Abstract
Background
Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein expressed predominantly on the proximal tubular epithelium.
Objective
We wanted to see if there was a critical time for increased tubular damage and its related biomarker, KIM-1 mRNA, and protein expressions during the first 24 h of ischemia-reperfusion injury.
Method
An Experimental research used five male Rattus Norvegicus rats in each group. Bulldog clamp was used to clamp renal arteries and veins to create renal ischemia. Immunohistochemistry was used for the analysis of KIM-1 protein expression. While Tubular Injury Score was examined by Histopathology. RT-PCR was used for KIM-1 mRNA expression.
Results
Tubular Injury Score (TIS) was significantly higher in ischemia than control. TIS remained similar after IR 30 min, peaked at IR 2 h, and decreased to the level of IR 30 min at IR 24 h.
The KIM-1 mRNA expression was also higher in ischemia than in control. Similarly, KIM-1 mRNA expression increased more after IR 30 min, IR 2 h, and IR 24 h.
The KIM-1 protein expression was higher in ischemia than in control. KIM-1 protein increased more after IR 30 min, IR for 2 h, and remained similar at IR for 24 h.
KIM-1 mRNA and protein expressions at IR 2 h were significantly different compared to ischemia but not significantly different compared to that in IR 24 h.
Conclusions
KIM-1 mRNA and protein expressions increased within 24 h IR with the critical time was in the 2 h IR.
Keywords: Ischemia-reperfusion injury (IRI), Kidney injury Molecule-1 (KIM-1)
Highlights
-
•
KIM-1 mRNA and protein expressions are high during renal ischemia reperfusion.
-
•
The critical time of KIM-1 mRNA and protein expressions in reperfusion is 2 h.
-
•
The critical time for kidney tubular damage in ischemic reperfusion is 2 h.
-
•
The levels of KIM-1 mRNA and protein correspond to tubular damage.
1. Introduction
The proximal renal tubular epithelium is the part of the kidney that is most susceptible to injury in ischemic conditions. This section requires the most ATP, but the glycolytic capacity of this epithelium is minimal, so the availability of ATP is highly dependent on aerobic mitochondrial respiration [1]. The proximal tubular epithelium is susceptible to injury due to ischemia [2].
Reperfusion results in the reoxygenation of cells, but the injured cells become more injured [3,4]. Therefore, renal ischemia-reperfusion injury (IRI) contributes to increased morbidity and mortality [5]. On the other hand, ischemia-reperfusion (IR) injury causes acute tubular necrosis (ATN) through oxidative stress mechanism [[6], [7], [8]], inflammation [[8], [9], [10], [11], [12], [13]], and the “no-reflow” phenomenon [[14], [15], [16], [17], [18]].
KIM-1 is a transmembrane glycoprotein that is localized to the apical membrane of the proximal tubular epithelium and is highly expressed in the injured epithelium [[19], [20], [21], [22], [23]] [[19], [20], [21], [22], [23]] [[19], [20], [21], [22], [23]]. KIM-1 level correlates with the severity of the tubular injury [24,25]. Furthermore, the duration of the IR affects the severity of the IR injury in animal and human studies [26]. Therefore, this study wanted to evaluate the crucial time for increased tubular damage and its associated biomarkers, KIM-1 mRNA, and protein expressions during 24 h of IR.
2. Materials and methods
2.1. Study design
An experimental animal study was conducted from August 2019 to January 2020. All experimental protocols employed in this study were approved by the Medical Research Ethics Committee of Hasanuddin University Makassar, Indonesia (Reference No. 1010/UN4.6.4.5.31/PP36/2019). In addition, the work has been reported according to the ARRIVE guidelines [27].
2.2. Experimental animals
Male Rattus Norvegicus, Wistar strain, bodyweight 200–300 mg, aged 10–12 weeks received from Animal Biopharmaceutical Laboratory, Faculty of Pharmacy, Hasanuddin University. A simple random sampling procedure was used to select the sample. According to Federer's formula, 25 samples were determined and were separated into five groups: negative control (n = 5), ischemia (n = 5), ischemia-reperfusion for 30 min (n = 5), ischemia-reperfusion for 2 h (n = 5), and ischemia-reperfusion for 24 h (n = 5). The animals were kept at a constant temperature of 25 °C, 12 h light-dark cycle, diet and water were provided ad libitum. The rats were acclimatized and treated under the Ethical principles of animal use and care.
2.3. Experimental procedures
Using all techniques and hands-on protocols at the Anatomy Laboratory, Gajah Mada University, and Hasanuddin University Medical Research Centre (HUMRC) Laboratory. Anesthesia was achieved by intraperitoneal injection of 0.1 mL per 20 g of body weight mixture of 1.75 mL of ketamine (100 mg/mL) + 0.25 mL of xylazine (100 mg/mL) + 8 mL of sterile water. The renal arteries and veins were clamped on the right and left kidneys using a non-traumatic vascular micro-clamp (bulldog clamp). If a uniform ischemic appearance were shown in both kidneys, the ischemia induction was considered successful and was maintained for 30 min. Renal arteries and veins were not clamped in the control group.
Release of arterial and venous clamps allowed for reperfusion. The induction of reperfusion was successful if the ischemic appearance in the kidney returned to normal appearance.
The rats were sacrificed by intraperitoneal injection 0.1 mL/20 g BW mixture of 1.75 mL ketamine (100 mg/mL) + 0.25 mL xylazine (100 mg/mL) + 8 mL sterile water. An incision was made from the median line to the lateral side of the abdominal wall to the peritoneal cavity after complete anesthesia. The ribs were cut from the lower to the higher lateral side to open the anterior thoracic wall. A needle connected to a perfusion pump machine hose was used to puncture the heart at the apex while still beating. A small incision was made in the right atrial wall (auricula) to relieve intracardiac pressure. The heart was then perfused with normal saline solution through the apex of the heart until the blood in the circulation was clear (marked by the liver, kidneys, and other organs being paler in color).
2.4. Experiment outcomes
Immunohistochemistry (IHC) for KIM-1 protein expression and histopathological studies for tubular injury score were examined using the right kidney's tissue. The left kidney was removed for RT-PCR analysis of KIM-1 mRNA expression.
3. Sample collection and examination
3.1. KIM-1 mRNA expression
The left kidney was removed and stored at −80 °C for RT-PCR to measure the quantity of KIM-1 mRNA. KIM-1 F CTGGAATGGCACTGTTGACATCC, KIM-1R GCAGATGCCAACATAGAAGCCC (Cat No. MCG0050); iSCRIPT cDNA Synthesis, 25R, BioRad Laboratories (Cat No. 1708890); EVA Green SMX 200R, BioRad Laboratories (Paint No. 1708890); EVA Green (Paint No. 1725200). All of these items were ordered from PT Scienwerke Jakarta. RT-PCR was performed using the PCR-Bio-Rad BR004129USA machine. A mixture of 22.5 μl PCR Mastermix and SYBR green QRT was prepared. DNA extract of 2.5 μl was added to a 22.5 μl mixture of PCR mix. First stage amplification was performed at 94 °C for 2s and continued up to 40 cycles 60s at 94 °C, and 45s at 57 °C. The expression of mRNA was calculated according to the previous study [28].
3.2. Tubular injury and KIM-1 protein expression
The right kidney was removed and stored/soaked in a 4% PFA (paraformaldehyde) solution for histological examination and immunohostochemistry (IHC). For histological examination, tissue sections were stained with HE using a Hemotoxylin-Eosin Staining kit. Histological alteration in the renal tubular interstitium was assayed according to the quantitative measurements of tubular damage, characterized by tubular epithelial swelling, degeneration, necrosis, tubular ectasia, inflammatory cell infiltration, and tubular interstitial fibrosis, evaluated at a magnification of 200x. The degree of tubular damage was scored using the following criteria: 0, normal; 1, damage area of 25% of tubules; 2, damage area of 25–50% of tubules; 3, damage area of 50–75% of tubules; 4, damage area of 75–100% of tubules. KIM-1 protein expression was analyzed using IHC with Novusbio TIM-1/KIM-1/HAVCR polyclonal antibodies, size 100 L, Catalog No.: NBP1-76701 were used. PT Vantagebio Scientific Solution in Jakarta was responsible for ordering the antibodies. Semiquantitative analysis was categorized as 0 (no staining or extremely weak expression), 1 (mild staining area, expression1-25%), 2 (expression 26–50%), 3 (expression 51–75%), 4 (expression >75%) [44].
3.3. Statistical analysis
As data were not normally distributed, the differential expression values of KIM-1 mRNA, KIM-1 protein, and histology were analyzed using the Kruskal Wallis and Mann-Whitney test. The data was presented as median ± interquartile range. The Spearman correlation test was used to examine the correlation between KIM-1 mRNA, KIM-1 protein, and Tubular Injury Score. p-value < 0.05 was considered significant. IBM SPSS 23 (IBM, New York, United States) was used for statistical analysis.
4. Results
4.1. Kidney tubular injury after ischemia and reperfusion
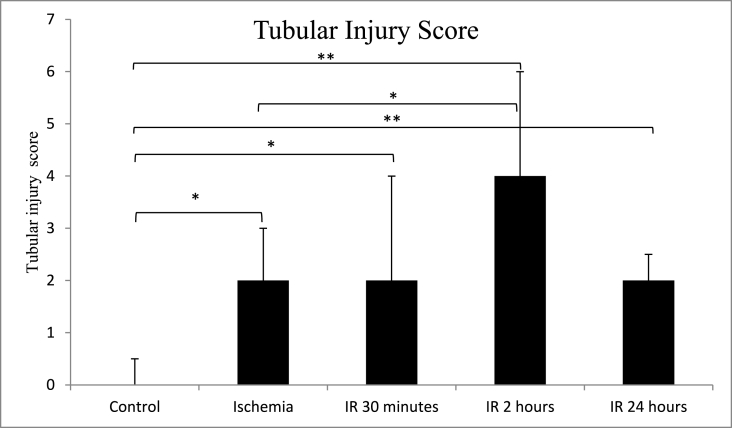
The median score of tubular injury was significantly higher (p = 0.011) in the ischemia group (2.00 ± 1.00) than in the control group (0.00 ± 0.50). In addition, tubular injury scores remained similar after 30 min of IR (2.00 ± 1.00) and increased after 2 h IR (4.00 ± 2.00) but subsequently decreased after 24 h IR (2.00 ± 0.50). Tubular injury scores increased after reperfusion IR for 30 min, IR for 2 h, and IR for 24 h, and they were significant when compared to ischemia and control (p < 0.05). The details are shown in Table 1, Table 2, and Fig. 1.
Table 1.
Comparison of KIM-1 mRNA and KIM-1 protein expressions and Tubular Injury Score.
|
Groups |
KIM-1 mRNA (fold change) Median ± IQR n = 5 |
KIM-1 mRNA (fold change) Mean ± SD |
KIM-1 protein (score) Median ± IQR n = 5 |
KIM-1 protein (score) Mean ± SD n = 5 |
Tubular Injury Score (score) Median ± IQR n = 5 |
Tubular Injury Score (score) Mean ± SD n = 5 |
|---|---|---|---|---|---|---|
| Control | 0.35 ± 0.51 | 0.42 ± 0.26 | 1.00 ± 1.00 | 0.60 ± 0.44 | 0.00 ± 0.50 | 0.20 ± 0.40 |
| Ischemia | 1.14 ± 0.41 | 1.28 ± 0.29 | 1.00 ± 1.50 | 0.80 ± 0.83 | 2.00 ± 1.00 | 1.60 ± 0.54 |
| IR 30 min | 3.14 ± 2.69 | 2.60 ± 1.41 | 2.00 ± 1.50 | 2.20 ± 0.83 | 2.00 ± 2.00 | 2.00 ± 1.00 |
| IR 2 Hours | 6.15 ± 2.72 | 5.12 ± 2.06 | 3.00 ± 1.00 | 2.60 ± 0.89 | 4.00 ± 2.00 | 3.20 ± 1.09 |
| IR 24 Hours | 9.01 ± 8.58 | 7.45 ± 4.74 | 3.00 ± 0.50 | 2.80 ± 0.44 | 2.00 ± 0.50 | 2.20 ± 0.44 |
| p-value* | 0.006 | 0.003 | 0.004 |
IR: Ischemia-Reperfusion, IQR: Interquartile range, *Kruskal Wallis test.
Table 2.
Comparison of Tubuar Injury Score, KIM-1 mRNA, and KIM-1 Protein Expressions amongst the groups.
| Variables | Groups | p-value∗ |
|---|---|---|
| Tubular Injury Score | Control vs. Ischemia | 0.011 |
| Control vs. 30 min IR | 0.012 | |
| Control vs. 2 h IR | 0.006 | |
| Control vs. 24 h IR | 0.005 | |
| Ischemia vs. 30 min IR | 0.502 | |
| Ischemia vs. 2 h IR | 0.031 | |
| Ischemia vs. 24 h IR | 0.093 | |
| 30 min IR vs. 2 h IR | 0.106 | |
| 30 min IR vs. 24 h IR | 0.734 | |
| 2 h IR vs. 24 h IR | 0.120 | |
| KIM-1 mRNA | Control vs. Ischemia | 0.009 |
| Control vs. 30 min IR | 0.009 | |
| Control vs. 2 h IR | 0.009 | |
| Control vs. 24 h IR | 0.076 | |
| Ischemia vs. 30 min IR | 0.251 | |
| Ischemia vs. 2 h IR | 0.016 | |
| Ischemia vs. 24 h IR | 0.117 | |
| 30 min IR vs. 2 h IR | 0.047 | |
| 30 min IR vs. 24 h IR | 0.117 | |
| 2 h IR vs. 24 h IR | 0.465 | |
| KIM-1 Protein | Control vs. Ischemia | 0.729 |
| Control vs. 30 min IR | 0.017 | |
| Control vs. 2 h IR | 0.014 | |
| Control vs. 24 h IR | 0.006 | |
| Ischemia vs. 30 min IR | 0.041 | |
| Ischemia vs. 2 h IR | 0.021 | |
| Ischemia vs. 24 h IR | 0.009 | |
| 30 min IR vs. 2 h IR | 0.343 | |
| 30 min IR vs. 24 h IR | 0.189 | |
| 2 h IR vs. 24 h IR | 0.881 |
Mann-Whitney test.
Fig. 1.
Comparison of kidney tubular injury score between control, ischemia, ischemia reperfusion (IR) 30 min, 2 h and 24 h groups. *p < 0.05, **p < 0.01 using Mann-Whitney test. Groups without asterisk mark (*) or (**) are not statistically significant. Data are expressed as Median ± IQR. n = 5 for each group.
4.2. KIM-1 mRNA and protein expression after ischemia and reperfusion
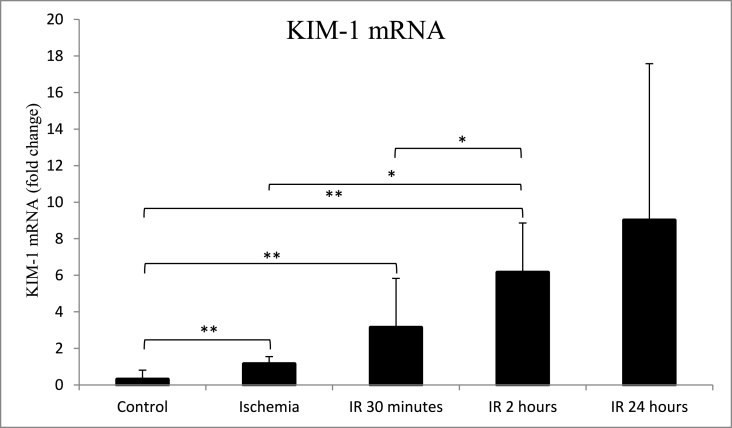
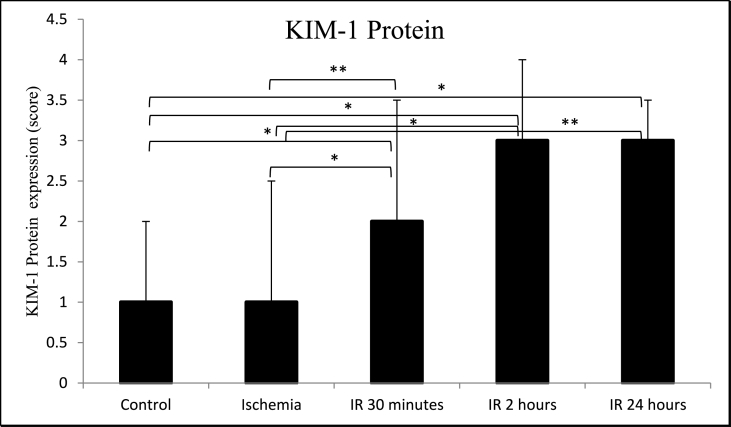
The average expression of KIM-1 mRNA was significantly higher (p = 0.009) in the ischemia group (1.14 ± 0.41) than in the control group (0.35 ± 0.51). KIM-1 mRNA further increased after IR for 30 min (3.14 ± 2.69), IR for 2 h (6.15 ± 2.72), and IR for 24 h (9.01 ± 8.58) (Table 1, Table 2 and Fig. 2). KIM-1 mRNA expression for IR 30 min was significantly different (p = 0.047) compared to IR 2 h but was not significantly different (p = 0.117) compared to IR 24 h (Fig. 2). The increase of KIM-1 mRNA expressions was accompanied by an increase of KIM-1 protein expressions. However, the KIM-1 protein expression was not significantly different (p = 0.729) in the ischemia group (1.00 ± 1.50) compared with the control group (1.00 ± 1.00). KIM-1 protein expression increased significantly (p = 0.017) after IR for 30 min (2.00 ± 1.50), IR for 2 h (3.00 ± 1.00, p = 0.014), and IR for 24 h (3.00 ± 0.50, p = 0.006) compared to the control group (Table 1, Table 2, Fig. 3, and Fig. 4). KIM-1 protein expression for IR 30 min was not significantly different compared to IR 2 h (p = 0.343) and IR 24 h (p = 0.189). Similarly, KIM-1 protein expression for IR 2 h was not significantly different compared to IR 24 h (p = 0.881).
Fig. 2.
Comparison of kidney mRNA KIM-1 expression between control, ischemia, ischemia reperfusion (IR) 30 min, 2 h and 24 h groups. *p < 0.05, **p < 0.01 using Mann-Whitney test. Groups without asterisk mark (*) or (**) are not statistically significant. Data are expressed as Median ± IQR. n = 5 for each group.
Fig. 3.
Comparison of kidney KIM-1 protein expression between control, ischemia, ischemia reperfusion (IR) 30 min, 2 h and 24 h groups. *p < 0.05, **p < 0.01 using Mann-Whitney test. Groups without asterisk mark (*) or (**) are not statistically significant. Data are expressed as Median ± IQR. n = 5 for each group.
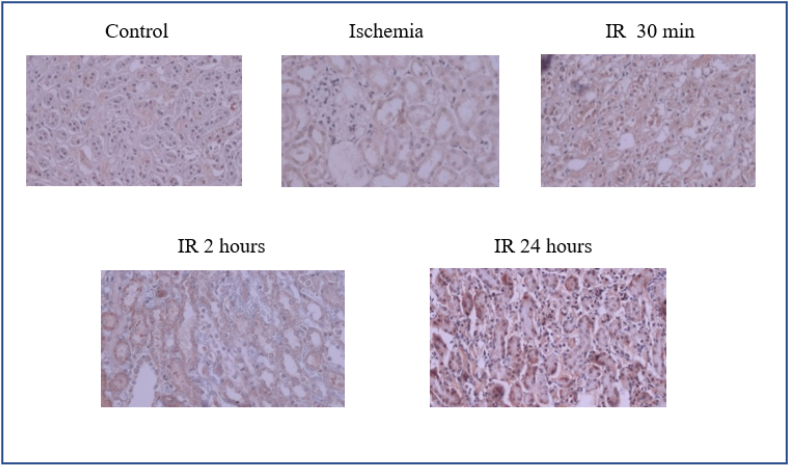
Fig. 4.
Level expression of kidney KIM-1 protein in control, ischemia, ischemia reperfusion for 30 min, 2 h, and 24 h groups. n = 5 for each group.
4.3. Correlation between KIM-1 gene expression, KIM-1 protein expression, and Tubular Injury Score on ischemia, IR 30 min, IR 2 h, and IR 24 h
A Spearman correlation test was used to perform a correlation analysis. Ischemia and reperfusion had a substantial influence on tubular damage, KIM-1 mRNA, and KIM-1 protein expression. Tubular damage was found to be substantially linked with KIM-1 mRNA (r = 0.751; p = 0.000) and protein levels (r = 0.645; p = 0.001). The expression of KIM-1 mRNA was shown to be substantially linked with KIM-1 protein (r = 0.805; p = 0.000). The correlation in detail is shown in Table 3.
Table 3.
Correlation amongst KIM-1 mRNA, KIM-1 protein expressions and Tubular Injury Score.
| Groups | r | Pa |
|---|---|---|
| Tubular injury vs. KIM-1 mRNA | 0.751 | 0.000 |
| Tubular injury vs. KIM-1 Protein | 0.645 | 0.001 |
| KIM-1 mRNA vs. KIM-1 Protein | 0.805 | 0.000 |
Spearman Correlation test.
5. Discussion
The availability of ATP in the renal proximal tubular epithelial cells is highly dependent on the availability of oxygen. Therefore, the tubular epithelium is highly susceptible to ischemic injury. Brief ischemia (10 min) will result in a 20% decrease in renal ATP levels. In reperfusion, injury is increased as a result of oxidative stress [[6], [7], [8]], inflammation [[9], [10], [11]] and “no-reflow” phenomenon [[14], [15], [16],18,26]. The causes of “no-reflow” are mainly endothelial oxidative stress, fibrinogen thromboembolic formation, neutrophil adhesion to capillary endothelium, endothelial dysfunction, increased vasoconstrictor, and decreased vasodilator. Thus, the “no-reflow” phenomenon results in prolonged ischemia during the reperfusion period [15,17,18,30]. In the present study, ischemia for 30 min caused significant tubular injury compared to no ischemia (control). After reperfusion for 30 min, the injury was not significantly different compared to that in ischemia. These findings were not in line with a previous study that found severe tubulointerstitial injury occurred after 30 min of reperfusion compared with controls [4]. A significant increase in reperfusion injury over ischemic injury occurred after 2 h of reperfusion, then decreased at 24 h of reperfusion. This shows that 2 h of reperfusion is a critical time for IR injury. This is due to a significant “no-reflow” phenomenon at 2 h of reperfusion, where the severity of this no-reflow correlates strongly with the severity of the microvascular injury. After 24 h, the level of injury was still higher than in control, although not significantly different than those in ischemia and 2 h after IR. This indicated that “no-reflow” might have been improved after 24 h of reperfusion or not as severe as at 2 h of reperfusion [26]. Cell death on reperfusion can be through the mechanisms of necrosis, apoptosis, necroptosis, and autophagic [12,31,32].
KIM-1 is a transmembrane glycoprotein that is found on the apical membrane of the proximal tubular epithelium and is highly expressed on injured epithelial cells [20,23,[33], [34], [35]]. The KIM-1 level was related to the severity of tubular injury [25,36]. Results of this study found that KIM-1 mRNA and protein expressions were increased after 30 min of reperfusion compared to ischemia (Table 1, Table 2, Fig. 2, Fig. 3). This study supports previous studies on KIM-1 expression that found reperfusion at 30 min of ischemia results in more progressive ischemia within 30–180 min of reperfusion, leading to persistent cell death and progressive atrophy [3]. A significant increase in KIM-1 expression occurred after 2 h and not 24 h of reperfusion. We support a previous study that found a progressively increased reperfusion injury with an increase in the extent of the injury after the first 2 h of reperfusion [26]. Elevated KIM-1 also indicates increased tubular inflammation [37]. Thus, increased KIM-1 mRNA and protein expressions at 2 h of reperfusion can increase the migration and proliferation of tubular epithelial cells for the recovery of injured cells [38,39]. This finding is essential for managing acute tubular injury in renal ischemia-reperfusion with the best therapeutic intervention prior to the first 2 h of ischemia-reperfusion.
The limitations of this study were that the sample size was small, and the expressions of KIM-1 and the level of tubular injury at the time points between 2 h and 24 h of reperfusion were not measured.
6. Conclusion
Tubular injury, KIM-1 mRNA, and protein expressions increased linearly up to 2 h and decreased after 24 h, indicating that the critical time may be the first 2 h of reperfusion.
Funding
This study was sponsored by Domestic Lecturer Excellence Scholarship. Education Fund Management Institute (LPDP).
Ethical approval
Ethical approval has been approved by the ethics commission of Faculty of Medicine, Hasanuddin University reference no. 1010/UN4.6.4.5.31/PP36/2019.
Consent
Not applicable to the study.
Author contribution
Jerny Dase (JD), Haerani Rasyid (HR), Rina Masadah (RM), Muhammad Husni Cangara (MHC), initiated and designed the study. JD, HR, RM, Agussalim Bukhari (AB), Mochammad Hatta (MH), Ressy Dwiyanti(RD), drafted and wrote the manuscript. MHC, Haerani Rasyid (HR), Rina Masadah (RM), contributed in the data processing. All authors have read and approved the final manuscript.
Registration of research studies
This study was not involving human participants. Registration was not applicable.
Guarantor
Jerny Dase.
Haerani Rasyid.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare there is no conflict of interest.
Contributor Information
Jerny Dase, Email: jerny.pdfi@gmail.com.
Haerani Rasyid, Email: haeraniabdurasyid@yahoo.com.
Rina Masadah, Email: rinamasadah@yahoo.com.
Muhammad Husni Cangara, Email: drhusni1977@gmail.com.
Agussalim Bukhari, Email: agussalim.bukhari@med.unhas.ac.id.
Ressy Dwiyanti, Email: ressy_chan@yahoo.co.id.
Mochammad Hatta, Email: hattaram@yahoo.com.
References
- 1.Li Z., Zhang G., Feil R., Han J., Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin α IIbβ3. Blood. 2006;107(3):965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David T.A.S., Basile P., Anderson Melissa D. Vol. 2. 2014. Pathophysiology of Acute Kidney Injury David; pp. 1303–1353. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holderied A., Kraft F., Marschner J.A., Weidenbusch M., Anders H.J. ‘Point of No return’ in unilateral renal ischemia reperfusion injury in mice. J. Biomed. Sci. 2020;27(1):1–15. doi: 10.1186/s12929-020-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y., et al. Ischemic duration and frequency determines AKI-to-CKD progression monitored by dynamic changes of tubular biomarkers in IRI mice. Front. Physiol. 2019;10:1–15. doi: 10.3389/fphys.2019.00153. FEB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015;4(2):20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath K.A., Norby S.M. Reactive oxygen species and acute renal failure OF REACTIVE OXYGEN SPECIES. Am. J. Med. 2000;109 doi: 10.1016/s0002-9343(00)00612-4. 9343. [DOI] [PubMed] [Google Scholar]
- 7.Sun M., Jin H., Sun X., Huang S., Zhang F., Guo Z. Vol. 2018. 2018. (Review Article Free Radical Damage in Ischemia-Reperfusion Injury : an Obstacle in Acute Ischemic Stroke after Revascularization Therapy). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K.H., Tseng W.C., Yang C.Y., Tarng D.C. The anti-inflammatory, anti-oxidative, and anti-apoptotic benefits of stem cells in acute ischemic kidney injury. Int. J. Mol. Sci. 2019;20(14) doi: 10.3390/ijms20143529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen H., Kreisel D., Goldstein D.R. Processes of sterile inflammation. J. Immunol. 2013;191(6):2857–2863. doi: 10.4049/jimmunol.1301539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalifian S., Broyles J.M., Tuffaha S.H., Alrakan M., Ibrahim Z., Sarhane K.A. Immune mechanisms of ischemia-reperfusion injury in transplantation. Open J. Immunol. 2013;3(3):158–164. doi: 10.4236/oji.2013.33020. [DOI] [Google Scholar]
- 11.Simone S., et al. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic. Biol. Med. 2014;74:263–273. doi: 10.1016/j.freeradbiomed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Kim C.R., Kim J.H., Park H.Y.L., Park C.K. Ischemia reperfusion injury triggers TNFα induced-necroptosis in rat retina. Curr. Eye Res. May 2017;42(5):771–779. doi: 10.1080/02713683.2016.1227449. [DOI] [PubMed] [Google Scholar]
- 13.Liu F., Ni W., Zhang J., Wang G., Li F., Ren W. Administration of curcumin protects kidney tubules against renal ischemia-reperfusion injury (RIRI) by modulating nitric oxide (NO) signaling pathway. Cell. Physiol. Biochem. 2017;44(1):401–411. doi: 10.1159/000484920. [DOI] [PubMed] [Google Scholar]
- 14.Alawi L.F., et al. Effects of angiotensin II type 1A receptor on ACE2, neprilysin and KIM-1 in two kidney one clip (2K1C) model of renovascular hypertension. Front. Pharmacol. 2021;11:1–16. doi: 10.3389/fphar.2020.602985. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sörensen-Zender I., et al. Role of fibrinogen in acute ischemic kidney injury. Am. J. Physiol. Ren. Physiol. 2013;305(5):777–785. doi: 10.1152/ajprenal.00418.2012. [DOI] [PubMed] [Google Scholar]
- 16.Shereif P.B., Rezkalla H., Kloner Robert A. 2015. No-Reflow Phenomenon; pp. 656–662. [DOI] [PubMed] [Google Scholar]
- 17.Reffelmann T., Kloner R.A. 2002. THE ‘NO-REFLOW’ PHENOMENON: BASIC SCIENCE AND CLINICAL CORRELATES. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galasso G., et al. No-reflow phenomenon: pathophysiology, diagnosis, prevention, and treatment. a review of the current literature and future perspectives. Angiology. 2014;65(3):180–189. doi: 10.1177/0003319712474336. [DOI] [PubMed] [Google Scholar]
- 19.van Timmeren M.M., van den Heuvel M.C., Bailly V., Bakker S.J.L., van Goor H., Stegeman C.A. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J. Pathol. Jun. 2007;212(2):209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 20.Bonventre J.V., Yang L. 2010. Kidney Injury Molecule-1; pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 21.Yang L., et al. Vol. 125. 2015. KIM-1 – Mediated Phagocytosis Reduces Acute Injury to the Kidney. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichimura T., et al. Vol. 2115. 2018. pp. 552–563. (Kidney Injury Molecule-1 : a Tissue and Urinary Biomarker for Nephrotoxicant-Induced Renal Injury). [DOI] [PubMed] [Google Scholar]
- 23.Xueying Zhao* G., Jiang Chen, Olufade Rebecca, Dong Liu, Nerimiah Emmett Department of Physiology, Morehouse School of Medicine, Atlanta . Vol. 231. 2018. pp. 896–907. (Kidney Injury Molecule-1 Enhances Endocytosis of Albumin in Renal Proximal Tubular Cells). 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Li A., Wen J., Zhen J., Hao Q., Zhang Y. Kidney Injury Molecule-1 Level is Associated with the Severity of Renal Interstitial Injury and Prognosis in Adult Henoch e Sch € onlein Purpura Nephritis. Arch. Med. Res. 2017:1–10. doi: 10.1016/j.arcmed.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Cai J., et al. Kidney injury molecule-1 expression predicts structural damage and outcome in histological acute tubular injury. Ren. Fail. 2019;41(1):80–87. doi: 10.1080/0886022X.2019.1578234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reffelmann T., Hale S.L., Li G., Kloner R.A. Relationship between no reflow and infarct size as influenced by the duration of ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2002;282(2 51–2):766–772. doi: 10.1152/ajpheart.00767.2001. [DOI] [PubMed] [Google Scholar]
- 27.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLoS Biol. 2010;8(6):6–10. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tommy T., et al. Effect of folinic acid on serum homocysteine, TNFα, IL-10, and HMGB1 gene expression in head injury model. Ann. Med. Surg. 2021;65 doi: 10.1016/j.amsu.2021.102273. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalogeris1 Theodore, Christopher P., Baines, Krenz Maike, Korthuis Ronald J. Vol. 7. 2017. pp. 113–170. (Ischemia/Reperfusion). 1. [DOI] [Google Scholar]
- 31.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Ischemia/reperfusion. Compr. Physiol. Jan. 2017;7(1):113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu M.Y., et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018;46(4):1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 33.Van Timmeren M.M., Van Den Heuvel M.C., Bailly V., Bakker S.J.L., Van Goor H., Stegeman C.A. 2007. Tubular Kidney Injury Molecule-1 (KIM-1) in Human Renal,” No. April; pp. 209–217. [DOI] [PubMed] [Google Scholar]
- 34.Starkov A.A. 2014. An Update on the Role of Mitochondrial α-ketoglutarate Dehydrogenase in Oxidative Stress Anatoly; pp. 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L., et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J. Clin. Invest. 2015;125(4):1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonventre J.V., Yang L. Kidney injury molecule-1. Curr. Opin. Crit. Care. Dec. 2010;16(6):556–561. doi: 10.1097/MCC.0b013e32834008d3. [DOI] [PubMed] [Google Scholar]
- 37.Song J., et al. Understanding kidney injury molecule 1: a novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019;11(3) [PMC free article] [PubMed] [Google Scholar]
- 38.Ismail O.Z., et al. Kidney injury molecule-1 protects against Gα12 activation and tissue damage in renal ischemia-reperfusion injury. Am. J. Pathol. May 2015;185(5):1207–1215. doi: 10.1016/j.ajpath.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z., Cai C.X. Kidney injury molecule-1 (KIM-1) mediates renal epithelial cell repair via ERK MAPK signaling pathway. Mol. Cell. Biochem. May 2016;416(1–2):109–116. doi: 10.1007/s11010-016-2700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]