Abstract
Ichthyosis refers to a comparatively rare group of skin disorders which may present with associated cardiomyopathy. We report a case of an 11-year-old female child who presented with ichthyosis and associated dilated cardiomyopathy. Genetic testing revealed mutation in the RBCK1 gene. She was successfully managed with heart transplantation. The purpose of the case report is to embark on the association between the skin and heart, the role of desmosomes, and the cutaneous manifestations of life-threatening cardiac disease. Cutaneous manifestations should not be escaped, as some of which could be a marker for sudden cardiac death and appropriate corrective actions can potentially save life.
Keywords: Dilated cardiomyopathy, Ichthyosis, Cardiac transplantation, Desmosomes
Introduction
The ichthyoses encompass a heterogeneous group of skin disorders associated with a characteristic clinical manifestation of localized and/or generalized scaling [1]. There are various types and syndromes associated with it. The severity of ichthyosis varies from its most common type (ichthyosis vulgaris) up to its life-threatening form (harlequin-type ichthyosis) [2]. The incidence of ichthyosis vulgaris is estimated to be 1 in 250 births. This disease can involve other organs including the skeletal, nervous, endocrine, and cardiovascular systems, depending on the mutation of a specific gene. Advanced next-generation sequencing technology has ensured a more rapid and cost-effective genetic analysis of ichthyosis. Retinoids have been used in the treatment of ichthyosis [3]. Dilated cardiomyopathy which is characterized by ventricular dilatation and decreased systolic contraction is the leading cause of pediatric heart transplantation [4]. Very scant reports are available on heart transplantation for ichthyosis-associated dilated cardiomyopathy [5]. We report a case of ichthyosis-associated dilated cardiomyopathy requiring cardiac transplantation.
Case report
An 11-year-old female child was referred for management of dilated cardiomyopathy with severe bi-ventricular dysfunction. She developed abdominal pain, swelling of lower limbs, and breathlessness on minimal exertion 4 and a half months back, for which she was tested and found to have developed idiopathic dilated cardiomyopathy. The skin of the child who was a known case of ichthyosis vulgaris appeared dry and there was mild scaling (Fig. 1a). The younger sibling who is now 2 years old was born with a severe form of ichthyosis (Fig. 1b) and is currently on symptomatic and supportive treatment with normal cardiac function. There was no history of a similar condition in any of the other immediate or extended family members. When she presented to us, she weighed 20.7 kg with a height of 130 cm and a body surface area (BSA) of 0.86 m2. Her electrocardiogram (ECG) was normal. Her left ventricular ejection fraction (LVEF) was 20%, with severe left ventricular systolic dysfunction, moderate mitral regurgitation, and pulmonary artery hypertension. Her cardiac catheterization study revealed central venous pressure (CVP) of 18 mm of Hg, PA (pulmonary arterial) pressure of 60/39 with a mean of 49 mm of Hg and wedge pressure of 40 mm of Hg. B-type natriuretic peptide (BNP) was 7358 pg/ml. She was offered cardiac transplantation and the child was registered in-state transplantation registry.
Fig. 1.
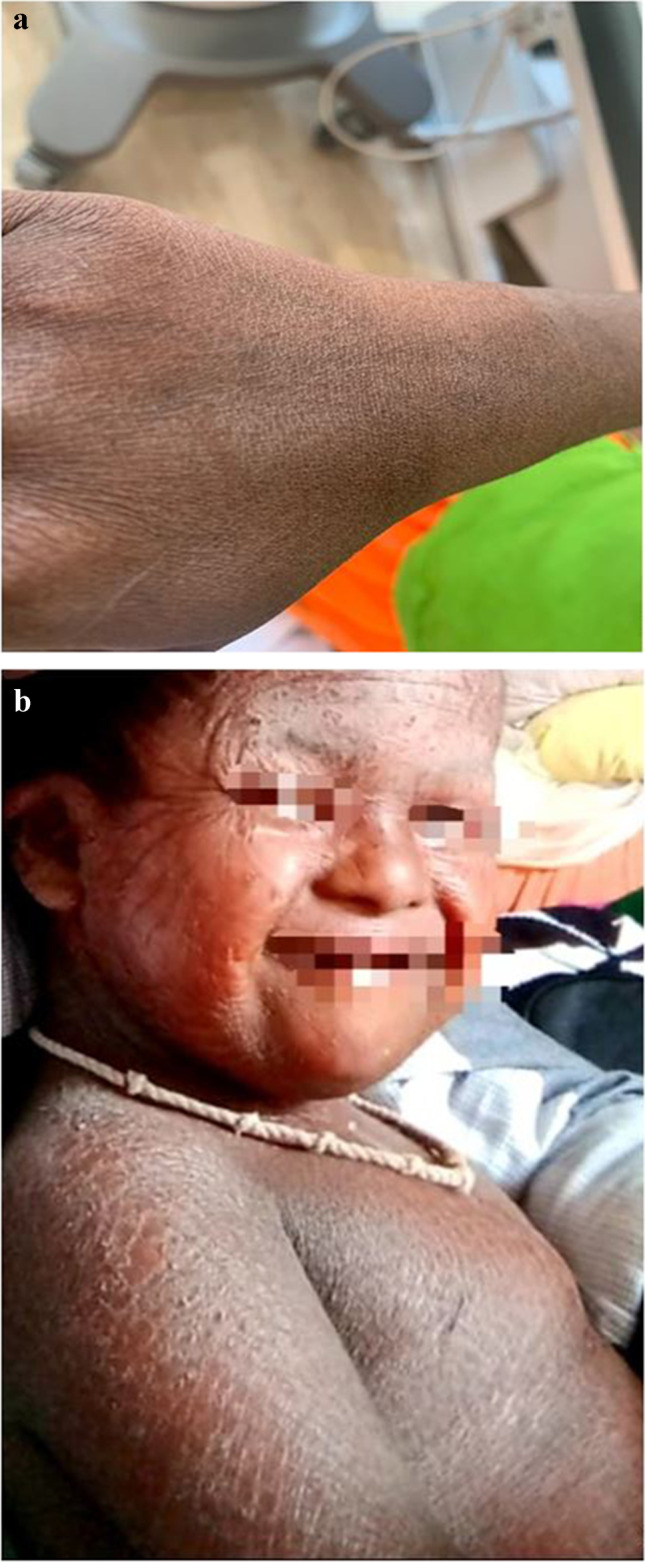
a Depicting dry skin of the patient with mild scaling. b Showing the skin of the patient’s younger sibling
Within 4 weeks, the child got a heart from a 17-year-old, 55-kg male donor. Donor heart harvesting was executed using histidine-tryptophan-glutarate (HTK) custodiol cardioplegia solution. The cold ischemic time was 210 min and transplantation was done using bicaval transplantation technique (BC). The recipient’s heart was grossly dysfunctional with biventricular dilatation. Histological examination of dilated cardiomyopathy of the explanted heart revealed variations in myocyte size, myocyte vacuolation, and fibrosis; increased myocyte nuclei size; and increased lymphocytes in the interstitial spaces (Hematoxylin Eosin stain, × 40). The intra-operative and postoperative courses were uneventful. Dry and scaly skin was not challenging in putting cannulation, catheterization, or post-operative wound healing. Posttransplantation period was uneventful and she was discharged after 3 weeks following endomyocardial biopsy which was negative for rejection.
Discussion
The skin and heart, though apparently disparate organs, have some ultrastructural and embryonic commonality which can partly explain some of the clinical findings seen in cardio-cutaneous syndromes. Hoeger and colleagues reported two children with ichthyosiform erythroderma associated with dilated cardiomyopathy, which was fatal in one and required heart transplantation in the other [5]. Skin and heart both face shear stress and desmosomes are the structures found in the junction which help them to adapt to these stresses and maintain structural integrity. Desmosomes meaning in Greek—Desmo means ‘bond’ and soma means ‘body’. The desmosomes present in the skin and heart are ultrastructurally similar and are made mainly of desmoplakin and plakoglobin. The desmosomes are complex structures and are made of the following:
The connector proteins to the intracellular cytoskeleton mainly the keratin
The Cadherin family of proteins which span the cell membranes
Desmocollin and desmoglein which function as extracellular linkage proteins
Mutations in the same genes can result in different phenotypes depending on several factors depending on the location, and the effect on the regulators, transcription factors and binding sites, and the interaction with environmental signals. Mutations of desmosomal components can lead to skin and heart disease, especially those associated with increased skin fragility including, ectodermal dysplasia syndrome, congenital epidermolysis bullosa, striate palmoplantar keratoderma, hypotrichosis, and wooly hair with arrhythmogenic cardiomyopathy [6].
It was not possible to enlist all the known cardio-cutaneous disorders in one article. Thus, some of the cardiocutaneous syndromes with associated plausible genes involved have been mentioned in Table 1.
Table 1.
Depicting genes involved in some of the cardiocutaneous syndromes
| Cardiocutaneous syndrome | Genetic mutations involved |
|---|---|
| Carney complex | CNC1, PRKAR1A, CNC2 |
| Costello syndrome (CS) | HRAS proto-oncogene on chromosome 11p13.3 |
| Cardiofaciocutaneous syndrome (CFCS) | BRAF, MEK 1, MEK 2 and KRAS encoding proteins downstream of RAS |
| Ellis van Creveld syndrome (EVC) | EVC1 and EVC2 genes on chromosome 4p16 |
| The H syndrome | SLC29A3 |
| Leopard syndrome (LS) | PTPN11 gene on chromosome 12q24.1 |
| Noonan syndrome (NS) | PTPN11, SOS1, RAF1, KRAS, NRAS and BRAF genes |
|
Pseudoxanthoma elasticum (PXE) Gröenblad-Strandberg syndrome |
ABCC6 (ATP-binding cassette subfamily C member 6) gene, on chromosome 16p |
| Naxos–Carvajal syndromes | Mutations in the plakoglobin and desmoplakin genes, respectively, on chromosome 17q21 |
| Ehlers-Danlos syndromes (EDS) | Collagen type V has been found to be mutated in the classic subtype (COL5A1 more often than COL5A2), |
| Marfan syndrome (MFS) | FBN1 gene encodes fibrillin-1 |
| Cutis laxa |
FBLN5 gene encoding fibulin-5 LTBP4 gene |
| CHIME syndrome | Mutations in the glycosylphosphatidylinositol gene PIGL Chromosome 17 |
| Refsum’s disease | Deficiency of Phytanoyl-coenzyme A hydroxylase |
| Turner’s syndrome | Monosomy X |
| Down syndrome | Trisomy 21 |
The common embryonic origin of cardiac and cutaneous melanocytes has recently been shown by using melanocyte-specific markers in atrial cells and atrioventricular endocardial cushions of the murine heart where they are < 0.1% of atrial cells. The cardiac melanocytes were found in anatomic locations from which atrial arrhythmias originate in humans including pulmonary veins, atrioventricular annulus, posterior left atrium, and foramen ovale. This could explain the existence of many syndromes that involve cardiac and melanocytic manifestations [7]. Additionally, the hazard of vitamin D inadequacy in managing patients with inherited ichthyosis has been discussed in the literature [8]. But, in our patient, the vitamin D levels and parathormone levels were within normal limits.
Massively parallel sequencing (next-generation sequencing) of our patient’s genomic deoxy ribonucleic acid (DNA) was executed. Paired-end sequencing was performed with 2 × 100/2 × 150 chemistry, on an Illumina platform. Genetic analysis revealed frameshift deletion in the RBCK1 gene on exon 5 near C-terminal which is known to be pathogenic and associated with cardiomyopathy. The frameshift deletion p.Q179Rfs*97 in RBCK1 (NM_031229.4) was detected which has not been reported previously as a pathogenic variant nor as a benign variant, to our knowledge. The patient was homozygous for truncating mutation in RBCK1. This variant is predicted to cause loss of normal protein function through protein truncation. The frame shifted sequence continues 97 residues until a stop codon is reached. For these reasons, this variant has been classified as likely pathogenic. Nilsson and colleagues also concluded that RBCK1 inadequacy is a frequent crusade of polyglucosan storage myopathy consociated with progressive muscle weakness and cardiomyopathy [9]. They described the requirement of heart transplantation in 4 out of 8 patients with rapidly progressive cardiomyopathy. Another study was documented among two cases carrying the same homozygous mutation in the middle part of the RBCK1 gene. These cases presented clinically with severe cardiomyopathy with additional immunological dysfunction [10]. RBCK1 inadequacy has been reported to exhibit cardio-cutaneous manifestations in the form of acute febrile neutrophilic dermatosis and psoriasis [9, 10]. The presentation of ichthyosis vulgaris with cardiomyopathy due to RBCK1 gene inadequacy is reported for the first time in our case.
Conclusion
It is important for clinicians to recognize cutaneous manifestations in a cardiac patient because some of which could be a marker for sudden cardiac death. The use of genetics in heart failure clinics should be promoted. Genetic testing can aid in identifying asymptomatic relatives who might be at risk of disease-related complications. Ongoing research on the importance of genetics in cardiomyopathy will facilitate improved clinical decision-making about the therapies offered. Further studies are warranted to establish such a different disease process.
Funding
None.
Declarations
Informed consent
Informed consent was obtained from the participant included in the study.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5:1497. [DOI] [PMC free article] [PubMed]
- 2.Okulicz JF, Schwartz RA. Hereditary and acquired ichthyosis vulgaris. Int J Dermatol. 2003;42:95–98. doi: 10.1046/j.1365-4362.2003.01308.x. [DOI] [PubMed] [Google Scholar]
- 3.Digiovanna JJ, Mauro T, Milstone LM, Schmuth M, Toro JR. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol Ther. 2013;26:26–38. doi: 10.1111/j.1529-8019.2012.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adwani SS, Whitehead BF, Rees PG, et al. Heart transplantation for dilated cardiomyopathy. Arch Dis Child. 1995;73:447–52. doi: 10.1136/adc.73.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeger PH, Adwani SS, Whitehead BF, Finlay AY, Harper JI. Ichthyosiform erythroderma and cardiomyopathy: report of two cases and review of the literature. Br J Dermatol. 1998;139:1055–9. doi: 10.1046/j.1365-2133.1998.02565.x. [DOI] [PubMed] [Google Scholar]
- 6.Kowalczyk AP, Green KJ. Structure, function and regulation of desmosomes. Prog Mol Biol Transl Sci. 2013;116:95–118. doi: 10.1016/B978-0-12-394311-8.00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brito FC, Kos L. Timeline and distribution of melanocyte precursors in the mouse heart. Pigment Cell Melanoma Res. 2008;21:464–470. doi: 10.1111/j.1755-148X.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 8.Frascari F, Dreyfus I, Rodriguez L, et al. Prevalence and risk factors of vitamin D deficiency in inherited ichthyosis: a French prospective observational study performed in a reference center. Orphanet J Rare Dis. 2014;9:127. doi: 10.1186/s13023-014-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson J, Schoser B, Laforet P, et al. Polyglucosan body myopathy caused by defective ubiquitin ligase RBCK1. Ann Neurol. 2013;74:914–919. [DOI] [PubMed]
- 10.Krenn M, Salzer E, Simonitsch-Klupp I, et al. Mutations outside the N-terminal part of RBCK1 may cause polyglucosan body myopathy with immunological dysfunction: expanding the genotype-phenotype spectrum. J Neurol. 2018;265:394–401. doi: 10.1007/s00415-017-8710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


