence rates of the examined mental and physical health outcomes to those with past-year but not past-month PDM or past-month PDM without co-ingestion. As such, co-ingestion seems to be a marker of increased likelihood of greater substance use, any SUD, and multiple SUDs specifically, while any PDM signifies increased odds of poorer self-reported health, greater healthcare utilization, and elevated rates of psychological distress, major depression, and suicidal ideation. These results are all generally consistent with the nationally representative research on PDM-involved co-ingestion in adolescents, which also found significantly higher rates of other substance use and recreational PDM motives in those engaged in co-ingestion, versus those without (McCabe et al., 2015; McCabe et al., 2012; Schepis et al., 2016). In contrast, Baggio et al. (2014) found poorer mental and physical health-related quality-of-life scores and greater physical health consequences among young Swiss males engaged in co-ingestion, versus those with only concurrent PDM-alcohol (i.e., both over the past year without any co-ingestion). Differences in our results are likely to result from the different populations and assessments, though future research should clarify the degree to which physical and mental health are related to co-ingestion in young adults.
When compared to the literature on more common forms of alcohol co-ingestion, namely alcohol-cannabis (Yurasek, Aston, & Metrik, 2017) and alcohol-tobacco co-ingestion, our results suggest that PDM-alcohol co-ingestion is less prevalent but associated with a poorer substance use profile. Alcohol-cannabis co-ingestion is linked to lower binge alcohol odds across adults than is PDM-alcohol co-ingestion but no differences in educational attainment, versus alcohol-only use (Subbaraman & Kerr, 2015). In 17 and 18 year-old secondary school students, alcohol-cannabis co-ingestion is linked to high rates of binge alcohol use, lower educational goals and lower parental educational attainment than those with alcohol-only use (Patrick et al., 2018). With that said, the risk was lower in the larger subset engaged in “lighter” or less frequent co-ingestion, which comprised nearly two-thirds of the co-ingestion group (Patrick et al., 2018).
Similarly, alcohol-tobacco co-ingestion is very common compared to alcohol-PDM co-ingestion, and alcohol-tobacco co-ingestion is associated with heavier alcohol use (McKee, Falba, O'Malley, Sindelar, & O'Connor, 2007). Notably, individuals with simultaneous alcohol-tobacco-cannabis-other drug use, with other drugs including PDM, had the most similar profiles to young adults engaged in co-ingestion in this study, and this group was also much less common than those with alcohol-cannabis co-ingestion (Davis, Slutske, Martin, Agrawal, & Lynskey, 2019). While differences in timeframes of co-ingestion (past-year versus past-month) and age groups examined preclude firm conclusions, it appears that alcohol co-ingestion involving PDM and/or illicit drugs is both less frequent than alcohol-marijuana or alcohol-tobacco co-ingestion and associated with greater engagement in other substance use and other concerning correlates.
Limitations and Future Directions
As the NSDUH is cross-sectional, causal inference cannot be inferred from these results. For instance, it may be that greater alcohol involvement predisposes co-ingestion among those with access to opioid, tranquilizer/sedative, or stimulant medication, or it may be that co-ingestion marks a greater propensity to engage in substance use generally because of higher-order influences (e.g., availability, genetics, externalizing behavior); these data cannot address those questions, and future, longitudinal research is needed to do so. Also, both self-report and self-selection bias are likely, given the nature of the survey and that a minority of approached individuals refused to participate. Nonetheless, data indicate that self-report substance use data are reliable and valid, with any misclassification likely to produce under-estimates of substance engagement (Johnston & O’Malley, 1985). While test-retest reliability kappa values were substantial or near perfect for many measures, a few measures had only moderate reliability (e.g., past-year major depression) and some were not assessed for reliability. The NSDUH also uses weighting to address non-response, ACASI interviewing, and visual cues (e.g., medication pictures) to promote accurate and honest reporting. These results are from young adults in the civilian, non-institutionalized U.S. population, and they cannot be applied to other populations. Finally, these data do not address PDM-involved co-ingestion with other substances or frequency or dose of co-ingestion, as such data are not available in the NSDUH.
Clinical Implications and Conclusions
These results emphasize the importance both of preventive counseling when opioids, tranquilizer/sedatives, or stimulant medications are prescribed and of screening for PDM and co-ingestion among those at risk. First, prescribers should counsel young adults about the risks of combining prescribed opioids, tranquilizer/sedatives, and/or stimulants with alcohol. This can be combined with more general counseling about appropriate medication use and potential side effects. Pharmacists also can engage in such counseling when medications are filled, with evidence that verbal counseling by pharmacists is effective, especially among those with low health literacy (Wali, Hudani, Wali, Mercer, & Grindrod, 2016). Counseling to enhance disposal and prevent diversion is also important, given evidence that peers are the leading medication source for young adults engaged in PDM (Hulme et al., 2018).
Second, screening for PDM in all U.S. young adults is warranted; this recommendation is consistent with the recent U.S. Preventive Services Task Force recommendation for substance use screening in all adults, including young adults (Krist et al., 2020). Such screening is also consistent with the goals of the Healthy People 2030 initiative to reduce the prevalence of alcohol use disorder, cannabis use disorder, and any other drug use disorder (Office of Disease Prevention and Health Promotion, n.d.). Given the very high rates of SUD in those with alcohol-PDM co-ingestion, identification of young adults via screening who are engaged in co-ingestion will also frequently identify those with SUD. This could combine more general tools that assess PDM, like the NIDA-Modified ASSIST (National Institute on Drug Abuse, 2015), with a drug screening measure that assesses co-ingestion, the DAST-10 (Skinner, 1982), and more intensive alcohol use screening tools, like the AUDIT (Reinert & Allen, 2002). While the treating clinician may have a complete picture of the mental health treatment needs of those engaged in co-ingestion, particular attention to suicidality and depressive symptoms is warranted. Finally, those with PDM-alcohol co-ingestion are likely to need both mental health and SUD treatment, and involvement of a multidisciplinary team to ensure all treatment needs are addressed is important.
Third, harm reduction techniques may be useful in limiting the most significant consequences of PDM-alcohol co-ingestion in young adults, such as overdose. For opioid PDM, Marshall and colleagues (2016) suggest peer outreach efforts through social networks and street- or nightlife-based efforts that include education and naloxone distribution. For stimulant PDM, Abelman (2017) notes that a focus on young adult college students is most likely to be effective and that education around the lack of academic benefits may be helpful. Mental health screening was also recommended (Abelman, 2017), which is consistent with our findings.
In conclusion, a substantial subpopulation of those engaged in past-month PDM have also co-ingested the prescription medication with alcohol: 32.7 to 44.6%, translating to over 585,000 young adults. Particularly for opioid or tranquilizer/sedative co-ingestion with alcohol, risk of SUD symptoms (McCabe et al., 2019), motor vehicular accidents (Dassanayake et al., 2011), and overdose (Dai et al., 2020; Fox et al., 2018) are increased. This study suggests that co-ingestion is most strongly associated with increased likelihood of binge alcohol use and greater alcohol use frequency, other substance use, any SUD, and multiple SUD diagnoses. Ultimately, PDM-alcohol co-ingestion signals a need for substance use treatment along with attention to potential psychopathology in this high-risk group of young adults.
Supplementary Material
PUBLIC SIGNIFICANCE STATEMENT:
Young adults who engage in prescription medication and alcohol simultaneous co-ingestion have significantly greater alcohol consumption and significantly higher rates of substance use disorders (SUDs), including multiple concurrent SUDs, than those engaged in prescription drug misuse (PDM) without co-ingestion. The estimated 585,000 U.S. young adults engaged in past month PDM-alcohol co-ingestion need comprehensive and multi-disciplinary treatment to reduce their risk for poor outcomes.
DISCLOSURES AND ACKNOWLEDGEMENTS:
This research was supported by grants R01DA043691 and R01DA031160 from the National Institute on Drug Abuse (NIDA). The NSDUH is sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA) and NIDA. The content is the authors’ responsibility and does not necessarily represent the views of NIDA or SAMHSA.
Footnotes
The authors declare no conflicts of interest.
The authors have no acknowledgements to make.
This manuscript was not posted to a preprint server or archive.
REFERENCES
- Abelman DD (2017). Mitigating risks of students use of study drugs through understanding motivations for use and applying harm reduction theory: a literature review. Harm Reduction Journal, 14(1), 68. doi: 10.1186/s12954-017-0194-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Arria AM, Wilcox HC, Caldeira KM, Vincent KB, Garnier-Dykstra LM, & O'Grady KE (2013). Dispelling the myth of "smart drugs": cannabis and alcohol use problems predict nonmedical use of prescription stimulants for studying. Addictive Behaviors, 38(3), 1643–1650. doi: 10.1016/j.addbeh.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio S, Deline S, Studer J, N'Goran A, Mohler-Kuo M, Daeppen JB, & Gmel G (2014). Concurrent versus simultaneous use of alcohol and non-medical use of prescription drugs: is simultaneous use worse for mental, social, and health issues? Journal of Psychoactive Drugs, 46(4), 334–339. doi: 10.1080/02791072.2014.921747 [DOI] [PubMed] [Google Scholar]
- Blevins CE, Stephens R, & Abrantes AM (2017). Motives for Prescription Stimulant Misuse in a College Sample: Characteristics of Users, Perception of Risk, and Consequences of Use. Substance Use and Misuse, 52(5), 555–561. doi: 10.1080/10826084.2016.1245338 [DOI] [PubMed] [Google Scholar]
- Bonar EE, Cunningham RM, Chermack ST, Blow FC, Barry KL, Booth BM, & Walton MA (2014). Prescription drug misuse and sexual risk behaviors among adolescents and emerging adults. Journal of Studies on Alcohol and Drugs, 75(2), 259–268. doi: 10.15288/jsad.2014.75.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier BA, Kinnard EN, Yedinak JL, Li Y, Elston B, Green TC, … Marshall BDL (2019). Prevalence and Correlates of Depressive Symptomology among Young Adults Who Use Prescription Opioids Non-medically. Journal of Psychoactive Drugs, 1–12. doi: 10.1080/02791072.2019.1654151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2017). 2016 National Survey on Drug Use and Health: Methodological resource book (Section 8, data collection final report). Rockville, MD: Substance Abuse and Mental Health Services Administration [Google Scholar]
- Dai Z, Abate MA, Long DL, Smith GS, Halki TM, Kraner JC, & Mock AR (2020). Quantifying enhanced risk from alcohol and other factors in polysubstance-related deaths. Forensic Science International, 313, 110352. doi: 10.1016/j.forsciint.2020.110352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassanayake T, Michie P, Carter G, & Jones A (2011). Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Safety, 34(2), 125–156. doi: 10.2165/11539050-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Davis CN, Slutske WS, Martin NG, Agrawal A, & Lynskey MT (2019). Identifying subtypes of cannabis users based on simultaneous polysubstance use. Drug and Alcohol Dependence, 205, 107696. doi: 10.1016/j.drugalcdep.2019.107696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazdowski TK, Kelly LM, & Kliewer WL (2020). Motivations for the nonmedical use of prescription drugs in a longitudinal national sample of young adults. Journal of Substance Abuse Treatment, 114, 108013. doi: 10.1016/j.jsat.2020.108013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JA, Pomykacz C, Szalewski A, McCabe SE, & Schepis TS (2020). Friends and relatives as sources of prescription opioids for misuse among young adults: The significance of physician source and race/ethnic differences. Substance Abuse, 41(1), 93–100. doi: 10.1080/08897077.2019.1635955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LM, Hoffman RS, Vlahov D, & Manini AF (2018). Risk factors for severe respiratory depression from prescription opioid overdose. Addiction, 113(1), 59–66. doi: 10.1111/add.13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chu A, Sigman R, Amsbary M, Kali J, Sugawara Y, … Goldstein R (2014). Source and accuracy statement: National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism [Google Scholar]
- Harrison LD, Martin SS, Enev T, & Harrington D (2007). Comparing drug testing and self-report of drug use among youths and young adults in the general population (DHHS Publication No. SMA 07-4249, Methodology Series M-7). Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies [Google Scholar]
- Hudgins JD, Porter JJ, Monuteaux MC, & Bourgeois FT (2019). Prescription opioid use and misuse among adolescents and young adults in the United States: A national survey study. PLoS Medicine, 16(11), e1002922. doi: 10.1371/journal.pmed.1002922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme S, Bright D, & Nielsen S (2018). The source and diversion of pharmaceutical drugs for non-medical use: A systematic review and meta-analysis. Drug and Alcohol Dependence, 186, 242–256. doi: 10.1016/j.drugalcdep.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Johnston LD, & O’Malley PM (1985). Issues of validity and population coverage in student surveys of drug use. NIDA Research Monograph, 57, 31–54. [PubMed] [Google Scholar]
- Kelly BC, Wells BE, Leclair A, Tracy D, Parsons JT, & Golub SA (2013). Prevalence and correlates of prescription drug misuse among socially active young adults. International Journal of Drug Policy, 24(4), 297–303. doi: 10.1016/j.drugpo.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, … Zaslavsky AM (2003). Screening for serious mental illness in the general population. Archives of General Psychiatry, 60(2), 184–189. [DOI] [PubMed] [Google Scholar]
- Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, … Wong JB (2020). Screening for Unhealthy Drug Use: US Preventive Services Task Force Recommendation Statement. Journal of the American Medical Association, 323(22), 2301–2309. doi: 10.1001/jama.2020.8020 [DOI] [PubMed] [Google Scholar]
- Marshall BD, Green TC, Yedinak JL, & Hadland SE (2016). Harm reduction for young people who use prescription opioids extra-medically: Obstacles and opportunities. International Journal on Drug Policy, 31, 25–31. doi: 10.1016/j.drugpo.2016.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Kim JH, Chen LY, Levin D, Keyes KM, Cerda M, & Storr CL (2015). Nonmedical prescription drug use among US young adults by educational attainment. Social Psychiatry and Psychiatric Epidemiology, 50(5), 713–724. doi: 10.1007/s00127-014-0980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Morales M, & Young A (2006). Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. Journal of Studies on Alcohol, 67(4), 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Wilens TE, & Schepis TS (2018). Sources of prescription medication misuse among young adults in the United States: The role of educational status. Journal of Clinical Psychiatry, 79(2), 17m11958. doi: 10.4088/JCP.17m11958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Veliz P, & Patrick ME (2017). High-intensity drinking and nonmedical use of prescription drugs: Results from a national survey of 12th grade students. Drug and Alcohol Dependence, 178, 372–379. doi: 10.1016/j.drugalcdep.2017.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Veliz PT, Boyd CJ, Schepis TS, McCabe VV, & Schulenberg JE (2019). A prospective study of nonmedical use of prescription opioids during adolescence and subsequent substance use disorder symptoms in early midlife. Drug and Alcohol Dependence, 194, 377–385. doi: 10.1016/j.drugalcdep.2018.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Schepis TS, & Teter CJ (2015). Simultaneous co-ingestion of prescription stimulants, alcohol and other drugs: a multi-cohort national study of US adolescents. Human Psychopharmacology: Clinical and Experimental, 30(1), 42–51. doi: 10.1002/hup.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Teter CJ, & Boyd CJ (2012). Co-ingestion of prescription opioids and other drugs among high school seniors: results from a national study. Drug and Alcohol Dependence, 126(1-2), 65–70. doi: 10.1016/j.drugalcdep.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Falba T, O'Malley SS, Sindelar J, & O'Connor PG (2007). Smoking status as a clinical indicator for alcohol misuse in US adults. Archives of Internal Medicine, 167(7), 716–721. doi: 10.1001/archinte.167.7.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. (2004). NIAAA Newsletter, Winter 2004. Bethesda, MD: Office of Research Translation and Communications, NIAAA [Google Scholar]
- National Institute on Drug Abuse. (2015). NIDA Screening Tool: NIDA-Modified ASSIST. Retrieved from https://www.drugabuse.gov/nmassist/
- Office of Disease Prevention and Health Promotion. (n.d.). Addiction. Healthy People 2030. Retrieved from https://health.gov/healthypeople/objectives-and-data/social-determinants-health [Google Scholar]
- Patrick ME, Kloska DD, Terry-McElrath YM, Lee CM, O'Malley PM, & Johnston LD (2018). Patterns of simultaneous and concurrent alcohol and marijuana use among adolescents. American Journal on Drug and Alcohol Abuse, 44(4), 441–451. doi: 10.1080/00952990.2017.1402335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert DF, & Allen JP (2002). The alcohol use disorders identification test (AUDIT): a review of recent research. Alcoholism: Clinical and Experimental Research, 26(2), 272–279. [PubMed] [Google Scholar]
- Schepis TS, Ford JA, Wilens TE, Teter CJ, & McCabe SE (2020a). Differences in Prescription Stimulant Misuse Motives across Adolescents and Young Adults in the United States. Journal of Clinical Psychiatry, 81(6), 20m13302. doi: 10.4088/JCP.20m13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Ford JA, Wilens TE, Teter CJ, & McCabe SE (2020b). Differences in Prescription Stimulant Misuse Motives Across Adolescents and Young Adults in the United States. Journal of Clinical Psychiatry, 81(6). doi: 10.4088/JCP.20m13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Teter CJ, & McCabe SE (2018). Prescription drug use, misuse and related substance use disorder symptoms vary by educational status and attainment in U.S. adolescents and young adults. Drug and Alcohol Dependence, 189, 172–177. doi: 10.1016/j.drugalcdep.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Wastila L, Ammerman B, McCabe VV, & McCabe SE (2020). Prescription Opioid Misuse Motives in US Older Adults. Pain Med, 21(10), 2237–2243. doi: 10.1093/pm/pnz304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Wastila L, & McCabe SE (2020). Prescription Tranquilizer/Sedative Misuse Motives Across the US Population. Journal of Addiction Medicine. doi: 10.1097/adm.0000000000000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, West BT, Teter CJ, & McCabe SE (2016). Prevalence and correlates of co-ingestion of prescription tranquilizers and other psychoactive substances by U.S. high school seniors: Results from a national survey. Addictive Behaviors, 52, 8–12. doi: 10.1016/j.addbeh.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA (1982). The drug abuse screening test. Addictive Behaviors, 7(4), 363–371. doi: 10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
- Sloan AR, McGovern R, & Buffalari DM (2016). Effects of concomitant methylphenidate and ethanol administration on working and reference memory in rats. Pharmacology, Biochemistry, and Behavior, 150-151, 134–137. doi: 10.1016/j.pbb.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Subbaraman MS, & Kerr WC (2015). Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcoholism: Clinical and Experimental Research, 39(5), 872–879. doi: 10.1111/acer.12698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2010). Reliability of Key Measures in the National Survey on Drug Use and Health (Office of Applied Studies, Methodology Series M-8, HHS Publication No. SMA 09-4425). Rockville, MD: Substance Abuse and Mental Health Services Administration; [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). Results from the 2018 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration [Google Scholar]
- Takiguchi A, Masuoka T, Yamamoto Y, Mikami A, & Kamei C (2006). Potentiation of ethanol in spatial memory deficits induced by some benzodiazepines. Journal of Pharmacological Sciences, 101(4), 325–328. doi: 10.1254/jphs.fpj06008x [DOI] [PubMed] [Google Scholar]
- Votaw VR, Geyer R, Rieselbach MM, & McHugh RK (2019). The epidemiology of benzodiazepine misuse: A systematic review. Drug and Alcohol Dependence, 200, 95–114. doi: 10.1016/j.drugalcdep.2019.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali H, Hudani Z, Wali S, Mercer K, & Grindrod K (2016). A systematic review of interventions to improve medication information for low health literate populations. Research in Social and Administrative Pharmacy, 12(6), 830–864. doi: 10.1016/j.sapharm.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Wells BE, Kelly BC, Rendina HJ, & Parsons JT (2015). Prescription Drug Misuse and Sexual Behavior Among Young Adults. Journal of Sex Research, 52(6), 659–668. doi: 10.1080/00224499.2014.918085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyandt LL, Gudmundsdottir BG, Holding EZ, Marraccini ME, Keith M, May SE, … Sweeney C (2020). Prescription opioid misuse among university students: A systematic review. Journal of American College Health, 1–19. doi: 10.1080/07448481.2020.1786095 [DOI] [PubMed] [Google Scholar]
- Yurasek AM, Aston ER, & Metrik J (2017). Co-use of Alcohol and Cannabis: A Review. Current Addiction Reports, 4(2), 184–193. doi: 10.1007/s40429-017-0149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, & Frankenburg FR (2001). Attainment and maintenance of reliability of axis I and II disorders over the course of a longitudinal study. Comprehensive Psychiatry, 42(5), 369–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


