Abstract
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is a systemic disorder that frequently affects the peripheral nervous system and consists of three distinct conditions: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA, previously Wegener’s granulomatosis), and eosinophilic granulomatosis with polyangiitis (EGPA, previously Churg-Strauss syndrome). The neuropathic features associated with this condition usually include mononeuritis multiplex, which reflects the locality of lesions. Findings suggestive of vasculitis are usually found in the epineurium and occur diffusely throughout the nerve trunk. Nerve fiber degeneration resulting from ischemia is sometimes focal or asymmetric and tends to become conspicuous at the middle portion of the nerve trunk. The attachment of neutrophils to endothelial cells in the epineurial vessels is frequently observed in patients with ANCA-associated vasculitis; neutrophils play an important role in vascular inflammation by binding of ANCA. The positivity rate of ANCA in EGPA is lower than that in MPA and GPA, and intravascular and tissue eosinophils appear to participate in neuropathy. Immunotherapy for ANCA-associated vasculitis involves the induction and maintenance of remission to prevent the relapse of the disease. A combination of glucocorticoids along with cyclophosphamide, rituximab, methotrexate, or mycophenolate mofetil is considered depending on the severity of the condition of the organ to induce remission. A combination of low-dose glucocorticoids and azathioprine, rituximab, methotrexate, or mycophenolate mofetil is recommended to maintain remission. The efficacy of anti-interleukin-5 therapy (i.e., mepolizumab) was demonstrated in the case of refractory or relapsing EGPA. Several other new agents, including avacopan, vilobelimab, and abatacept, are under development for the treatment of ANCA-associated vasculitis. Multidisciplinary approaches are required for the diagnosis and management of the disorder because of its systemic nature. Furthermore, active participation of neurologists is required because the associated neuropathic symptoms can significantly disrupt the day-to-day functioning and quality of life of patients with ANCA-associated vasculitis.
Keywords: Complement, Degranulation, Electron microscopy, Endothelium, NETosis, Neutrophil extracellular trap, Pathogenesis, Pathology, Ultrastructure, Vasculitic neuropathy
Key Summary Points
| Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is a systemic disorder that frequently affects the peripheral nervous system and comprises three main conditions: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). |
| The neuropathic features are usually characterized by mononeuritis multiplex, which reflects the locality of the lesions associated with vasculitis. |
| The attachment of neutrophils to endothelial cells in the epineurial vessels is frequently observed in nerve biopsy specimens obtained from patients with ANCA-associated vasculitis, suggesting that neutrophils play an important role in this disease. |
| The positivity rate of ANCA in EGPA is lower than that in MPA and GPA, and eosinophils also seem to participate in the mechanism of tissue damage. |
| Multidisciplinary approaches are needed for the diagnosis and management of ANCA-associated vasculitis because of the systemic nature of the disorder. |
Introduction
Vasculitis is a group of disorders that can affect a variety of vessels (from the aorta to the capillaries) and a variety of organs (including the lungs, heart, kidneys, gastrointestinal tract, skin, and nervous system) [1]. The involvement of the peripheral nervous system is caused by different types of vasculitis and can disrupt the day-to-day functioning and quality of life of patients because of weakness or pain in the extremities [2, 3]. According to the definition in the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitis (CHCC2012), vasculitides that are not associated with any particular systemic disease or probable etiology are categorized on the basis of the size of the affected vessels [1]. Because of the absence of large vessels in the peripheral nervous system [1], the relationship between neuropathy and vasculitis has been examined in medium- to small-vessel vasculitis. Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is a small-vessel vasculitis that predominantly affects the capillaries, venules, arterioles, and small arteries, particularly in the peripheral nervous system. This disorder comprises three main conditions: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA, previously known as Wegener’s granulomatosis), and eosinophilic granulomatosis with polyangiitis (EGPA, previously known as Churg-Strauss syndrome) [1, 3], and according to previous large-scale studies, neuropathy was reported in 58%, 17%, and 77% of patients with these conditions, respectively [4–6]. Although a recent large multinational observational case–control study reported lower frequency (23%) of vasculitic neuropathy in patients with MPA [7], ANCA-associated vasculitis is one of the most important causes of vasculitic neuropathy from the standpoint of neurologists [2, 3].
Multidisciplinary approaches are needed for the diagnosis and management of ANCA-associated vasculitis because of its systemic nature. Hence, neurologists, physicians, and researchers need to share information about the neuropathic aspects of the disorder. From this viewpoint, this review describes the characteristics of peripheral neuropathy in ANCA-associated vasculitis by focusing on the pathological aspects. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Clinical Characteristics
Characteristics of MPA, GPA, and EGPA
Although MPA, GPA, and EGPA share common systemic features, each entity also has a distinct profile. For example, MPA is associated with ANCA directed against myeloperoxidase (MPO-ANCA) and necrotizing vasculitis with few or no immune deposits [1, 6]. Alternatively, GPA is characterized by granulomatous inflammation of the respiratory tract and ANCA directed against proteinase 3 (PR3-ANCA) [8]. The positivity rate of ANCA in sera from patients is approximately 80% in both MPA and GPA [6, 8]. There is a strong association between EGPA and allergic diathesis, particularly asthma and eosinophilia [5]. Although EGPA is also associated with ANCA, in which MPO-ANCA is predominant, the positivity rate is lower (only 30–40%) [9, 10]. Several studies have shown differences in the clinical characteristics of patients with EGPA based on their ANCA status [9–13]. Cardiac involvement is more frequent, whereas ear, nose, and throat manifestations, peripheral nerve involvement, and renal involvement are less frequent, in ANCA-negative EGPA patients when compared with ANCA-positive EGPA patients.
According to a study of 955 patients with ANCA-associated vasculitis, including MPA, GPA, and EGPA, vasculitic neuropathy was associated with MPO-ANCA positivity and skin, musculoskeletal, and cardiovascular involvement [7]. Additionally, patients with vasculitic neuropathy were less likely to have renal, ocular, or gastrointestinal involvement [7].
Neuropathic Features
The characteristics of neuropathies caused by vasculitides that predominantly affect the small vessels, including ANCA-associated vasculitis, are similar [13–17]. Generally, tingling or painful paresthesia in the distal portions of the limbs, particularly the lower limbs, is the initial symptom of neuropathy [18]. The symptoms suddenly develop in a single nerve territory followed by the gradual involvement of other nerves [19]. The distribution of the sensory impairment tends to be similar to the pattern observed in mononeuritis multiplex, which reflects the focal lesions of the vasculitis. However, multiple lesions may yield a symmetric or asymmetric distally accentuated polyneuropathy pattern [20]. According to studies focusing on neuropathic features of ANCA-associated vasculitis, mononeuritis multiplex occurred in approximately 70–90% of patients, whereas the others manifested symmetric or asymmetric polyneuropathy [4, 13, 14]. Muscle weakness corresponding to the affected nerves might be evident, resulting in muscle atrophy. Therefore, patients with this disorder experience sensory or sensorimotor neuropathy, whereas pure motor neuropathy is an exclusion criterion for vasculitic neuropathy [2]. Deep tendon reflexes are reduced or absent depending on the affected nerves. The incidence of cranial neuropathy is low, except for GPA, in which granuloma formation in the cranial region is common [4, 21]. It should be noted that central nervous system manifestations resulting from ischemia hemorrhage, or granuloma formation may occur and complicate neuropathic features in patients with ANCA-associated vasculitis [22].
Cerebrospinal fluid protein levels and cell counts are usually normal [15, 16]. Electrophysiological studies indicate the presence of axonal neuropathy characterized by a decrease in the compound muscle action potential and sensory nerve action potential but the preservation of the motor and sensory conduction velocities and distal motor latency [13–17]. These findings are predominantly observed in the lower extremities.
Pathological Findings
Findings in Biopsy Specimens
The pathological findings of neuropathy resulting from vasculitis are characterized by the axonal degeneration of the nerve fibers and inflammation of the epineurial vessels accompanied by the destruction of vascular structures, such as the disruption of the vascular layers and/or obstruction of the lumen, with or without fibrinoid necrosis (Fig. 1) [2]. Both myelinated and unmyelinated fibers are affected [18]. These findings are commonly observed in patients with MPA, GPA, and EGPA [1, 20]. Although vessel wall inflammation accompanied by vascular structural damage is required for the pathological diagnosis of vasculitic neuropathy, the sensitivity of this finding is not high [2]. Furthermore, focal or asymmetric nerve fiber loss reflecting the region of the blood supply by the affected vessels is considered a supportive finding [2]. After axonal degeneration, the debris is cleared by the macrophages [23]; the macrophages can be seen penetrating the basement membrane, surrounding the myelinated fibers, and phagocytizing the myelin in the degenerated fibers (Fig. 2). After the clearance of the myelin, subunits of Schwann cells indicative of remnants of the myelinated fibers (i.e., bands of Büngner) remain in the endoneurium [24]. The macrophages that phagocytize the myelin during the process of axonal neuropathy are morphologically similar to those that participate in demyelination in patients with demyelinating neuropathies, such as Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy [25, 26]. However, the structures in the axon, such as the neurofilaments and microtubules, are well preserved in patients with demyelinating neuropathies.
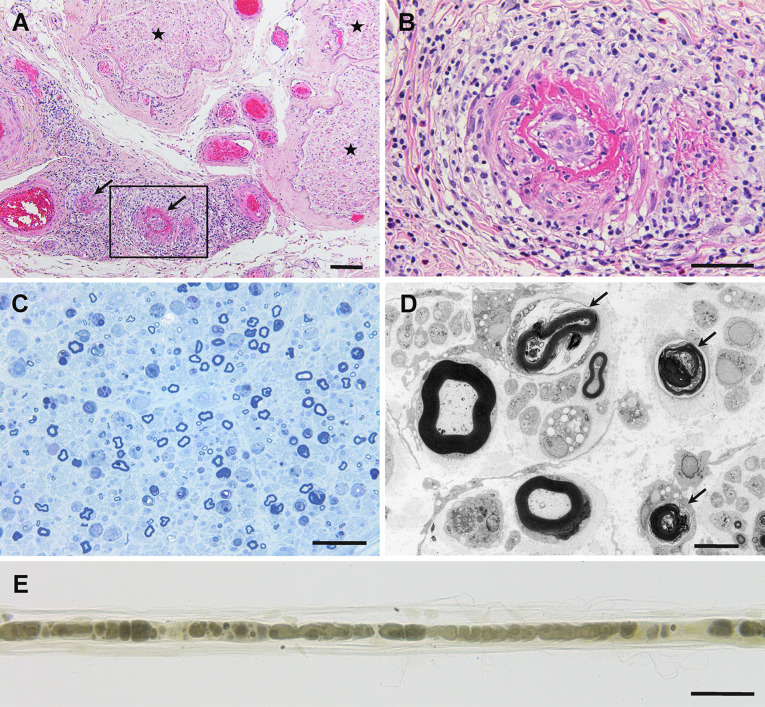
Fig. 1.
Representative photographs showing neuropathy in anti-neutrophil cytoplasmic antibody-associated vasculitis. Cross-sections (a–d) and a teased-fiber preparation (e) of sural nerve biopsy specimens obtained from patients with microscopic polyangiitis. a Epineurial vessels indicated by arrows show fibrinoid necrosis. Massive inflammatory cell infiltration was observed around these vessels. The endoneurium, where nerve fibers are located, is indicated by asterisks. b A high-powered view of the region in the box in (a). c The density of myelinated fibers is reduced. d Degeneration of myelinated fibers is evident (arrows) via electron microscopy. e Teased-fiber preparations also demonstrated myelinated fiber degeneration. Hematoxylin and eosin staining (a, b), toluidine blue staining (c), uranyl acetate and lead citrate staining (d), and osmium staining (e). Scale bars = 100 μm (a), 50 μm (b, c, e), and 5 μm (d)
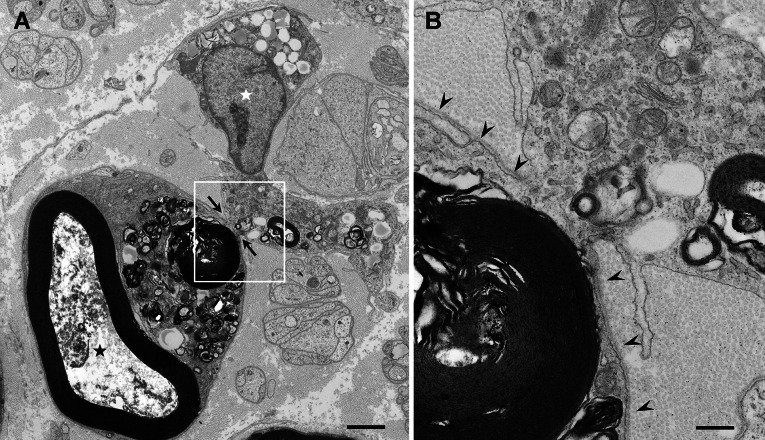
Fig. 2.
Clearance of degenerated nerve fibers by macrophages. Cross-sections of sural nerve biopsy specimens obtained from a patient with microscopic polyangiitis. a A macrophage penetrating the basement membrane surrounding myelinated fibers at sites indicated by arrows and phagocytizing myelin. Axonal structures, such as neurofilaments and microtubules, are lost because of degeneration (black asterisk). A nucleus of the macrophage is located outside the basement membrane tube (white asterisk). b A high-powered view of the region in the box in (a). The basement membrane is indicated by arrowheads. Uranyl acetate and lead citrate staining. Scale bars = 2 μm (a) and 0.5 μm (b)
The prevalence of ANCA in EGPA is lower than that in MPA and GPA, and infiltration of eosinophils is an important pathological feature included in the diagnostic criteria for EGPA (Fig. 3A) [27]. A recent study demonstrated that findings suggestive of necrotizing vasculitis, such as disruption of the vascular structures and fibrinoid necrosis, were more frequently observed in EGPA patients positive for ANCA than in those negative for ANCA [13]. By contrast, vessels filled with eosinophils, despite the preservation of the structures in the vascular wall, were more frequently observed in patients negative for ANCA than in those who were positive for ANCA (Fig. 3B) [13]. Furthermore, eosinophils were more frequently found in the extravascular space of the endoneurium in patients who were negative for ANCA compared with those who were positive for ANCA (Fig. 3C) [13]. The extent of nerve fiber degeneration in ANCA-negative patients was similar to that in the ANCA-positive patients, despite the scarcity of necrotizing vasculitis [13]; thus, tissue damage induced by eosinophils might be involved in the mechanisms of neuropathy in EGPA, as described later.
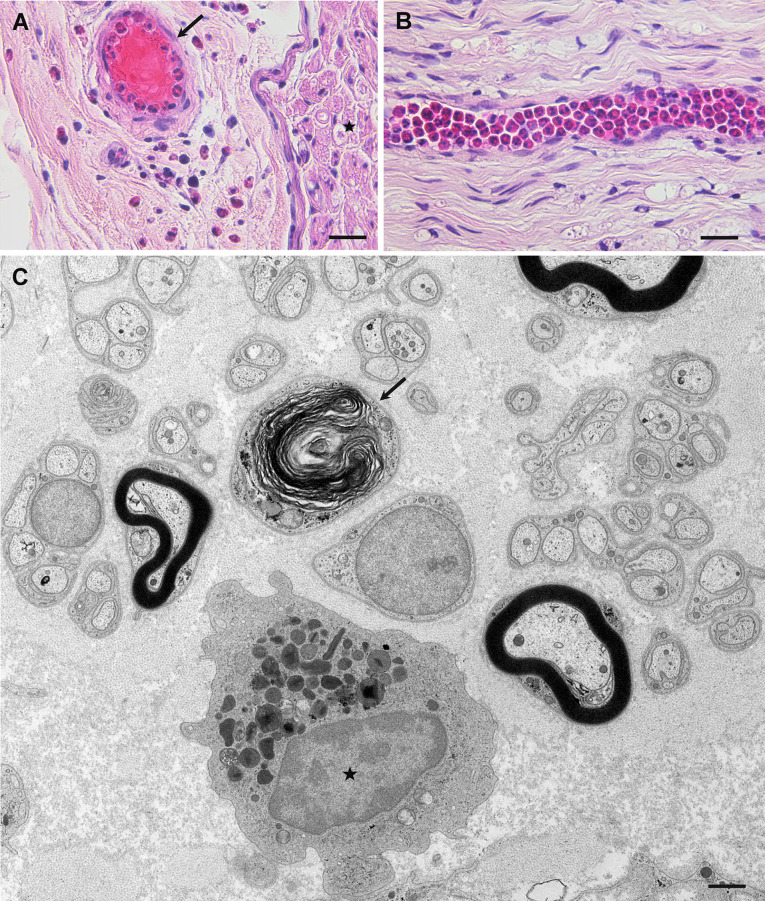
Fig. 3.
Pathological findings of eosinophilic granulomatosis with polyangiitis. Sural nerve biopsy specimens obtained from patients negative for anti-neutrophil cytoplasmic antibody. Cross-sections (a, c) and a longitudinal section (b). a Infiltration of eosinophils into the extravascular space in the epineurium is observed. Eosinophils are seen in the lumen of a vessel (arrow). An endoneurium, where the nerve fibers are located, is indicated by an asterisk. b Many eosinophils are packed inside an endoneurial vessel. c An eosinophil indicated by an asterisk is located in the extravascular space of the endoneurium. The arrow indicates a degenerated myelinated fiber. Hematoxylin and eosin staining (a, b); uranyl acetate and lead citrate staining (c). Scale bars = 20 μm (a, b) and 1 μm (c)
Distribution of Vasculitis and Nerve Fiber Degeneration
Axonal degeneration of nerve fibers in patients with ANCA-associated vasculitis has been believed to result from ischemia that is induced by vasculitis. According to a study of autopsy specimens of the peripheral nerve obtained from eight patients with MPA, necrotizing vasculitis was diffusely observed extending from the proximal to the distal portions of the sciatic-tibial and median nerve trunks [28]. Findings suggestive of vasculitis were not observed, and neurons were preserved in the parenchyma of the dorsal root ganglia and sympathetic ganglia [28]. Additionally, the nerve fibers in the anterior and posterior spinal roots were preserved [28]. By contrast, the loss of myelinated fibers resulting from axonal degeneration tended to become conspicuous at the middle to distal portions of the sciatic-tibial and median nerve trunk [28]. A similar distribution of nerve fiber damage was reported in a patient with vasculitic neuropathy due to rheumatoid arthritis [29], suggesting the presence of sites particularly vulnerable to ischemic damage at the middle portion of the nerve trunk.
An injection of polystyrene microspheres, which selectively occludes the capillaries, into the arterial supply of rat sciatic nerves induced a characteristic distribution of nerve fiber degeneration beginning from the core of the fascicles of the distal sciatic nerve and was termed central fascicular fiber degeneration [30]. Additionally, ligation of the femoral artery reduced the blood flow in the sciatic nerve, particularly at the core of the fascicles in the proximal posterior tibial branch [31]. These findings suggest that central fascicular fiber degeneration can be considered direct evidence of the presence of nerve ischemia [32]. In patients with MPA, central fascicular fiber degeneration was observed only at the proximo-middle portions of the sciatic-tibial and median nerve trunks, suggesting that these portions were watershed zones of blood supply and vulnerable to ischemia (Fig. 4A) [28]. Additionally, other patterns of focal nerve fiber degeneration were frequently seen around these portions (Fig. 4B) [28].
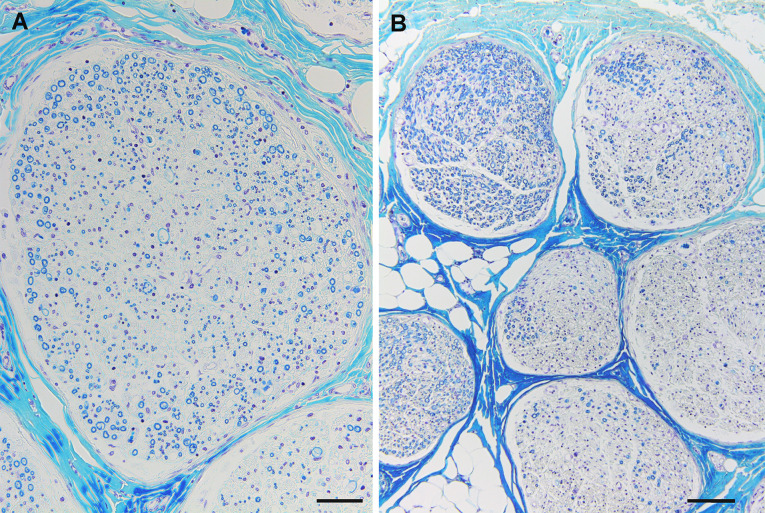
Fig. 4.
The distribution of nerve fiber degeneration suggestive of vasculitis. Autopsy specimens obtained from a patient with microscopic polyangiitis. Cross-sections of the proximo-middle portions of the median nerve (a) and sciatic-tibial nerve (b). a Central fascicular fiber degeneration, defined as a more conspicuous loss of nerve fibers at the core when compared with that in the periphery of the fascicles, is observed. b Interfascicular or intrafascicular variations in the extent of nerve fiber loss are conspicuous. Klüver–Barrera staining. Scale bars = 50 μm (a) and 100 μm (b)
Pathophysiology of ANCA-Associated Vasculitis
Role of ANCA
ANCA comprises IgG antibodies that mainly target myeloperoxidase (MPO) or proteinase 3 (PR3) in neutrophils and monocytes [33]. MPO-ANCA shows a perinuclear staining pattern in the immunofluorescence assay and is alternatively called P-ANCA [33]. Alternatively, PR3-ANCA is known as C-ANCA because of its granular and cytoplasmic staining pattern [33].
Although ANCA is not positive in 100% of patients using the current methods for examination [16], it is widely accepted that it plays an important role in ANCA-associated vasculitis. For example, mice that received anti-MPO IgG developed pauci-immune necrotizing glomerulonephritis [34]. In another study using rats immunized with human MPO, anti-MPO antibodies directed against rat leukocytes were generated and the interaction between leukocytes and vascular endothelial cells was enhanced, resulting in the development of vasculitis [35]. The complement system was not initially thought to be associated with the development of ANCA-associated vasculitis because this disorder was considered a “pauci-immune” vasculitis [1, 16]. However, accumulating evidence from animal models and clinical observations suggests that activation of the complement system, particularly the alternative complement system, plays an important role [36–40]. Indeed, the complement activation product C5a and the neutrophil C5a receptor were demonstrated to compose an amplification loop for neutrophil activation mediated by ANCA [41].
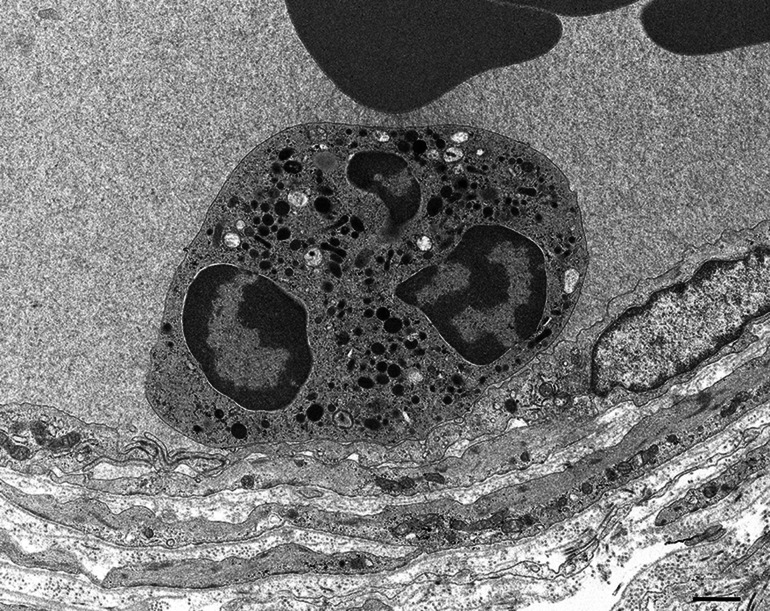
A recent study using sural nerve biopsy specimens obtained from patients with MPA demonstrated the attachment of leukocytes, particularly neutrophils, to epineurial vascular endothelial cells (Fig. 5) [16]. Additionally, an increase in the number of cytoplasmic organelles within the endothelial cells attached to the neutrophils was occasionally observed. The attachment of neutrophils to endothelial cells was the initial morphological evidence of vasculitis because it was also observed in vessels with otherwise preserved morphologies [16]. This finding was scarce or absent in patients with nonsystemic vasculitic neuropathy [16], which is distinct from ANCA-associated vasculitis [2, 15, 42]. Neutrophils do not interact with resting endothelial cells under physiological conditions; therefore, the binding of ANCA to neutrophils is believed to promote the firm adhesion of these cells to the endothelial cells via adhesion molecules [43]. Neutrophils primed by cytokines express MPO on their surfaces, which enables the binding of ANCA in the bloodstream [44]. Although the pathogenesis of ANCA-associated vasculitis is complex, these neutrophils are considered to participate in the inflammation of the blood vessels. In vitro studies suggest that neutrophils mediate vascular inflammation via the degranulation and production of oxygen radicals in ANCA-associated vasculitis [44]. In addition to the release of cytoplasmic granules, recent studies have suggested that nuclear chromatins are extruded to the extracellular space as neutrophil extracellular traps (NETs), a phenomenon called NETosis, during the process of vasculitis associated with ANCA [45, 46]. Recent studies suggest that stimulation of neutrophils with ANCA and C5a not only results in the neutrophil respiratory burst, degranulation, and NETosis, but also activates the coagulation system and generates thrombin [38, 47].
Fig. 5.
A sural nerve biopsy specimen obtained from a patient with microscopic polyangiitis showing the attachment of neutrophils to endothelial cells in the epineurial vessel. Firm adhesion of neutrophils to endothelial cells is frequently observed in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Uranyl acetate and lead citrate staining. Scale bar = 1 μm
Role of Eosinophils in EGPA
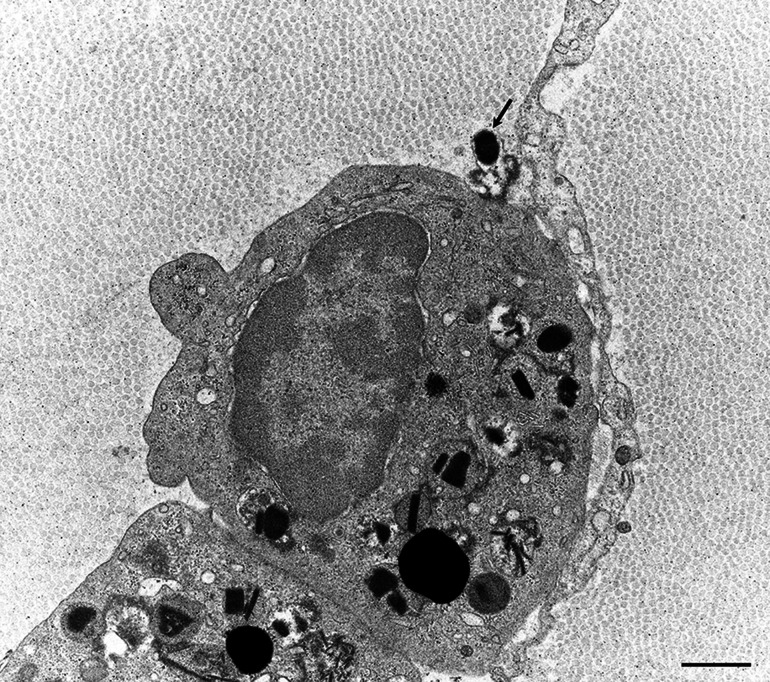
Although EGPA has been considered a disease in the spectrum of ANCA-associated vasculitis, tissue damage induced by eosinophils appears to be involved in the disease process, as described earlier. Eosinophils exert their toxicity by releasing proteins within the eosinophil-specific granules into the extracellular space via multiple mechanisms, including classical exocytosis, cytolysis, and piecemeal degranulation [48, 49]. Among these, cytolysis and piecemeal degranulation are considered important in eosinophil-associated diseases [49]. Recent studies suggested that nuclear chromatins expelled into the extracellular space participate in the mechanism of tissue damage by forming eosinophil extracellular traps, a phenomenon called eosinophil ETosis [50]. Degranulation, resulting from cytolysis of eosinophils that migrate into the extravascular interstitium of the epineurium, was demonstrated in a study of nerve biopsy specimens from patients with EGPA (Fig. 6) [13]. Another study showed findings suggestive of eosinophil ETosis in nerve specimens from EGPA patients [51]. The presence of eosinophil infiltration not only in the epineurium but also in the endoneurium [13, 17], where nerve fibers are located, indicates that eosinophils might directly induce axonal degeneration.
Fig. 6.
Degranulation of eosinophils. A sural nerve biopsy specimen obtained from a patient with eosinophilic granulomatosis with polyangiitis. An eosinophil granule released to the extracellular space is indicated by an arrow. Uranyl acetate and lead citrate staining. Scale bar = 1 μm
Another possibility is that eosinophils interrupt blood circulation by clogging the small vessels. Studies on nerve biopsy specimens obtained from patients with EGPA demonstrated the presence of epineurial vessels packed with eosinophils [13, 17]. The structures of the vessel walls tended to be preserved despite the abundance of eosinophils; thus, the disturbance of blood flow within the packed vessel might be independent of the ANCA-associated vasculitic processes [13, 17]. For example, eosinophils were suggested to aid in the development of a thrombus by interacting with platelets [52]. An increased risk of arterial and venous thrombotic events has been reported in patients with EGPA [53].
Treatment
General Considerations
Specific treatment for neuropathy resulting from ANCA-associated vasculitis has not yet been established, and immunotherapy is usually based on pieces of evidence obtained from ordinary ANCA-associated vasculitides. Patients with neuropathy generally present with concomitant involvement of other organs, such as the kidneys and lungs [28], which can lead to a fatal course if left untreated [54]. Hence, although the patients are initially referred to a neurologist, close collaboration between the neurologist and other experts, including rheumatologists, nephrologists, and internists, is necessary [19]. Peripheral neuropathy can significantly disrupt the day-to-day functioning and quality of life of patients because of weakness or pain in the extremities; therefore, supportive therapies for these neuropathic symptoms are important. Rehabilitation can involve both physical and occupational therapists. Lower limb orthosis may be needed for patients with foot drop. Thus, the management of ANCA-associated vasculitic neuropathy requires a multidisciplinary approach.
It should be noted that patients with ANCA-associated vasculitis are generally compromised because long-term immunotherapies are required to maintain remission. Trimethoprim-sulfamethoxazole is an important treatment adjunct used to prevent Pneumocystis jirovecii pneumonia in patients with ANCA-associated vasculitis [55]. The American Thoracic Society statement recommends treatment with trimethoprim-sulfamethoxazole in patients who receive 20 mg/day or more of prednisone for more than 1 month [56]. Additionally, the review and completion of appropriate vaccinations should be considered [55]. Osteoporosis medications, such as vitamin D and bisphosphonate, are recommended for patients who receive glucocorticoids [57].
Therapeutic Strategy for ANCA-Associated Vasculitis
The general approach to immunotherapy for ANCA-associated vasculitis involves the induction and maintenance of remission to prevent relapse [55]. According to recommendations of the European Alliance of Associations for Rheumatology (EULAR, formerly the European League Against Rheumatism) and the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA), treatment with a combination of glucocorticoids and either cyclophosphamide or rituximab is generally recommended for the induction of remission in patients with organ- or life-threatening ANCA-associated vasculitis, particularly MPA and GPA [58]. In patients with mild, non-organ-threatening disease, a combination of glucocorticoids and either methotrexate or mycophenolate mofetil may be considered [58]. Although plasma exchange was considered another therapeutic option for patients with severe renal involvement or severe diffuse alveolar hemorrhage in the EULAR/ERA-EDTA recommendations [58], a recent study could not demonstrate its effectiveness in terms of the incidence of end-stage kidney disease and death [59].
For maintenance of remission, a combination of low-dose glucocorticoids and azathioprine, rituximab, methotrexate, or mycophenolate mofetil is recommended [58]. The use of cyclophosphamide for more than 3 to 6 months is generally not recommended because of potential toxicities that can limit its applicability for longer-term therapy [58]. Azathioprine was reported to be a more effective maintenance agent compared with mycophenolate mofetil [60]. A recent expert consensus guideline recommended the use of rituximab for maintenance of remission in patients who receive a rituximab-based regimen for the induction of remission [61]. They also suggested that rituximab should be considered for maintenance in patients who received a cyclophosphamide-based regimen for remission.
Therapeutic strategies to suppress eosinophils have been considered in patients with EGPA because eosinophil-associated tissue damage is known to participate in the disease process. Interleukin-5 (IL-5) produced by Th2 cells and type 2 innate lymphoid cells is a good candidate for targeted therapy because it is a potent activator of eosinophils [62]. A phase 3 trial involving 136 patients with refractory or relapsing EGPA demonstrated the efficacy of mepolizumab, a humanized monoclonal antibody against IL-5, for EGPA [63]. A recent study demonstrated an improvement of neuropathy in patients treated with mepolizumab [64].
Future Therapies
Although the use of immunosuppressive agents improved the survival of patients, a meta-analysis reported a 2.7-fold increase in mortality among patients with ANCA-associated vasculitis when compared with that in the general population (95% confidence interval [CI], 2.26–3.24) [65]. A study comprising patients with MPA and GPA suggested that the severity of initial disease, age, number of relapses, and duration of glucocorticoid use were indicative of the extent of long-term vasculitis damage [66]. Thus, an improved strategy tailored to individual patients is warranted.
At present, several new therapeutic agents for ANCA-associated vasculitis are being developed. As described earlier, complements have not received any attention as therapeutic targets for ANCA-associated vasculitis because this disorder was considered a “pauci-immune” vasculitis until recently [1, 16]. However, accumulating evidence suggests that activation of the complement system plays an important role in the mechanism of ANCA-associated vasculitis [36–40]. A phase 3 trial involving 331 patients with MPA and GPA was conducted to investigate the efficacy of avacopan, an orally administered small-molecule C5a receptor antagonist [67]. The patients were randomized to receive either avacopan or prednisone during the induction of remission with either cyclophosphamide or rituximab; the results suggested that the efficacy of avacopan was similar to that of prednisone at 26 weeks and superior to that of prednisone (concerning sustained remission) at 52 weeks [67]. IFX-1, now called vilobelimab [68], is a chimeric monoclonal IgG4 antibody against the soluble form of C5a and is being considered as another agent that can inhibit complement activation in patients with ANCA-associated vasculitis. Two phase 2 trials for MPA and GPA are currently in progress (NCT03712345 and NCT03895801) [69]. Abatacept is a fusion protein of the Fc region of IgG1 and the extracellular domain of cytotoxic T lymphocyte antigen 4. It inhibits CD28-mediated T cell co-stimulation by binding to CD80 and CD86 on antigen-presenting cells, thereby blocking its binding to CD28 on T cells [70]. An open-label study demonstrated efficacy of abatacept in terms of the frequency of disease remission and prednisone discontinuation in patients with non-severe relapsing GPA [71]. At present, a phase 3 trial involving patients with non-severe relapsing GPA is ongoing (NCT02108860) [69].
Practical Information on the Investigation and Management of ANCA-Associated Vasculitic Neuropathies that the Clinician Should Be Aware of
The diagnosis of neuropathy resulting from ANCA-associated vasculitis is established by a nerve biopsy. Considering the accessibility and residual deficits after the procedure, the sural nerve, superficial peroneal nerve, and superficial radial nerve are usually chosen for biopsy [19]. However, these nerves may not be involved in patients with vasculitic neuropathy, because mononeuritis multiplex reflecting the locality of the lesions is a common feature. Indeed, the diagnostic sensitivity of nerve biopsy for definite vasculitis in patients with vasculitic neuropathy is considered to be no more than 50–60% [2, 7]. To increase the diagnostic sensitivity, muscle or skin tissues can be simultaneously obtained from the site of the nerve biopsy [72, 73]. ANCA-associated vasculitis is a systemic disorder; hence, biopsies from other organs might prove useful for diagnostic purposes.
Although most patients report reduced pain and improved strength within several weeks to months after initiation of immunotherapies, maximum improvement can take considerably longer [19]. According to a study of patients with MPA and GPA, no neuropathy was recorded on the vasculitis damage index within 6 months in 14 (35%) of 40 patients who had active vasculitic neuropathy at baseline [74]. However, patients may suffer from residual neuropathic symptoms for a long time even after remission has been achieved from the viewpoint of disease activity [14, 75]. The effectiveness of intravenous immunoglobulin on these residual neuropathic symptoms in patients with EGPA has been demonstrated [75]. As for mortality, a study of patients with MPA and GPA demonstrated that 10 (25%) of 40 patients with vasculitic neuropathy at baseline died within 5 years, compared with 80 (18%) of 450 without neuropathy, which was not statistically significant [74].
Summary and Conclusion
ANCA-associated vasculitis comprising MPA, GPA, and EGPA frequently affects the peripheral nervous system [1, 3]. The neuropathic features are usually characterized by mononeuritis multiplex, which reflects the locality of the lesions [13–17]. Findings suggestive of vasculitis are usually found in the epineurium and observed diffusely throughout the nerve trunk [28]. Nerve fiber degeneration resulting from ischemia is sometimes focal or asymmetric and tends to become conspicuous at the middle portion of the nerve trunk [28]. The attachment of neutrophils to endothelial cells in the epineurial vessels is frequently observed in nerve biopsy specimens from patients with ANCA-associated vasculitis [16]. This finding is scarce or absent in patients with nonsystemic vasculitic neuropathy who are negative for ANCA; hence, primed neutrophils expressing the target antigens of ANCA on their surfaces might play an important role in vascular inflammation by binding to ANCA in patients with ANCA-associated vasculitis [43, 44]. The positivity rate in patients with EGPA is lower than that in patients with MPA and GPA. Moreover, intravascular and tissue eosinophils are abundant, particularly in patients negative for ANCA, suggesting that eosinophils also participate in the tissue damage process [13].
The general approach to immunotherapy of ANCA-associated vasculitis consists of the induction and maintenance of remission [55]. A combination of glucocorticoids and cyclophosphamide, rituximab, methotrexate, or mycophenolate mofetil is considered depending on the severity of the organ involvement for remission [58]. Furthermore, a combination of low-dose glucocorticoids and azathioprine, rituximab, methotrexate, or mycophenolate mofetil is recommended for the maintenance of remission [58]. The efficacy of anti-IL-5 therapy (i.e., mepolizumab) in suppressing the activation of eosinophils has been demonstrated in patients with refractory or relapsing EGPA [63]. Several other new agents, including avacopan, vilobelimab, and abatacept, are under development for the treatment of ANCA-associated vasculitis [67, 69].
Although neuropathic symptoms may significantly disrupt the day-to-day functioning and quality of life of patients with ANCA-associated vasculitis, an approach specific for neuropathy has not been fully established so far. Therefore, active participation of neurologists, in terms of research studies, is warranted.
Acknowledgements
Funding
This work was supported in part by the Health and Labour Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan (20FC1030) and JSPS KAKENHI (20K07882). No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Haruki Koike and Atsuro Chiba developed the concept of the article. Haruki Koike carried out the literature review. Haruki Koike wrote the first draft, and all authors critically evaluated the manuscript.
Disclosures
Haruki Koike is a member of the journal’s Editorial Board. Ryoji Nishi, Ken Ohyama, Saori Morozumi, Yuichi Kawagashira, Soma Furukawa, Naohiro Mouri, Yuki Fukami, Masahiro Iijima, Gen Sobue, and Masahisa Katsuno have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Collins MP, Dyck PJ, Gronseth GS, Guillevin L, Hadden RD, Heuss D, Léger JM, Notermans NC, Pollard JD, Said G, Sobue G, Vrancken AF, Kissel JT. Peripheral Nerve Society Peripheral Nerve Society Guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst. 2010;15(3):176–184. doi: 10.1111/j.1529-8027.2010.00281.x. [DOI] [PubMed] [Google Scholar]
- 3.Koike H, Sobue G. Clinicopathological features of neuropathy in anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Nephrol. 2013;17(5):683–685. doi: 10.1007/s10157-012-0767-3. [DOI] [PubMed] [Google Scholar]
- 4.Nishino H, Rubino FA, DeRemee RA, Swanson JW, Parisi JE. Neurological involvement in Wegener's granulomatosis: an analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol. 1993;33(1):4–9. doi: 10.1002/ana.410330103. [DOI] [PubMed] [Google Scholar]
- 5.Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine. 1999;78(1):26–37. doi: 10.1097/00005792-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Guillevin L, Durand-Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, Amouroux J, Casassus P, Jarrousse B. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42(3):421–430. doi: 10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Bischof A, Jaeger VK, Hadden RDM, Luqmani RA, Pröbstel AK, Merkel PA, Suppiah R, Craven A, Collins MP, Daikeler T. Peripheral neuropathy in antineutrophil cytoplasmic antibody-associated vasculitides: Insights from the DCVAS study. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e615. doi: 10.1212/NXI.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone JH; Wegener's Granulomatosis Etanercept Trial Research Group Limited versus severe Wegener's granulomatosis: baseline data on patients in the Wegener's granulomatosis etanercept trial. Arthritis Rheum. 2003;48(8):2299–2309. doi: 10.1002/art.11075. [DOI] [PubMed] [Google Scholar]
- 9.Sinico RA, Di Toma L, Maggiore U, Bottero P, Radice A, Tosoni C, Grasselli C, Pavone L, Gregorini G, Monti S, Frassi M, Vecchio F, Corace C, Venegoni E, Buzio C. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005;52(9):2926–2935. doi: 10.1002/art.21250. [DOI] [PubMed] [Google Scholar]
- 10.Sablé-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, Blockmans D, Cordier JF, Delaval P, Puechal X, Lauque D, Viallard JF, Zoulim A, Guillevin L. French Vasculitis Study Group. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005;143(9):632–638. doi: 10.7326/0003-4819-143-9-200511010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Healy B, Bibby S, Steele R, Weatherall M, Nelson H, Beasley R. Antineutrophil cytoplasmic autoantibodies and myeloperoxidase autoantibodies in clinical expression of Churg-Strauss syndrome. J Allergy Clin Immunol. 2013;131(2):571–576. doi: 10.1016/j.jaci.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, Maurier F, Jouneau S, Bienvenu B, Puéchal X, Aumaître O, Le Guenno G, Le Quellec A, Cevallos R, Fain O, Godeau B, Seror R, Dunogué B, Mahr A, Guilpain P, Cohen P, Aouba A, Mouthon L, Guillevin L. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270–281. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 13.Nishi R, Koike H, Ohyama K, Fukami Y, Ikeda S, Kawagashira Y, Iijima M, Katsuno M, Sobue G. Differential clinicopathologic features of EGPA-associated neuropathy with and without ANCA. Neurology. 2020;94(16):e1726–e1737. doi: 10.1212/WNL.0000000000009309. [DOI] [PubMed] [Google Scholar]
- 14.Hattori N, Mori K, Misu K, Koike H, Ichimura M, Sobue G. Mortality and morbidity in peripheral neuropathy associated Churg-Strauss syndrome and microscopic polyangiitis. J Rheumatol. 2002;29(7):1408–1414. [PubMed] [Google Scholar]
- 15.Sugiura M, Koike H, Iijima M, Mori K, Hattori N, Katsuno M, Tanaka F, Sobue G. Clinicopathologic features of nonsystemic vasculitic neuropathy and microscopic polyangiitis-associated neuropathy: a comparative study. J Neurol Sci. 2006;241(1–2):31–37. doi: 10.1016/j.jns.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Koike H, Ikeda S, Kawagashira Y, Iijima M, Hashizume A, Katsuno M, Sobue G. Distinct pathogenesis in nonsystemic vasculitic neuropathy and microscopic polyangiitis. Neurol Neuroimmunol Neuroinflamm. 2017;4(6):e407. doi: 10.1212/NXI.0000000000000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishi R, Koike H, Ohyama K, Fukami Y, Iijima M, Sobue G, Katsuno M. Association between IL-5 levels and the clinicopathologic features of eosinophilic granulomatosis with polyangiitis. Neurology. 2021;96(5):226–229. doi: 10.1212/WNL.0000000000011142. [DOI] [PubMed] [Google Scholar]
- 18.Hattori N, Ichimura M, Nagamatsu M, Li M, Yamamoto K, Kumazawa K, Mitsuma T, Sobue G. Clinicopathological features of Churg-Strauss syndrome-associated neuropathy. Brain. 1999;122(Pt 3):427–439. doi: 10.1093/brain/122.3.427. [DOI] [PubMed] [Google Scholar]
- 19.Gwathmey KG, Burns TM, Collins MP, Dyck PJ. Vasculitic neuropathies. Lancet Neurol. 2014;13(1):67–82. doi: 10.1016/S1474-4422(13)70236-9. [DOI] [PubMed] [Google Scholar]
- 20.Said G, Lacroix C. Primary and secondary vasculitic neuropathy. J Neurol. 2005;252(6):633–641. doi: 10.1007/s00415-005-0833-9. [DOI] [PubMed] [Google Scholar]
- 21.André R, Cottin V, Saraux JL, Blaison G, Bienvenu B, Cathebras P, Dhote R, Foucher A, Gil H, Lapoirie J, Launay D, Loustau V, Maurier F, Pertuiset E, Zénone T, Seebach J, Costedoat-Chalumeau N, Puéchal X, Mouthon L, Guillevin L, Terrier B. French Vasculitis Study Group (FVSG) Central nervous system involvement in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Report of 26 patients and review of the literature. Autoimmun Rev. 2017;16(9):963–969. doi: 10.1016/j.autrev.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M. Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol. 2019;10(9):1166. doi: 10.3389/fneur.2018.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989;18(5):671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- 24.Park HT, Kim JK, Tricaud N. The conceptual introduction of the "demyelinating Schwann cell" in peripheral demyelinating neuropathies. Glia. 2019;67(4):571–581. doi: 10.1002/glia.23509. [DOI] [PubMed] [Google Scholar]
- 25.Koike H, Nishi R, Ikeda S, Kawagashira Y, Iijima M, Katsuno M, Sobue G. Ultrastructural mechanisms of macrophage-induced demyelination in CIDP. Neurology. 2018;91(23):1051–1060. doi: 10.1212/WNL.0000000000006625. [DOI] [PubMed] [Google Scholar]
- 26.Koike H, Fukami Y, Nishi R, Kawagashira Y, Iijima M, Katsuno M, Sobue G. Ultrastructural mechanisms of macrophage-induced demyelination in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2020;91(6):650–659. doi: 10.1136/jnnp-2019-322479. [DOI] [PubMed] [Google Scholar]
- 27.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33(8):1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 28.Morozumi S, Koike H, Tomita M, Kawagashira Y, Iijima M, Katsuno M, Hattori N, Tanaka F, Sobue G. Spatial distribution of nerve fiber pathology and vasculitis in microscopic polyangiitis-associated neuropathy. J Neuropathol Exp Neurol. 2011;70(5):340–348. doi: 10.1097/NEN.0b013e3182172290. [DOI] [PubMed] [Google Scholar]
- 29.Dyck PJ, Conn DL, Okazaki H. Necrotizing angiopathic neuropathy. Three-dimensional morphology of fiber degeneration related to sites of occluded vessels. Mayo Clin Proc. 1972;47(7):461–475. [PubMed] [Google Scholar]
- 30.Nukada H, Dyck PJ. Microsphere embolization of nerve capillaries and fiber degeneration. Am J Pathol. 1984;115(2):275–287. [PMC free article] [PubMed] [Google Scholar]
- 31.Sladky JT, Greenberg JH, Brown MJ. Regional perfusion in normal and ischemic rat sciatic nerves. Ann Neurol. 1985;17(2):191–195. doi: 10.1002/ana.410170215. [DOI] [PubMed] [Google Scholar]
- 32.Nukada H, van Rij AM, Packer SG, McMorran PD. Pathology of acute and chronic ischaemic neuropathy in atherosclerotic peripheral vascular disease. Brain. 1996;119(Pt 5):1449–1460. doi: 10.1093/brain/119.5.1449. [DOI] [PubMed] [Google Scholar]
- 33.Radice A, Bianchi L, Sinico RA. Anti-neutrophil cytoplasmic autoantibodies: methodological aspects and clinical significance in systemic vasculitis. Autoimmun Rev. 2013;12(4):487–495. doi: 10.1016/j.autrev.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110(7):955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106(6):2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- 36.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huugen D, van Esch A, Xiao H, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71(7):646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 38.Chen M, Jayne DRW, Zhao MH. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol. 2017;13(6):359–367. doi: 10.1038/nrneph.2017.37. [DOI] [PubMed] [Google Scholar]
- 39.Hilhorst M, van Paassen P, van Rie H, Bijnens N, Heerings-Rewinkel P, van Breda VP, Cohen Tervaert JW. Limburg renal registry complement in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2017;32(8):1302–1313. doi: 10.1093/ndt/gfv288. [DOI] [PubMed] [Google Scholar]
- 40.Moiseev S, Lee JM, Zykova A, Bulanov N, Novikov P, Gitel E, Bulanova M, Safonova E, Shin JI, Kronbichler A, Jayne DRW. The alternative complement pathway in ANCA-associated vasculitis: further evidence and a meta-analysis. Clin Exp Immunol. 2020;202(3):394–402. doi: 10.1111/cei.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20(2):289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dyck PJ, Benstead TJ, Conn DL, Stevens JC, Windebank AJ, Low PA. Nonsystemic vasculitic neuropathy. Brain. 1987;110(Pt 4):843–853. doi: 10.1093/brain/110.4.843. [DOI] [PubMed] [Google Scholar]
- 43.Radford DJ, Luu NT, Hewins P, Nash GB, Savage CO. Antineutrophil cytoplasmic antibodies stabilize adhesion and promote migration of flowing neutrophils on endothelial cells. Arthritis Rheum. 2001;44(12):2851–2861. doi: 10.1002/1529-0131(200112)44:12<2851::aid-art473>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura H, Mii A, Shoji J, Arakawa Y, Shimizu A. Immunohistochemical detection of citrullinated histone H3-positive neutrophils is useful for identifying active glomerular and interstitial lesions in antineutrophil cytoplasmic antibody-associated vasculitis. Histopathology. 2021;78(4):520–531. doi: 10.1111/his.14247. [DOI] [PubMed] [Google Scholar]
- 46.O'Sullivan KM, Holdsworth SR. Neutrophil extracellular traps: a potential therapeutic target in MPO-ANCA associated vasculitis? Front Immunol. 2021;12:635188. doi: 10.3389/fimmu.2021.635188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YM, Wang H, Wang C, Chen M, Zhao MH. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 2015;67(10):2780–2790. doi: 10.1002/art.39239. [DOI] [PubMed] [Google Scholar]
- 48.Saffari H, Hoffman LH, Peterson KA, Fang JC, Leiferman KM, Pease LF, 3rd, Gleich GJ. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;133(6):1728–1734. doi: 10.1016/j.jaci.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17(12):746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukuchi M, Miyabe Y, Furutani C, Saga T, Moritoki Y, Yamada T, Weller PF, Ueki S. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol Int. 2021;70(1):19–29. doi: 10.1016/j.alit.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuchi M, Kamide Y, Ueki S, Miyabe Y, Konno Y, Oka N, Takeuchi H, Koyota S, Hirokawa M, Yamada T, Melo RCN, Weller PF, Taniguchi M. Eosinophil ETosis-mediated release of galectin-10 in eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol. 2021;73(9):1683–1693. doi: 10.1002/art.41727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marx C, Novotny J, Salbeck D, Zellner KR, Nicolai L, Pekayvaz K, Kilani B, Stockhausen S, Bürgener N, Kupka D, Stocker TJ, Weckbach LT, Pircher J, Moser M, Joner M, Desmet W, Adriaenssens T, Neumann FJ, Gerschlick AH, Ten Berg JM, Lorenz M, Stark K. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134(21):1859–1872. doi: 10.1182/blood.2019000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bettiol A, Sinico RA, Schiavon F, Monti S, Bozzolo EP, Franceschini F, Govoni M, Lunardi C, Guida G, Lopalco G, Paolazzi G, Vacca A, Gregorini G, Leccese P, Piga M, Conti F, Fraticelli P, Quartuccio L, Alberici F, Salvarani C, Bettio S, Negrini S, Selmi C, Sciascia S, Moroni G, Colla L, Manno C, Urban ML, Vannacci A, Pozzi MR, Fabbrini P, Polti S, Felicetti M, Marchi MR, Padoan R, Delvino P, Caporali R, Montecucco C, Dagna L, Cariddi A, Toniati P, Tamanini S, Furini F, Bortoluzzi A, Tinazzi E, Delfino L, Badiu I, Rolla G, Venerito V, Iannone F, Berti A, Bortolotti R, Racanelli V, Jeannin G, Padula A, Cauli A, Priori R, Gabrielli A, Bond M, Tedesco M, Pazzola G, Tomietto P, Pellecchio M, Marvisi C, Maritati F, Palmisano A, Dejaco C, Willeit J, Kiechl S, Olivotto I, Willeit P, Prisco D, Vaglio A, Emmi G. Italian EGPA Consortium Risk of acute arterial and venous thromboembolic events in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) Eur Respir J. 2021;57(5):2004158. doi: 10.1183/13993003.04158-2020. [DOI] [PubMed] [Google Scholar]
- 54.Walton EW. Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis) Br Med J. 1958;2(5091):265–270. doi: 10.1136/bmj.2.5091.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace ZS, Miloslavsky EM. Management of ANCA associated vasculitis. BMJ. 2020;368:m421. doi: 10.1136/bmj.m421. [DOI] [PubMed] [Google Scholar]
- 56.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA. American Thoracic Society Fungal Working Group An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183(1):96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 57.Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017 American College of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–1537. doi: 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 58.Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, Hellmich B, Holle JU, Laudien M, Little MA, Luqmani RA, Mahr A, Merkel PA, Mills J, Mooney J, Segelmark M, Tesar V, Westman K, Vaglio A, Yalçındağ N, Jayne DR, Mukhtyar C. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 59.Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, Hawley CM, Khalidi N, Floßmann O, Wald R, Girard LP, Levin A, Gregorini G, Harper L, Clark WF, Pagnoux C, Specks U, Smyth L, Tesar V, Ito-Ihara T, de Zoysa JR, Szczeklik W, Flores-Suárez LF, Carette S, Guillevin L, Pusey CD, Casian AL, Brezina B, Mazzetti A, McAlear CA, Broadhurst E, Reidlinger D, Mehta S, Ives N, Jayne DRW. PEXIVAS Investigators plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–631. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, Hauser T, Neumann I, Tesar V, Wissing KM, Pagnoux C, Schmitt W, Jayne DR. European Vasculitis Study Group (EUVAS) Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304(21):2381–2388. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 61.Tieu J, Smith R, Basu N, Brogan P, D'Cruz D, Dhaun N, Flossmann O, Harper L, Jones RB, Lanyon PC, Luqmani RA, McAdoo SP, Mukhtyar C, Pearce FA, Pusey CD, Robson JC, Salama AD, Smyth L, Watts RA, Willcocks LC, Jayne DRW. Rituximab for maintenance of remission in ANCA-associated vasculitis: expert consensus guidelines. Rheumatology (Oxford) 2020;59(4):e24–e32. doi: 10.1093/rheumatology/kez640. [DOI] [PubMed] [Google Scholar]
- 62.Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int. 2020;69(2):178–186. doi: 10.1016/j.alit.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, Merkel PA, Moosig F, Specks U, Cid MC, Luqmani R, Brown J, Mallett S, Philipson R, Yancey SW, Steinfeld J, Weller PF, Gleich GJ. EGPA mepolizumab study team mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bettiol A, Urban ML, Dagna L, Cottin V, Franceschini F, Del Giacco S, Schiavon F, Neumann T, Lopalco G, Novikov P, Baldini C, Lombardi C, Berti A, Alberici F, Folci M, Negrini S, Sinico RA, Quartuccio L, Lunardi C, Parronchi P, Moosig F, Espígol-Frigolé G, Schroeder J, Kernder AL, Monti S, Silvagni E, Crimi C, Cinetto F, Fraticelli P, Roccatello D, Vacca A, Mohammad AJ, Hellmich B, Samson M, Bargagli E, Cohen Tervaert JW, Ribi C, Fiori D, Bello F, Fagni F, Moroni L, Ramirez GA, Nasser M, Marvisi C, Toniati P, Firinu D, Padoan R, Egan A, Seeliger B, Iannone F, Salvarani C, Jayne D, Prisco D, Vaglio A, Emmi G. European EGPA Study Group mepolizumab for eosinophilic granulomatosis with polyangiitis (EGPA): a European multicenter observational study. Arthritis Rheumatol. 2021 doi: 10.1002/art.41943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan JA, Dehghan N, Chen W, Xie H, Esdaile JM, Avina-Zubieta JA. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann Rheum Dis. 2017;76(9):1566–1574. doi: 10.1136/annrheumdis-2016-210942. [DOI] [PubMed] [Google Scholar]
- 66.Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, Jayne D, Mahr A, Westman K, Luqmani R. Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: long-term data from the European Vasculitis Study Group trials. Rheumatology (Oxford) 2015;54(3):471–481. doi: 10.1093/rheumatology/keu366. [DOI] [PubMed] [Google Scholar]
- 67.Jayne DRW, Merkel PA, Schall TJ, Bekker P. ADVOCATE Study Group Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384(7):599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 68.Vlaar APJ, de Bruin S, Busch M, Timmermans SAMEG, van Zeggeren IE, Koning R, Ter Horst L, Bulle EB, van Baarle FEHP, van de Poll MCG, Kemper EM, van der Horst ICC, Schultz MJ, Horn J, Paulus F, Bos LD, Wiersinga WJ, Witzenrath M, Rueckinger S, Pilz K, Brouwer MC, Guo RF, Heunks L, van Paassen P, Riedemann NC, van de Beek D. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020;2(12):e764–e773. doi: 10.1016/S2665-9913(20)30341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prendecki M, McAdoo SP. New therapeutic targets in antineutrophil cytoplasm antibody-associated vasculitis. Arthritis Rheumatol. 2021;73(3):361–370. doi: 10.1002/art.41407. [DOI] [PubMed] [Google Scholar]
- 70.Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5(3):185–186. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 71.Langford CA, Monach PA, Specks U, Seo P, Cuthbertson D, McAlear CA, Ytterberg SR, Hoffman GS, Krischer JP, Merkel PA. Vasculitis Clinical Research Consortium An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener's) Ann Rheum Dis. 2014;73(7):1376–1379. doi: 10.1136/annrheumdis-2013-204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collins MP, Mendell JR, Periquet MI, Sahenk Z, Amato AA, Gronseth GS, Barohn RJ, Jackson CE, Kissel JT. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology. 2000;55(5):636–643. doi: 10.1212/wnl.55.5.636. [DOI] [PubMed] [Google Scholar]
- 73.Masuda H, Misawa S, Arai K, Oide T, Shibuya K, Isose S, Sekiguchi Y, Nasu S, Mitsuma S, Kuwabara S. Combined nerve/muscle/skin biopsy could increase diagnostic sensitivity for vasculitic neuropathy. Clin Exp Neuroimmunol. 2015;6:312–317. [Google Scholar]
- 74.Suppiah R, Hadden RD, Batra R, Arden NK, Collins MP, Guillevin L, Jayne DR, Luqmani RA. European Vasculitis Study Group Peripheral neuropathy in ANCA-associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology (Oxford) 2011;50(12):2214–2222. doi: 10.1093/rheumatology/ker266. [DOI] [PubMed] [Google Scholar]
- 75.Koike H, Akiyama K, Saito T, Sobue G. Research Group for IVIg for EGPA/CSS in Japan Intravenous immunoglobulin for chronic residual peripheral neuropathy in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a multicenter, double-blind trial. J Neurol. 2015;262(3):752–759. doi: 10.1007/s00415-014-7618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.