Abstract
Introduction
Endovascular treatment’s (EVT) safety and efficacy have been proven in treating acute ischemic stroke (AIS) due to large vessel occlusion (LVO). However, limited data exist in different stroke subtypes. We aimed to investigate the differences in efficacy and safety of EVT for acute LVO according to different stroke subtypes.
Methods
A total of 1635 AIS patients with LVO undergoing EVT from a prospective cohort of the Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke (ANGEL-ACT) registry were classified into three types according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. We compared the primary outcome: 90-day modified Rankin Scale (mRS) score, the secondary outcomes: 90-day mRS (0–1, 0–2, and 0–3), successful recanalization (mTICI 2b/3), and complete recanalization (mTICI 3), and the safety outcomes: death within 90 days, parenchymal hemorrhage (PH), and symptomatic intracranial hemorrhage (SICH) among the three subtypes of stroke patients. Then, multivariable logistic regression models adjusting for potential baseline-confounding variables to determine the associations between stroke subtypes and safety and efficacy endpoints were performed. Finally, we performed subgroup analyses to explore discrepancies in the relationships.
Results
EVT of cardioembolic LVO (CE-LVO) had a higher rate of mTICI 3 (71.7% vs. 65.9% and 63.2%; P = 0.024) and a higher rate of PH (13.8% vs. 5.4% and 6.7%; P < 0.001) when compared to other stroke subtypes. Even multivariable analysis demonstrated that CE-LVO was associated with mTICI 3 [adjusted odds ratio (OR), 1.50 (95% CI 1.04–2.17)] and PH [adjusted OR, 1.97 (95% CI 1.09–3.55)]. However, the 90-day mRS distribution and 90-day mRS (0–1, 0–2, and 0–3) did not differ among the stroke subtypes, and nor did the SICH (P > 0.05).
Conclusions
Functional outcomes were similar among different stroke subtypes. Despite a higher rate of complete recanalization, there is an increased risk of parenchymal hemorrhage in CE-LVO.
Trial Registration
Clinical trial registration number: NCT03370939.
Keywords: Endovascular treatment, TOAST classification, Safety, Efficacy, Outcomes
Key Summary Points
| Functional outcomes were similar among different stroke subtypes. |
| Endovascular treatment for large vessel occlusion due to cardioembolism (CE-LVO) had a higher rate of complete recanalization and parenchymal hemorrhage than other stroke subtypes. |
| CE-LVO patients were older, had a higher rate of atrial fibrillation, and presented with a higher National Institutes of Health Stroke Scale (NIHSS) score which implies a larger ischemic area. |
Introduction
Endovascular treatment (EVT) has become the standard management for acute ischemic stroke caused by large vessel occlusions (LVO) [1]. However, different stroke subtypes have different risk factors, clinical features, and prognoses [2–6]. Determining the stroke subtypes is crucial to optimizing and improving the safety and efficacy of EVT. Due to the ease of use and the reliability in the clinic, the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification has been widely used to classify the ischemic stroke subtype [5, 7]. Many researchers have undertaken a range of studies to evaluate the clinical features and prognosis of acute ischemic stroke (AIS) patients undergoing EVT based upon the TOAST classification [2–4, 8, 9]. However, those studies were mainly in the western population, and there is still a lack of data from Asian populations, and the proportions of stroke etiologic subtypes are different among different ethnicities and countries [10, 11]
Therefore, in this large registry study of an Asian population, mainly Chinese, we evaluate the safety and efficacy of EVT in different stroke subtypes to provide further information to supplement the global data.
Methods
Study Population
The present study enrolled 1793 consecutive patients with AIS caused by acute large vessel occlusion undergoing EVT in 111 hospitals in China between November 2017 and March 2019. The inclusion and exclusion criteria followed the previous study [12]. Patients who underwent EVT were included in the study. The exclusion criteria were as follows: (1) patients without an EVT record; (2) patients without a TOAST assessment; (3) patients with small-artery occlusion lacunar (SAA). The study protocol was approved by the Ethics Committees of Beijing Tiantan Hospital and the ethics committees of all participating centers. The number of the approval: KY2017-048–01. The study procedures were in accordance with the 1964 Helsinki declaration and its later amendments. Subjects or their legally authorized representatives provided written informed consent.
Data Collection and Outcomes Measurement
Information on demographics, risk factors, medical history, National Institutes of Health Stroke Scale (NIHSS) score on admission, location of infarct cerebral tissue (anterior/posterior circulation), procedural characteristics (general anesthesia, GPIIb/IIIa receptor inhibitor, stent retriever, intra-arterial thrombolysis, balloon angioplasty, and stenting), number of mechanical thrombectomies, and the time points of working flow were prospectively collected. Baseline computed tomography (CT)/magnetic resonance (MR), CTA/MRA, DSA images during EVT, and follow-up head CT or MRI were evaluated by an imaging core laboratory blinded to clinical data and outcomes. All imaging was independently assessed by two neuroradiologists, with a third available for adjudication when needed. Alberta Stroke Program Early CT Score (ASPECTS) for anterior circulation strokes and posterior circulation Alberta Stroke Program Early CT Score for posterior circulation strokes were assessed on baseline CT [13, 14]. Final modified thrombolysis in cerebral ischemia score (mTICI) were assessed on the DSA [15].
We considered functional outcome at the 90-day (90-day mRS) primary endpoint. Meanwhile, we considered mRS 0–1, mRS 0–2, and mRS 0–3, successful recanalization (mTICI 2b/3), and complete recanalization (mTICI 3) as the secondary outcomes. Any symptomatic ICH (SICH) per Heidelberg Bleeding Classification within 24 h post-EVT [16], and parenchymal hemorrhage (PH) according to ECASS II Classification [17], and death within 90 days were considered safety endpoints.
Classification of the Stroke Subtypes
We categorized the stroke subtypes according to the TOAST classification [18]. Based on imaging and angiographic findings, we classified the stroke subtypes into large artery atherosclerosis (LAA), CE cardioembolism, and SUE/SOE stroke of unknown etiology/stroke of other determined etiology. We determined the classification through reconstructed images acquired from preprocedural CT angiography (CTA) or MR angiography (MRA), confirmed by intraprocedural digital subtraction angiography (DSA). We defined LAA as the presence of a lesion with significant stenosis (> 50%) or occlusion of the involved artery due to atherosclerosis during the EVT procedure. A history of intermittent claudication, transient ischemic attack in the same vascular territory, a carotid bruit, or diminished pulses help to support the clinical diagnosis. We defined CE as the arterial occlusions caused by embolus arising from the cardiac as either a high- or medium-risk. Potential large-artery atherosclerotic sources of thrombosis or embolism should be eliminated. In SUE, the stroke etiology could not be determined even after extensive evaluation was performed. Patients with two or more potential causes of stroke, which lead to physicians being unable to make final diagnoses, were also included in this group. Meanwhile, patients with rare causes of stroke, such as nonatherosclerotic vasculopathy, hypercoagulable states, or hematologic disorders, were categorized as SOE. These diagnoses had to be confirmed by diagnostic studies such as angiography or blood tests. In addition, cardiac sources of embolism and large-artery atherosclerosis had to be excluded by other studies [18].
Statistical Analysis
We used proportions for categorical variables, and median with interquartile range (IQR) for the continuous variables. We compared the baseline characteristics among groups using the Pearson χ2 test or the Kruskal–Wallis test. Variables with P < 0.05 in the univariable analysis were selected as the confounders into the multivariable logistic regression model. Then, we performed logistic regression to calculate the odds ratios (OR) or common OR with 95% confidence intervals (CI). We performed further subgroup analysis to discriminate the relationship between stroke subtypes and efficacy and safety outcomes in a different stratification. Significance level was set to P = 0.05 (2-sided). We used SAS software v.9.4 (SAS Institute, Cary, NC, USA) to conduct the statistical analyses.
Results
Baseline Characteristics
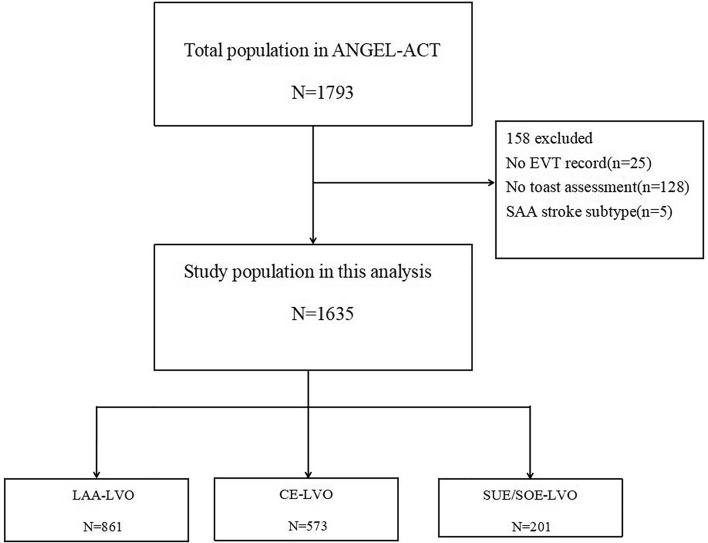
We enrolled 1793 AIS patients who underwent EVT. Of these, 158 patients were eliminated based on the exclusion criteria (Fig. 1). The baseline characteristics, procedure characteristics, and outcomes of the enrolled patients are presented in Table 1. LAA-LVO was the most prevalent etiology (n = 861) group, followed by CE-LVO (n = 573) and SUE/SOE-LVO (n = 201). Patients with CE-LVO were older, more likely to be female, and had higher NIHSS scores on admission than those in the LAA-LVO and SUE/SOE-LVO groups (Table 1). Patients with LAA-LVO had higher comorbidities, such as current smoking habits, hypertension, diabetes mellitus, hyperlipidemia, and prior stroke compared with patients with CE-LVO and SUE/SOE-LVO. Atrial fibrillation was highest in the CE-LVO group, and systolic blood pressure (SBP) on admission was highest in the LAA-LVO group. We identified higher anticoagulants in the CE-LVO group than the other two groups, but we noted higher use of antiplatelet agents in the SUE/SOE-LVO group. CE-LVO was more frequently found in the anterior circulation, while LAA-LVO and SUE/SOE-LVO were more frequent in the posterior circulation.
Fig. 1.
Flow chart of patient selection. EVT endovascular treatment, SAA small-artery occlusion lacunar, LAA large-artery atherosclerosis, CE cardioembolism, SUE/SOE stroke of unknown etiology/stroke of other determined etiology, LVO large vessel occlusion
Table 1.
Baseline characteristics and outcome of different stroke subtypes
| Variables | Total (n = 1635) | LAA-LVO (n = 861) | CE-LVO (n = 573) | SUE/SOE-LVO (n = 201) | P |
|---|---|---|---|---|---|
| Baseline clinical parameters | |||||
| Age, median(IQR) | 65 (55–73) | 64 (55–71) | 69 (62–77) | 59 (48–67) | < 0.001 |
| Male, n (%) | 1097 (67.1) | 682 (79.2) | 277 (48.3) | 138 (68.7) | < 0.001 |
| Premorbid mRSa, n (%) | 0.481 | ||||
| mRS 0 | 1414 (86.5) | 737 (85.6) | 498 (86.9) | 179 (89.5) | |
| mRS 1 | 193 (11.8) | 109 (12.7) | 64 (11.2) | 20 (10.0) | |
| mRS 2 | 27 (1.7) | 15 (1.7) | 11 (1.9) | 1 (0.5) | |
| Current smoking, n (%) | 550 (33.6) | 377 (43.8) | 97 (16.9) | 76 (37.8) | < 0.001 |
| SBP, median(IQR) | 145 (130–160) | 149 (134–165) | 145 (130–160) | 140 (127–158) | < 0.001 |
| Admission NIHSSb, median (IQR) | 16 (11–21) | 15 (11–21) | 17 (13–21) | 14 (11–20) | < 0.001 |
| ASPECTSc, median (IQR) | 9 (7–10) | 8 (7–10) | 10 (7–10) | 10 (8–10) | < 0.001 |
| Comorbidities | |||||
| Hypertension, n (%) | 930 (56.9) | 530 (61.6) | 313 (54.6) | 87 (43.3) | < 0.001 |
| Diabetes mellitus, n (%) | 283 (17.3) | 169 (19.6) | 88 (15.4) | 26 (12.9) | 0.024 |
| Hyperlipidemia, n (%) | 148 (9.1) | 91 (10.6) | 47 (8.2) | 10 (5.0) | 0.031 |
| Atrial fibrillation, n (%) | 500 (30.6) | 63 (7.3) | 418 (73.0) | 19 (9.5) | < 0.001 |
| Prior stroke, n (%) | 360 (22.0) | 196 (22.8) | 122 (21.3) | 42 (20.9) | 0.740 |
| Pretreatment | |||||
| Prior use of antiplatelet agents, n (%) | 270 (16.5) | 147 (17.1) | 78 (13.6) | 45 (22.4) | 0.013 |
| Prior use of anticoagulants, n (%) | 66 (4.0) | 10 (1.2) | 51 (8.9) | 5 (2.5) | < 0.001 |
| Bridging IVT, n (%) | 474 (29.0) | 243 (28.2) | 169 (29.5) | 62 (30.9) | 0.722 |
| Procedural parameters | |||||
| Occlusion location, n (%) | < 0.001 | ||||
| Anterior circulation | 1273 (77.9) | 620 (72.0) | 503 (87.8) | 150 (74.6) | |
| Posterior circulation | 362 (22.1) | 241 (28.0) | 70 (12.2) | 51 (25.4) | |
| General anesthesia, n (%) | 651 (39.8) | 364 (42.3) | 212 (37.0) | 75 (37.3) | 0.100 |
| GP IIb/IIIa receptor inhibitor, n (%) | 858 (52.5) | 586 (68.1) | 204 (35.6) | 68 (33.8) | < 0.001 |
| Stent retriever as first-line, n (%) | 1092 (66.8) | 534 (62.0) | 411 (71.7) | 147 (73.1) | < 0.001 |
| Direct aspiration as first-line, n (%) | 100 (6.1) | 39 (4.5) | 50 (8.7) | 11 (5.5) | 0.005 |
| Direct aspiration + stent retriever as first-line | 168 (10.3) | 74 (8.6) | 80 (14.0) | 14 (7.0) | 0.001 |
| IAT, n (%) | 136 (8.3) | 75 (8.7) | 31 (5.4) | 30 (14.9) | < 0.001 |
| Rescue balloon/stenting angioplasty, n (%) | 234 (14.3) | 191 (22.2) | 18 (3.1) | 25 (12.4) | < 0.001 |
| MT times, median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–3) | 1 (1–2) | < 0.001 |
| FPR, n (%) | 803 (49.1) | 424 (49.3) | 278 (48.5) | 101 (50.3) | 0.909 |
| Intraprocedural embolization, n (%) | 80 (4.9) | 29 (3.4) | 43 (7.5) | 8 (4.0) | 0.002 |
| Time-metric parameters | |||||
| OTDd, median (IQR), min | 150 (63–287) | 164.5 (68–305) | 135 (60–260) | 160 (66–275) | 0.030 |
| DTPe, median (IQR), min | 120 (80–178) | 127 (89–190) | 115 (75–161) | 115.5 (78–170) | < 0.001 |
| Procedure durationf, median (IQR), min | 85 (53–128) | 88 (53–130) | 80 (50–118) | 90 (57.5–144.5) | 0.018 |
| Primary outcome | |||||
| 90-day mRS, median (IQR) | 3 (0–5) | 3 (0–4) | 3 (0–5) | 2 (0–4) | 0.002 |
| Secondary outcomes | |||||
| 90-day mRS 0–1g, n (%) | 688 (42.6) | 388 (45.4) | 204 (36.2) | 96 (49.0) | < 0.001 |
| 90-day mRS 0–2g, n (%) | 753 (46.7) | 419 (49.1) | 232 (41.1) | 102 (52.0) | 0.004 |
| 90-day mRS 0–3g, n (%) | 919 (56.9) | 499 (58.4) | 296 (52.5) | 124 (63.3) | 0.014 |
| mTICI, n (%) | 0.006 | ||||
| mTICI 0–1 | 106 (6.5) | 58 (6.7) | 31 (5.4) | 17 (8.5) | |
| mTICI 2a | 68 (4.2) | 27 (3.1) | 32 (5.6) | 9 (4.5) | |
| mTICI 2b | 356 (21.8) | 209 (24.3) | 99 (17.3) | 48 (23.9) | |
| mTICI 3 | 1105 (67.6) | 567 (65.9) | 411 (71.7) | 127 (63.2) | |
| Successful recanalization, n (%) | 1461 (89.4) | 776 (90.1) | 510 (89.0) | 175 (87.1) | 0.423 |
| Complete recanalization, n (%) | 1105 (67.6) | 567 (65.9) | 411 (71.7) | 127 (63.2) | 0.024 |
| Safety outcomes | |||||
| Death within 90 days, n (%) | 211 (13.1) | 97 (11.4) | 85 (15.1) | 29 (14.8) | 0.095 |
|
PHh n (%) |
135 (8.5) | 45 (5.4) | 77 (13.8) | 13 (6.7) | < 0.001 |
| SICHh, n (%) | 102 (6.5) | 40 (4.8) | 52 (9.5) | 10 (5.2) | 0.002 |
| SAHI, n (%) | 2 (0.1) | 1 (0.1) | 1 (0.2) | 0 | 0.845 |
| IVHI, n (%) | 7 (0.4) | 3 (0.4) | 3 (0.5) | 1 (0.5) | 0.654 |
LAA Large-artery atherosclerosis, CE cardioembolism, SOE stroke of other determined etiology, SUE stroke of undetermined etiology, LVO large vessel occlusion, SD standard deviation, SBP systolic blood pressure, IQR interquartile range, NIHSS National Institutes of Health Stroke Scale score, ASPECTS Alberta Stroke Program Early CT score, OTD onset-to-door, DTP door-to-puncture, PTR puncture-to-recanalization, IVT intravenous thrombolysis, IAT intraarterial thrombolysis, MT menchanial thrombectomy, FPR first pass recanalization, SICH symptomatic intracranial hemorrhage, PH parenchymal hemorrhage, mRS modified Rankin score, IVH intraventricular hemorrhage, OTD onset to door, DTP door to puncture
a1 missing data
b 7 missing data
c12 missing data
d36 missing data
e125 missing data
f1 missing data
g21 missing data
h55 missing data
I40 missing data
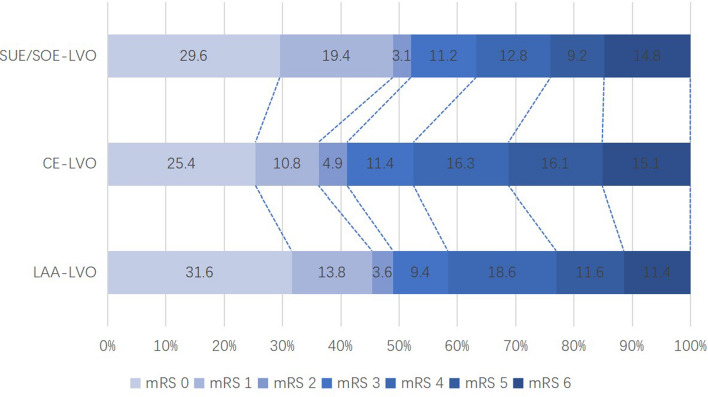
EVT of CE-LVO had a higher rate of complete recanalization (71.7% vs. 65.9% and 63.2%; P = 0.024) compared to LAA-LVO and SUE/SOE-LVO. Moreover, we observed higher PH (13.8% vs. 5.4% and 6.7%; P < 0.001) and SICH (9.5% vs. 4.8% and 5.2%; P = 0.002) rate in the CE-LVO group compared to LAA-LVO and SUE/SOE-LVO. However, no significant difference regarding the incidence of subarachnoid hemorrhage (SAH) and intraventricular hemorrhage among groups (P > 0.05). We also identified lower mRS 0–1, 0–2, and 0–3 at 90 days in the CE-LVO group than with the other two groups (P < 0.05 for all) (Fig. 2). Stent retriever as first-line, direct aspiration as first-line and direct aspiration + stent retriever as first-line were higher in this group (P < 0.001). We also noted that CE-LVO required more retrieval attempts than the other two-stroke subtypes (2 vs. 1 and 1; P < 0.001). However, we observed a higher rate of used GP IIb/IIIa receptor inhibitors in LAA-LVO (68.1% vs. 35.6% and 33.8%; P < 0.001). Time from onset to door was longer in patients with LAA-LVO than CE-LVO and SUE/SOE-LVO (164.5 vs. 135 and 160 min; P = 0.03), as was the time from door to puncture (127 vs. 115 and 115 min; P < 0.001). However, the time from puncture to recanalization was longer in SUE/SOE-LVO than LAA-LVO and CE-LVO (90 vs. 88 and 80 min; P = 0.018).
Fig. 2.
Shift on 90-day mRS score stratified by TOAST classification. LAA large-artery atherosclerosis, CE cardioembolism, SUE/SOE stroke of unknown etiology/stroke of other determined etiology, LVO large vessel occlusion, mRS modified Rankin Scale
CE-LVO was associated with complete recanalization after adjustment for potential confounders compared with LAA-LVO [adjusted OR, 1.50 (95% CI 1.04–2.17), P = 0.031]. Consistently, CE-LVO was also associated with parenchymal hemorrhage when compared to LAA-LVO [adjusted OR, 1.97 (95% CI 1.09–3.55), P = 0.025]. However, we did not observe any association between stroke subtypes and 90-day mRS and mRS0-1, mRS0-2, and mRS0-3 even after adjustment for potential confounders (P > 0.05 for all). Subgroup analyses showed no significant association between stroke subtypes and 90-day mRS score and SICH when we stratified the age, gender, NIHSS score on admission, the use of anesthesia, the involved circulation system, time workflow, and successful recanalization status (P > 0.05 for all) (Tables 2, 3, 4).
Table 2.
Adjusted OR/HR of safety and efficacy outcome according to different stroke subtypes
| Outcomes | Groups | unadjusted OR/HR (95% CI) | P value | Adjusted OR/HR (95% CI) | P value |
|---|---|---|---|---|---|
| 90-day mRSa, median (IQR) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 1.39(1.15–1.68) | 0.006 | 1.05(0.79–1.41) | 0.727 | |
| SUE/SOE-LVO | 0.99(0.75–1.30) | 0.943 | 0.95(0.69–1.30) | 0.746 | |
| 90-day mRS 0–1a, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 0.68(0.55–0.85) | 0.001 | 0.91(0.64–1.30) | 0.603 | |
| SUE/SOE-LVO | 1.15(0.85–1.57) | 0.369 | 1.19(0.82–1.73) | 0.366 | |
| 90-day mRS 0–2a, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 0.73(0.59–0.90) | 0.003 | 1.05(0.74–1.49) | 0.775 | |
| SUE/SOE-LVO | 1.13(0.83–1.54) | 0.452 | 1.23(0.84–1.78) | 0.288 | |
| 90-day mRS 0–3a, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 0.79(0.63–0.97) | 0.027 | 1.31(0.89–1.93) | 0.946 | |
| SUE/SOE-LVO | 1.23(0.89–1.69) | 0.215 | 0.99(0.70–1.40) | 0.174 | |
| Successful recanalization, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 2.82(1.92–4.14) | < 0.001 | 1.02(0.58–1.79) | 0.951 | |
| SUE/SOE-LVO | 1.26(0.67–2.39) | 0.475 | 0.91(0.50–1.64) | 0.743 | |
| Complete recanalization, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 1.32(1.05–1.66) | 0.020 | 1.50(1.04–2.17) | 0.031 | |
| SUE/SOE-LVO | 0.89(0.65–1.23) | 0.474 | 0.96(0.65–1.41) | 0.830 | |
| Death within 90 days‖, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 1.39(1.01–1.89) | 0.041 | 1.09(0.67–1.79) | 0.724 | |
| SUE/SOE-LVO | 1.36(0.87–2.12) | 0.183 | 1.47(0.86–2.50) | 0.155 | |
| SICHb, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 2.08(1.36–3.19) | 0.001 | 1.24(0.63–2.45) | 0.531 | |
| SUE/SOE-LVO | 1.08(0.53–2.20) | 0.829 | 0.87(0.38–1.98) | 0.741 | |
| PHc, n (%) | LAA-LVO | Ref | Ref | ||
| CE-LVO | 2.82(1.92–4.14) | < 0.001 | 1.97(1.09–3.55) | 0.025 | |
| SUE/SOE-LVO | 1.26(0.67–2.39) | 0.475 | 1.04(0.51–2.13) | 0.919 |
LAA Large-artery atherosclerosis, CE cardioembolism, SOE stroke of other determined etiology, SUE stroke of undetermined etiology, LVO large vessel occlusion, SICH symptomatic intracranial hemorrhage, PH parenchymal hemorrhage, mRS modified rankin score
a21 missing data
b55 missing data
b40 missing data
Table 3.
Subgroup analysis regarding 90-day mRSa of different stroke subtypes
| Variables | Num | Adjusted OR and 95% CI | P for interaction | ||
|---|---|---|---|---|---|
| LAA-LVO (reference) | CE-LVO | SUE/SOE-LVO | |||
| Age | |||||
| Age < 65 | 771 | 1 | 0.79(0.50–1.26) | 0.89(0.60–1.31) | 0.169 |
| Age ≥ 65 | 843 | 1 | 1.22(0.84–1.78) | 0.78(0.46–1.31) | |
| Gender | |||||
| Male | 1085 | 1 | 1.04(0.72–1.50) | 0.94(0.65–1.36) | 0.430 |
| Female | 529 | 1 | 1.02(0.63–1.65) | 0.91(0.50–1.65) | |
| NIHSS | |||||
| NIHSS ≤ 15 | 773 | 1 | 1.06(0.67–1.65) | 1.00(0.64–1.59) | 0.532 |
| NIHSS > 15 | 841 | 1 | 0.99(0.68–1.45) | 0.84(0.54–1.30) | |
| Anesthesia | |||||
| GA | 640 | 1 | 0.97(0.60–1.57) | 0.95(0.56–1.60) | 0.552 |
| LA | 974 | 1 | 1.08(0.75–1.55) | 0.91(0.61–1.37) | |
| Occlusion location | |||||
| Anterior circulation | 1258 | 1 | 1.04(0.76–1.42) | 0.90(0.63–1.30) | 0.244 |
| Posterior circulation | 356 | 1 | 1.27(0.55–2.94) | 0.76(0.39–1.51) | |
| OTD (min) | |||||
| OTD ≤ 270 | 1192 | 1 | 1.10(0.78–1.54) | 0.94(0.65–1.35) | 0.906 |
| OTD > 270 | 422 | 1 | 0.97(0.56–1.68) | 0.84(0.44–1.60) | |
| DTP (min) | |||||
| DTP ≤ 90 | 580 | 1 | 0.84(0.54–1.30) | 0.79(0.48–1.30) | 0.422 |
| DTP > 90 | 1034 | 1 | 1.20(0.83–1.74) | 1.19(0.81–1.76) | |
| Procedure duration (min) | |||||
| Procedure duration ≤ 90 | 884 | 1 | 0.90(0.61–1.32) | 0.83(0.53–1.30) | 0.484 |
| Procedure duration > 90 | 730 | 1 | 1.40(0.89–2.18) | 1.16(0.73–1.82) | |
| Successful recanalization | |||||
| Yes | 1447 | 1 | 1.01(0.74–1.38) | 0.96(0.69–1.35) | 0.159 |
| No | 167 | 1 | 2.83(1.16–6.91) | 1.47(0.54–4.02) | |
LAA Large-artery atherosclerosis, CE cardioembolism, SOE stroke of other determined etiology, SUE stroke of undetermined etiology, LVO large vessel occlusion, NIHSS National Institutes of Health Stroke Scale score, GA general anesthesia, LA local anesthesia, OTD onset to door, DTP door to puncture
a21 missing data
Table 4.
Subgroup analysis regarding SICHa of different stroke subtypes
| Variables | Num | Adjusted OR and 95% CI | P for interaction | ||
|---|---|---|---|---|---|
| LAA-LVO (reference) | CE-LVO | SUE/SOE-LVO | |||
| Age | |||||
| Age < 65 | 760 | 1 | 1.16(0.39–3.42) | 0.45(0.15–1.34) | 0.586 |
| Age ≥ 65 | 820 | 1 | 1.37(0.54–3.46) | 1.38(0.35–5.41) | |
| Gender | |||||
| Male | 1068 | 1 | 1.23(0.50–3.03) | 0.63(0.21–1.89) | 0.571 |
| Female | 512 | 1 | 1.54(0.46–5.13) | 1.80(0.44–7.35) | |
| NIHSS | |||||
| NIHSS ≤ 15 | 761 | 1 | 1.39(0.49–3.97) | 0.67(0.21–2.16) | 0.960 |
| NIHSS > 15 | 819 | 1 | 0.93(0.37–2.40) | 1.01(0.32–3.60) | |
| Anesthesia | |||||
| GA | 623 | 1 | 0.76(0.29–2.01) | 0.82(0.23–2.98) | 0.895 |
| LA | 954 | 1 | 1.67(0.62–4.46) | 0.81(0.24–2.74) | |
| Occlusion location | |||||
| Anterior circulation | 1235 | 1 | 0.06(0.01–3.41) | 1.90(0.03–105.68) | 0.775 |
| Posterior circulation | 345 | 1 | 1.40(0.69–2.84) | 0.86(0.36–2.05) | |
| OTD (min) | |||||
| OTD ≤ 270 | 1161 | 1 | 1.94(0.88–4.30) | 1.31(0.53–3.24) | 0.256 |
| OTD > 270 | 419 | 1 | 0.38(0.08–1.69) | 0.12(0.01–1.40) | |
| DTP (min) | |||||
| DTP ≤ 90 | 564 | 1 | 2.86(0.81–10.09) | 1.24(0.28–5.39) | 0.275 |
| DTP > 90 | 1016 | 1 | 1.05(0.45–2.46) | 1.04(0.40–2.73) | |
| Procedure duration (min) | |||||
| Procedure duration ≤ 90 | 858 | 1 | 1.41(0.44–4.51) | 1.47(0.35–6.13) | 0.517 |
| Procedure duration > 90 | 722 | 1 | 1.15(0.47–2.80) | 0.95(0.34–2.66) | |
| Successful recanalization | |||||
| Yes | 1410 | 1 | 1.27(0.58–2.78) | 0.97(0.39–2.43) | 0.896 |
| No | 170 | 1 | 0.89(0.07–10.72) | 0.35(0.02–7.51) | |
LAA Large-artery atherosclerosis, CE cardioembolism, SOE stroke of other determined etiology, SUE stroke of undetermined etiology, LVO large vessel occlusion, NIHSS National Institutes of Health Stroke Scale score, GA general anesthesia, LA local anesthesia, OTD onset to door, DTP door to puncture, PTR puncture to recanalization
a55 data were missing
Discussion
In our study, the functional outcome from EVT did not differ among stroke subtypes. Different characteristics in each stroke subtype might affect the outcome of EVT. We also found an increasing risk of bleeding despite complete recanalization in a patient with the CE stroke subtype. Several factors might account for this finding, such as CE-LVO patients were older, had a higher rate of atrial fibrillation, and presented with a higher NIHSS score, which implies a larger ischemic area.
Our result was not in line with the previous study, which reported that EVT showed different efficacy in different stroke subtypes [2]. Our study showed that the stroke subtype did not withhold the benefit of EVT. This result might be attributed to the higher successful recanalization rate among stroke subtypes in the present study compared with Tiedt et al. [2], although we did not assess this difference statistically. The previous study reported higher efficacy of EVT in CE-LVO, which might be attributed to several factors, including (1) thrombus composition, which may determine the success of thrombectomy [19], and (2) the complexity of the atherosclerotic lesion, which may impede technical access to the occlusion site [2, 20]. In addition, CE-LVO was presented more in their study when compared with non-CE or LAA-LVO, which may bias the actual result. However, the higher number of LAA-LVO in the current study may also contribute to a biased result. However, the significant difference between groups regarding the baseline has been adjusted in our multivariate analysis, which can reduce the probability of a biased result due to the unproportionate number of patients between groups.
Different nature characteristics of the lesion among stroke subtypes need different EVT strategies. LAA-LVO are relatively more complex than CE-LVO [21, 22], as our study, and previously reported literature demonstrated that LAA-LVO often presented with a longer duration of the recanalization time than CE-LVO [23–31]. Nevertheless, the complexity of the atherosclerotic lesion did not withhold achieving successful recanalization, as rescue therapy, such as the use of GP IIb/IIIa receptor inhibitors, could be given during EVT. The efficacy of GP IIb/IIIa receptor inhibitors in AIS has been reported previously [32–34]. Consistently, we noted higher use of GP IIb/IIIa receptor inhibitors in the LAA-LVO group. Furthermore, this might also explain the higher recanalization rate in LAA-LVO in the present study.
A noteworthy finding from the current study is an increased risk of bleeding despite complete recanalization in the CE-LVO group. The underlying mechanism remains unclear. However, the greater severity of strokes in CE-LVO presenting with higher NIHSS scores than other stroke subtypes may partially explain this finding. Moreover, CE-LVO is often related to a more extensive core infarct and less penumbra [35, 36]. In addition, early reperfusion might also contribute to an increase in PH in CE-LVO [37], as the onset to door and door to puncture time was shorter compared with other stroke subtypes.
Although recanalization represents a powerful predictor of stroke outcomes [38], this should also warn us of the possibility of reperfusion injury, which might exacerbate the outcomes. Thus, precaution and intensive management are needed to reduce the mortality and morbidity risks. There is still an ongoing debate whether “complete” recanalization (TICI 3) or “successful” recanalization (TICI 2b/3) should be achieved. This finding also has an important message: restoring brain perfusion with recanalization does not mean that the occluded vessel should be completely recanalized. Further studies to evaluate the extent of recanalization in CE-LVO are urgently needed.
Our study has several limitations. First, the retrospective nature of the present study, and the lack of a control group. A further randomized controlled trial is needed to assess any intention to treat. Second, this classification only can be applied after the complete diagnostic work-up. Therefore, the value of such a classification to assess acute stroke therapeutic options remains to be elucidated. Third, the present study lacked further angiographic assessment in different stroke subtypes, such as the collateral status and ratio of the core ischemic area to the penumbra. However, the strengths of our study included a large sample size and the use of nationwide collected data. Nevertheless, this study was limited to Chinese populations, and the stroke subtype’s proportion differs among ethnicities and countries. Therefore, this result cannot be generalized to the global population.
Conclusions
Our nationwide real-world registry data provide evidence for a higher rate of complete recanalization and increased risk of PH in CE-LVO and confirmed the stroke subtypes as a determinant of EVT safety and efficacy. Further research should focus on the extent of EVT in this stroke subtype.
Acknowledgements
Funding
This Study funded by the National Key Research and Development Program of China, grant number 2016YFC1301500 and China Postdoctoral Science Foundation, grant number 2020-YJ-008. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors' Contributions
Zhongrong Miao, Zeguang Ren planned the study; Raynald, Dapeng Sun, Xiaochuan Huo interpreted the findings and wrote the manuscript; Anxin Wang analysed the data, Baixue Jia, Xu Tong, Gaoting Ma, contributed to data collection; Dapeng Mo, Ning Ma, Feng Gao, Sheyar Amin provided critical comments / revisions of the manuscript. Zhongrong Miao, Raynald, Dapeng Sun, Xiaochuan Huo are responsible for the overall content.
Disclosures
Xiaochuan Huo, Dapeng Sun, Raynald, Baixue Jia, Xu Tong, Anxin Wang, Ning Ma, Feng Gao, Dapeng Mo, Gaoting Ma, Sheyar Amin, Zeguang Ren and Zhongrong Miao declared that they have no conflict of interests.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethics Committees of Beijing Tiantan Hospital and the ethics committees of all participating centers. The number of the approval: KY2017-048-01. The study procedures were in accordance with the 1964 Helsinki declaration and its later amendments. Subjects or their legally authorized representatives provided written informed consent.
Data Availability
Anonymous data that support the findings of this study are available on reasonable request from the corresponding author.
Footnotes
Xiaochuan Huo and Dapeng Sun contributed equally to this work.
Contributor Information
Zeguang Ren, Email: renzem@gmail.com.
Zhongrong Miao, Email: doctorzhongrongm@126.com.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 2.Tiedt S, Wollenweber FA. Response by tiedt and wollenweber to letter regarding article, "stroke etiology modifies the effect of endovascular treatment in acute stroke". Stroke. 2020;51:e159–e160. doi: 10.1161/STROKEAHA.120.030266. [DOI] [PubMed] [Google Scholar]
- 3.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 4.Mustanoja S, Meretoja A, Putaala J, et al. Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke. 2011;42:102–106. doi: 10.1161/STROKEAHA.110.597534. [DOI] [PubMed] [Google Scholar]
- 5.Wei W, Li S, San F, et al. Retrospective analysis of prognosis and risk factors of patients with stroke by TOAST. Medicine (Baltimore) 2018;97:e0412. doi: 10.1097/MD.0000000000010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan YF, Zhan LX, Chen XH, Guo JJ, Qin C, Xu E. Risk factors, clinical features and prognosis for subtypes of ischemic stroke in a chinese population. Curr Med Sci. 2018;38:296–303. doi: 10.1007/s11596-018-1878-1. [DOI] [PubMed] [Google Scholar]
- 7.McArdle PF, Kittner SJ, Ay H, et al. Agreement between TOAST and CCS ischemic stroke classification: the NINDS SiGN study. Neurology. 2014;83:1653–1660. doi: 10.1212/WNL.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang Q, Zhou M, Feng H, Guo J, Chen N, He L. Research on the relationship between fibrinogen level and subtypes of the TOAST criteria in the acute ischemic stroke. BMC Neurol. 2013;13:207. doi: 10.1186/1471-2377-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ois A, Cuadrado-Godia E, Rodriguez-Campello A, et al. Relevance of stroke subtype in vascular risk prediction. Neurology. 2013;81:575–580. doi: 10.1212/WNL.0b013e31829e6f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao Z, Huo X, Gao F, et al. Endovascular therapy for Acute ischemic Stroke Trial (EAST): study protocol for a prospective, multicentre control trial in China. Stroke Vasc Neurol. 2016;1:44–51. doi: 10.1136/svn-2016-000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo X, Ma N, Mo D, et al. Acute Ischaemic Stroke Cooperation Group of Endovascular Treatment (ANGEL) registry: study protocol for a prospective, multicentre registry in China. Stroke Vasc Neurol. 2019;4:57–60. doi: 10.1136/svn-2018-000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia B, Ren Z, Mokin M, et al. Current status of endovascular treatment for acute large vessel occlusion in China: a real-world nationwide registry. Stroke. 2021;52:1203–1212. doi: 10.1161/STROKEAHA.120.031869. [DOI] [PubMed] [Google Scholar]
- 13.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 14.Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. doi: 10.1161/STROKEAHA.107.511162. [DOI] [PubMed] [Google Scholar]
- 15.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. Am J Neuroradiol. 2008;29:582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerventional Surg. 2018;10:34–38. doi: 10.1136/neurintsurg-2016-012721. [DOI] [PubMed] [Google Scholar]
- 20.Tsang ACO, Lau KK, Tsang FCP, Tse MMY, Lee R, Lui WM. Severity of intracranial carotid artery calcification in intracranial atherosclerosis-related occlusion treated with endovascular thrombectomy. Clin Neurol Neurosurg. 2018;174:214–216. doi: 10.1016/j.clineuro.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Park H, Baek JH, Kim BM. Endovascular treatment of acute stroke due to intracranial atherosclerotic stenosis-related large vessel occlusion. Front Neurol. 2019;10:308. doi: 10.3389/fneur.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JS, Lee SJ, Yoo JS, et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J Stroke. 2018;20:394–403. doi: 10.5853/jos.2018.01627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek JH, Kim BM, Kim DJ, et al. Importance of truncal-type occlusion in stentriever-based thrombectomy for acute stroke. Neurology. 2016;87:1542–1550. doi: 10.1212/WNL.0000000000003202. [DOI] [PubMed] [Google Scholar]
- 24.Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37:350–355. doi: 10.1159/000362435. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. 2016;18:96–101. doi: 10.5853/jos.2015.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek JH, Kim BM, Yoo J, et al. Predictive value of computed tomography angiography-determined occlusion type in stent retriever thrombectomy. Stroke. 2017;48:2746–2752. doi: 10.1161/STROKEAHA.117.018096. [DOI] [PubMed] [Google Scholar]
- 27.Baek JH, Kim BM, Heo JH, Kim DJ, Nam HS, Kim YD. Outcomes of endovascular treatment for acute intracranial atherosclerosis-related large vessel occlusion. Stroke. 2018;49:2699–2705. doi: 10.1161/STROKEAHA.118.022327. [DOI] [PubMed] [Google Scholar]
- 28.Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery. 2015;76:680–686. doi: 10.1227/NEU.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Hong JM, Lee KS, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis vs. embolism. J Stroke Cerebrovasc Dis. 2015;24:2074–2080. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Matias-Guiu JA, Serna-Candel C, Matias-Guiu J. Stroke etiology determines effectiveness of retrievable stents. J Neurointerventional Surg. 2014;6:e11. doi: 10.1136/neurintsurg-2012-010395. [DOI] [PubMed] [Google Scholar]
- 31.Gascou G, Lobotesis K, Machi P, et al. Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. Am J Neuroradiol. 2014;35:734–740. doi: 10.3174/ajnr.A3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun C, Li X, Zhao Z, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol. 2019;10:1100. doi: 10.3389/fneur.2019.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XG, Zhang LQ, Liao XL, et al. Unfavorable outcome of thrombolysis in chinese patients with cardioembolic stroke: a prospective cohort study. CNS Neurosci Ther. 2015;21:657–661. doi: 10.1111/cns.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48:3289–3294. doi: 10.1161/STROKEAHA.117.019193. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Seok JM, Bang OY, et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan YT, Lee JD, Lin YH, et al. Comparisons of outcomes in stroke subtypes after intravenous thrombolysis. Springerplus. 2016;5:47. doi: 10.1186/s40064-016-1666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 38.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42:S16–19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymous data that support the findings of this study are available on reasonable request from the corresponding author.