Abstract
Evidence on the role of depression and anxiety in patients undergoing surgical treatment for symptomatic degenerative lumbar spinal stenosis (DLSS) is conflicting. We aimed to assess the association between depression and anxiety with symptoms and function in patients undergoing surgery for DLSS. Included were patients with symptomatic DLSS participating in a prospective multicentre cohort study who underwent surgery and completed the 24-month follow-up. We used the hospital anxiety and depression scale (HADS) to assess depression/anxiety. We used mixed-effects models to quantify the impact on the primary outcome change in the spinal stenosis measure (SSM) symptoms/function subscale from baseline to 12- and 24-months. Logistic regression analysis was used to quantify the odds of the SSM to reach a minimal clinically important difference (MCID) at 24 months follow-up. The robustness of the results in the presence of unmeasured confounding was quantified using a benchmarking method based on a multiple linear model. Out of 401 patients 72 (17.95%) were depressed and 80 anxious (19.05%). Depression was associated with more symptoms (β = 0.36, 95% confidence interval (CI) 0.20 to 0.51, p < 0.001) and worse function (β = 0.37, 95% CI 0.24 to 0.50, p < 0.001) at 12- and 24-months. Only the association between baseline depression and SSM symptoms/function was robust at 12 and 24 months. There was no evidence for baseline depression/anxiety decreasing odds for a MCID in SSM symptoms and function over time. In patients undergoing surgery for symptomatic DLSS, preoperative depression but not anxiety was associated with more severe symptoms and disability at 12 and 24 months.
Subject terms: Medical research, Outcomes research
Introduction
Degenerative lumbar spinal stenosis (DLSS) is a common indication for spine surgery in in the elderly1,2. In symptomatic patients, the narrowing of spinal canal and compression of neural roots result in neurogenic claudication, a pain in the gluteal region and/or lower extremity during walking, which relieves through rest and lumbar flexion; with or without low back pain3,4. Pain and functional limitations results in a strong negative influence on health-related quality of life5. In symptomatic patients, treatment options include watchful waiting, physiotherapy, pain medications, injection of analgesics and steroids, and decompression surgery6.
Although surgery has been shown to be effective, not all patients will benefit from the treatment7. Approximately one-third of patients undergoing surgery will eventually not achieve a clinically relevant improvement7. Further, imaging studies in asymptomatic adults aged 60 years or older showed in up to 20% a stenosis without pain8. Various factors may influence treatment success in elderly patients. Age, sex, and comorbidities have been shown to result in longer hospital stay and more peri- and postoperative complications9–12. Psychological factors such as depression and anxiety may be also relevant. Depression has been shown to negatively influence treatment efficacy in patients with DLSS13. The evidence on anxiety and the interaction between anxiety and depression is less well studied and conflicting14,15.
The aim of this study is to evaluate whether depression and/or anxiety at baseline are associated with symptom severity and functional disability in patients with DLSS undergoing surgery. A novel methodological approach was used to assess the robustness of the results in the presence of unmeasured confounding16.
Methods
Data source and patient selection
Secondary analysis of patients included in a multicentre prospective cohort study conducted in eight hospitals in German-speaking Switzerland. The details of the study have been described previously3,17–19. In summary, patients were included into the Lumbar Stenosis Outcome Study (LSOS) if they (1) were ≥ 50 years old, (2) suffered from neurogenic claudication, (3) had a clinically and radiologically verified diagnosis of DLSS, (4) had a life-expectancy of > 1 year, (5) provided informed consent, and (6) follow-up assessment were feasible. Excluded were patients requiring urgent surgery, acute fractures or infection, excessive lumbar scoliosis (> 15 degrees), verified peripheral arterial disease19. For the current study, we included patients who underwent surgery up to 6 months of study inclusion and completed a 24-month follow-up. Excluded were patients who underwent non-surgical treatments.
The study was approved by the ethical committee (canton Zurich, Switzerland, KEK-ZH-NR: 2010-0395/0). The study was reported according to the STROBE guidelines20.
Surgical procedure
The choice of the surgical procedures was at the discretion of the treating physician. Procedures included decompression surgery alone or with fusion techniques. Decompression surgery consisted of a standard open posterior lumbar decompression of affected level(s) with or without decompression of the lateral recessus and the foramina where appropriate. Fusion surgery consisted of additional implantation of pedicle screws with rods, and intersomatic fusion and cage(s) at the affected level(s). All procedures were performed or supervised by senior orthopaedic/neurosurgeons (> 10 years of experience after board certification).
Depression and anxiety definition
Anxiety and depressive symptoms were assessed using the self-rated, validated, and reliable hospital anxiety and depression scale (HADS)21. The HADS consists of an anxiety and a depression subscale with a score-range of 0 (best) to 21 (worst) for each subscale22,23. Patients were categorized as depressed if the score was ≥ 8 points on the HADS depression subscale (HADS-D)21. Anxiety was defined as ≥ 8 points on the HADS anxiety subscale (HADS-A)21. Sensitivity and specificity of a cut-off of ≥ 8 points for both HADS subscales to identify patients with depression/anxiety has been shown to be between 0.70 and 0.9021.
Outcomes
The primary outcomes were symptoms (pain) and physical function assessed using the spinal stenosis measure (SSM) symptoms and function subscale at 12 and 24 months. Secondary outcomes were meaningful clinically important difference (MCID) in the SSM symptoms and function at 24 months, complications, and reoperation rates. The SSM German version is a reliable and validated brief self-administered questionnaire (Supplementary Appendix 1)24,25. The symptom subscale ranges from 1 to 5 points (best–worst) and the function subscale from 1 to 4 (best–worst). The MCID in the SSM symptoms subscale was a 0.48 point improvement (decrease) and in the SSM function subscale score 0.52 points24,25. In patients with baseline values below the thresholds (SSM symptoms < 1.48, SSM function < 1.52), and remaining low SSM scores at all time points, an MCID of 1 was imputed at 24 months follow-up.
Sample size justification
The number of patients available for this study was large enough for fitting multiple linear mixed-effects regression models with a time trend, including the parameters of interest (depression and anxiety) as well as eight other baseline characteristics to adjust for confounding.
Confounder variables
Patients completed a set of self-reported questionnaires at baseline and additional information on comorbidities and smoking were collected during a structured interviews to complete the Cumulative illness rating scale (CIRS)26. Each patient was asked whether he/she was currently smoking. In case a patient was a non-smoker, we additionally asked for smoking in the past. Smoker was defined as a patient that reported currently to smoke. Low back and buttock pain were assessed in self-reported questions at the baseline evaluation.
Statistical analysis
Descriptive statistics included mean and standard deviation (SD) (continuous/ordinal variables) and counts and percentages (categorical variables). Groups (depression/no depression and anxiety/no anxiety) were compared with exploratory p-values from χ2 and t tests. A predefined set of confounders18 was used including age, gender, body mass index (BMI) (≥ 25 kg/m2), civil risk (living alone, or single/divorced/widowed and living in a nursing/residential home), duration of symptoms (> 6 months), cumulative illness rating scale (CIRS), epidural injections within 90 days before baseline, and necessity of fusion surgery. Time was additionally included as a categorical variable, to account for overall trends at 12 and 24 months. We used multiple linear mixed effects regression models with continuous SSM symptom and function scores (separately) over time. Repeated observations in SSM symptoms and function scores were captured in patient-specific random intercept terms. The effects of depression and anxiety were adjusted for overall change from baseline to 12 and 24 months in these outcomes. We evaluated whether there was the necessity for an interaction term of depression and anxiety, and we included the interaction term in the model, if the corresponding p-value of the interaction was < 0.05. Results were presented as beta-coefficients and corresponding 95% confidence intervals (95% CI).
For the binary outcomes MCID at 24 months for SSM symptom and SSM function, multiple logistic regression models (adjusted for the same confounders) were used to estimate odds ratios (OR) and the corresponding 95% CI. In all multiple regression models, missing values were excluded listwise.
Sensitivity analysis
A common concern in observational research is the presence of unmeasured confounding. Unmeasured confounding could affect the estimate of the variable of interest in principle in both directions (in- or decrease) of the estimated effect. In a first step, we adjusted the estimated effects of depression and anxiety for a set of eight pre-defined confounders. Because we could not exclude that other unmeasured confounders affect the results, we used a benchmarking method recently proposed by Cinelly and Hazlett16. We used a multiple linear model to assess the robustness of the estimated effects of baseline depression/anxiety on continuous outcomes SSM symptoms/function at 24 months. The idea was to quantify and assess visually how strong an unmeasured confounder would have to be to explain away the original effect16. In our study, the adjusted estimated effects of depression and anxiety from multiple linear mixed-effects regression models was benchmarked against gender, representing the most relevant confounder of clinical importance in this context, if there was strong evidence for an association. The proposed method used contour plots of the original estimate against its reduction in the direction of no effect with the magnitude of multiples of the gender effect. Estimated effects that are robust up to multiple times the benchmarking variable are considered less likely to be explained away by unmeasured confounding.
All analyses were performed with the statistical software R27 in combination with dynamic reporting.
Results
Patient characteristics
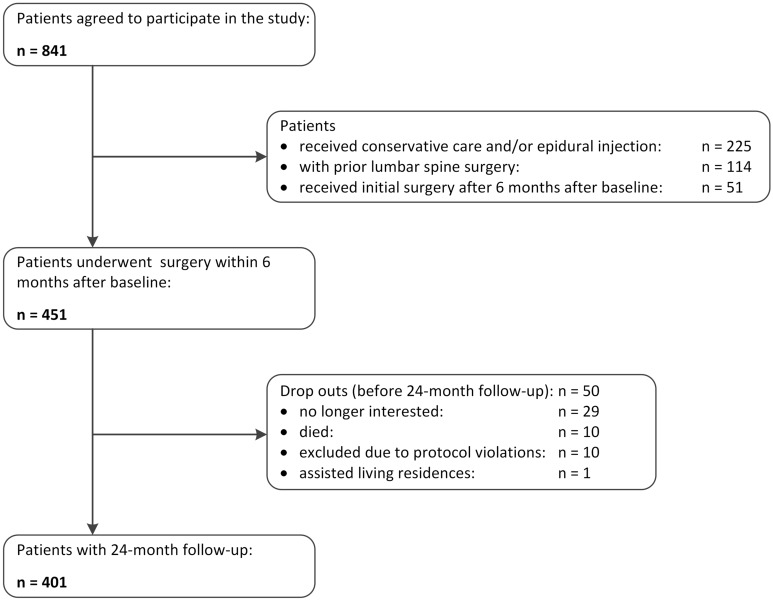
Out of 451 patients who were eligible for the current study, 401 patients (88.91%) completed their 24-month follow-up assessment after surgery and were included in the analysis (Fig. 1). Reasons for missing follow-up evaluations included drop-out or moving to a residential home (n = 30, 6.65%), death (n = 10, 2.22%), and exclusion due to protocol violations (n = 10, 2.22%).
Figure 1.
Study flow.
In total 50.37% were female, the mean age was 72.50 years (SD 8.41), mean BMI 27.30 kg/m2 (SD 4.61), and symptom duration was > 6 months in 76.25% (Table 1). Compared to patients without depressive symptoms, depressed patients (n = 72, 17.95%) were more often women (63.89% vs. 47.42%) and had a higher mean score in the HADS anxiety subscale (8.49 vs. 3.69). Compared to patients without anxiety, patients with anxiety (n = 80, 19.05%) were younger (mean age 70.54 vs. 72.98 years), more likely female (63.75% vs. 47.04%), had more buttocks pain (88.75% vs 76.64%), complained about more worsening symptoms during the last three months (90.0% vs. 76.88%) and had higher mean score in the HADS depression subscale (8.15 vs. 3.81). Although not statistically significant, the proportion of decompression surgery only was higher in patients with depression compared to non-depressed patients. The proportion of fusion surgery was somewhat higher in patients with anxiety (31.25% vs. 21.50%, p = 0.09).
Table 1.
Baseline characteristics of the study population.
| Variable | Overall | No depression§ | Depression | Exploratory p-values | No anxiety‡ | Anxiety | Exploratory p-values |
|---|---|---|---|---|---|---|---|
| Number of patients (%) | 401 | 329 (82.05) | 72 (17.95) | 321 (80.05) | 80 (19.05) | ||
| Age, mean (SD) | 72.50 (8.41) | 72.56 (8.19) | 72.22 (9.44) | 0.76 | 72.98 (8.23) | 70.54 (8.92) | 0.02 |
| Female, n (%) | 202 (50.37) | 156 (47.42) | 46 (63.89) | 0.02 | 151 (47.04) | 51 (63.75) | 0.01 |
| BMI kg/m2, mean (SD) | 27.30 (4.61) | 27.41 (4.60) | 26.78 (4.66) | 0.29 | 27.40 (4.68) | 26.92 (4.36) | 0.41 |
| BMI ≥ 25 kg/m2, n (%) | 269 (67.08) | 223 (67.78) | 46 (63.89) | 0.62 | 213 (66.36) | 56 (70.00) | 0.63 |
| Civil risk*, n (%) | 133 (33.17) | 105 (31.91) | 28 (38.89) | 0.32 | 109 (33.96) | 24 (30.00) | 0.59 |
| Compulsory education, n (%) | 101 (25.19) | 85 (25.84) | 16 (22.22) | 0.62 | 79 (24.61) | 22 (27.50) | 0.70 |
| Back pain, n (%) | 344 (86.22) | 278 (85.02) | 66 (91.67) | 0.20 | 271 (84.95) | 73 (91.25) | 0.20 |
| Buttocks pain, n (%) | 317 (79.05) | 257 (78.12) | 60 (83.33) | 0.41 | 246 (76.64) | 71 (88.75) | 0.03 |
| Duration of symptoms > 6 months, n (%) | 305 (76.25) | 250 (76.22) | 55 (76.39) | 1.00 | 244 (76.25) | 61 (76.25) | 1.00 |
| Problem getting better/worse during last 3 months, n (%) | 0.16 | 0.03 | |||||
| Getting better | 22 (5.50) | 19 (5.79) | 3 (4.17) | 20 (6.25) | 2 (2.50) | ||
| Staying about the same | 58 (14.50) | 53 (16.16) | 5 (6.94) | 53 (16.56) | 5 (6.25) | ||
| Getting worse | 318 (79.50) | 254 (77.44) | 64 (88.89) | 246 (76.88) | 72 (90.00) | ||
| Don’t know | 2 (0.50) | 2 (0.61) | 0 (0.00) | 1 (0.31) | 1 (1.25) | ||
| CIRS, mean (SD) | 9.32 (3.93) | 9.12 (3.97) | 10.24 (3.67) | 0.40 | 9.37 (4.03) | 9.15 (3.55) | 0.73 |
| Diabetes, n (%) | 47 (11.72) | 36 (10.94) | 11 (15.28) | 0.73 | 39 (12.15) | 8 (10.00) | 0.19 |
| Smoker, n (%) | 64 (16.00) | 51 (15.55) | 13 (18.06) | < 0.001 | 47 (14.64) | 17 (21.52) | < 0.001 |
| HADS anxiety subscale, mean (SD) | 4.55 (3.52) | 3.69 (2.80) | 8.49 (3.80) | < 0.001 | 3.19 (2.20) | 10.03 (2.31) | < 0.001 |
| HADS depression subscale, mean (SD) | 4.67 (3.36) | 3.44 (2.03) | 10.31 (2.35) | < 0.001 | 3.81 (2.68) | 8.15 (3.58) | < 0.001 |
| Epidural injections within 90 days before baseline, n (%) | 129 (32.17) | 103 (31.31) | 26 (36.11) | 0.50 | 99 (30.84) | 30 (37.50) | 0.31 |
| Surgery | 0.18 | 0.09 | |||||
| Decompression surgery only, n (%) | 307 (76.56) | 247 (75.08) | 60 (83.33) | 252 (78.50) | 55 (68.75) | ||
| Decompression with fusion, n (%) | 94 (23.44) | 82 (24.92) | 12 (16.67) | 69 (21.50) | 25 (31.25) | ||
IQR interquartile range, CIRS cumulative illness rating scale (range from 0 points (best) to 56 points (worst)), HADS hospital anxiety and depression scale (each subscale range from 0 points (best) to 21 points (worst)), SSM spinal stenosis outcome measure.
§Depression defined as HADS depression subscale score ≥ 8 (score-range of 0 (best) to 21 (worst)).
‡Anxiety defined as HADS anxiety subscale score ≥ 8 (score-range of 0 (best) to 21 (worst)).
*Living alone, or single/divorced/widowed and living in a nursing/residential home.
χ2 and t-tests were used for group comparison.
Surgical treatment types, surgical complications, and reoperations
Surgical procedures included decompression alone (n = 307, 76.56%) and decompression and fusion surgery (n = 94, 23.44%). Table 2 summarizes intra- and postoperative complications and reoperations. Intra- and postoperative complications occurred in 70 (17.46%) index operations with dural tear (6.22%) being the most common intraoperative complication. There was no difference in complication rates, reoperation rates, and reasons for reoperation in patients with/without depression and anxiety (Table 2).
Table 2.
Surgical complications, and reoperations.
| Variable | Overall | No depression | Depression | Exploratory p-values | No anxiety | Anxiety | Exploratory p-values |
|---|---|---|---|---|---|---|---|
| n | 401 | 329 | 72 | 321 | 80 | ||
| Complications | |||||||
| Intraoperative bleeding | 4 (1.00) | 3 (0.91) | 1 (1.39) | 1.00 | 4 (1.25) | 0 (0.00) | 0.71 |
| Intraoperative dural tear | 25 (6.22) | 24 (7.29) | 1 (1.39) | 0.11 | 25 (7.79) | 0 (0.00) | 0.02 |
| Postoperative wound infection | 5 (1.24) | 3 (0.91) | 2 (2.78) | 0.48 | 3 (0.93) | 2 (2.50) | 0.57 |
| Postoperative osseous infection | 1 (0.25) | 1 (0.30) | 0 (0.00) | 1.00 | 1 (0.31) | 0 (0.00) | 1.00 |
| Postoperative other* | 35 (8.71) | 26 (7.90) | 9 (12.50) | 0.31 | 26 (8.10) | 9 (11.25) | 0.50 |
| Number of reoperations: n | 53 | 34 | 19 | 37 | 16 | ||
| 1 reoperation, patients: n (%) | 42 (79.25) | 28 (82.35) | 14 (73.68) | 0.37 | 30 (81.08) | 12 (75.00) | 0.63 |
| 2 reoperations, patients: n (%) | 10 (18.87) | 6 (17.65) | 4 (21.05) | 6 (16.22) | 4 (25.00) | ||
| 3 reoperations, patients: n (%) | 1 (1.89) | 0 (0.00) | 1 (5.26) | 1 (2.70) | 0 (0.00) | ||
| Type of reoperation: fusion | 32 (60.38) | 19 (55.88) | 13 (68.42) | 0.55 | 21 (56.76) | 11 (68.75) | 0.61 |
| Indication: restenosis | 38 (71.70) | 22 (64.71) | 16 (84.21) | 0.23 | 26 (70.27) | 12 (75.00) | 0.99 |
| Indication: infection | 1 (1.89) | 1 (2.94) | 0 (0.00) | 1.00 | 0 (0.00) | 1 (6.25) | 0.66 |
| During the 1. year | 36 (67.92) | 21 (61.76) | 15 (78.95) | 0.33 | 26 (70.27) | 10 (62.50) | 0.81 |
| During the 2. year | 16 (30.19) | 12 (35.29) | 4 (21.05) | 0.44 | 10 (27.03) | 6 (37.50) | 0.66 |
| During the 3. year | 1 (1.89) | 1 (2.94) | 0 (0.00) | 1.00 | 1 (2.70) | 0 (0.00) | 1.00 |
| Median days baseline operation to 1. reoperation, (IQR) | 199.50 [92.25, 469.75] | 213.00 [56.00, 483.50] | 193.50 [152.50, 415.00] | 0.94 | 190.00 [56.00, 441.25] | 369.50 [153.50, 473.25] | 0.20 |
| Median days 1. reoperation to 2. reoperation, (IQR) | 200.50 [81.75, 322.75] | 207.00 [113.00, 374.50] | 149.00 [12.50, 294.50] | 0.29 | 288.50 [159.75, 374.50] | 63.50 [12.50, 166.25] | 0.14 |
| Median days 2. Reoperation to 3. reoperation, (IQR) | 86.00 [86.00, 86.00] | NA [NA, NA] | 86.00 [86.00, 86.00] | NA | 86.00 [86.00, 86.00] | NA [NA, NA] | NA |
NA not applicable.
*Example, urosepsis, hemorrhage, wound healing deficit.
Improvement in symptoms and function between baseline and 24-month
Table 3 summarizes the mean SSM symptoms and function scores at baseline, 12- and 24-months follow-up. Depressed patients expressed higher SSM symptoms compared to patients with no depression (3.39 vs. 3.08, p < 0.001) and function scores (2.55 vs. 2.18, p < 0.001). Baseline anxiety was associated with higher SSM symptoms (3.42 vs. 3.07, p < 0.001) and function scores (2.51 vs. 2.18, p < 0.001).
Table 3.
Symptoms and function at baseline and follow-up.
| Variable | Overall | No depression | Depression | No anxiety | Anxiety |
|---|---|---|---|---|---|
| SSM symptoms, mean (SD) | |||||
| Baseline | 3.14 (0.59) | 3.08 (0.58) | 3.39 (0.59) | 3.07 (0.59) | 3.42 (0.52) |
| 12-months | 2.10 (0.80) | 1.99 (0.74) | 2.61 (0.84) | 2.02 (0.77) | 2.38 (0.85) |
| 24-months | 2.15 (0.84) | 2.05 (0.82) | 2.63 (0.77) | 2.09 (0.84) | 2.41 (0.81) |
| SSM function, mean (SD) | |||||
| Baseline | 2.24 (0.66) | 2.18 (0.64) | 2.55 (0.64) | 2.18 (0.66) | 2.51 (0.58) |
| 12-months | 1.52 (0.60) | 1.43 (0.53) | 1.91 (0.73) | 1.49 (0.59) | 1.64 (0.64) |
| 24-months | 1.55 (0.62) | 1.46 (0.56) | 1.93 (0.70) | 1.52 (0.62) | 1.64 (0.61) |
SSM spinal stenosis outcome measure.
On average, patients improved over time in both outcome scores (Tables 3, 4). Baseline depression was associated with worse SSM symptoms (β = 0.36, 95% CI 0.20 to 0.51, p < 0.001, Table 4a) and SSM function (β = 0.37, 95% CI 0.24 to 0.50, p < 0.001, Table 4b) scores at 24-month follow-up. Baseline anxiety was associated with worse SSM symptoms (β = 0.18, 95% CI 0.03 to 0.33, p = 0.02) without evidence for an association with SSM function (β = 0.03, 95% CI − 0.09 to 0.16, p = 0.63). Whereas each effect size was smaller than the 0.48 points for SSM symptoms and 0.52 points for SSM function score, effects of anxiety and depression combined were clinically relevant. There was no significant interaction between anxiety and depression for SSM symptoms (p = 0.48) and function (p = 0.47).
Table 4.
Summary of the multiple linear mixed effects regression models for SSM symptom and function at 12 and 24 months in patients with depression and anxiety.
| Variable | Estimate | Lower bound of 95% CI | Upper bound of 95% CI | p-value |
|---|---|---|---|---|
| (A) SSM symptoms | ||||
| (Intercept) | 2.28 | 1.76 | 2.81 | < 0.001 |
| Depression§ | 0.36 | 0.20 | 0.51 | < 0.001 |
| Anxiety‡ | 0.18 | 0.03 | 0.33 | 0.02 |
| Time 12 months follow-up | − 1.04 | − 1.12 | − 0.96 | < 0.001 |
| Time 24 months follow-up | − 0.99 | − 1.07 | − 0.91 | < 0.001 |
| Age | 0.003 | − 0.004 | 0.01 | 0.34 |
| Female | 0.20 | 0.08 | 0.32 | 0.001 |
| BMI ≥ 25 kg/m2 | 0.14 | 0.02 | 0.25 | 0.02 |
| Civil risk* | − 0.02 | − 0.15 | 0.10 | 0.71 |
| Duration of symptoms > 6 months | 0.07 | − 0.06 | 0.20 | 0.28 |
| CIRS | 0.03 | 0.02 | 0.05 | < 0.001 |
| Epidural injections within 90 days before baseline | − 0.02 | − 0.13 | 0.10 | 0.76 |
| Fusion surgery | − 0.06 | − 0.19 | 0.07 | 0.39 |
| (B) SSM function | ||||
| (Intercept) | 1.48 | 1.05 | 1.92 | < 0.001 |
| Depression§ | 0.37 | 0.24 | 0.50 | < 0.001 |
| Anxiety‡ | 0.03 | − 0.09 | 0.16 | 0.63 |
| Time 12 months follow-up | − 0.73 | − 0.79 | − 0.66 | < 0.001 |
| Time 24 months follow-up | − 0.70 | − 0.76 | − 0.63 | < 0.001 |
| Age | 0.005 | − 0.001 | 0.01 | 0.12 |
| Female | 0.19 | 0.10 | 0.29 | < 0.001 |
| BMI ≥ 25 kg/m2 | 0.19 | 0.10 | 0.29 | < 0.001 |
| Civil risk* | − 0.03 | − 0.14 | 0.07 | 0.54 |
| Duration of symptoms > 6 months | 0.01 | − 0.10 | 0.11 | 0.91 |
| CIRS | 0.02 | 0.01 | 0.03 | 0.01 |
| Epidural injections within 90 days before baseline | 0.03 | − 0.07 | 0.13 | 0.55 |
| Fusion surgery | − 0.12 | − 0.23 | − 0.01 | 0.03 |
A negative value corresponds to reduction of symptom severity/functional disability.
CIRS cumulative illness rating scale (range from 0 points (best) to 56 points (worst)).
§Depression defined as HADS depression subscale score ≥ 8 (score-range of 0 (best) to 21 (worst)).
‡Anxiety defined as HADS anxiety subscale score ≥ 8 (score-range of 0 (best) to 21 (worst)).
*Living alone, or single/divorced/widowed and living in a nursing/residential home.
Significance values are given in bold.
Sensitivity analysis
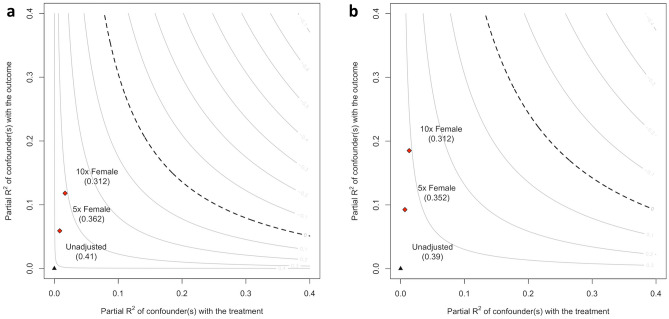
The sensitivity analysis was performed for depression, as there was strong evidence for an association with SSM symptoms and SSM function. The sensitivity analysis revealed that the estimated effect of depression on SSM symptoms and SSM function was robust even if an unmeasured confounder of the magnitude of at least 10 times gender was present in our study (Fig. 2a,b).
Figure 2.
(a) Contour plot of robustness of depression for SSM symptoms. The partial R2 gives the percentage of variation in a multiple linear model that cannot be explained by the confounders. The unadjusted estimate 0.41 represents the estimate for depression in a multiple linear model with SSM symptoms as outcome, and with the confounders age, gender female, BMI ≥ 25, civil risk, duration of symptoms > 6 months, CIRS, epidural injections within 90 days before baseline, and necessity of fusion surgery. The estimate of 0.36 represents the estimate for depression on SSM symptoms, if there was an unmeasured confounders with the strength of association of five times female gender present. Accordingly, the estimate of 0.31 shows the estimate for depression on SSM symptom if there was an unmeasured confounder with the strength of association of ten times gender female present. The sensitivity analysis shows that even in the presence of a strong unmeasured confounder, the estimate of depression on SSM symptom would remain robust. (b) Contour plot of robustness of depression for SSM function. The partial R2 gives the percentage of variation in a multiple linear model that cannot be explained by the confounders. The unadjusted estimate 0.39 represents the estimate for depression in a multiple linear model with SSM function as outcome, and with the confounders age, gender female, BMI ≥ 25, civil risk, duration of symptoms > 6 months, CIRS, epidural injections within 90 days before baseline, and necessity of fusion surgery. The estimate of 0.35 represents the estimate for depression on SSM function, if there was an unmeasured confounders with the strength of association of five times female gender present. Accordingly, the estimate of 0.31 shows the estimate for depression on SSM function if there was an unmeasured confounder with the strength of association of ten times gender female present. The sensitivity analysis shows that even in the presence of a strong unmeasured confounder, the estimate of depression on SSM function would remain robust.
Clinical relevance of depression and anxiety
The number of patients with clinically meaningful improvement (MCID) in SSM symptoms was 280 (69.83%; depression 63.89%, no depression 71.12%, anxiety 68.75%, and no anxiety 70.09%) over 2 years follow-up. The corresponding numbers for patients with MCID in SSM function were 254 (63.34%; depression 56.94%, no depression 64.74%, anxiety 67.50%, and no anxiety 62.31%). There was no evidence that depression was associated with decreased odds for a MCID in SSM symptoms (OR 0.70, 95% CI 0.37 to 1.34, p = 0.28) and function (OR 0.59, 95% CI 0.32 to 1.11, p = 0.10) at 24-month follow-up. There was also no evidence that anxiety was associated with a decreased odds for a MCID in SSM symptoms (OR 1.01, 95% CI 0.54 to 1.96, p = 0.97) and SSM function (OR 1.47, 95% CI 0.80 to 2.79, p = 0.22) scores at 24-month follow-up (Table 5).
Table 5.
Summary of the multiple logistic regression models for minimal clinical important difference (MCID) in the SSM symptoms and function at 24 months in patients with depression, anxiety.
| Variable | OR** | Lower bound of 95% CI | Upper bound of 95% CI | p |
|---|---|---|---|---|
| (A) SSM symptoms | ||||
| Depression§ | 0.70 | 0.37 | 1.34 | 0.28 |
| Anxiety‡ | 1.01 | 0.54 | 1.96 | 0.97 |
| Age | 0.97 | 0.94 | 0.10 | 0.02 |
| Female | 1.00 | 0.61 | 1.66 | 1.00 |
| BMI ≥ 25 kg/m2 | 0.77 | 0.46 | 1.27 | 0.31 |
| Civil risk* | 0.76 | 0.45 | 1.28 | 0.29 |
| Duration of symptoms > 6 months | 0.55 | 0.30 | 0.97 | 0.04 |
| CIRS | 0.98 | 0.92 | 1.04 | 0.44 |
| Epidural injections within 90 days before baseline | 1.69 | 1.02 | 2.85 | 0.05 |
| Fusion surgery | 1.02 | 0.58 | 1.81 | 0.96 |
| (B) SSM function | ||||
| Depression§ | 0.59 | 0.32 | 1.11 | 0.10 |
| Anxiety‡ | 1.47 | 0.80 | 2.79 | 0.22 |
| Age | 0.97 | 0.94 | 1.00 | 0.05 |
| Female | 0.87 | 0.54 | 1.39 | 0.55 |
| BMI ≥ 25 kg/m2 | 0.70 | 0.44 | 1.12 | 0.14 |
| Civil risk* | 0.94 | 0.57 | 1.56 | 0.81 |
| Duration of symptoms > 6 months | 0.47 | 0.27 | 0.82 | 0.01 |
| CIRS | 0.95 | 0.90 | 1.00 | 0.07 |
| Epidural injections within 90 days before baseline | 1.56 | 0.98 | 2.53 | 0.07 |
| Fusion surgery | 0.84 | 0.50 | 1.43 | 0.52 |
§Depression defined as HADS depression subscale score ≥ 8 (score-range of 0 (best) to 21 (worst)).
‡Anxiety defined as HADS anxiety subscale score ≥ 8 (score-range of 0 (best) to 21 (worst)).
*Living alone, or single/divorced/widowed and living in a nursing/residential home.
**A value smaller than 1 corresponds to a reduced chance to achieve minimal important difference of SSM symptoms/function subscale.
Significance values are given in bold.
Discussion
In this study of 401 patients undergoing surgery for symptomatic DLSS, we found a robust association between baseline depression with more severe symptoms and worse function even after accounting for the potential presence of strong unmeasured confounding. Although the effect of anxiety alone was clinically not relevant, baseline depression and anxiety combined were associated with a clinically relevant increase in the severity of symptoms over the study period. Baseline depression and anxiety did not reduce the odds for a clinical meaningful improvement in symptoms and function scores and were not associated with complication rates and reoperation.
Results in the light of existing literature
The results from our study expands on the conclusion of a systematic review published in 201413. Although our study supports the authors conclusion that depression is a prognostic indicator in patients undergoing surgery for lumbar spinal stenosis13 for symptoms and function, depression did not result in a reduced odds for a clinical meaningful improvement after surgery. Lebow et al.28 argued that pain from disc herniation might contribute to preoperative depression and anxiety and that discectomy improves mental health and well-being during the postoperative period over months with improvement in anxiety and depression. Three studies showed level of depression measured preoperatively did not predict the surgical outcome15,28,29 and that depression improved postoperatively in patients with a favorable outcome and did not improve in patients with a poor treatment outcome, suggesting that psychological disorders may be the result of DLSS related complaints29,30. However, other studies found preoperative depression to be associated with poorer life satisfaction after surgery31 and poorer treatment outcome (symptom severity, disability and walking capacity) after surgery31,32. Whether depression alone or as a result of a general life-dissatisfaction, as suggested by a study following patients over 10 years after surgery for DLSS33, warrants further investigation to elucidate whether preoperative depression should be addressed as a modifiable factor.
A systematic review concluded that anxiety and catastrophizing may be a relevant and modifiable factor to consider, in particular, in musculoskeletal surgery34. In one study preoperative anxiety was associated with postoperative anxiety and physical complaints after lumbar spinal surgery35. Pain related anxiety and avoidance behavior are physiological responses after an acute incidence. However, over time avoidance may result in disuse and disability and may increase the disease burden36,37. Therefore, anxiety and avoidance may be potentially modifiable coping styles38–40 and relevant to consider after spine surgery. Indeed, patients with persistent fear avoidance beliefs after decompression surgery for DLSS reported poorer treatment outcome after 1 year than patients with decreased or no fear avoidance beliefs after surgery41,42. In the current study, we observed an additive effect of anxiety to depression which reached clinical relevance for symptoms which may warrant further studies.
Implications for research
Depression and anxiety may be relevant and modifiable factors to consider during the postoperative phase and should be further studied. In particular, in patients with persistent depression despite improvement after surgery, concomitant psychological interventions should be studied. Further, the importance of pre- and perioperative stress on postoperative complications is not well understood. Starkweather et al.43 showed that psychological stress had an immunosuppressive effect that may result in an increased risk for complications.
Implications for clinical practice
Although patients with preoperative depression and anxiety may report more symptoms after a 2-year follow-up, they are equally likely to experience a clinical meaningful improvement as patients without depression or anxiety.
Strength and limitations
A strength of the study include the large number of patients undergoing in surgery for symptomatic DLSS, the prospective recording of known prognostic factors, and the use of a novel method to benchmark the estimated effect of depression against the clinically most relevant confounder gender. A strength of our study is that we used new methods to assess the relevance of the findings accounting for unmeasured confounding. There are several limitations to discuss. First, depression and anxiety were based on self-reported measures (the validated HADS questionnaire) and we had no information on the clinical diagnosis and treatment of depression. Therefore, we were not able to determine the severity and nature of depression and anxiety. Further, we do not know whether some patients received treatment for depressive symptoms that may influent treatment outcome. Second, we did not collect information on anxiety and depression during the follow-up period. Therefore, we were not able to assess the interaction between treatment, depression/anxiety, and outcome.
Conclusion
In patients undergoing surgery for symptomatic DLSS, we observed a robust association between preoperative depression and more symptoms and disability at 12- and 24-months. Anxiety was associated with more symptoms at 12- and 24-months but the effect was small. Both, baseline depression and anxiety did not influence the odds for clinically meaningful improvement in symptoms and function scores.
Supplementary Information
Abbreviations
- DLSS
Degenerative lumbar spinal stenosis
- LSOS
Lumbar Stenosis Outcome Study
- HADS
Hospital anxiety and depression scale
- CIRS
Cumulative illness rating scale
- SSM
Spinal stenosis measure
- MCID
Minimal clinically important difference
Author contributions
J.S., M.W. and U.H. designed the study. J.B. and G.P. were responsible for data management and data extraction. J.B., U.H. and M.D. were responsible for data analysis. M.W., J.B. and U.H. wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Funding
The funding was provided by Helmut Horten Stiftung, Baugarten Foundation, OPO-Foundations, Stiftung Symphasis and also by Pfizer-Foundation for geriatrics and research in geriatrics.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: U. Held and J. M. Burgstaller.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06797-1.
References
- 1.Deyo RA. Treatment of lumbar spinal stenosis: A balancing act. Spine J. 2010;10:625–627. doi: 10.1016/j.spinee.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Ravindra VM, Senglaub SS, Rattani A, Dewan MC, Härtl R, Bisson E, Park KB, Shrime MG. Degenerative lumbar spine disease: Estimating global incidence and worldwide volume. Glob. Spine J. 2018;8:784–794. doi: 10.1177/2192568218770769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steurer J, Nydegger A, Held U, Brunner F, Hodler J, Porchet F, Min K, Mannion AF, Michel B. LumbSten: The lumbar spinal stenosis outcome study. BMC Musculoskelet. Disord. 2010;11:254. doi: 10.1186/1471-2474-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the spine patient outcomes research trial. Spine. 2010;35:1329–1338. doi: 10.1097/BRS.0b013e3181e0f04d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otani K, Kikuchi S, Yabuki S, Igarashi T, Nikaido T, Watanabe K, Konno S. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: An epidemiological cross-sectional study of 1862 community-dwelling individuals. Sci. World J. 2013;2013:590652. doi: 10.1155/2013/590652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, Cho CH, DePalma MJ, Dougherty P, 2nd, Fernand R, Ghiselli G, Hanna AS, Lamer T, Lisi AJ, Mazanec DJ, Meagher RJ, Nucci RC, Patel RD, Sembrano JN, Sharma AK, Summers JT, Taleghani CK, Tontz WL, Jr, Toton JF. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14:180–191. doi: 10.1016/j.spinee.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Wertli MM, Buletti FC, Held U, Rasmussen-Barr E, Weiser S, Burgstaller JM, Steurer J. A comparison between different outcome measures based on "meaningful important differences" in patients with lumbar spinal stenosis. Eur. Spine J. 2017;26:450–461. doi: 10.1007/s00586-016-4587-0. [DOI] [PubMed] [Google Scholar]
- 8.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 1990;72:403–408. doi: 10.2106/00004623-199072030-00013. [DOI] [PubMed] [Google Scholar]
- 9.Marengoni A, Angleman S, Meinow B, Santoni G, Mangialasche F, Rizzuto D, Fastbom J, Melis R, Parker M, Johnell K, Fratiglioni L. Coexisting chronic conditions in the older population: Variation by health indicators. Eur. J. Intern. Med. 2016;31:29–34. doi: 10.1016/j.ejim.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Bouras T, Stranjalis G, Loufardaki M, Sourtzis I, Stavrinou LC, Sakas DE. Predictors of long-term outcome in an elderly group after laminectomy for lumbar stenosis. J. Neurosurg. Spine. 2010;13:329–334. doi: 10.3171/2010.3.SPINE09487. [DOI] [PubMed] [Google Scholar]
- 11.Basques BA, Varthi AG, Golinvaux NS, Bohl DD, Grauer JN. Patient characteristics associated with increased postoperative length of stay and readmission after elective laminectomy for lumbar spinal stenosis. Spine. 2014;39:833–840. doi: 10.1097/BRS.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JM, Choi MK, Kim SB. Perioperative results and complications after posterior lumbar interbody fusion for spinal stenosis in geriatric patients over than 70 years old. J. Korean Neurosurg. Soc. 2017;60:684–690. doi: 10.3340/jkns.2017.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKillop AB, Carroll LJ, Battie MC. Depression as a prognostic factor of lumbar spinal stenosis: A systematic review. Spine J. 2014;14:837–846. doi: 10.1016/j.spinee.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Heikkinen J, Honkanen R, Williams L, Leung J, Rauma P, Quirk S, Koivumaa-Honkanen H. Depressive disorders, anxiety disorders and subjective mental health in common musculoskeletal diseases: A review. Maturitas. 2019;127:18–25. doi: 10.1016/j.maturitas.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Carreon LY, Djurasovic M, Dimar JR, 2nd, Owens RK, 2nd, Crawford CH, 3rd, Puno RM, Bratcher KR, McGraw KE, Glassman SD. Can the anxiety domain of EQ-5D and mental health items from SF-36 help predict outcomes after surgery for lumbar degenerative disorders? J. Neurosurg. Spine. 2016;25:352–356. doi: 10.3171/2016.2.spine151472. [DOI] [PubMed] [Google Scholar]
- 16.Cinelli C, Hazlett C. Making sense of sensitivity: Extending omitted variable bias. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2020;82:39–67. doi: 10.1111/rssb.12348. [DOI] [Google Scholar]
- 17.Held U, Steurer J, Pichierri G, Wertli MM, Farshad M, Brunner F, Guggenberger R, Porchet F, Fekete TF, Schmid UD, Gravestock I, Burgstaller JM. What is the treatment effect of surgery compared with nonoperative treatment in patients with lumbar spinal stenosis at 1-year follow-up? J. Neurosurg. Spine. 2019 doi: 10.3171/2019.1.spine181098. [DOI] [PubMed] [Google Scholar]
- 18.Held U, Burgstaller JM, Wertli MM, Pichierri G, Winklhofer S, Brunner F, Porchet F, Farshad M, Steurer J. Prognostic function to estimate the probability of meaningful clinical improvement after surgery—Results of a prospective multicenter observational cohort study on patients with lumbar spinal stenosis. PLoS ONE. 2018;13:e0207126. doi: 10.1371/journal.pone.0207126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steurer J, Nydegger A, Held U, Brunner F, Hodler J, Porchet F, Min K, Mannion AF, Michel B, Collaboration LR. LumbSten: The lumbar spinal stenosis outcome study. BMC Musculoskelet. Dis. 2010;11:254. doi: 10.1186/1471-2474-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (London) 2007;370:1453–1457. doi: 10.1016/s0140-6736(07)61602-x. [DOI] [PubMed] [Google Scholar]
- 21.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J. Psychosom. Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich NH, et al. The impact of incidental durotomy on the outcome of decompression surgery in degenerative lumbar spinal canal stenosis: Analysis of the Lumbar Spinal Outcome Study (LSOS) data—A Swiss prospective multi-center cohort study. BMC Musculoskelet. Disord. 2016;17:170. doi: 10.1186/s12891-016-1022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stucki G, Daltroy L, Liang MH, Lipson SJ, Fossel AH, Katz JN. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine. 1996;21:796–803. doi: 10.1097/00007632-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Wertli MM, Steurer J, Wildi LM, Held U. Cross-cultural adaptation of the German version of the spinal stenosis measure. Eur. Spine J. 2014;23:1309–1319. doi: 10.1007/s00586-014-3245-7. [DOI] [PubMed] [Google Scholar]
- 26.Salvi F, Miller M, Grilli A, Giorgi R, Towers A, Morichi V, Spazzafumo L, Mancinelli L, Espinosa E, Rappelli A, Dessì Fulgheri P. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J. Am. Geriatr. Soc. 2008;56:1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team . R: A language and environment for statistical computing. In: Core Team R, editor. R Foundation for Statistical Computing. R Core Team; 2021. [Google Scholar]
- 28.Lebow R, Parker SL, Adogwa O, Reig A, Cheng J, Bydon A, McGirt MJ. Microdiscectomy improves pain-associated depression, somatic anxiety, and mental well-being in patients with herniated lumbar disc. Neurosurgery. 2012;70:306–311. doi: 10.1227/NEU.0b013e3182302ec3. [DOI] [PubMed] [Google Scholar]
- 29.Falavigna A, Righesso O, Teles AR, Conzati LP, Bossardi JB, da Silva PG, Cheng JS. Responsiveness of depression and its influence on surgical outcomes of lumbar degenerative diseases. Eur. J. Orthop. Surg. Traumatol. 2015;25(Suppl 1):S35–S41. doi: 10.1007/s00590-015-1651-0. [DOI] [PubMed] [Google Scholar]
- 30.Havakeshian S, Mannion AF. Negative beliefs and psychological disturbance in spine surgery patients: A cause or consequence of a poor treatment outcome? Eur. Spine J. 2013;22:2827–2835. doi: 10.1007/s00586-013-2822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinikallio S, Aalto T, Koivumaa-Honkanen H, Airaksinen O, Herno A, Kroger H, Viinamaki H. Life dissatisfaction is associated with a poorer surgery outcome and depression among lumbar spinal stenosis patients: A 2-year prospective study. Eur. Spine J. 2009;18:1187–1193. doi: 10.1007/s00586-009-0955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinikallio S, Aalto T, Airaksinen O, Lehto SM, Kroger H, Viinamaki H. Depression is associated with a poorer outcome of lumbar spinal stenosis surgery: A two-year prospective follow-up study. Spine. 2011;36:677–682. doi: 10.1097/BRS.0b013e3181dcaf4a. [DOI] [PubMed] [Google Scholar]
- 33.Pakarinen M, Tuomainen I, Koivumaa-Honkanen H, Sinikallio S, Lehto SM, Airaksinen O, Viinamaki H, Aalto T. Life dissatisfaction is associated with depression and poorer surgical outcomes among lumbar spinal stenosis patients: A 10-year follow-up study. Int. J. Rehabil. Res. 2016 doi: 10.1097/mrr.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 34.Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: A systematic review and meta-analysis of the association with chronic postsurgical pain. Clin. J. Pain. 2012;28:819–841. doi: 10.1097/AJP.0b013e31824549d6. [DOI] [PubMed] [Google Scholar]
- 35.de Groot KI, Boeke S, van den Berge HJ, Duivenvoorden HJ, Bonke B, Passchier J. The influence of psychological variables on postoperative anxiety and physical complaints in patients undergoing lumbar surgery. Pain. 1997;69:19–25. doi: 10.1016/s0304-3959(96)03228-9. [DOI] [PubMed] [Google Scholar]
- 36.Vlaeyen JW, Linton SJ. Are we "fear-avoidant"? Pain. 2006;124:240–241. doi: 10.1016/j.pain.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 37.Pincus T, Smeets RJ, Simmonds MJ, Sullivan MJ. The fear avoidance model disentangled: Improving the clinical utility of the fear avoidance model. Clin. J. Pain. 2010;26:739–746. doi: 10.1097/AJP.0b013e3181f15d45. [DOI] [PubMed] [Google Scholar]
- 38.Wertli MM, Rasmussen-Barr E, Held U, Weiser S, Bachmann LM, Brunner F. Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: A systematic review. Spine J. 2014;14:2658–2678. doi: 10.1016/j.spinee.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 39.Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing-a prognostic factor for outcome in patients with low back pain: A systematic review. Spine J. 2014;14:2639–2657. doi: 10.1016/j.spinee.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: A systematic review. Spine J. 2014;14:816–836. doi: 10.1016/j.spinee.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 41.Burgstaller JM, et al. The influence of pre- and postoperative fear avoidance beliefs on postoperative pain and disability in patients with lumbar spinal stenosis: Analysis of the Lumbar Spinal Outcome Study (LSOS) Data. Spine. 2017;42:E425–E432. doi: 10.1097/BRS.0000000000001845. [DOI] [PubMed] [Google Scholar]
- 42.Archer KR, Seebach CL, Mathis SL, Riley LH, 3rd, Wegener ST. Early postoperative fear of movement predicts pain, disability, and physical health six months after spinal surgery for degenerative conditions. Spine J. 2014;14:759–767. doi: 10.1016/j.spinee.2013.06.087. [DOI] [PubMed] [Google Scholar]
- 43.Starkweather AR, Witek-Janusek L, Nockels RP, Peterson J, Mathews HL. Immune function, pain, and psychological stress in patients undergoing spinal surgery. Spine. 2006;31:E641–E647. doi: 10.1097/01.brs.0000231795.85409.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.