Abstract
Objectives
The diagnosis of interstitial lung disease is based on clinical and biological analysis associated with computed tomography (CT) pattern after discussion in multidisciplinary discussion. Lung transbronchial cryobiopsy has emerged with acceptable diagnostic reliability and low morbidity and mortality. The goal of our work is to describe our experience with lung cryobiopsy.
Methods
This is monocentric and retrospective analysis of prospectively collected data on epidemiological, clinical, biological, CT, respiratory and histological features of patients with lung transbronchial cryobiopsy between January 1st, 2017, and July 1st, 2020.
Results
Lung transbronchial cryobiopsy has been done for 23 patients with sex ratio M/F of 1.1, majority of smoker/former smoker. Thirty-nine percent of procedures were complicated by pneumothorax. On the haemorrhagic level, we reported 1 grade 2 bleeding (no serious bleeding). An histological diagnosis was obtained for 19 patients (82%). Only 4 patients needed to discuss surgical lung biopsy: 2 (8.6%) surgical lung biopsy, 1 refused surgical lung biopsy and 1 patient lost to follow-up.
Conclusion
Our results suggest that transbronchial lung cryobiopsy may be considered the first diagnostic modality instead of surgical lung biopsy for interstitial lung disease in appropriate patients. Larger studies are, however, needed to confirm our observations.
Keywords: Lung transbronchial cryobiospy, Interstitial lung disease, Multidisciplinary discussion
Introduction
The group of respiratory diseases known as interstitial lung disease (ILD) present as a wide, heterogenous range of pathophysiologies with diverse aetiologies. Diagnosis depends heavily on multidisciplinary discussions (MDD) and access to pattern on computed tomography (CT) scan, in addition to an aetiological assessment that is as exhaustive as possible. However, if the diagnosis is uncertain or difficult, as is often the case due to the presence of another disease, such as usual interstitial pneumonia (UIP), then a lung biopsy may be indicated, especially if a histological diagnosis would modify either the prognosis or the treatment [1].
The current gold standard for obtaining a sample for histology is a surgical lung biopsy (SLB) via video-thorascopy. This is an invasive procedure that has an appreciable level of associated morbimortality; it has a mortality rate of 2% [2–4]. In recent years, however, a technique that uses a cryoprobe to perform a transbronchial biopsy has emerged [5]. The diagnostic performance of this test is slightly lower than SLB (80%), but it is far less invasive and associated morbidity and mortality are also considerably lower: it has a mortality rate of 0.3% [5–8]. Furthermore, when used as part of a MDD approach, the diagnostic performance of cryobiopsy appears to be almost the same to that of samples obtained by SLB [9].
In the case of fibrosing ILD, in particular idiopathic pulmonary fibrosis which has a poor prognosis and often occurs in older individuals and those with comorbidities, the use of a minimally invasive technique represents an improvement in diagnosis technique. As a result, since 2016, we started offering cryobiopsy to eligible patients as the first choice of biopsy technique.
The primary objective of this work, therefore, was to present our experience in using cryobiopsy in regular practice as the first choice of biopsy method. The second objective was to describe the performance of cryobiopsy in terms of its morbidity and mortality. Since SLB was reserved for cases where cryobiopsy was unsuitable or a first attempt had failed, as a third aim, we therefore also wished to investigate the number of times that a second (surgical) biopsy was required.
Patients and methods
This was a single-centre, retrospective analysis of CT scan, functional respiratory measurements and epidemiological, clinical, biological and histological data that were collected from patients who underwent cryobiopsy between 1st January 2017 and 1st July 2020.
Aim
To describe our experience with lung cryobiopsy
Objectives
The diagnostic yield rate
Analyse the complications of cryobiopsy
Analyse the need for surgical lung biopsy
Methods to explain biopsy methods
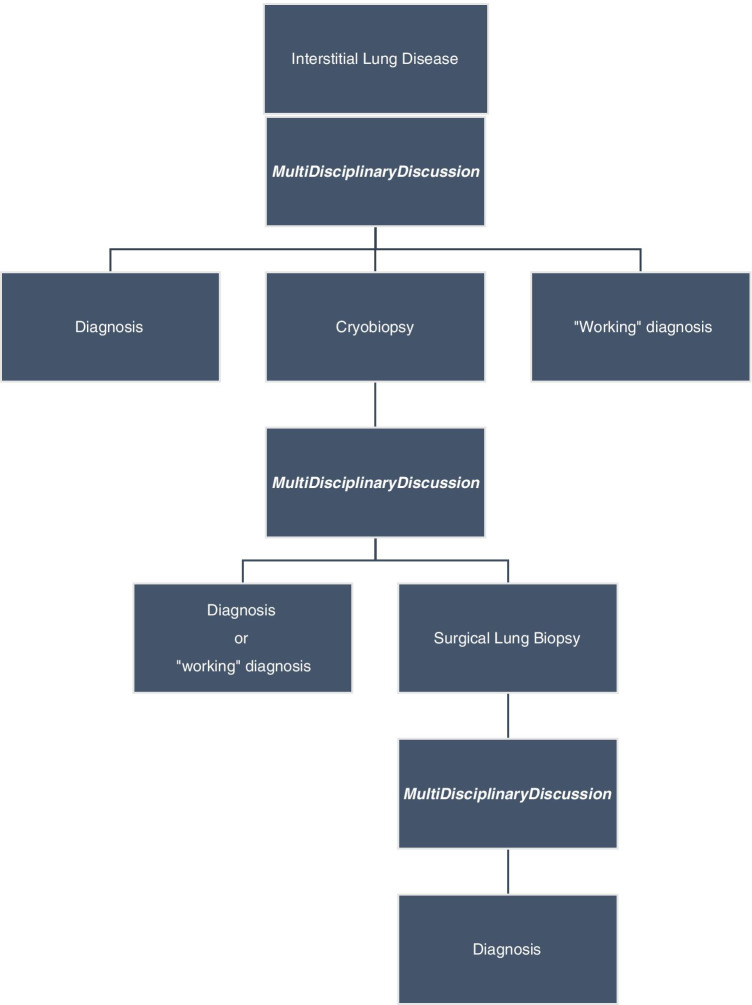
All patient histological indications had been discussed in MDD. The MDD met monthly and composed of pulmonologist, thoracic radiologist and thoracic surgeon (given its distance, the discussion with pathologist takes place by telephone outside this meeting). This discussion has explained to patient in consultation during which their treatment options and the MDD decisions were presented (Fig. 1). Patients received personalised, clear, detailed and impartial information regarding the modalities, advantages and complications associated with pulmonary biopsy. Patients were informed about both SLB and cryobiopsy options.
Fig. 1.
Diagnostic algorythm of ILD with lung transbronchial cryobiopsy in 1st intention if lung biopsy decided in MDD
Inclusion and exclusion criteria
This was a non-interventional, retrospective study that did not require ethical committee approval.
The inclusion criteria were as follows: all patients who were treated in Percy Military Hospital and who underwent cryobiopsy during this 3-year period. For transbronchial lung cryobiopsy, minimum respiratory functional parameters were identical to those recommended for SLB (FVC>55%Th and DLCO>35%Th).
The exclusion criterion was the lack of documented acceptance from the patient regarding the use of their data. Patient data were not included until they had provided informed consent regarding the use of their anonymised data. Patients were free to undergo a biopsy using the technique of their choice.
Transbronchial cryobiopsy realisation
Each cryobiopsy was carried out under general anaesthesia using jet ventilation and a rigid bronchoscope with a Cryoprobe ERBE© 2.4mm probe. Systematic radioscopy allowed the position of the probe to be monitored, particularly its proximity to the bronchial wall. A radioprotection report was documented in each patient’s file. In order to obtain a quality sample, the tissue was frozen 7 s. After this time, the flexible fibroscope/probe with frozen biopsy could be extracted from the rigid bronchoscope. The balloon of previously inserted Fogarty N°6 probe was then inflated to prevent haemorrhage. As recommended, whenever possible, at least two samples from at least two different segments were taken [10].
After each biopsy, the sample was slowly defrosted by immersion in isotonic saline at room temperature before then being fixed with 4% formaldehyde solution. Samples were sent to a local histopathology centre (Service d’Anatomopathologie, Bichat Hospital, Paris) which had considerable experience in performing such analyses. Once the results were received back, they were reviewed as part of an MDD and, where a conclusion was possible, used to decide upon a diagnosis.
Data analysis
The following epidemiological (age, sex, smoking, professional or environmental exposure) and functional respiratory data were collected from each patient: total lung capacity (TLC), forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), forced expiratory volume in 1 s/forced vital capacity ratio (Tiffeneau ratio) and forced expiratory volume in 1 s (FEV1). Pulmonary hypertension has been searched with cardiac echography and defined by tricuspid regurgitation velocity >2.8m/s.
Cryobiopsy procedure performance data were also gathered, including the number of biopsies undertaken, number of segments, mortality, diagnosis following the MDD, occurrence of pneumothorax and, if the pneumothorax required draining, the number of days this took and the duration of hospitalisation. The occurrence of bleeding during the procedure was also noted and was graded as follows:
Grade 1: minimal bleeding that stopped spontaneously after several seconds.
Grade 2: moderate bleeding that required bronchial obstruction but that stopped spontaneously after several minutes.
Grade 3: severe bleeding that required specific treatment such as embolisation or haemostatic surgery.
Grade 4: fatal bleeding.
Statistical analysis
A descriptive analysis was performed. Categorical variables are given as numbers (and percentages); continuous variables as medians [with interquartile ranges]. Data were compiled and analysed using Excel® (Microsoft, WA, USA) and XLSTAT® (version 19.4, Addinsoft, Paris, France).
Results
Population characteristics with cryobiopsy in first indication
Patient characteristics are summarised in Table 1. During the period studied, 23 patients underwent cryobiopsy, including 11 women and 12 men. The majority were smokers or ex-smokers. The majority had grade 1 restrictive ventilatory defect (RVD) (TLC <80% and FVC >80%) and impaired DLCO. No patients had pulmonary hypertension based on cardiac ultrasound results.
Table 1.
Characteristics of population and procedure of cryobiospy
| Characteristics | N=23 |
|---|---|
| Sex (W/M) | 11/12 |
| Average age (year) | 63.8 [61; 74] |
| Smoking (yes/no/former) | 3/9/11 |
| Average pack-year | 31.8 |
| Average length of hospital stay (days) | 4.14 [2–5] |
| Median TLC (%theorical) | 77 [71.5; 81.5] |
| Median FVC (L) | 2.3 [1.91; 2.89] |
| Median FVC (%theorical) | 77 [59; 87.5] |
| Median Tiffeneau (%theorical) | 82 [71.5; 86.5] |
| Median FEV1 (L) | 2.02 [1.55; 2.41] |
| Median FEV1 (%theorical) | 77 [68.5; 92] |
| Median TLCO (%theorical) | 56 [50; 64] |
| Average samples number per patient | 4.2 [3; 5] |
| Number of different lobe or segment | 2 |
| Pneumothorax (N) | 9 (39%) |
| Pneumothorax needs drainage (N) | 7 (77.8%) |
| Drainage duration (average days) | 4 [2–4] |
| Bleeding | |
| Grade 1 | 0 |
| Grade 2 | 1 (5.5%) |
| Grade 3 | 0 |
| Grade 4 | 0 |
| Histological diagnosis | 19 (82.6%) |
| Surgical lung biopsy | 2 (9%) |
TLC total lung capacity, FVC forced vital capacity, FEV1 forces expiratory velocity in 1 second, TLCO transfer CO
Hospitalization (days), samples number (average), segments number (average) and drainage duration (days) were expressed in average (range). TLC, FVC, Tiffeneau, FEV1 and TLCO were expressed in median [standard deviation]
Diagnostic yield
The diagnoses in MDD before and with biopsy results are resumed in Tables 2 and 3. The MDD with biopsy results established 14 diagnostic confirmations, 6 modifications and 3 adjustment. The majority were UIP and non-specific interstitial pneumonia (NSIP). Histological diagnosis data were available from 19/23 patients (82.6%).
Table 2.
Histological diagnoses of cryobiospy
| Diagnostic | N=23 (cryobiopsy) | N=2 (SLB) |
|---|---|---|
| UIP pattern | 6 (26%) | 1 (50%) |
| NSIP pattern | 5 (21.7%) | |
| fHP | 4 (17.4%) | |
| DIP or RB-ILD | 2 (8.7%) | |
| IAH | 1 (4.4%) | |
| Sarcoïdosis | 1 (4.4) | |
| Inclassable ILD | 3 (13%) | 1 (50%) |
| No diagnosis | 1 (4.4%) |
UIP usual interstitial pneumonia, NSIP non-specific interstitial pneumonia, fHP fibrotic chronic hypersensibility pneumonia, DIP desquamative interstitial pneumonia, IAH intra-alveolar hemorrhage, RB-ILD bronchiolitis with ILD, ILD interstitial lung disease, SLB surgical lung biopsy
Table 3.
Comparison between MDD diagnoses before and with cryobiopsy results
| Diagnosis in MDD before biopsy | Diagnosis in MDD with biopsy |
|---|---|
| Probable UIP | IPF |
| fHP | fHP |
| Probable UIP | IPF |
| Idiopathic NSIP | Idiopathic NSIP |
| Probable UIP | Inclassable ILD |
| Idiopathic NSIP | fHP |
| Indeterminate for UIP | Inclassable ILD |
| Indeterminate for UIP | fHP |
| Idiopathic NSIP | DIP |
| Indeterminate for UIP | IPF |
| Probable UIP | IPF |
| Adenocarcinoma relapse | IAH |
| Indeterminate for UIP | NSIP |
| Probable UIP | IPF |
| Idiopathic NSIP | NSIP |
| Probable UIP | IPF |
| Indeterminate for UIP | Indetermined ILD |
|
Idiopathic NSIP Idiopathic NSIP |
NSIP NSIP |
|
fHP Indeterminate for UIP Indeterminate for UIP Sarcoïdosis |
fHP RB-ILD NSIP Sarcoïdosis |
MDD multidisciplinary discussion, IPF idiopathic pulmonary fibrosis, NSIP non-specific interstitial pneumonia, fHP fibrosis hypersensitivity pneumonia, DIP desquamative interstitial pneumonia, IAH intra-alveolar hamorraege, ILD interstitial lung disease, RB-ILD bronchiolitis with interstitial lung disease
Need for SLB
Of the 4 without histological diagnosis, 3 were diagnosed with unclassifiable ILD and 1 indetermined ILD. Two of these 4 patients underwent a further SLB. After this second biopsy, a diagnosis could only be made with certainty in one patient and they were diagnosed with UIP. The other patient, who also had a SLB, had their original diagnosis of a non-classifiable ILD reconfirmed. The 2 others refused SLB and so received a working diagnosis of idiopathic pulmonary fibrosis following cryobiopsy.
Cryobiopsy complications
The mean duration of hospitalisation for all patients in our cohort was 4.14 days and 2 days for 14 patients whose cryobiopsy was not associated with any complications. Each patient had 4.2 biopsies which were carried out in 2 different segments/lobes for each patient. Nine procedures (39%) were complicated by pneumothorax that required drainage with drainage lasting in 7 cases with average drainage duration of 4 days. With regard to haemorrhagic complications, there was only 1 case of grade 2 bleeding; it was confined to the biopsied segment by use of Fogarty probe balloon and it stopped spontaneously after 5 min. Neither severe bleeding (grades 3–4) nor any deaths occurred.
Discussion
The results from this study indicated that cryobiopsy in context of MDD effectively provided a diagnosis in 82.6% of cases (19/23). Although this was only a small study, this figure agreed with other reports in the literature regarding the diagnostic efficacy of cryobiopsy (72–85%) [9, 11–14]. In addition, our data also confirmed published figures regarding complications associated with cryobiopsies, i.e. 39% of patients developed pneumothorax, one patient out of 23 (5.5%) had grade 2 bleeding. There were no deaths following the procedure [13, 14]. Our results were the same as previously published data in terms of performance, complication and safety of cryobiopsy. The important rate of pneumothorax could be explained by the learning curve because 7 of 9 pneumothorax occurred in the 12 first procedures and explained by the high rate of ILD with distal lung involvement on CT scan needing distal biopsy (7 indeterminate for UIP).
For all patients, their stay in hospital was shorter than the hospitalisations previously reported for patients following SLB, in particular if a pneumothorax occurred as a complication. Patient’s hospital stays following cryobiopsies were further reduced if there were no complications after the cryobiopsy. The mean duration of hospitalisation was comparable to that found in the literature [14, 15].
The distribution of diagnoses following MDD with cryobiopsy was found to be the same as the distribution described following SLB, i.e. UIP, NSIP and chronic fibrotic hypersensitivity pneumonitis (cHP) [16]. SLB was only indicated in four of the 23 cases (17.4%) following failure of the cryobiopsy procedure to produce histological samples. Of these three cases, only two SLB were actually undertaken and, even in these few cases, the results only ultimately changed the diagnosis for one patient.
Data published over the past few years regarding cryobiopsy are interesting and tend to support the viewpoint that it is a valid substitute for SLB [5, 9, 10, 17, 18]. Based on published data and our experiences, we propose that cryobiopsy should be fully integrated into the decision processes for the diagnosis of ILD, not as alternative to SLB, but as the first choice of sampling method when a multidisciplinary team has decided that a pulmonary biopsy is necessary. SLB should instead be considered the second choice of sampling method, should the cryobiopsy fail to produce useable histological data.
In addition to its comparable efficacy in diagnosing ILD conditions, cryobiopsy is also considerably less invasive than SLB and represents a superior procedure in terms of both technical and anaesthetic aspects. Cryobiopsy uses an endoscopic approach and does not require a drain (except in cases of complications) and is associated with no post-operative pain, fewer complications and practically no mortality and has a high rate of successful outcomes in terms of the number of valid histological results it produces and subsequent diagnoses that it facilitates (72–82%) [14, 19].
We therefore feel it is essential to also emphasise the importance of discussion between healthcare colleagues during ILD diagnosis, and also to stress that these discussions should always involve the histopathologist who examined the sample. The expert viewpoint from the pathologist is even more important if cryobiopsy is used as a biopsy technique since this method results in smaller samples being taken than during SLB, and these can be harder to analyse. The specialist expertise of the pathologist is thus required, in addition to their knowledge of ILD.
The importance of collegiate discussion has been confirmed by a larger (n=65), randomised, blinded study [16] where all patients underwent both procedures which compared SLB with cryobiopsy and integrated the expert pathologist as an essential element in MDD. Their results confirmed and corroborated our findings: not only did cryobiopsy provide sufficient, high-quality tissue samples for a good diagnostic performance but also there was strong agreement between cryobiopsy and SLB findings (70% with κ of 0.70). These encouraging data corroborate our experience and the role of cryobiopsy as a 1st intention technique.
Another recent study by Raghu et al. showed the potential value in detection of a molecular signature on a classic transbronchial biopsy sample as a method for the diagnosis of UIP versus non-UIP [19]. This technique needs to be validated in a larger, randomised study but nevertheless it provides compelling support for importance of pulmonary biopsy techniques evolving towards less invasive methods (such as cryobiopsy). In addition, this also opens up new possibilities for histological and molecular analysis which would be compatible with the high-quality samples that can be obtained by cryobiopsy.
Limitations
This study has several limitations. The first is the small sample size and the lack of a control or comparison group, but it was a real-life study and SLB was performed as a method of 2nd intention in our centre. A direct comparison of cryobiopsy and SLB has not been done and that further studies are recommended in this direction to provide the evidence that cryobiopsy is superior. A second limitation, based on our collective opinion regarding cryobiopsy, is that it was possible that a selection bias existed which meant patients were more likely to opt for the cryobiopsy method when offered the choice. Nevertheless, we believe that the information given to the patients regarding the different techniques (cryobiopsy and SLB) was honest, precise and impartial. It is also important to note that a thoracic surgery unit was available on site in the centre and so SLB was readily available, if desired or necessary. A final limitation to the current study was related to the current lack of standardisation in terms of anaesthesia, ventilation, use of a rigid or non-rigid bronchoscope and the size of the probe to be used during cryobiopsy procedures. These inherent variabilities could all have affected the comparability of the results between our data and the different published studies. In order to improve comparability and to standardise the methodology, a first draft of recommendations has been published in order to attempt to harmonise cryobiopsy practices [10].
Conclusion
In summary, therefore, the aims for this study have been achieved: we have presented our method and experiences in using cryobiopsy in regular practice as the primary biopsy method. We conclude that cryobiopsy, a minimally invasive technique, has a good diagnostic performance in context of MDD. We have described the performance of cryobiopsy in all eligible patients in our clinic terms of its morbimortality and it is this low morbidity and, in this study, no mortality, that leads us to propose that cryobiopsy should be used as standard when pulmonary biopsy is considered necessary. We believe that it could, or should, replace the current reference technique of SLB. The third aim was to investigate the number of times that a second (surgical) biopsy was required revealed that it was an uncommon event; it occurred in only 4 out of 23 cases. SLB therefore remains as an important method for diagnosing ILD but we believe it should become the second choice, used only if histological data are inadequate or unavailable following cryobiopsy. The published data is booming in this area and further work is needed but those studies that exist have similar findings to ours. They also emphasise the importance of the multidisciplinary approach to diagnosing ILD and in particular for the relevance of conclusion of ILD expert pathologist.
Author contribution
Concept: FR
Data collection: FR, AC
Analysis: FR
Writer: FR
Critical review and approved: FR, AC, OB, HLF, WG, MAC, FC, JM, FG
All authors have read and approved this manuscript.
Funding
None.
Declarations
Ethics approval
Approval was granted from the protocol evaluation committee for observational research from the French Pneumologist Society (CEPRO 2018-035).
Human and animal rights statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Taken.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161–1167. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JH, Shapera S, To T, Marras TK, Gershon A, Dell S. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur Respir J. 2019;53:1801164. [DOI] [PubMed]
- 4.Ravaglia C, Bonifazi M, Wells AU, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91:215–227. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 5.Casoni GL, Tomassetti S, Cavazza A. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poletti V, Ravaglia C, Tomassetti S. Transbronchial cryobiopsy in diffuse parenchymal lung diseases. Curr Opin Pulm Med. 2016;22:289–96. [DOI] [PubMed]
- 7.Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration. 2009;78:203–208. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 8.Lentz RJ, Taylor TM, Kropski JA, et al. Utility of flexible bronchoscopic cryobiopsy for diagnosis of diffuse parenchymal lung diseases. J Bronchology Interv Pulmonol. 2018;25:88–96. doi: 10.1097/LBR.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:745–52. [DOI] [PubMed]
- 10.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95:188–200. doi: 10.1159/000484055. [DOI] [PubMed] [Google Scholar]
- 11.Kropski JA, Pritchett JM, Mason WR, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One. 2013;8:e78674. doi: 10.1371/journal.pone.0078674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramaswamy A, Homer R, Killam J, et al. Comparison of transbronchial and cryobiopsies in evaluation of diffuse parenchymal lung disease: J Bronchology Interv Pulmonol. 2016;23:14–21. [DOI] [PMC free article] [PubMed]
- 13.Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. a systematic review and metaanalysis. Ann Am Thorac Soc. 2016;13:1828–1838. doi: 10.1513/AnnalsATS.201606-461SR. [DOI] [PubMed] [Google Scholar]
- 14.Dhooria S, Sehgal IS, Aggarwal AN, Behera D, Agarwal R. Diagnostic yield and safety of cryoprobe transbronchial lung biopsy in diffuse parenchymal lung diseases: systematic review and meta-analysis. Respir Care. 2016;61:700–712. doi: 10.4187/respcare.04488. [DOI] [PubMed] [Google Scholar]
- 15.Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med. 2020;8:171–181. doi: 10.1016/S2213-2600(19)30342-X. [DOI] [PubMed] [Google Scholar]
- 16.Dhooria S, Mehta RM, Srinivasan A, et al. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J. 2018;12:1711–20. [DOI] [PubMed]
- 17.Fruchter O, Fridel L, Rosengarten D, Abed-el Rahman N, Kramer MR. Transbronchial cryobiopsy in immunocompromised patients with pulmonary infiltrates: a pilot study. Lung 2013;191:619–24. [DOI] [PubMed]
- 18.Poletti V, Benzaquen S. Transbronchial cryobiopsy in diffuse parenchymal lung disease. A new star in the horizon. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:178–181. [PubMed] [Google Scholar]
- 19.Raghu G, Flaherty KR, Lederer DJ, et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: a prospective validation study. Lancet Respir Med. 2019;7:487–496. doi: 10.1016/S2213-2600(19)30059-1. [DOI] [PubMed] [Google Scholar]