Abstract
The development and application of nucleic acid sequence-based amplification (NASBA) assays for the detection of West Nile (WN) and St. Louis encephalitis (SLE) viruses are reported. Two unique detection formats were developed for the NASBA assays: a postamplification detection step with a virus-specific internal capture probe and electrochemiluminescence (NASBA-ECL assay) and a real-time assay with 6-carboxyfluorescein-labeled virus-specific molecular beacon probes (NASBA-beacon assay). The sensitivities and specificities of these NASBA assays were compared to those of a newly described standard reverse transcription (RT)-PCR and TaqMan assays for SLE virus and to a previously published TaqMan assay for WN virus. The NASBA assays demonstrated exceptional sensitivities and specificities compared to those of virus isolation, the TaqMan assays, and standard RT-PCR, with the NASBA-beacon assay yielding results in less than 1 h. These assays should be of utility in the diagnostic laboratory to complement existing diagnostic testing methodologies and as a tool in conducting flavivirus surveillance in the United States.
West Nile (WN) and St. Louis encephalitis (SLE) viruses are arthropod-borne viruses (family Flaviviridae, genus Flavivirus) within the Japanese encephalitis virus serocomplex (15). As with other members of this complex, WN and SLE viruses possess a single-stranded plus-sense RNA genome of approximately 11,000 nucleotides. Both WN and SLE viruses circulate in natural transmission cycles involving primarily Culex species mosquitoes and birds; humans and other mammals are thought to be incidental hosts (14). Severe human disease caused by both WN and SLE viruses has been reported and is commonly associated with old age (14). Endemic SLE virus transmission in nature is silent, with no reports of avian mortality, whereas in the Western Hemisphere and Israel WN virus infections have been reported to cause high rates of mortality among domestic and wild birds as well as equines (9, 14).
Historically, WN virus has circulated primarily in Africa, Asia, southern Europe, and Australia and has been responsible for several significant epidemics, notably, in Israel (1950s), France (1962), South Africa (1974), and Romania (1996) (6, 17, 21). In 1999 and 2000, WN virus was responsible for epidemics and epizootics in the northeastern United States, in which there were human fatalities and extensive avian mortality (1, 3, 4, 11). SLE virus is endemic throughout the United States and has also been isolated from several South American countries (14). Over the past 70 years, SLE virus has been responsible for numerous epidemics throughout the United States; the largest occurred in 1975, with approximately 2,000 cases reported (14). The appropriate public health responses for both WN and SLE virus epidemics are identical and involve public education and mosquito control programs. In both instances, however, timely implementation of these interventions is critical to reduce the risk to humans; therefore, surveillance programs for WN and SLE viruses must ensure rapid detection of virus activity. Typical means of surveillance for these viruses have involved the testing of field-collected mosquitoes and, in the case of WN virus, the testing of dead birds for the presence of virus by isolation in cell culture. However, virus isolation followed by identification through immunofluorescence assays can take over a week to complete. TaqMan assays for the rapid detection of WN virus from mosquito pools and avian tissues have been described, but no such approach exists for SLE virus (10, 13, 18).
In the diagnostic laboratory, human WN and SLE virus infections can be inferred by immunoglobulin M (IgM) capture and IgG enzyme-linked immunosorbent assays (ELISAs); however, confirmation of the type of infecting virus is possible only by detection of a fourfold or greater rise in virus-specific neutralizing antibody titers in either cerebrospinal fluid (CSF) or serum by performing the plaque reduction neutralization assay (PRNT) with several flaviviruses (7, 13). Virus isolation in cell culture from CSF or serum has generally been unsuccessful, likely due to the low level and short-lived viremia associated with infections with these viruses (14, 20). Recently, several investigators have reported on TaqMan assays for the detection of WN virus from human CSF specimens for which cell culture assays were negative, suggesting that nucleic acid-based assays hold greater promise for the detection of these viruses in human specimens (2, 10).
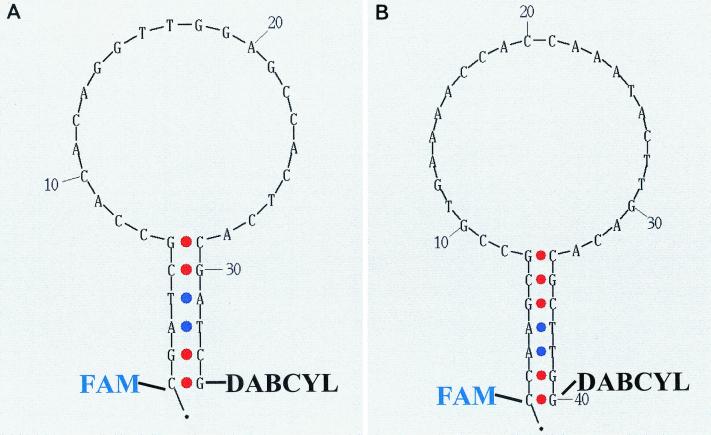
Nucleic acid sequence-based amplification (NASBA) is a robust amplification technology that has been used to detect a number of pathogens, including RNA viruses (5, 8, 12, 16, 19, 22). The amplification methodology involves the use of three enzymes, reverse transcriptase, T7 RNA polymerase, and RNase H; and the final amplification product is single-stranded RNA with a polarity opposite that of the target. The amplified RNA product can be detected through the use of a target-specific capture probe bound to magnetic particles in conjunction with a ruthenium-labeled detector probe and an instrument (NucliSens Reader; bioMérieux) capable of measuring electrochemiluminescence (ECL) (5). Alternatively, RNA amplified by NASBA can specifically be detected in real time through the use of molecular beacon probes included in the amplification reaction (16). Molecular beacon probes possess a 5′ fluorescent dye and a 3′ quencher molecule (typically, 4-dimethylaminophenylazobenzoyl [DABCYL]) and are designed to form stem-loop structures that bring into close proximity the 5′ and 3′ ends of the probe, resulting in minimal fluorescence (Fig. 1). In the presence of a complementary target sequence, the probe will hybridize to the target, separating the reporter dye from the quencher, resulting in a measurable increase in fluorescence.
FIG. 1.
Primary nucleotide sequence and predicted secondary structure of the WN (A) and SLE (B) virus molecular beacon probes. The folding algorithm used to generate the figure was designed by D. Stewart and M. Zucker, and the software program is available at the Zucker Group's website (RNA mfold; http://bioinfo.math.rpi.edu/∼zukerm/rna/).
We report here on the development of NASBA assays for the detection of WN and SLE viruses that use both ECL and molecular beacon detection technologies. We compared the sensitivities and specificities of these assays to those of virus isolation, TaqMan assay, and standard reverse transcription (RT)-PCR. These newly developed NASBA assays display sensitivities similar to or even greater than the sensitivity of our previously developed TaqMan assay. In addition, the NASBA assays provide a more rapid means of amplification and detection, with positive results available in less than 1 h.
MATERIALS AND METHODS
Virus strains.
All virus strains were obtained from the reference collection maintained at the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention (CDC). WN virus strain NY99 (flamingo 382-99) and SLE virus strain TBH 28 were titrated in Vero cells by a standard plaque assay.
RNA extraction.
Viral RNA was isolated from virus seeds, mosquito pools, homogenized avian tissues, and human CSF by using the QIAamp viral RNA kit (Qiagen, Valencia, Calif.). Mosquito pools and avian tissues were first homogenized as described previously (10), and total RNA was extracted from 100 μl (virus seeds) or 140 μl (mosquito and avian samples). RNA was eluted from the Qiagen columns in a final volume of 100 μl of elution buffer and was stored at −70°C until use.
Primer design.
The WN virus RT-PCR and the TaqMan assay primer-probe design methodology and sequences have been published previously (10). SLE virus RT-PCR primers were designed for the present study by using the PrimerSelect software program (DNASTAR Inc., Madison, Wis.) and the published sequence of the Mississippi 1975 SLE strain-MSI.7 (GenBank accession number M16614). The sequences of the PrimerSelect-derived primer pairs were compared to an alignment of 13 SLE virus structural region sequences, and two primer pairs that demonstrated maximum homology to all SLE virus strains were selected (Table 1). The two SLE virus-specific RT-PCR primer pairs performed equally in the sensitivity and specificity experiments; therefore, only data for primer pair 727c-1119c are shown (Table 2). The SLE virus-specific TaqMan assay primers and probe were designed with the PrimerExpress software package (PE Applied Biosystems, Foster City, Calif.), and the selection of the two primer-probe sets was based upon homology to aligned SLE virus structural region sequences as described above. Both SLE virus-specific TaqMan assay primer-probe sets performed equivalently, and only data for the 834-905c set are shown. The SLE virus-specific TaqMan assay probes were 5′ labeled with the reporter dye 6-carboxyfluorescein (FAM) and labeled at the 3′ end with the quencher dye 6-carboxytetramethylrhodamine (TAMRA). WN virus- and SLE virus-specific primers and probes for the NASBA assays were designed by following the primer design guidelines described in the NucliSens Basic Kit Application Manual (bioMérieux, Durham, N.C.). The reverse primers for the NASBA assays incorporate the T7 promoter sequence at the 5′ end of the primer, and the forward primers contain a generic capture sequence complementary to the ruthenium-labeled detection probe (generic ECL probe) at the 5′ end of the primer (Table 1). The virus-specific capture probes for the NASBA-ECL assay were labeled with biotin at the 5′ end and were immobilized onto avidin-coated magnetic particles by following the protocol described in the NucliSens Basic Kit Application Manual. Molecular beacon probes for the NASBA assays were designed with the help of the NucliSens Basic Kit World Wide Web-hosted help desk. The virus-specific capture probes for the NASBA-ECL assays were flanked with a sequence of seven (SLE virus) or six (WN virus) nucleotides capable of forming a self-complementary stem, such that the beacon probes would assume a stem-loop structure (Fig. 1). Molecular beacon probes were synthesized with a FAM fluorophore label at the 5′ end and a DABCYL molecule as a quencher at the 3′ end.
TABLE 1.
Oligonucleotide primers and probes in the NASBA, RT-PCR, and TaqMan assaysa
| Primer | Genome position | Sequence (5′-3′) | Product size (bp) |
|---|---|---|---|
| SLE virus-specific standard RT-PCR primers | |||
| SLE727 | 727–750b | GTAGCCGACGGTCAATCTCTGTGC | 393 |
| SLE1119c | 1119–1096 | ACTCGGTAGCCTCCATCTTCATCA | |
| SLE1637 | 1637–1659 | GACGAGCCCTGCCACAACTGATT | 495 |
| SLE2131c | 2131–2108 | GTGCCTCTTCCGACGACGATGTAA | |
| SLE virus-specific TaqMan assay primers and probes | |||
| SLE2420 | 2420–2439 | CTGGCTGTCGGAGGGATTCT | 68 |
| SLE2487c | 2487–2468 | TAGGTCAATTGCACATCCCG | |
| SLE2444-probe | 2444–2466 | TCTGGCGACCAGCGTGCAAGCCG | |
| SLE834 | 834–852 | GAAAACTGGGTTCTGCGCA | 72 |
| SLE905c | 905–889 | GTTGCTGCCTAGCATCCATCC | |
| SLE857-probe | 857–880 | TGGATATGCCCTAGTTGCGCTGGC | |
| SLE virus-specific NASBA assay primers and probes | |||
| SLE708 | 708–729 | gatgcaaggtcgcatatgag-CGCATGGGACATTCGAGGCGTAc | 234 |
| SLE941c | 941–919 | aattctaatacgactcactatagggagaagg-CATAAGCATGATCACAAAGACCA | |
| SLE802-ECL probe | 802–827 | CCGTGAAAACCACCAAATACTTGACA | |
| SLE802-Beacon probe | 802–827 | ccaagcg-CCGTGAAAACCACCAAATACTTGACA-cgcttgg | |
| WN virus-specific NASBA assay primers and probes | |||
| WN 1333 | 1333–1354 | gatgcaaggtcgcatatgag-ACCAAGGCAATAGGAAGAACCA | 162 |
| WN1494c | 1494–1472 | aattctaatacgactcactatagggagaagg-GTATGAAGGCGCCGCAGGAGTGA | |
| WN1432-ECL probe | 1432–1456 | TCCACACAGGTTGGAGCCACTCAGG | |
| WN1433-Beacon probe | 1433–1454 | cgatcg-CCACACAGGTTGGAGCCACTCA-cgatcg |
WN virus-specific standard RT-PCR and TaqMan assay primers have been published previously (10).
SLE virus-specific primer genome positions are according to SLE MSI.7 sequence in GenBank (accession number M16614).
NASBA forward primers have a 5′ ECL sequence (lowercase, bold, and italic); reverse primers have a T7 promoter sequence (lowercase, bold, and italic); beacon probes have stem sequences shown in lowercase-bold.
TABLE 2.
Sensitivities and specificities of the SLE virus NASBA assays compared to those of Vero cell culture, TaqMan assay, and standard RT-PCRa
| Sample | Quantity (no. of PFU) | RT-PCR with 727-1119c | TaqMan assayb
|
NASBA-ECL assayc
|
NASBA-beacon assayd
|
|||
|---|---|---|---|---|---|---|---|---|
| CT | Int. | ECL units | Int. | Beacon (min) | Int. | |||
| Titrated SLE virus (TBH 28) seede | ||||||||
| SLE-1 | 150,000 | POS | 13.2 | POS | 5,280,120 | POS | 17.5 | POS |
| SLE-2 | 15,000 | POS | 17.7 | POS | 4,437,851 | POS | 19.8 | POS |
| SLE-3 | 1,500 | POS | 20.6 | POS | 2,484,647 | POS | 23.7 | POS |
| SLE-4 | 150 | POS | 24.2 | POS | 2,199,690 | POS | 26.4 | POS |
| SLE-5 | 15 | POS | 28.1 | POS | 2,292,637 | POS | 27.5 | POS |
| SLE-6 | 1.5 | POS | 32.0 | POS | 625,447 | POS | 34.1 | POS |
| SLE-7 | 0.15 | POS | 36.5 | POS | 4,506 | POS | 59.0 | POS |
| SLE-8 | 0.015 | NEG | 38.7 | NEG | 260 | NEG | 90.0 | NEG |
| SLE-9 | 0.0015 | NEG | 45.0 | NEG | 118 | NEG | 90.0 | NEG |
| SLE-10 | 0.00015 | NEG | 45.0 | NEG | ND | NEG | ND | NEG |
| SLE virus strains | ||||||||
| Maryland 1975 | ND | POS | 12.3 | POS | 307,577 | POS | 11.3 | POS |
| Guatemala 1969 | ND | POS | 10.6 | POS | 18,995 | POS | 24.9 | POS |
| Panama 1973 | ND | POS | 20.2 | POS | 460,798 | POS | 15.0 | POS |
| Ecuador 1976 | ND | POS | 11.8 | POS | 381,882 | POS | 11.6 | POS |
| Texas 1966 | ND | POS | 16.7 | POS | 688,404 | POS | 9.9 | POS |
| Florida 1979 | ND | POS | 14.4 | POS | 1,209,079 | POS | 9.1 | POS |
| Illinois 1979 | ND | POS | 14.1 | POS | 1,154,617 | POS | 9.3 | POS |
| Mississippi 1975 | ND | POS | 14.2 | POS | 1,494,960 | POS | 8.4 | POS |
| California 1963 | ND | POS | 12.3 | POS | 530,166 | POS | 9.4 | POS |
| Texas 1999-29a | ND | POS | 25.2 | POS | 1,113,905 | POS | 9.1 | POS |
| Texas 1999-29b | ND | POS | 16.6 | POS | 37,173 | POS | 29.3 | POS |
| Texas 1999-30a | ND | POS | 23.4 | POS | 623,510 | POS | 10.7 | POS |
| Texas 1999-30b | ND | POS | 16.0 | POS | 338,634 | POS | 19.8 | POS |
| Other viruses | ||||||||
| EEE | ND | NEG | 45 | NEG | 99 | NEG | 90 | NEG |
| WEE | ND | NEG | 45 | NEG | 88 | NEG | 90 | NEG |
| VEE | ND | NEG | 45 | NEG | 114 | NEG | 90 | NEG |
| HJ | ND | NEG | 45 | NEG | 101 | NEG | 90 | NEG |
| LAC | ND | NEG | 45 | NEG | 85 | NEG | 90 | NEG |
| DEN-2 | ND | NEG | 45 | NEG | 101 | NEG | 90 | NEG |
| YF | ND | NEG | 45 | NEG | 104 | NEG | 90 | NEG |
| POW | ND | NEG | 45 | NEG | 106 | NEG | 90 | NEG |
| JE | ND | NEG | 45 | NEG | 73 | NEG | 90 | NEG |
| WNV | ND | NEG | 45 | NEG | 78 | NEG | 90 | NEG |
Abbreviations: POS, positive; NEG, negative; Int., interpretation; ND, not determined; EEE, eastern equine encephalitis virus; WEE, western equine encephalitis virus; VEE, Venezuelan equine encephalitis virus; HJ, Highlands J virus; LAC, La crosse virus; DEN-2, dengue virus type 2; YF, yellow fever virus, POW, Powassan virus; JE, Japanese encephalitis virus.
CT value of <37 was positive; for an explanation of TaqMan assay data interpretation, see Materials and Methods.
NASBA-ECL assay units >300 are interpreted as positive.
The values shown are the times at which fluorescence crosses the threshold; values <60 min are interpreted as positive. For a complete explanation of real-time NASBA-beacon assay data interpretation, see Materials and Methods.
All TaqMan CT, NASBA-ECL, and NASBA-beacon values were calculated by averaging the values for samples tested in duplicate.
RT-PCR and TaqMan assays.
Standard RT-PCRs were performed with the TITAN One-Tube RT-PCR kit (Roche Molecular Biochemicals, Indianapolis, Ind.) by using 5 μl of RNA and 50 pmol of each primer in a total reaction volume of 50 μl as described previously (10). After the RT-PCR was performed, a 5-μl portion was analyzed by agarose gel electrophoresis on a 3% NuSieve 3:1 agarose gel (FMC Bioproducts, Rockland, Maine), and the DNA was visualized by ethidium bromide staining. The TaqMan assays for WN and SLE viruses were performed as described previously with 5 μl of RNA per 50-μl reaction mixture by using the TaqMan RT-PCR Ready-Mix kit (PE Applied Biosystems) (10). The samples were subjected to 45 cycles of amplification in an ABI Prism 7700 sequence detection system instrument (PE Applied Biosystems) by the manufacturer's protocol for TaqMan RT-PCR cycling conditions. Positive results by the TaqMan assays were calculated by taking into account the real-time cycle number at which fluorescence increases above the threshold value (CT; threshold fixed at 0.1) and the relative increase in fluorescence (Rn) calculated by the end-point plate read function of the instrument. A sample was interpreted as positive if both the CT value was ≤37 and the Rn value was two or more times the average of the Rn values for the eight negative control wells. The results for samples that met one of the two criteria for positivity were interpreted as equivocal.
NASBA-ECL and NASBA-beacon assays.
All NASBA amplification reactions were performed with the NucliSens Basic Kit amplification reagents (bioMérieux). For the ECL detection assay, amplification reactions were set up by combining 5 μl of RNA with 50 pmol of each primer in a 10-μl amplification cocktail. The mixture was heated to 65°C for 5 min and then placed in a 41°C water bath for 5 min, followed by the addition of the enzyme mixture. After the 90-min amplification reaction, a 5-μl portion was removed and was diluted to 100 μl with the detection diluent (1:20 dilution) supplied with the NucliSens kit. The diluted amplification product was combined with the virus-specific capture probe bound to magnetic beads and the generic ECL detection probe, and the mixture was then incubated at 41°C for 30 min by the manufacturer's protocol. Samples were read in a NucliSens reader (bioMérieux), which detects the amplified RNA-capture probe complex through the electrochemiluminescence emitted by the bound generic ECL probe. The NucliSens reader calculates positive results on the basis of the values for the positive and negative controls included in the assay. For the real-time molecular beacon assay (NASBA-beacon assay), the NASBA reactions used the same amplification reagents described above, with the addition of the molecular beacon probe at a final concentration of 0.2 μM and 0.6 μM 5-6-carboxy-x-rhodamine as a reference dye. The amplification mixture was heated to 65°C for 5 min, followed by cooling to 41°C, and enzyme was added as described above. The amplification and real-time detection were performed at 41°C for 120 min in an ABI Prism 7700 sequence detection system instrument (PE Applied Biosystems). The results for the samples were interpreted as positive if they met the two criteria described in the interpretation of the TaqMan assay results, with the exception that time to positivity (Tp; in minutes) was used in the interpretation instead of CT values. Tp values ≤60 min were considered positive.
Avian tissues, mosquito pools, and clinical specimens.
Avian tissues and mosquito pool specimens that had been collected during the 1999 WN virus outbreak and previously tested for WN virus by virus isolation, RT-PCR, and TaqMan assays were coded for blind testing and tested by the WN virus NASBA assays. CSF specimens were obtained from patients presenting with fever and/or viral encephalitis during the time frame of the WN virus epidemic in New York State. Patient positivity for WN virus by serology was determined by a positive IgM capture ELISA and the presence of detectable WN virus-specific neutralizing antibody, as measured by the plaque reduction neutralization assay. These specimens were also previously tested by virus isolation and RT-PCR assays. No similar panel of field-collected or human specimens was available for testing by the SLE virus assays.
RESULTS
Sensitivities and specificities of NASBA assays.
To ascertain the detection limits of the WN and SLE virus NASBA assays, we tested 10-fold dilutions of seed viruses that had previously been quantitated by plaque titration. For comparison, these same virus seed dilutions were also tested by standard RT-PCR and TaqMan assays (Fig. 2; Tables 2 and 3). Both formats of the SLE NASBA assay (ECL and molecular beacon) detected less than 1 PFU of SLE virus (0.15 PFU), which was the same level of detection achieved in the standard RT-PCR and TaqMan assays (Table 2). The WN virus NASBA-ECL assay was 10-fold more sensitive than the NASBA-beacon assay and the TaqMan assay, detecting 0.01 PFU of WN virus, whereas the other assays detected 0.1 PFU of WN virus (Table 3).
FIG. 2.
NASBA amplification with real-time molecular beacon detection of dilutions of WN NY 1999 virus. The amplification plot was generated in an ABI Prism 7700 sequence detection system instrument (PE Applied Biosystems). The x axis is the time from the initiation of amplification; the y axis is the increase in fluorescence (ΔRn); threshold fluorescence is shown as the bold horizontal line. Tenfold virus dilutions (Table 3), ranging from 100,000 to 0.0001 PFU, were tested.
TABLE 3.
Sensitivities and specificities of WN virus NASBA assays compared to those of Vero cell culture, TaqMan assay, and standard RT-PCRa
| Sample | Quantity (no. of PFv) | RT-PCR with 233-640 | TaqMan assayb
|
NASBA-ECL assayc
|
NASBA-beacon assayd
|
|||
|---|---|---|---|---|---|---|---|---|
| CT | Int. | ECL units | Int. | Beacon (min) | Int. | |||
| Titrated WN virus (NY99) seede | ||||||||
| WNV-1 | 100,000 | POS | 17.9 | POS | 1,653,417 | POS | 15.2 | POS |
| WNV-2 | 10,000 | POS | 20.9 | POS | 1,187,613 | POS | 16.6 | POS |
| WNV-3 | 1,000 | POS | 24.2 | POS | 1,810,790 | POS | 20.2 | POS |
| WNV-4 | 100 | POS | 27.8 | POS | 1,666,084 | POS | 22.8 | POS |
| WNV-5 | 10 | POS | 31.2 | POS | 1,211,426 | POS | 28.0 | POS |
| WNV-6 | 1 | POS | 34.1 | POS | 1,209,491 | POS | 31.7 | POS |
| WNV-7 | 0.1 | NEG | 36.8 | POS | 326,954 | POS | 35.6 | POS |
| WNV-8 | 0.01 | NEG | 45.0 | NEG | 5,782 | POS | 90.0 | NEG |
| WNV-9 | 0.001 | NEG | 45.0 | NEG | 110 | NEG | 90.0 | NEG |
| WNV-10 | 0.0001 | NEG | 45.0 | NEG | 91 | NEG | ND | NEG |
| WN virus strains | ||||||||
| WNV-Romania-1996M | ND | POS | 29.02 | POS | 313,605 | POS | 32.8 | POS |
| WNV-Egypt-1951 | ND | POS | 25.54 | POS | 437,541 | POS | 19.0 | POS |
| WNV-Italy 1998 | ND | POS | 23.82 | POS | 237,753 | POS | 18.1 | POS |
| WNV-Kenya 1998 | ND | POS | 21.38 | POS | 226,175 | POS | 26.1 | POS |
| Kunjin | ND | POS | 20.58 | POS | 109 | NEG | 90 | NEG |
| Other viruses | ||||||||
| DEN-2 | ND | NEG | 45 | NEG | 27 | NEG | 90 | NEG |
| YF | ND | NEG | 45 | NEG | 8 | NEG | 90 | NEG |
| SLE | ND | NEG | 45 | NEG | 1 | NEG | 90 | NEG |
| JE | ND | NEG | 45 | NEG | 7 | NEG | 90 | NEG |
| MVE | ND | NEG | 45 | NEG | 2 | NEG | 90 | NEG |
| EEE | ND | NEG | 45 | NEG | 1 | NEG | 90 | NEG |
| WEE | ND | NEG | 45 | NEG | 12 | NEG | 90 | NEG |
| POW | ND | NEG | 45 | NEG | 29 | NEG | 90 | NEG |
| LAC | ND | NEG | 45 | NEG | 1 | NEG | 90 | NEG |
Abbreviations: POS, positive; NEG, negative; Int., interpretation; ND, not determined; DEN-2, dengue virus type 2; YF, yellow fever virus, JE, Japanese encephalitis virus; MVE, Murrey Valley encephalitis virus; EEE, equine encephalitis virus; WEE, western equine encephalitis virus; POW, Powassan virus; LAC, La Crosse virus.
A CT value of <37 was positive; for an explanation of TaqMan assay data interpretation, see Materials and Methods.
NASBA-ECL assay units >300 are interpreted as positive.
Values shown are the times at which fluorescence crosses the threshold; values <60 min are interpreted as positive. For a complete explanation of real-time NASBA-beacon assay data interpretation, see Materials and Methods.
All TaqMan CT, NASBA-ECL, and NASBA-beacon assay values were calculated by averaging the values from samples tested in duplicate.
The SLE virus-specific primer pairs shown in Table 1 were tested for their specificities by performing the NASBA, TaqMan, and RT-PCR assays with viral RNAs extracted from 13 geographically and temporally distinct SLE virus strains, including both North and South American isolates (Table 2). The primer pairs were also evaluated for their specificities by performing the assays with RNAs extracted from five serologically related flaviviruses (Japanese encephalitis, WN, dengue type 2, yellow fever, and Powassan viruses) and five arthropod-borne viruses that circulate in North and South America (eastern equine encephalitis, western equine encephalitis, Venezuelan equine encephalitis, Highlands J, and La Crosse viruses). All of the SLE virus-specific primer pairs were highly specific for SLE virus strains; they detected all of the SLE virus strains and yielded negative results for all of the arthropod-borne flaviviruses or other Western Hemisphere arthropod-borne viruses (Table 2). A similar strategy was used to evaluate the specificities of the WN virus-specific primers; the primers were tested by using RNAs extracted from various WN virus strains and other arthropod-borne viruses. The WN virus-specific primers used for the TaqMan and RT-PCR assays have previously been evaluated for their specificities, and the data are reproduced in Table 3 for comparison (10). Both NASBA assays for WN virus demonstrated a high degree of specificity for WN virus strains, detecting all WN strains tested (with one exception; see below) and yielding negative results for all other viruses tested. Kunjin virus was not detected by the primers used for the NASBA assays; however, these results are not unexpected. Kunjin virus, which has been detected only in Australia, is taxonomically classified as a subtype of WN virus, yet it demonstrates only 87% nucleotide identity with the WN virus strains that are circulating in the United States and Europe.
NASBA assay detection of WN virus in field-collected mosquito pools and avian tissues.
A coded panel of 68 specimens consisting of a random combination of mosquito pool specimens and avian tissues obtained from collections retrieved in New York and New Jersey during the 1999 WN epidemic-epizootic (September to November 1999) were tested by virus isolation in Vero cell culture and by the NASBA, TaqMan, and RT-PCR assays. Due to sample depletion, the NASBA-beacon assay was performed with only a subset of these samples (32 samples), with the results being identical to those obtained by the ECL detection assay. WN virus was isolated from 32 of the 68 samples (Table 4). All 32 of these culture-positive specimens were also positive by the NASBA assay. Two NASBA assay-positive specimens were culture negative, and these specimens had equivocal results by both the TaqMan and the RT-PCR assays, suggesting that these samples contained low levels of WN virus. The TaqMan assay detected WN virus RNA in 31 of the 32 culture-positive samples; the single TaqMan assay-negative, culture-positive specimen was also positive by the NASBA assay. As stated above, the two samples with equivocal results by the TaqMan assay also had equivocal results by RT-PCR and positive results by the NASBA assay. The RT-PCR assay detected WN virus RNA in 24 of the 32 culture-positive specimens. Five of the samples with equivocal results by RT-PCR (faint bands) were positive by all other methods.
TABLE 4.
Detection of WN virus in mosquito pools and avian tissues by Vero cell culture, NASBA assay, TaqMan assay, and RT-PCR
| Result | No. of samples
|
|||
|---|---|---|---|---|
| Vero cell culture | NASBA-ECL assaya | TaqMan assay | RT-PCR | |
| Positive | 32 | 34 | 31 | 24 |
| Negative | 36 | 34 | 35 | 37 |
| Equivocalb | 0 | 0 | 2 | 7 |
The real-time NASBA-beacon assay was performed with a subset (n = 32) of these coded specimens and yielded results identical to those of the NASBA-ECL assay; the remaining samples were not tested due to sample depletion.
Equivocal indicates that the samples satisfied only one of two required criteria for positive interpretation; for complete explanation of equivocal results, see Materials and Methods.
NASBA assay detection of WN virus in human specimens.
Twenty CSF specimens from patients classified as being either non-WN virus infected or confirmed to be infected with WN virus, as determined by serological testing (IgM ELISA and PRNT assay), were tested by the NASBA, TaqMan, and RT-PCR assays (Table 5). Virus isolation was performed with most of these specimens, and no WN virus was isolated (data not shown). Seven of the 10 CSF samples from patients with serologically confirmed WN virus infections were positive by the NASBA-ECL assay, and none of these were positive by RT-PCR (Table 5). Interestingly, three specimens had equivocal results by the TaqMan assay, but all of these specimens were positive by the NASBA assay. Finally, one specimen positive for WN virus by the TaqMan assay was negative by the NASBA assay.
TABLE 5.
Detection of WN virus in human CSF specimens by NASBA, TaqMan, and RT-PCR assaysa
| Sample no. | WN virus serologyb | NASBA-ECL assay result
|
TaqMan assay
|
RT-PCR result | ||
|---|---|---|---|---|---|---|
| ECL units | Interpretationc | CT value | Interpretationd | |||
| 1 | POS | 578,934 | POS | 35.7 | POS | NEG |
| 2 | POS | 65,745 | POS | 36.2 | POS | NEG |
| 3 | POS | 39 | NEG | 37 | POS | NEG |
| 4 | POS | 219,583 | POS | 38.3 | EQUIV | NEG |
| 5 | POS | 2,176 | POS | 39.3 | EQUIV | NEG |
| 6 | POS | 222,392 | POS | 45 | NEG | NEG |
| 7 | POS | 189,660 | POS | 39.67 | EQUIV | NEG |
| 8 | POS | 18,395 | POS | 35.2 | POS | NEG |
| 9 | POS | 35 | NEG | 45 | NEG | NEG |
| 10 | POS | 10 | NEG | 45 | NEG | NEG |
| 11–20 | All NEG | All <50 | All NEG | All 45 | All NEG | All NEG |
Abbreviations: POS, positive; NEG, negative; EQUIV, equivocal.
WN virus serology positive is defined as positivity by IgM and PRNT assay.
NASBA-ECL assay units >300 are interpreted as positive.
CT values <37 and Rn values greater than two times the average fluorescence for negative control samples is interpreted as a positive TaqMan assay result. For complete explanation of TaqMan assay: data interpretation, see Materials and Methods.
DISCUSSION
This report describes the development of NASBA assays for the rapid detection of WN and SLE viral RNAs. The NASBA assays used two formats for the detection of virus-specific amplification: either the postamplification ECL detection system or a real-time system with virus-specific molecular beacon probes. The NASBA assays demonstrated a level of detection similar to or greater than those of virus isolation and TaqMan assays. The NASBA assay for SLE virus, in both detection formats, was able to detect 0.15 PFU of SLE virus, the same level of detection achieved by the TaqMan and standard RT-PCR assays (Table 2). The NASBA-ECL assay for WN virus was consistently 10-fold more sensitive than either the TaqMan assay or the NASBA-beacon assay, detecting 0.01 PFU of WN virus (Table 3). The NASBA assays also demonstrated a high degree of specificity; no false-positive results were obtained with any of the serologically related flaviviruses tested or with any of the other domestic arthropod-borne viruses tested (Tables 2 and 3).
The NASBA assays for WN virus were able to detect WN virus in mosquito pools, avian tissue specimens, and human CSF specimens with sensitivities similar to or greater than that of a previously described TaqMan RT-PCR assay for WN virus (10). The NASBA-ECL assay for WN virus detected WN virus in two specimens that were Vero cell culture negative, and these two specimens had equivocal results by the TaqMan and RT-PCR assays. Our accumulated experience with the TaqMan assay for WN virus strongly suggests that in most cases specimens with equivocal results by the TaqMan assay actually possess low levels of viral RNA. We have consistently observed that equivocal results are reproducible with other primer-probe combinations and that increasing the cycle number reveals sustained amplification. In this instance, the results for two culture-negative, NASBA assay-positive specimens were equivocal by a TaqMan assay with an independent primer-probe set, suggesting that these samples had low levels of WN viral RNA rather than false-positive NASBA assay results. The NASBA assay for WN virus was also able to detect WN virus in three human CSF specimens that had equivocal results by the TaqMan assay. Again, it is likely that these samples had low levels of WN viral RNA rather than false-positive results. Alternatively, one TaqMan assay-positive CSF specimen (Table 5, sample 3) was NASBA assay negative. Unfortunately, these samples were depleted and no further testing by the NASBA or TaqMan assay was possible. The lack of a corresponding panel of human and/or field-collected specimens infected with SLE virus abrogated the ability to use the NASBA assays for SLE virus with real-world specimens. However, the sensitivity and specificity data generated with laboratory SLE virus-infected specimens give a clear indication that the test should perform similarly to the NASBA assay for WN virus for the detection of SLE virus in field-collected and clinical specimens.
The introduction of WN virus into the northeastern United States creates the distinct possibility that two serologically related flaviviruses will cocirculate in the same geographical region. Therefore, rapid and accurate surveillance assays for detection of these viruses are needed throughout the Western Hemisphere. Rapid detection of these viruses in field-collected specimens can accelerate appropriate public education and mosquito control measures that could prevent transmission and disease among humans. The ability of the NASBA assay to rapidly detect WN virus in human clinical specimens is also significant, given the nonspecificity of the IgM ELISA and the time required to serologically confirm WN virus infection by the PRNT assay. The difficulty in isolating WN virus from human specimens in tissue culture also necessitates the need for a reliable virus detection assay. The high cost of instrumentation capable of performing real-time TaqMan assays ($50,000 to $100,000) is, in many cases, prohibitive to the establishment of these assays in the clinical laboratory. The NASBA-ECL assay uses instrumentation that costs much less than TaqMan assay instruments (NASBA-ECL assay instruments are approximately $20,000), or alternatively, the NucliSens reader can also be leased from the manufacturer. In addition, in-house NASBA assays are easily developed with the NucliSens Basic Kit (bioMérieux), which contains standardized reagents for nucleic acid isolation, NASBA, and ECL detection. The NASBA-beacon assay can be performed with any instrument capable of maintaining a constant temperature while measuring fluorescence, and such instruments are also generally less costly than real-time TaqMan assay instruments (NASBA-beacon assay instruments cost approximately $20,000). As reported here, the NASBA-beacon assay can also be performed in real-time TaqMan assay instruments (i.e., the ABI Prism 7700 instrument or the Bio-Rad iCycler instrument). For laboratories that use TaqMan assays, the NASBA-beacon assay could thus be a primary or confirmatory test by a unique amplification method with the same instrument.
Of particular importance is the substantial reduction in time required for the confirmation of results by NASBA assays: less than 1 h. The NASBA-beacon assay data reveal that the NASBA assay is an inherently more rapid amplification technology than the TaqMan RT-PCR. The accumulation of amplified RNA, as detected by fluorescence values that exceed a threshold, can be detected as early as 14 min after the addition of enzyme, and for most samples amplification is essentially complete in approximately 45 min (Fig. 2). Taken together, the data reported here indicate that the NASBA assays are extremely rapid, highly sensitive, and specific and could be used along with TaqMan assays and/or virus isolation for a comprehensive WN virus detection system in the diagnostic laboratory.
ACKNOWLEDGMENTS
We thank Denise Martin, Alison Johnson, and Jason Velez for serological characterization of the human CSF samples used in this project; Roger Nasci, Marvin Godsey, Carl Mitchell, Harry Savage, Nicholas Komar, Nicholas Panella, Kristy Gottfried, and Chris Happ for mosquito pool and avian tissue preparation and Vero cell culture assay; Grant Campbell (CDC) and Marci Layton, Annie Fine, Dennis Nash, Alex Ramon, and Iqbal Poshni (all New York City Department of Health) for providing human specimens for testing; Brian Holloway (CDC) and the staff at the CDC Scientific Resources Program for assistance in designing the TaqMan assay primers and probes and for oligonucleotide synthesis; and Pierre van Aarle, Birgit Deiman, Lynell Grosso, and Mike Cronin (bioMérieux) for assistance in designing the NASBA-beacon assay probes and NASBA assay design.
REFERENCES
- 1.Anderson J F, Andreadis T G, Vossbrinck C R, Tirrell S, Wakem E M, French R A, Garmendia A E, Van Kruiningen H J. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science. 1999;286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 2.Briese T, Glass W G, Lipkin I W. Detection of West Nile virus sequences in cerebrospinal fluid. Lancet. 2000;355:1614–1615. doi: 10.1016/s0140-6736(00)02220-0. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Outbreak of West Nile-like viral encephalitis—New York, 1999. Morb Mortal Wkly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: West Nile virus activity—eastern United States, 2000. Morb Mortal Wkly Rep. 2000;49:1044–1047. [PubMed] [Google Scholar]
- 5.Chan A B, Fox J D. NASBA and other transcription-based amplification methods for research and diagnostic microbiology. Rev Med Microbiol. 1999;10:185–196. [Google Scholar]
- 6.Hayes C G. West Nile fever. In: Monath T P, editor. The arboviruses: epidemiology and ecology. V. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 59–88. [Google Scholar]
- 7.Johnson A J, Martin D A, Karabatsos N, Roehrig J T. Detection of antiarboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:1827–1831. doi: 10.1128/jcm.38.5.1827-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kievits T, van Gemen B, van Strijp D, Schukkink R, Dircks M, Adriaanse H, Malek L, Sooknanan R, Lens P. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods. 1991;35:273–286. doi: 10.1016/0166-0934(91)90069-c. [DOI] [PubMed] [Google Scholar]
- 9.Komar N. West Nile viral encephalitis. Rev Sci Tech Off Int Epiz. 2000;19:166–176. doi: 10.20506/rst.19.1.1201. [DOI] [PubMed] [Google Scholar]
- 10.Lanciotti R S, Kerst A J, Nasci R S, Godsey M S, Mitchell C J, Savage H M, Komar N, Panella N A, Allen B C, Volpe K E, Davis B S, Roehrig J T. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanciotti R S, Roehrig J T, Deubel V, Smith J, Parker M, Steele K, Volpe K E, Crabtree M B, Scherret J H, Hall R A, MacKenzie J S, Cropp C B, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage H M, Stone W, McNamara T, Gubler D J. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern U.S. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 12.Leone G, van Schijndel H, van Gemen B, Kramer F R, Schoen C D. Molecular beacon probes combined with amplification by NASBA enable homogeneous, real-time detection of RNA. Nucleic Acids Res. 1998;26:2150–2155. doi: 10.1093/nar/26.9.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin D A, Muth D A, Brown T, Johnson A J, Karabatsos N, Roehrig J T. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, editor. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 978–984. [Google Scholar]
- 15.Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D. Virus taxonomy, classification and nomenclature of viruses. Arch Virol. 1995;10(Suppl.):1–586. [Google Scholar]
- 16.Romano J W, van Gemen B, Kievits T. NASBA: a novel, isothermal detection technology for qualitative and quantitative HIV-1 RNA measurements. Clin Lab Med. 1996;16:89–103. [PubMed] [Google Scholar]
- 17.Savage H M, Ceianu C, Nicolescu G, Karabatsos N, Lanciotti R, Vladimirescu A, Laiv L, Ungureanu A, Romanca C, Tsai T F. Entomologic and avian investigations of an epidemic of West Nile fever in Romania, 1996, with serological and molecular characterization of a virus from mosquitoes. Am J Trop Med Hyg. 1999;61:600–611. doi: 10.4269/ajtmh.1999.61.600. [DOI] [PubMed] [Google Scholar]
- 18.Shi P, Kauffman E B, Ren P, Felton A, Tai J H, Dupuis A P, Jones S A, Ngo K A, Nicholas D C, Maffei J, Ebel G D, Bernard K A, Kramer L D. High-throughput detection of West Nile virus RNA. J Clin Microbiol. 2001;39:1264–1271. doi: 10.1128/JCM.39.4.1264-1271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sillikens P T. Qualitative and quantitative NASBA for detection of human immunodeficiency virus type 1 and hepatitis C virus infection. Transplant Proc. 1996;28:2941–2944. [PubMed] [Google Scholar]
- 20.Southam C M, Moore A E. Induced virus infections in man by the Egypt isolates of West Nile virus. Am J Trop Med Hyg. 1954;3:19–50. doi: 10.4269/ajtmh.1954.3.19. [DOI] [PubMed] [Google Scholar]
- 21.Tsai T F, Popovici F, Cernescu C, Campbell G L, Nedelcu N I. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 22.Van Gemen B, van Beuningen R, Nabbe A, van Strijp D, Jurriaans S, Lens P, Kievits T. A one-tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labeled probes. J Virol Methods. 1994;49:157–167. doi: 10.1016/0166-0934(94)90040-x. [DOI] [PubMed] [Google Scholar]