Abstract
Objectives:
ABRUPT was a prospective, non-interventional, observational study of resuscitation practices at 21 burn centers. The primary goal was to examine burn resuscitation with albumin or crystalloids alone, in order to design a future prospective randomized trial.
Summary Background Data:
No modern prospective study has determined whether to use colloids or crystalloids for acute burn resuscitation.
Methods:
Patients ≥ 18 years with burns ≥ 20% total body surface area (TBSA) had hourly documentation of resuscitation parameters for 48 hours. Patients received either crystalloids alone or had albumin supplemented to crystalloid based on center protocols.
Results:
Of 379 enrollees, two-thirds (253) were resuscitated with albumin and one-third (126) were resuscitated with crystalloid alone. Albumin patients received more total fluid than Crystalloid patients (5.2±2.3 versus 3.7±1.7 mL/kg/% TBSA burn/24 hours) but patients in the Albumin Group were older, had larger burns, higher admission Sequential Organ Failure Assessment (SOFA) scores, and more inhalation injury. Albumin lowered the in-to-out (I/O) ratio and was started ≤ 12 hours in patients with the highest initial fluid requirements, given >12 hours with intermediate requirements, and avoided in patients who responded to crystalloid alone.
Conclusion:
Albumin use is associated with older age, larger and deeper burns, and more severe organ dysfunction at presentation. Albumin supplementation is started when initial crystalloid rates are above expected targets and improves the I/O ratio. The fluid received in the first 24 hours was at or above the Parkland Formula estimate.
Mini-Abstract
A prospective, non-interventional, observational study of burn resuscitative practices of 21 burn centers revealed that albumin was used instead of crystalloid alone in two-thirds of patients. Albumin was used for patients with higher-than-expected fluid requirements, especially if they were older or had more severe burns. Albumin rapidly reduced fluid requirements and improved urine output.
Introduction
The Parkland Formula1,2 is the most used burn resuscitation formula, providing 4 mL/kg/% TBSA burn of lactated Ringer’s solution (LR) for 24 hours. Years ago, over-resuscitation, coined “fluid creep”, was declared a major problem by Basil Pruitt and others.3-6 The Modified Brooke Formula, providing 2 mL/kg/% TBSA burn/24 hours, was supported by him and the American Burn Association’s (ABA) Advanced Burn Life Support (ABLS) course.8 A contributor to “fluid creep” may be lack of fidelity to titration of fluids to the urine output goal of 0.5-1.0 mL/kg/hour.4, 9,10 Unfortunately, using hemodynamic endpoints derived from invasive and semi-invasive techniques results in inconsistent reductions in fluid delivery, and occasionally, more fluid administration.11, 12 Some clinicians speculate that abandonment of colloids contributed to the phenomenon of fluid creep.4 Colloids should reduce fluid requirements by exerting an osmotic effect. However, burns damage the endothelial barrier to increase capillary leakage.13 Studies in animals suggest that while colloids cannot limit intravascular fluid loss, colloids can maintain an effective colloid osmotic gradient that limits intravascular fluid leakage in unburned tissues after 8- 12 hours.14-22 Compared to crystalloids, there is animal evidence that colloids reduce resuscitation volumes and improve cardiac output.23, 24 Older human studies suggest that using albumin lowers fluid resuscitation volumes.25-31 Thus, there is both animal and clinical evidence that colloid administration reduces resuscitation volumes and limits over-resuscitation. An international survey reported that half of responders administered albumin during the first 24 hours post-burn.32 In the two ABA “State of the Science Meetings” it was clear that albumin is commonly used.9,10
The effectiveness of albumin in moderating fluid resuscitation volumes has not been rigorously studied in human trials. Answering the question of whether albumin reduces resuscitation volumes in major burns will require a large multicenter randomized trial. To facilitate such a trial, we conducted this prospective, multicenter, observational trial on the use of albumin or crystalloids for burn resuscitation in burn centers of the United States and Canada.
Methods
The study was registered (ClinTrials.gov #NCT03144427) and approved by the University of California, Davis Institutional Review Board (Protocol #922669-1), Department of Defense Human Research Protection Office (Log #A-19700.a) and at each participating center’s Institutional Review Board. Since it was a noninterventional trial, no consent was required. All data was collected through REDCap™ (Research Electronic Data Capture, Vanderbilt University, Nashville, TN) and housed in the secure Data Coordination Center at the University of California, Davis.
Trial Design
The trial was a prospective, non-interventional, observational study of consecutive burn patients admitted to 21 burn centers in the United States and Canada. The primary objective was to obtain detailed information on the use of albumin and crystalloids during the first 48 hours of acute burn resuscitation. Hour-by-hour documentation of fluid infusion rates, vital signs and laboratory values of patients receiving fluid resuscitation was collected. All aspects of patient care including resuscitation was performed according to the participating center’s regular protocol.
Participants
All patients admitted to each participating burn center were screened. Inclusion criteria included age ≥18 years, acute burn ≥20% total body burn size (TBSA), and admission ≤12 hours after injury. Exclusion criteria included significant associated trauma, high voltage (≥1000 volts) electrical burn, surgical burn excision within 48 hours; death or institution of comfort care within 48 hours; or the presence at admission of severe cardiovascular, renal or hepatic disease. The use of other colloids (fresh frozen plasma [FFP], hydroxyethyl starch), hypertonic saline or high dose vitamin C also led to exclusion.
Data Collection
The time of burn injury was designated as “time 0” and subsequent hourly collection was based on that starting time. Typical patient demographics were recorded (definitions in Supplemental File 1). The mechanism of burn injury; % total body surface area (TBSA) burn, full-thickness (FT) and partial-thickness burn sizes, presence of smoke inhalation injury (based on bronchoscopy) and mechanical ventilation were also recorded. Crystalloid and/or albumin volumes prior to admission were also documented. Other admission data included Sequential Organ Failure Assessment (SOFA) scores33, Acute Kidney Injury Network (AKIN) stages34, and all admission laboratories.
On an hourly basis, the following data was collected: volume of crystalloid, volume and concentration of albumin, volume of red blood cells, oral fluids, urine output, amount and type of any bolus provided, use of vasopressors or inotropes, vital signs (heart rate [HR], mean arterial pressure [MAP]) and ventilator settings.
Outcomes
The primary outcome measure was the total fluid resuscitation volumes in mL/kg/%TBSA burn at 24 and 48 hours. Secondary outcomes included the albumin proportion (defined as volume of albumin/total fluid volume), the in-to-out (I/O) ratio (defined as volume of fluid in mL/kg/%TBSA burn divided by total urinary output in mL/kg), time to complete resuscitation (Supplemental Table 1). Complications (escharotomies, fasciotomies, abdominal compartment syndrome, AKIN and SOFA scores), duration of mechanical ventilation, length of hospital stay, 28-day and in-hospital survival were also recorded.
Statistical Analysis
Sample Size
The sample size was determined using a two-sample t-test to compare fluid resuscitation volumes between patients receiving and not receiving albumin. The sample size was adjusted to account for within-center correlation using an intra-class correlation (ICC) of 0.05.35 Assuming a standard deviation (SD) of 2 mL/kg/% burn to detect a difference in 48 hour fluid resuscitation volumes of at least 1.0 mL/kg/% burn, a total sample size of 366 subjects (183/group) was estimated to provide 90% power at a significance level of 0.05. We planned to enroll 400 subjects at 21 sites to account for withdrawals or exclusions post-enrollment.
Statistical Analyses
Quantitative traits were summarized as means ± SD, or medians and interquartile range (IQR). Two-sample t-tests or Wilcoxon Rank Sum tests were used to compare quantitative patient and injury characteristics between Albumin and Crystalloids groups, and between Early and Late-start of Albumin groups. Patients with albumin started <12 hours of injury were categorized as Early and Late as ≥12 hours. Chi-square tests were used for categorical variables. Kaplan-Meier curves were used to characterize time to initiation of albumin as well as to completion of fluid resuscitation.
A mixed-effect logistic regression was used to relate the log odds of receiving albumin within 48 hours and presence of inhalation injury, age, %full-thickness burn, %TBSA burn, and admission SOFA score as fixed effects. A random effect for each center was included to account for within-center correlation. A mixed-effect linear regression model was used to model crystalloid use per hour versus albumin use as a time varying predictor, time since injury, and the interaction between albumin use and time. A random patient effect was included to account for within-subject correlation. Using only patients without inhalation injury, a linear mixed effect model was used to evaluate the effect of mechanical ventilation on total fluids given (mL/kg/%TBSA) in 48 hours with inclusion of age, TBSA, full thickness burn, and albumin administration as covariates. A random effect for each center was included to account for within-center correlation.
Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Hypothesis tests were two-sided and evaluated at a significance level of 0.05. As this was a descriptive and exploratory study, no adjustment for multiple testing was employed.
Results
Patient characteristics
After enrolling the target 400 subjects (4/2017-6/2020), 21 were excluded (2 early excision, 7 received FFP; cardiac, liver, renal disease [6, 1, 1, respectively]; 2 comfort care, 2 other). Of the remaining 379 subjects, age was 46.3±15.9 (mean ± SD) years, weight 90.0±24.8 kg, height 175.1±10 cm, BSA 2.1±0.3 m2, BMI 29.2±7.3 kg/m2 and predicted body weight 69.5±10.4 kg. The time from burn to admission was 2.9±2.6 hours. The median %TBSA (IQR) was 31.6 (18.0) and %FT was 8.0 (22.0). As expected, most burns were <40% TBSA with decreasing numbers with increasing burn size (Supplemental File 2). Only 48 (12.7%) were diagnosed with inhalation injury but bronchoscopy rates were low, so the number is an underrepresentation. The median admission SOFA score was 2.0 (6.0), lactate 3.0 (2.7) mmol/L, and mean base deficit was 5.3 ± 3.6 mmol/L.
Characteristics of Crystalloid and Albumin Patients
Two-thirds of the cohort (N=253) received supplemental albumin (Albumin Group) and one-third (N=126) were resuscitated with crystalloids only (Crystalloid Group). When comparing the characteristics of the Albumin to the Crystalloid Group (Supplemental File 3) there were no significant differences in body habitus. Patients in the Albumin group were older (48±16.2 versus 42.9±14.7 years, p=0.003), had larger total burn size [36.0 (19.5)% TBSA versus 24.7 (11.0)%TBSA, p<0.0001] and full-thickness burns [15.0 (26.0)% TBSA versus 0.0 (7.5)%TBSA FTB, p<0.0001], but smaller partial-thickness burns [19.0 (22.0)% TBSA versus 21.3 (10.5)% TBSA, p=0.03]; and more prevalent inhalation injury (17.4% versus 3.2%, p<0.0001). Only patients in the Albumin Group received vasopressors (29 [11.5%] versus zero, p<0.0001), and they had higher admission SOFA scores (4.0 [5.0] versus 1.0 [2.0], p<0.0001). Baseline serum creatinine, lactate, and base deficit were similar between groups. Multiple logistic regression analysis evaluating factors predicting albumin use indicated that increasing age (OR 1.03, 95% CI: 1.02-1.05), larger total burn size (OR 1.08, 95% CI: 1.04-1.11) greater full thickness burn size (OR 1.04, 95% CI: 1.01-1.07), and higher admission SOFA score (OR 1.26, 95% CI: 1.11-1.42) were all significantly associated with use of albumin. The odds of albumin use was higher with inhalation injury but not statistically significant (OR 2.85, 95% CI: 0.85-9.59).
Fluid resuscitation characteristics
Prior to burn center arrival, patients received 1553±1782 [95% CI: 1374, 1732] mL of fluid (Crystalloid Group: 1271±1463 [95% CI: 1016, 1527] mL, and Albumin Group: 1694±1908 [95% CI: 1459, 1929] mL) (Table 2). At 24 hours, total fluids in the Crystalloid Group was 9207.9±4392.5 [95% CI 8535.6, 9880.2] mL and 16,827.5±7867.0 [95% CI: 15,775.9, 17,879.1] mL for the Albumin Group; and 13,617.4±6347.5 [95% CI: 12,509.1, 14,725.7] mL and 25,171.1±10,886.7 [95% CI: 23,829.6, 26,512.6] mL, respectively at 48 hours. Total fluids, indexed to weight and %TBSA burn at 24 hours were 4.6±2.2 mL/kg/% TBSA burn for the whole cohort; and 3.7±1.7 mL/kg/% TBSA burn and 5.2±2.3 mL/kg/% TBSA burn, for the Crystalloid and Albumin Groups, respectively. Hourly urine output for 24 hours was 0.87±0.51 mL/kg/hour for the whole cohort, while the Crystalloid Group produced 0.96±0.57 mL/kg/hour and the Albumin Group produced 0.80±0.46 mL/kg/hour.
Table 2:
Comparison of patient characteristics and fluid dynamics prior to administration of albumin by those who received albumin ≤12 hours and >12 hours post burn. Values are shown as mean ± SD or median (IQR) as indicated.
| Albumin started ≤ 12 hours N=118 |
Albumin started >12 hours N=135 |
P value | |
|---|---|---|---|
| Age (years) | 49.8 ± 17.4 | 46.5 ± 15 | 0.11 |
| % TBSA burn | 39.3 ± 20.0 | 34.0 ± 18.0 | 0.01 |
| % full thickness burn | 18.5 ± 29.5 | 10.0 ± 22.5 | 0.01 |
| Inhalation Injury | 28 (23.7) | 16 (11.9) | 0.01 |
| Cumulative Crystalloids (mL) | 5014.9 ± 3327.7 | 10894.4 ± 6190.4 | <0.0001 |
| Cumulative Crystalloids (mL/kg/%TBSA burn) | 1.51 ± 0.98 | 3.53 ± 1.81 | <0.0001 |
| Urine (mL/kg/hour)* | 0.45 (0.70) | 0.64 (0.49) | <0.0001 |
| Input:Output Ratio** | 0.49 (1.04) | 0.34 (0.32) | 0.01 |
% full-thickness burn, % TBSA burn, Urine (mL/kg/hour), and Input:Output Ratio were compared with a Wilcoxon-Mann-Whitney Test. Age, Cumulative Crystalloids (mL), and Cumulative Crystalloids (ml/kg/% TBSA burn) were compared with a two-sample t-test. Inhalation Injury was evaluated with a Chi-Square Test.
Note: Urine (mL/kg/hour) did not include pre burn-center urine output.
Note: For 21 patients, albumin was started upon admission or did not have any urine output before being administered albumin. They are listed as ‘missing’ and not included in the analysis.
Albumin was started 15.3±8.4 hours after injury and for a duration of 21.9±13.4 hours. The majority (162, 64.0%) received 5% albumin, 41 (16.2%) had 25% albumin and 48 (19.0%) received a combination of both. Albumin was started within 8 hours of injury in 36 patients (14.2%), 118 (46.6%) at 12 hours and 215 (85.0%) received albumin by 24 hours (Supplemental File 4). The I/O ratio at 24 hours was lower in the Crystalloid Group (0.27±0.46) than the Albumin Group (0.41±0.63) but identical at 48 hours (0.23 in each, Table 1). The graph of the I/O over time revealed that the Albumin Group I:O ratio had an upward spike at 8 hours post burn, then a rapid decline, eventually meeting that for Crystalloid patients (Figure 1A). This pattern persisted whether albumin was started before or after 12 hours (Figure 1B). The I/O ratio for patients who received albumin, shown in the hours preceding and following albumin initiation (Figure 2) shows a steep rise in the I/O ratio in the hours prior to starting albumin, followed by a precipitous fall in the first few hours after albumin initiation.
Table 1:
Fluid resuscitation characteristics for the whole cohort, patients in the Crystalloid Group and patients in the Albumin Group. Values shown are means ± SD.
| Whole Cohort (N = 379) |
Crystalloid Group (N = 126) |
Albumin Group (N = 253) |
|
|---|---|---|---|
| First 24 hours | N = 164 | N = 215 | |
| Total crystalloid volume in first 24 hours (mL) | 11894.4 ± 7351.1 | 7999.2 ± 4609.4 | 14865.6 ± 7666.3 |
| Total albumin volume in first 24 hours (mL) 38 Missing |
1599.7 ± 1887.8 | 0 | 1599.7 ± 1887.8 |
| Total volume of fluids at 24 hours (mL) | 13530.4 ± 7592.6 | 9207.9 ± 4392.5 | 16827.5 ± 7867.0 |
| Total fluid (mL/kg/% TBSA burn) | 4.6 ± 2.2 | 3.7 ± 1.7 | 5.2 ± 2.3 |
| Total urine output in first 24 hours (mL) | 1647.5 ± 934.1 | 1769.3 ± 926.0 | 1554.7 ± 931.7 |
| Urine output (mL/kg/hour) | 0.87 ± 0.51 | 0.96 ± 0.57 | 0.80 ± 0.46 |
| I:O ratio in first 24 hours – 2 missing | 0.35 ± 0.57 | 0.27 ± 0.46 | 0.41 ± 0.63 |
| At 48 hours | N=126 | N = 253 | |
| Total crystalloid volume at 48 hours (mL) | 17225.1 ± 10558.7 | 9998.8 ± 6009.4 | 20823.9 ± 10497.8 |
| Total albumin volume at 48 hours (mL) | 2676.7 ± 2882.9 | 0 | 2676.7 ± 2882.9 |
| Total volume of fluids at 48 hours (mL) | 21330.1 ± 11047.2 | 13617.4 ± 6347.5 | 25171.1 ± 10886.7 |
| Total fluid (mL/kg/% TBSA burn) | 7.4 ± 3.7 | 5.9 ± 3.2 | 8.1 ± 3.7 |
| Total urine output at 48 (mL) | 3645.6 ± 1622.6 | 3739.2 ± 1628.9 | 3598.9 ± 1620.6 |
| Urine output (mL/kg/hour) | 0.94 ± 0.47 | 0.96 ± 0.52 | 0.93 ± 0.44 |
| I:O ratio at 48 hours – 2 missing | 0.23 ± 0.41 | 0.23 ± 0.67 | 0.23 ± 0.18 |
| Peak lactate by 48 hours – 165 missing | 3.93 ± 2.14 | 3.52 ± 2.15 | 4.04 ± 2.14 |
| Change to peak lactate – 165 missing | 0.49 ± 1.05 | 0.28 ± 0.88 | 0.55 ± 1.09 |
| Peak base deficit by 48 hours – 181 missing | 6.40 ± 3.83 | 4.84 ± 3.00 | 6.69 ± 3.91 |
| Change to peak base deficit – 181 missing | 1.05 ± 1.70 | 0.62 ± 1.63 | 1.13 ± 1.71 |
Figure 1:
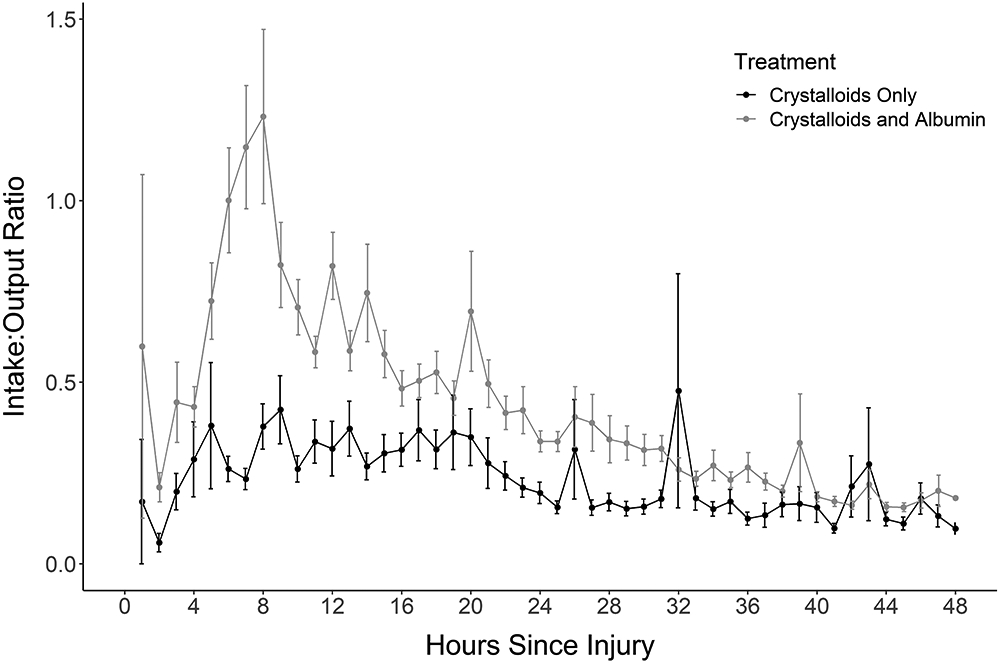
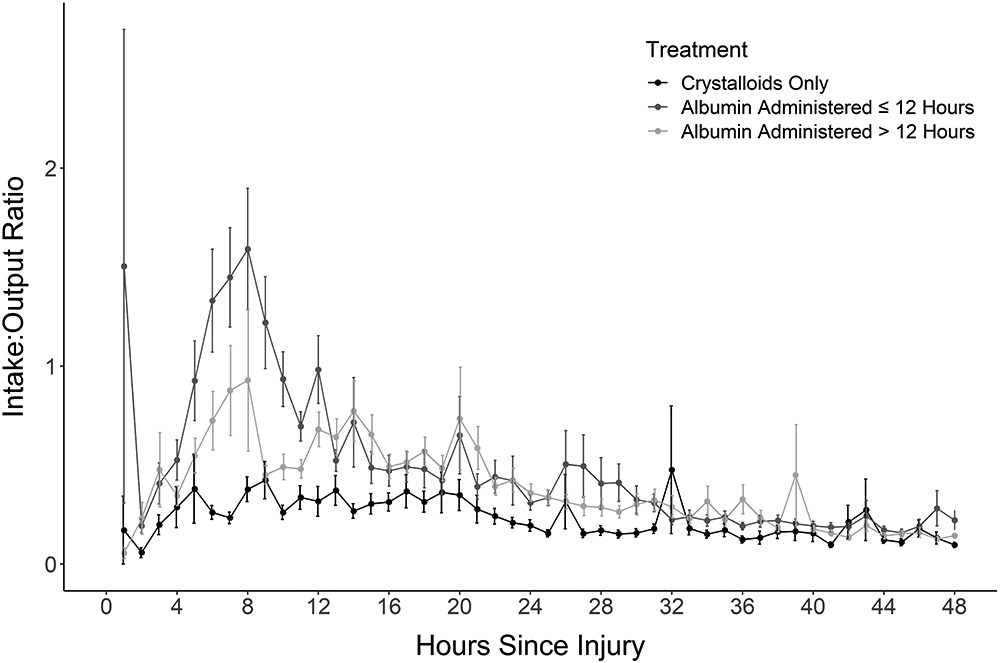
A) The Input/Output ratio for crystalloid versus albumin treatment reveals a much higher ratio for patients treated with albumin. B) The pattern persists when breaking the albumin group into albumin ≤ 12 hours, albumin > 12 hours and crystalloid.
Figure 2:
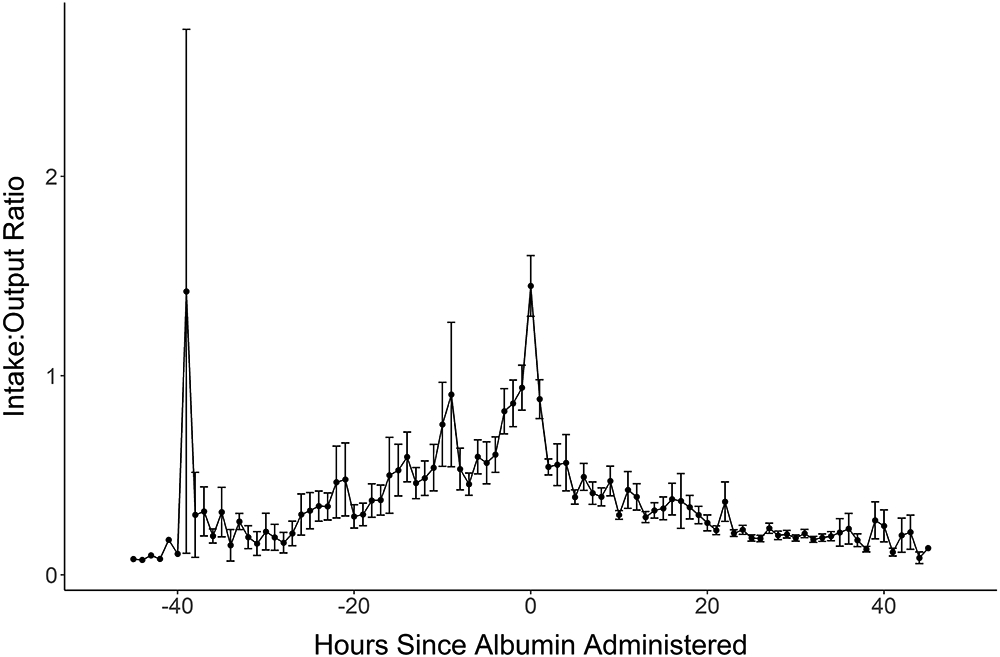
The I/O ratio in patients who were given albumin. Hour 0 is the point of albumin initiation. Hours with a negative value are the hours before albumin was started and hours with a positive value are the hours after albumin starts.
We compared those patients who received crystalloid alone with those who received albumin ≤12 hours, or >12 hours post-injury (Table 2). Patients who received albumin ≤12 hours had significantly larger total and full thickness burns, and more inhalation injury. Up to the point of initiating albumin, significantly more cumulative crystalloid fluid was administered in the >12-hour group than the ≤12-hour group (3.53±1.81 versus 1.51±0.98 mL/kg/%TBSA burn, respectively). The higher crystalloid volume reflects the longer duration of treatment. There is a higher crystalloid infusion rate in the ≤12-hour Albumin Group. This finding is supported by the higher I/O ratio in the ≤12-hour group compared to the >12-hour group (0.49 [1.04] versus 0.34 [0.32], median [IQR]). The hourly crystalloid volume for crystalloid and albumin at ≤12 and >12 hours is shown in Figure 3. A linear mixed effects regression model (Supplemental File 5) found that while crystalloid volumes were initially higher in patients who received albumin (prior to the start of albumin) than crystalloid patients, the crystalloid volumes declined much faster once albumin was started than for crystalloid-only patients.
Figure 3:
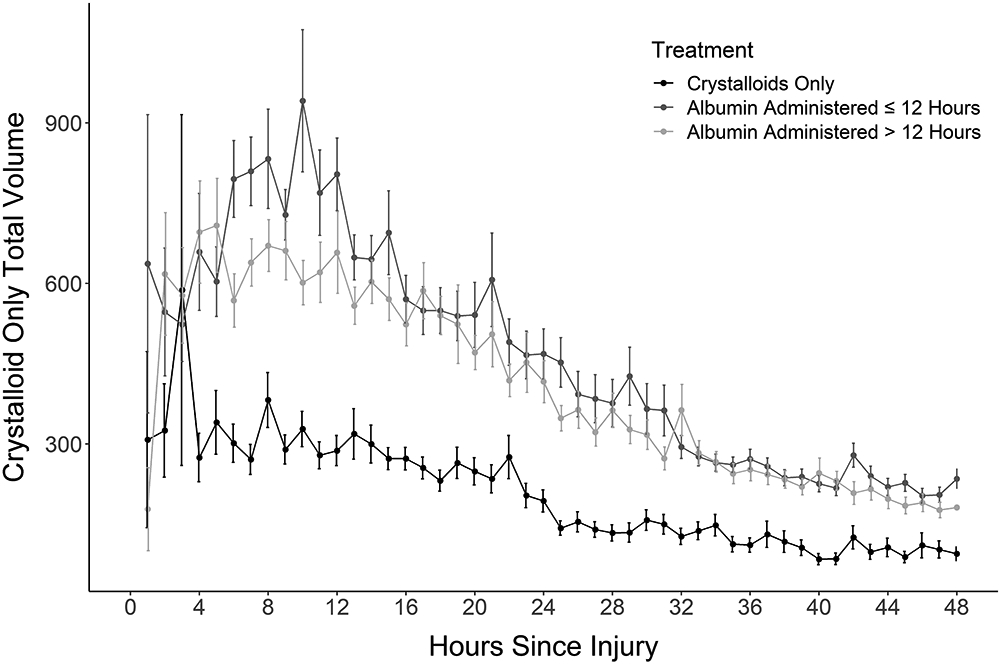
The total crystalloid volume provided for the three groups (crystalloid, albumin ≤ 12 hours and albumin > 12 hours). Greater volumes were provided in the albumin ≤ 12 hours group, followed by the > 12 hours and least in the crystalloid only group.
Inhalation injury did not influence total fluids but, since bronchoscopy was not required, the diagnosis accuracy is in doubt. We wondered if the presence of a ventilator influenced 48-hour fluid totals. In a secondary analysis of patients without inhalation injury (N=331) we found ventilated patients received, on average, 1.32 ml/kg/% TBSA burn (p=0.001) more total fluids in 48 hours than unventilated patients after adjusting for %TBSA burn, %FT burn, age and albumin administration (Supplemental File 6).
For hemodynamic changes, the Crystalloid Group had the lowest heart rate during the first 24 hours. Treatment with albumin (albumin ≤12 hours) dropped the heart rate at 10-12 hours compared to the >12-hour group. After 24 hours, the heart rates equalized (Supplementary File 7). A similar pattern was seen for mean arterial pressures (MAP) with albumin ≤12 hours being the lowest and crystalloid having the highest throughout the 48 hours (Supplementary File 8). The albumin ≤12-hour group had the lowest MAP mean for the first 12 hours but caught up to the >12-hour albumin group at 12 hours.
Outcomes
Outcomes were worse in the Albumin Group compared to the Crystalloid Group (Table 3). All fasciotomies and abdominal compartment syndromes were in the Albumin Group. All but one patient who received dialysis also received albumin. The Albumin Group had lower 28-day and in-hospital survivals; and longer duration of mechanical ventilation, length of stay, and length of stay per %TBSA burn. There was more renal compromise (Supplemental File 9), and worse SOFA scores in the Albumin Group (Supplemental File 10). The length of stay per %TBSA burn was, on average, close to the expected value of 1. The longer length of stay per %TBSA burn for the Albumin Group fits the findings of a previous study that larger and deeper burns tend to remain longer in respect to the burn size.36 Finally, we defined the time to completion of resuscitation as the point when the fluid rate equaled daily basal requirements plus evaporative loss. We found that resuscitation was completed for 50% of patients receiving Crystalloids within 8 hours of injury compared to about 24 hours for patients receiving Albumin (Supplemental File 11).
Table 3:
Summary of patient characteristics for the whole cohort, Crystalloid Group and Albumin Group. Values shown are counts (%), or as median [IQR].
| Variable | Whole Cohort (N = 379) |
Crystalloid Group (N = 126) |
Albumin Group (N = 253) |
|---|---|---|---|
| Incidence of limb fasciotomies (24 hours) | 7 (1.9%) | 0 (0%) | 7 (2.8%) |
| Incidence of limb fasciotomies (48 hours) | 9 (2.4%) | 0 (0%) | 9 (3.6%) |
| Occurrence of abdominal compartment syndrome (24 hours) | 1 (0.3%) | 0 (0%) | 1 (0.4%) |
| Occurrence of abdominal compartment syndrome (48 hours) | 2 (0.5%) | 0 (0%) | 2 (0.8%) |
| Initiation of renal replacement therapy (96 hours) | 16 (4.2%) | 1 (0.8%) | 15 (5.9%) |
| 28-Day Survivala | 341 (90.0%) | 123 (97.6%) | 218 (86.2%) |
| In Hospital Survivala | 326 (86.2%) | 123 (97.6%) | 203 (80.6%) |
| Ventilated in first 48 hours | 218 (57.5%) | 32 (25.4%) | 186 (73.5%) |
| Duration of Mechanical Ventilationa | 4.0 [20.0] | 0.0 [1.0] | 11.5 [26.0] |
| Duration of Mechanical Ventilation For Patients ever on a Ventilatorb |
17 [26.0] | 2.0 [4.5] | 19.0 [29.0] |
| Length of Hospital Staya | 28.0 [33.0] | 19.0 [13.0] | 39.0 [40.5] |
| Length of Hospital Stay/%TBSAa | 1.0 [0.9] | 0.7 [0.5] | 11.1 [0.9] |
1 Missing because a patient is still in the hospital under burn service care
162 Missing because they were never on a ventilator
Discussion
We did not plan to solve the “crystalloid versus colloid” question, but the debate has existed for decades.9 Colloids were initially used but, the modified Brooke and Parkland formulas (1960s-1970s) suggested that colloid was unnecessary during the first 24 hours.1,2,37 It was felt that since proteins were leaking across the capillaries, any resuscitative colloid would also leak to “pull” more fluid across the endothelium.17,18,38 At that time, colloids were expensive and not as safe so crystalloid resuscitation was in vogue.39, 40 In addition, a Cochrane Review suggested that albumin was harmful.41 However, studies from Pittsburgh42 and Israel43 suggested that protein-containing fluids (FFP) reduced resuscitation volumes. Subsequently, several trials demonstrated that using albumin for septic patients was advantageous.44-46 Over-resuscitation (“fluid creep”) became a concern.3-6 Albumin crept back into the practices of many burn teams. In a world-wide survey half of the respondents admitted using albumin during resuscitation.32
There are two strategies for the use of albumin during burn resuscitation. One strategy is to provide colloid at the initiation of resuscitation.25-28 Older studies were not supportive,27 but recent studies suggest that early albumin is beneficial.25,26,28 The second practice is to add albumin as a “rescue agent” when the volume of crystalloid exceeds expected resuscitation goals.29-31 If the fluid rate is greater than the expected 4 ml/kg/% TBSA burns, then albumin is added to reduce fluid volumes. Two studies in Utah poignantly demonstrated that patients with excessive crystalloid rates responded to albumin with rapid decreases in fluid requirements.29,30 Our observations parallel their findings (Figures 1 and 2). A rising I/O ratio prompted the initiation of albumin, followed by a precipitous drop in fluid requirements. Our results clearly demonstrate that when crystalloid resuscitation is excessive, physicians add albumin. With very high crystalloid requirements, albumin is started ≤12 hours. More moderate fluid rates are treated with albumin >12 hours. Caregivers clearly consider albumin to be an effective rescue agent for patients who are exceeding expected resuscitation volumes. Post-hoc proportional hazards analysis modeling of time to albumin administration revealed the likelihood of albumin administration significantly increased with increasing I/O ratios (OR 1.10 95% CI 1.06-1.14). Patients with severe burns are more likely to require colloid to stay within target.
It would be erroneous to conclude that albumin increased fluid requirements. While they received more fluid than the Crystalloid Group, the Albumin Group had older patients, larger and deeper burns, more inhalation injury, and more organ dysfunction. Furthermore, albumin initiation was prompted by rising fluid requirements and diminishing urine output (rising I/O ratio). Finally, a relatively small albumin dose (95 grams by 24 hours and 163 grams by 48 hours) was provided. An unanswered question is whether earlier intervention with higher doses of albumin would decrease overall fluid requirements, as suggested by other studies.25-31,47,48
ABRUPT provides valuable information about current concepts of burn resuscitation. For instance, what are the best indicators of adequacy of resuscitation?9,10 Currently, urine output is used as the standard indicator. Our results reveal that caregivers stay within the urine output goal (0.5-1 mL/kg/hour, Table 2). Peak lactate and base deficit were both higher in the Albumin Group, but again, this would be expected based on the greater severity of injury. Similarly, more advanced monitoring approaches were not documented, but other studies suggest that they are not helpful.11,12
Another ongoing debate is whether the Parkland Formula (4 mL/kg/% TBSA for 24 hours) as the starting fluid rate is too high or a reduced rate based on the modified Brooke formula (2-3 mL/kg/% TBSA) and ABLS is better.8 Despite the goal to reduce resuscitative volumes, the ABRUPT study revealed that 4.6 mL/kg/% TBSA was the 24-hour total (Table 2). The Crystalloid Group received close to the Parkland goal (3.7 mL/kg/%) but patients in the Albumin Group with more severe burns required significantly more fluid (5.2 mL/kg/%). These findings suggest that attaining the goal of 2 mL/kg/% may not be feasible.
A frequent question has been, when is burn resuscitation completed? If one uses the concept that resuscitation is complete when resuscitation volumes reach the calculated basal and evaporative losses, then half of the Crystalloid Group was resuscitated around 10 hours, while half of the Albumin Group was resuscitated around 24 hours (Supplemental Figure 2). These results suggest that resuscitation is completed quite rapidly in less severe burns while lasting >24 hours in more severe burns.
Finally, we found that mechanical ventilation, without inhalation injury, increased 48-hour fluid requirements. This finding supports the supposition that the use of a ventilator increases fluid requirements by increasing intrathoracic pressures to decrease venous return. More fluid is required to overcome the increased intrathoracic pressures.49 Anxiolytic agents used during mechanical ventilation have hemodynamic effects that may also contribute to higher fluid requirements.
The primary objective of this study was to inform the design of a prospective, randomized trial to compare the efficacy of crystalloid alone versus crystalloid with albumin for burn resuscitation. The results of this observational study suggest that a prospective, randomized trial is required. Albumin is commonly used and is typically initiated to trim rising fluid infusion rates. In the prospective trial, we will aim for albumin to be given within 12 hours in a ratio of one-third albumin to two-thirds crystalloid.31,50 Since small burns did not require albumin, the inclusion criteria for the prospective trial will be increased to >25% TBSA and >20% FT burns.
The major limitation to this study is that since it is an observational study, the choice of whether to use crystalloid or albumin was determined by the local clinician. There was bias for using albumin in larger, deeper burns, and for those patients not responding to crystalloid. The question of whether a crystalloid or colloid solution is better for acute burn resuscitation is therefore still unanswered. The ABRUPT study did, however, improve the design for the upcoming prospective, randomized, multicenter trial.
Conclusion
The ABRUPT trial provides an extensive view of acute burn resuscitative practices throughout North America. Crystalloids are more likely to be used for smaller burns and colloids for older patients with larger burns. If crystalloid volumes become excessive, clinicians add albumin with good effect to reduce total fluid rates. The study suggests that for burns >20% TBSA, the Parkland Formula target of 4 mL/kg/% TBSA burn for 24 hours is accurate. The urine output target of 0.5-1 mL/kg/hour seems to be a useful indicator for resuscitation. Finally, the results justify the initiation of a prospective, randomized, multicenter trial comparing crystalloid to albumin.
Supplementary Material
Key Points.
Caregivers in the United States and Canada use albumin for acute resuscitation in two-thirds of their burn patients
Albumin tends to be used in patients who are older, and with larger, deeper burns, and with more severe organ failure
The twenty-four-hour resuscitation volume remains around the Parkland Formula of 4 ml/kg/% TBSA burn
The use of albumin is dictated by the trajectory of fluid delivered. When too much crystalloid is required most caregivers add albumin to the resuscitation fluid
Albumin supplementation rapidly lowered fluid requirements relative to urinary output
Acknowledgements
We thank the following Research Coordinators, who made this study possible: Katrina Falwell, Marybeth Lawless, Elizabeth Calvieri, Claudia Islas, Kaelin Grant, Jennifer Levin, Sharada Manchikanti, Brianna Arko, Shreedar Reddy, D'Ann Hershel, David Roggy, Ben Kelly, Elsa Coates, Yan Zhai, Filip Wilk, Elizabeth Morgan, Sara Samborn, Celeste Finnerty, Paula Alem, Than Tran, Sarah Fischer
Grant Support:
Supported by the American Burn Association and funded by the Department of Defense USAMRMC Award W81XWH-16-2-0048, and National Center for Advancing Translational Sciences, National Institutes of Health, UL1 TR001860
Footnotes
Conflict of Interest: Dr. Gibson: Consultant for Mallinckrodt, Inc.; Dr. Wibbenmeyer: Research support from Avita, Inc., MEDIWound, Inc., Mallincrodt, Inc.; Dr. Holmes: Equity in Abbott Labs, AbbVie, Change Healthcare, Imbed Biosciences, and consultant for Avita, Inc. and Mallinckrodt, Inc.; Dr. Foster: Consultant for Baxter, Integra, Inc., Skingenix; Dr. Khandelwal: Consultant for Avita, Inc.; The remaining authors report no conflicts of interest
References
- 1.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci 1968;150:874–94. [DOI] [PubMed] [Google Scholar]
- 2.Baxter CR, Marvin J, Curreri PW. Fluid and electrolyte therapy of burn shock. Heart Lung 1973;2:707–13. [PubMed] [Google Scholar]
- 3.Pruitt BA Jr. Protection from excessive resuscitation: pushing the pendulum back. J Trauma 2000;49:567–568. [DOI] [PubMed] [Google Scholar]
- 4.Saffle JR. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care and Res 2007;28:382–95. [DOI] [PubMed] [Google Scholar]
- 5.Cartotto R, Zhou A. Fluid Creep: The Pendulum Hasn’t Swung Back Yet! J Burn Care and Res 2010; 31:551–559. [DOI] [PubMed] [Google Scholar]
- 6.Engrav LH, Colescott PL, Kemalyan N et al. A biopsy of the use of the Baxter Formula to resuscitate burns or do we do it like Charlie did? J Burn Care and Rehabil 2002;23:258–65. [DOI] [PubMed] [Google Scholar]
- 7.Pruitt BA Jr. Fluid and electrolyte replacement in the burned patient. Surg Clin North Am 1978;58:1291–312. [DOI] [PubMed] [Google Scholar]
- 8.Advanced Burn Life Support (ABLS: Emergency care for burn injury). A product of the American Burn Association, 2020. [Google Scholar]
- 9.Greenhalgh DG. Burn Resuscitation. J Burn Care Res 2007;28: 555–565. [DOI] [PubMed] [Google Scholar]
- 10.Cartotto R, Greenhalgh DG, Cancio C. Burn State of the Science: Fluid Resuscitation. J Burn Care Res 2017;38:e596–e604. [DOI] [PubMed] [Google Scholar]
- 11.Barton RG, Saffle JR, Morris SE, Mone M, Davis B, Shelby J. Resuscitation of thermally injured patients with oxygen transport criteria as goals of therapy. J Burn Care Rehabil 1997;18:1–9. [DOI] [PubMed] [Google Scholar]
- 12.Paratz JD, Stockton K, Paratz ED, et al. Burn resuscitation hourly urine output versus alternative endpoints: a systematic review. Shock 2014;42:295–306. [DOI] [PubMed] [Google Scholar]
- 13.Dull RO, Hahn RG. Transcapillary refill: The physiology underlying fluid resorption. J Trauma Acute Care Surg 2021;90:e31–e39. [DOI] [PubMed] [Google Scholar]
- 14.Cope O, Graham JB. The nature of the shift of plasma protein to the extravascular space following thermal trauma. Ann Surg 1948;128:1041–55. [PubMed] [Google Scholar]
- 15.Arturson G Microvascular permeability to macro- molecules in thermal injury. Acta Physiol Scand Suppl 1979;463:111–22. [PubMed] [Google Scholar]
- 16.Pitt RM, Parker JC, Jurkovich GJ, Taylor AE, Curreri PW. Analysis of altered capillary pressure and permeability after thermal injury. J Surg Res 1987;42:693–702. [DOI] [PubMed] [Google Scholar]
- 17.Demling RH, Kramer GC, Gunther R, Nerlich M. Effect of nonprotein colloid on postburn edema formation in soft tissues and lung. Surgery 1984;95:593–602. [PubMed] [Google Scholar]
- 18.Demling RH, Kramer G, Harms B. Role of thermal in- jury-induced hypoproteinemia on fluid flux and protein permeability in burned and nonburned tissue. Surgery 1984;95:136–44. [PubMed] [Google Scholar]
- 19.Guha SC, Kinsky MP, Button B, et al. Burn resuscitation: crystalloid versus colloid versus hypertonic saline hyperon- cotic colloid in sheep. Crit Care Med 1996;24:1849–57. [DOI] [PubMed] [Google Scholar]
- 20.Demling RH, Smith M, Bodai B, et al. Comparison of post- burn capillary permeability in soft tissue and lung. J Burn Care Rehabil 1981;15:86–92. [Google Scholar]
- 21.Harms BA, Bodai BI, Kramer GC, Demling RH. Microvascular fluid and protein flux in pulmonary and systemic circulations after thermal injury. Microvasc Res 1982;23:77–86. [DOI] [PubMed] [Google Scholar]
- 22.Onarheim H, Reed RK. Thermal skin injury: effect of fluid therapy on the transcapillary colloid osmotic gradient. J Surg Res 1991;50:272–8. [DOI] [PubMed] [Google Scholar]
- 23.Mehrkens HH, Ahnefeld FW. Volume and fluid replacement in the early post burn period: an animal experimental study. Burns 1979;5:113–15. [Google Scholar]
- 24.Asch MJ, Feldman RJ, Walker HL, et al. Systemic and pulmonary hemodynamic changes accompanying thermal injury. Ann Surg 1973;178:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recinos PR, Hartford CA, Ziffren SE. Fluid resuscitation of burn patients comparing a crystalloid with a colloid containing solution: a prospective study. J Iowa Med Soc 1975;65:426–32. [PubMed] [Google Scholar]
- 26.Jelenko C 3rd, Williams JB, Wheeler ML, et al. Studies in shock and resuscitation, I: use of a hypertonic, albumin-containing, fluid demand regimen (HALFD) in resuscitation. Crit Care Med 1979;7:157–67. [PubMed] [Google Scholar]
- 27.Goodwin CW, Dorethy J, Lam V, Pruitt BA Jr. Randomized trial of efficacy of crystalloid and colloid resuscitation on hemodynamic response and lung water following thermal injury. Ann Surg 1983;197:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper AB, Cohn SM, Zhang HS, Hanna K, Stewart TE, Slutsky AS; ALBUR Investigators. Five percent albumin for adult burn shock resuscitation: lack of effect on daily multiple organ dysfunction score. Transfusion 2006;46:80–9. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence A, Faraklas I, Watkins H, et al. Colloid administration normalizes resuscitation ratio and ameliorates “fluid creep.” J Burn Care Res 2010;31:40–7. [DOI] [PubMed] [Google Scholar]
- 30.Faraklas I, Lam V, Cochran A, Stoddard G, Saffle J. Colloid normalizes resuscitation ratio in pediatric burns. J Burn Care and Research 2011;32:91–7. [DOI] [PubMed] [Google Scholar]
- 31.Park SH, Hemilla MR, Whal WL. Early albumin use improves mortality in difficult to resuscitate burn patients. J Trauma 2012;73:1294–1297. [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh DG. Burn Resuscitation: The results of the ISBI/ABA survey. Burns 2010;36:176–182. [DOI] [PubMed] [Google Scholar]
- 33.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 34.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kul S, Vanhaecht K, Panella M. Intraclass correlation coefficients for randomized trials in care pathways and usual care: hospital treatment for heart failure. BMC Health Services Research 2014;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor SL, Sen S, Greenhalgh DG, Lawless M, Curri T, Palmieri TL. Real-time prediction for burn length of stay via median residual hospital length of stay methodology. J Burn Care Res 2016;37:e476–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pruitt BA Jr. Fluid and electrolyte replacement in the burned patient. Surg Clin North Am 1978;58:1291–312. [DOI] [PubMed] [Google Scholar]
- 38.Demling RH. The burn edema process: Current concepts. J Burn Care Rehabil 2005;26:207–227. [PubMed] [Google Scholar]
- 39.Cartotto R, Greenhalgh D. Colloids in acute burn resuscitation. Crit Care Clin 2016;32:507–523. [DOI] [PubMed] [Google Scholar]
- 40.Cartotto R, Callum J. A review of the use of human albumin in burn patients. J Burn Care and Research 2012;33:702–717. [DOI] [PubMed] [Google Scholar]
- 41.Cochrane Injuries Group. Human albumin administration in critically ill patients: systematic review of randomized controlled trials. BMJ 1998;317:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du G, Slater H, Goldfarb IW. Influences of different resuscitation regimens on acute early weight gain in extensively burned patients. Burns 1991;17:147–150. [DOI] [PubMed] [Google Scholar]
- 43.Ullmann Y, Kremer R, Ramon Y, et al. Evaluation of the validity of the Haifa formula for fluid resuscitation in burn patients at the Rambam Medical Centre. Ann Burns Fire Disasters 2000;13:1–10. [Google Scholar]
- 44.Finfer S, and the The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247–2256. [DOI] [PubMed] [Google Scholar]
- 45.Charpentier J, Mira JP, EARSS study group. Efficacy and tolerance of hyperoncotic albumin administration in septic shock patients: The EARSS Study. Intensive Care Med 2011;37:suppl 1:S115. [Google Scholar]
- 46.Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. New Engl J Med 2014;370:1412–1421. [DOI] [PubMed] [Google Scholar]
- 47.Navickis RJ, Greenhalgh DG, Wilkes MM. Albumin in burn shock resuscitation: A meta-analysis of controlled clinical studies. J Burn Care Res 2016;37:e268–e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eljaiek R, Heylbroeck C, Dubois M-J. Albumin administration for fluid resuscitation in burn patients: A systemic review and meta-analysis. Burns 2017;43:17–24. [DOI] [PubMed] [Google Scholar]
- 49.Mackie DP. Inhalation injury or mechanical ventilation: Which is the true killer in burn patients? Burns 2013;39:1329–1330. [DOI] [PubMed] [Google Scholar]
- 50.Cochran A, Morris SE, Edelman LS, Saffle JR. Burn patient characteristics and outcomes following resuscitation with albumin. Burns 2007;33:25–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


