Abstract
Background and aim
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucose-lowering drugs that increase urinary glucose excretion have been shown to reduce CV events in patients with type 2 diabetes (T2D). Furthermore, several studies have demonstrated that treatment with SGLT2 inhibitors affect calcium and phosphate homeostasis, but the effect of empagliflozin on these biomarkers is hitherto not investigated in detail. Therefore, this analysis of the EMPA hemodynamics study examined effects of empagliflozin on calcium and phosphate homeostasis.
Methods
In this placebo-controlled, randomized, double-blind study patients with T2D were randomized to empagliflozin 10 mg (n = 20) or placebo (n = 22). Biomarkers of calcium and phosphate homeostasis were assessed before, and after 3 days and 3 months of treatment.
Results
After 3 days of treatment empagliflozin significantly increased serum levels of phosphate (baseline: 1.10 ± 0.21 mmol/L; day 3: 1.25 ± 0.23 mmol/L; p = 0.036), parathyroid hormone (PTH) (baseline: 57.40 ± 30.49 pg/mL; day 3: 70.23 ± 39.25 pg/mL; p = 0.025), fibroblast growth factor 23 (FGF23) (baseline: 77.92 ± 24.31 pg/mL; day 3: 109.18 ± 58.20 pg/mL; p = 0.001) and decreased 1,25-dihydroxyvitamin D (baseline: 35.01 ± 14.01 ng/L; day 3: 22.09 ± 10.02 mg/L; p < 0.001), while no difference of these parameters was recorded after 3 months of treatment. Empagliflozin had no significant effects on serum calcium and markers of bone resorption (collagen type 1 β-carboxy-telopeptide = β-CTX) or formation (osteocalcin) after 3 days and 3 months of treatment.
Conclusions
Empagliflozin treatment of patients with T2D transiently increases serum phosphate, PTH and FGF23, and decreases 1,25-dihydroxyvitamin D. This might reflect a temporal increase of sodium driven phosphate reabsorption in the proximal tubule of the kidney caused by increased sodium availability in response to SGLT2 inhibition.
Keywords: Type 2 diabetes, SGLT2 inhibitors, Empagliflozin, Serum phosphate, FGF23, PTH
Highlights
-
•
Empagliflozin transiently increases serum phosphate.
-
•
This might reflect an increase of Na+ driven phosphate reabsorption in the kidney.
-
•
Empagliflozin had no effects on markers of bone resorption or formation.
1. Introduction
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are glucose-lowering drugs used to treat patients with type 2 diabetes (T2D). These agents act by inhibiting SGLT2 in the proximal tubule of the kidney leading to reduced tubular glucose and sodium reabsorption with subsequent increase of urinary glucose excretion, natriuresis, as well as osmotic diuresis (Gallo et al., 2015). In patients with T2D, several placebo-controlled cardiovascular outcome trials (CVOTs) with SGLT2 inhibitors (EMPA-REG OUTCOME with empagliflozin (Zinman et al., 2015), the CANVAS program (Neal et al., 2017) and CREDENCE (Perkovic et al., 2019) with canagliflozin, DECLARE with dapagliflozin (Wiviott et al., 2019), VERTIS with ertugliflozin (Cannon et al., 2020)) demonstrated a reduction in CV events and/or a reduction in hospitalisation for heart failure (HHF) in patients with atherosclerotic CV disease (ASCVD), multiple CV risk factors, or chronic kidney disease (McGuire et al., 2021).
Irrespective, some studies have demonstrated adverse effects of SGLT2 inhibitors on bone metabolism. Treatment with dapagliflozin increased bone fractures in patients with T2D (Kohan et al., 2014) while canagliflozin was associated with increased incidence of fractures in patients with T2D in the CANVAS trial (Watts et al., 2016). In addition, in the EMPA-REG OUTCOME trial upper extremity fractures were numerically higher under treatment with empagliflozin (FDA, 2016). Previous studies further demonstrated effects of SGLT2 inhibitors on bone and mineral homeostasis. Serum levels of phosphate increased under treatment with dapagliflozin (de Jong et al., 2019), while levels of fibroblast growth factor 23 (FGF23) – which decreases the reabsorption of calcium and increases excretion of phosphate – were induced during canagliflozin treatment (Blau et al., 2018).
It currently remains unknown whether effects of SGLT2 inhibition on bone metabolism are compound specific and whether this is a transient or persistent phenomena. Therefore, we investigated the effect of empagliflozin on serum levels of calcium, phosphate, parathyroid hormone (PTH), 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, FGF23, collagen type 1 β-carboxy-telopeptide (β-CTX) and osteocalcin at baseline, and after 3 days and 3 months of treatment in a secondary analysis of the randomized, placebo-controlled, double-blind EMPA hemodynamics study in patients with T2D (Rau et al., 2021).
2. Methods
2.1. Study population and study design
In this placebo-controlled, double-blind, randomized, 2-arm parallel, interventional study 44 patients with T2D were randomized into 2 groups. The randomization list was computer generated using a permuted block randomization with block size of 4. The sequence generation method and the block size were concealed from the investigators. An independent pharmacist labelled the study medications according to the randomization list. Study participants received empagliflozin 10 mg or placebo for a period of 3 months in addition to their concomitant medication. Blood chemistry were performed at baseline (day 0), day 1, day 3 and after 3 months. Participants were recruited from the Department of Internal Medicine I at University Hospital Aachen, RWTH Aachen University, Germany. Inclusion criteria were as follows: type 2 diabetes, HbA1c ≥ 6.5% and age ≥ 18 years. Exclusion criteria were type 1 diabetes, uncontrolled hypertension, age ≥ 75 years, pregnancy, renal impairment (eGFR < 30 mL/min/1.73 m2), liver disease (serum levels of AST, ALT or AP more than three times the upper limit of normal), uncontrolled thyroid disease, endocrinopathies like Graves' disease, akromegaly, Cushings' disease, secondary hypertension due to renal artery stenosis, pheochromocytoma or hyperaldosteronism, hypertensive retinopathy or encephalopathy, acute coronary syndrome, stroke or transient ischemic attack in last 6 weeks prior to randomization. The study protocol was approved by the local ethic committee and all subjects gave written informed consent. The trial was registered: EudraCT number: 2016-000172-19.
2.2. Laboratory measurement
All serum samples of our study patients have been collected in the morning and in a fasting state in accordance with the study protocol. Serum chemistry including calcium, phosphate, PTH, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were performed at baseline, day 3 and after 3 months of treatment. We used commercially available assays to determine FGF23, β-CTX and osteocalcin at baseline, day 3 and after 3 months of treatment. FGF23 was measured using a human ELISA kit (2nd Generation) of Immutopics, Inc. (San Clemente, CA, USA). The sensitivity of the ELISA is 1.5 pg/mL. According to the manufacturer's specifications, coefficients of variation for intra-assay precision are 4.1% (mean value: 43 pg/mL) respectively 2.0% (mean value: 426 pg/mL) (calculated from 20 duplicate determinations in a single assay). To assess inter-assay precision means (46 pg/mL respectively 441 pg/mL) and coefficients of variation (9.1% respectively 3.5%) were calculated from duplicate determinations in 20 assays by the manufacturer. An ELISA kit of Immunodiagnostic Systems Holdings PLC (Boldon Colliery, Tyne and Wear, UK) was used to determine β-CTX (assay range: 0–3.380 ng/mL). The sensitivity of the ELISA is 0.020 ng/mL. According to the manufacturer's specifications, coefficients of variation for intra-assay precision are 1.7–3.0% and 2.5–10.9% for inter-assay precision, respectively. Osteocalcin was measured using an ELISA kit of Quidel Corporation (San Diego, CA, USA). According to the manufacturer's specifications, limit of detection is 0.45 ng/mL, and coefficients of variation are 4.8–10.0% (for intra-assay precision) and 4.8–9.8% (for inter-assay precision). We collected 24 h urine at baseline, day 3 and after 3 months to measure renal excretion of glucose, sodium, calcium, phosphate and creatinine.
2.3. Statistical analysis
Statistical analyses were performed using SAS 9.4 Software (SAS Institute Inc., Cary, NC, USA). We investigated the influence of empagliflozin as compared to placebo with regard to all parameters described above. For each of the outcome variables measured at day 3 and month 3, we fitted a repeated linear mixed effects model (Proc Mixed in SAS). The baseline value of the respective variable as well as the treatment group, the time of the visit in days and the interaction between treatment and days were included in the model. The days were included as repeated effect and a random intercept was modelled. The variance components covariance structure was used. All investigated variables and the corresponding baseline values were log-transformed before being entered into the model to improve the model fit. The model fit was examined by visual inspection of the residual plots. The pairwise differences of least square means between empagliflozin and placebo and p-values were calculated for each time point (day 3 and month 3).
For the graphical illustration of the courses over time of selected outcome variables, means and standard errors are shown for empagliflozin and placebo for each time point. Graphical illustrations were created using the statistical software R (Version 4.0.2). For all statistical analyses p-values ≤ 0.05 were categorized as statistically significant.
3. Results
3.1. Baseline characteristics
From May 2017 to January 2019 a total of 44 patients underwent randomization. Data analysis was performed on 42 patients with 2 patients in the empagliflozin group being excluded because of protocol violations (concomitant intake of SGLT2 inhibitors at baseline and throughout study). As reported previously (Rau et al., 2021), comparable baseline characteristics were observed between empagliflozin and placebo treated patients (Supplementary table). Mean age of study participants was 62 ± 6.8 years, 81% were male, with a mean glycated hemoglobin of 7.7 ± 1.1%, a mean BMI of 31.3 ± 4.6 kg/m2, a mean eGFR of 83 ± 19 mL/min/1.73 m2 and a history of CVD in 71% of all patients. Patients had a baseline blood pressure of 135/81 mmHg (SD 16.9/13.2). Baseline values for 25-hydroxyvitamin D were significant different between the empagliflozin and placebo group (Fig. 1 and Table 1). Baseline medication was comparable in both groups including anti-diabetic drugs, RAAS-inhibition, beta blockers and statins (Supplementary table).
Fig. 1.
Changes in biomarker levels during the study. Serum calcium [A], serum phosphate [B], 25(OH)D [C], 1,25(OH)2D [D], PTH [E], FGF23 [F], β-CTX [G], osteocalcin [H], urine calcium/creatinine ratio [I] and urine phosphate/creatinine ratio [J] in patients with type 2 diabetes treated with empagliflozin (n = 20; black line) or placebo (n = 22; blue dotted line). Data are shown as mean ± standard error at baseline, after 3 days and after 3 months. p-values were calculated from the respective linear mixed effects models for the log-transformed value of day 3 and month 3, adjusting for the baseline value. p-values ≤ 0.05 were categorized as statistically significant.
Table 1.
Changes in biomarker levels during the study.
| Biomarker | Baseline |
Day 3 |
Month 3 |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Empagliflozin | Placebo | Empagliflozin | p | Placebo | Empagliflozin | p | |
| Serum calcium – mmol/L | 2.33 ± 0.10 | 2.35 ± 0.11 | 2.32 ± 0.11 | 2.35 ± 0.10 | p = 0.588 | 2.33 ± 0.09 | 2.40 ± 0.15 | p = 0.104 |
| Serum phosphate – mmol/L | 1.09 ± 0.15 | 1.10 ± 0.21 | 1.11 ± 0.19 | 1.25 ± 0.23 | p = 0.036 | 1.09 ± 0.15 | 1.17 ± 0.25 | p = 0.225 |
| 25(OH)D – ng/mL | 19.95 ± 9.07 | 15.62 ± 7.41 | 19.74 ± 9.05 | 15.77 ± 8.27 | p = 0.674 | 18.15 ± 10.25 | 17.42 ± 9.02 | p = 0.049 |
| 1,25(OH)2D – ng/L | 36.76 ± 12.39 | 35.01 ± 14.01 | 36.62 ± 14.79 | 22.09 ± 10.02 | p < 0.001 | 34.17 ± 13.95 | 31.51 ± 16.56 | p = 0.629 |
| PTH – pg/mL | 48.69 ± 25.10 | 57.40 ± 30.49 | 48.77 ± 24.99 | 70.23 ± 39.25 | p = 0.025 | 52.59 ± 16.38 | 67.82 ± 44.70 | p = 0.984 |
| FGF23 – pg/mL | 70.34 ± 14.28 | 77.92 ± 24.31 | 75.34 ± 17.60 | 109.18 ± 58.20 | p = 0.001 | 71.22 ± 13.44 | 90.29 ± 23.09 | p = 0.089 |
| β-CTX – ng/mL | 0.05 ± 0.03 | 0.07 ± 0.13 | 0.04 ± 0.03 | 0.07 ± 0.10 | p = 0.419 | 0.05 ± 0.03 | 0.05 ± 0.03 | p = 0.922 |
| Osteocalcin – ng/mL | 1.65 ± 0.03 | 1.65 ± 0.02 | 1.63 ± 0.02 | 1.64 ± 0.02 | p = 0.583 | 1.63 ± 0.02 | 1.63 ± 0.03 | p = 0.552 |
| Urine calcium/creatinine ratio | 0.29 ± 0.24 | 0.25 ± 0.13 | 0.29 ± 0.23 | 0.22 ± 0.13 | p = 0.098 | 0.25 ± 0.19 | 0.27 ± 0.16 | p = 0.684 |
| Urine phosphate/creatinine ratio | 1.88 ± 0.58 | 2.04 ± 0.54 | 2.08 ± 0.66 | 2.28 ± 0.60 | p = 0.351 | 2.13 ± 0.61 | 2.22 ± 0.49 | p = 0.640 |
Values are mean ± SD. p-values were calculated from the respective linear mixed effects models for the log-transformed value of day 3 and month 3, adjusting for the baseline value. p-values ≤ 0.05 were categorized as statistically significant.
1,25 (OH)2D = 1,25-dihydroxyvitamin D, 25(OH)D = 25-hydroxyvitamin D, β-CTX = collagen type 1 β-carboxy-telopeptide, FGF23 = fibroblast growth factor 23, PTH = parathyroid hormone.
3.2. Effects of empagliflozin on calcium and phosphate homeostasis
After 3 days of treatment, empagliflozin significantly increased serum levels of phosphate (baseline: 1.10 ± 0.21 mmol/L; day 3: 1.25 ± 0.23 mmol/L; p = 0.036), PTH (baseline: 57.40 ± 30.49 pg/mL; day 3: 70.23 ± 39.25 pg/mL; p = 0.025) and FGF23 (baseline: 77.92 ± 24.31 pg/mL; day 3: 109.18 ± 58.20 pg/mL; p = 0.001) while decreasing 1,25-dihydroxyvitamin D (baseline: 35.01 ± 14.01 ng/L; day 3: 22.09 ± 10.02 mg/L; p < 0.001) (Table 1). This occurred without calcium, 25-hydroxyvitamin D nor β-CTX and osteocalcin levels being affected (Table 1). The modulation of phosphate metabolism was transient and no longer present after 3 months of treatment (Fig. 1 and Table 1). Further, empagliflozin did not affect urinary phosphate nor calcium excretion after 3 days and 3 months of treatment (Fig. 1 and Table 1).
4. Discussion
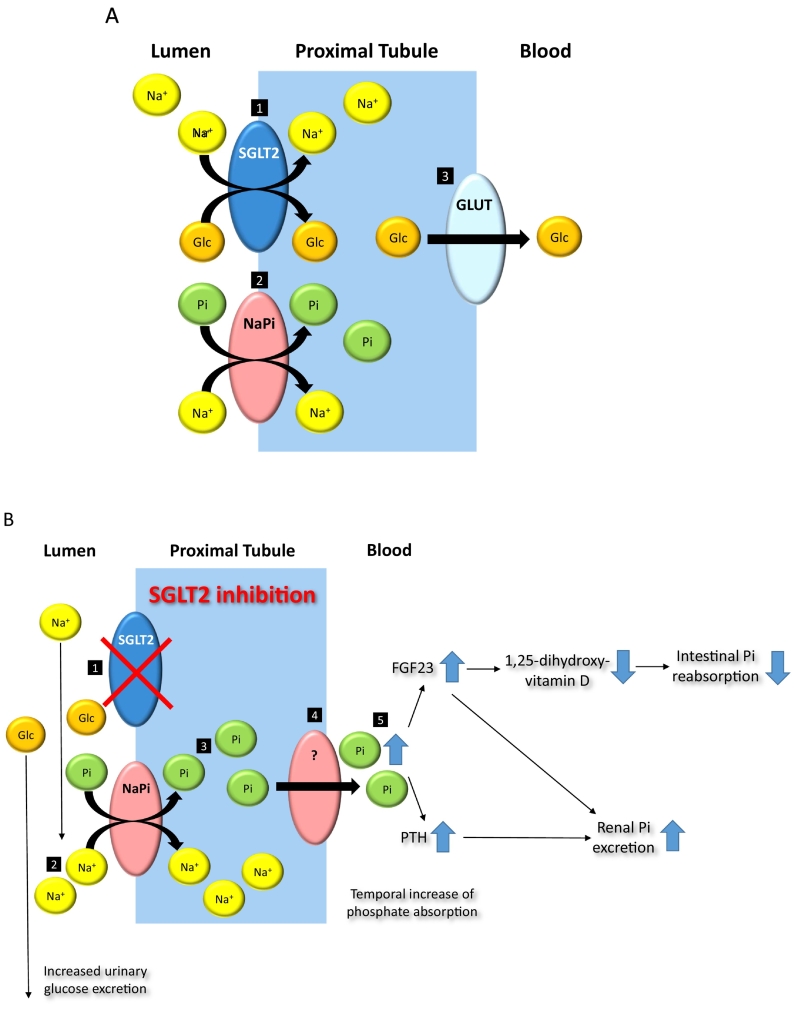
The most striking observation of our study was an early and significant increase of serum phosphate levels in empagliflozin treated patients after 3 days which was no longer apparent at the later 3 month time point. Renal reabsorption of inorganic phosphate (Pi) is a key process in the control of the extracellular phosphate concentration. Phosphate reabsorption mostly happens in the proximal tubule of the kidney which is the same location relevant for glucose reabsorption by SGLT2 transporters (Fig. 2A). Similar to glucose reabsorption by SGLT2 the reabsorption of phosphate occurs in conjunction with sodium mediated by the sodium-dependent phosphate (NaPi) cotransporters. Initial binding of one Na+ ion to the NaPi transporter is followed by connection to inorganic phosphate and a subsequent Na+ ion required for isoelectrical cell membrane translocation (Berndt and Knox, 1992). Increased renal phosphate reabsorption might therefore be a counter regulatory mechanism to prevent urinary loss of Na+ in response to SGLT2 inhibition and/or be mediated by increased local urinary sodium availability in the same tubular segment (Fig. 2B). Consistently SGLT2 inhibition causes an early but not sustained increase of urinary sodium excretion (Sha et al., 2014; Ferrannini et al., 2017), which was by trend also found in our study at the early 3 day but not 3 month time point.
Fig. 2.
Effects of empagliflozin on phosphate homeostasis and related hormones. A. Under physiological conditions sodium-glucose cotransporter-2 (SGLT2) mediates reabsorption of filtered glucose (Glc) via active cotransport with sodium (Na+) in the proximal tubule [1], while reabsorption of phosphate is mediated by sodium-dependent phosphate cotransporters (NaPi) [2]. Glucose transporter (GLUT) mediates passive diffusion of glucose out of proximal tubule [3]. B. SGLT2 inhibition [1] increases availability of sodium in the proximal tubule [2] with consecutive increase of phosphate (Pi) absorption by NaPi as a possible counter regulatory mechanism to prevent urinary loss of Na+ in response of SGLT2 inhibition [3]. Mechanisms of phosphate transport from proximal tubule into blood are unknown [4]. Elevated serum phosphate levels [5] trigger secretion of PTH, and FGF23 with subsequent inhibition of 1,25-dihydroxyvitamin D synthesis with consecutively reduced intestinal Pi reabsorption. FGF23 and PTH synergistically increase renal Pi excretion by downregulating NaPi.
Given the requirement of the organism to tightly control phosphate homeostasis the observed increase of FGF23 and PTH serum levels in empagliflozin treated patients in our study most likely reflects a contra regulatory response to maintain ambient phosphate concentrations (Fig. 2B). FGF23 and PTH both inhibit renal phosphate absorption by downregulating renal sodium-dependent phosphate (NaPi) cotransporters (Fig. 2B). This might be reflected by the normalization of phosphate serum levels at the 3 month time point suggesting successful recalibration of phosphate homeostasis in presence of SGLT2 inhibition in our study. Although FGF23 and PTH synergistically increase renal phosphate excretion, both hormones antagonistically modulate renal 1,25(OH)2D synthesis as the relevant mediator of intestinal phosphate absorption (Fig. 2B). While FGF23 is known to inhibit 1,25(OH)2D synthesis, PTH stimulates 1,25(OH)2D synthesis. The observed decrease of 1,25(OH)2D serum concentrations in our study suggests a dominating relevance of FGF23 over PTH for 1,25(OH)2D synthesis aiming for a reduction of circulating phosphate concentrations (Fig. 2B).
The increase of serum phosphate, PTH and FGF23 by SGLT2 inhibition in our study is in line with previous reports using dapagliflozin (de Jong et al., 2019; List et al., 2009) and canagliflozin (Blau et al., 2018; Taylor et al., 2015). Moreover, canagliflozin increased FGF23 and PTH while decreasing 1,25(OH)2D in healthy volunteers after 5 days of treatment (Blau et al., 2018). A previous study with dapagliflozin reported a nonsignificant trend in reduction of 1,25(OH)2D (de Jong et al., 2019). The interpretation of PTH and FGF23 secretion being an compensatory response to maintain phosphate homeostasis under SGLT2 inhibition (Vervloet et al., 2011; Perwad et al., 2005) was supported by Blau et al. who reported peak levels of FGF23 12 h after peak levels of serum phosphate in healthy volunteers with canagliflozin treatment (Blau et al., 2018).
Importantly, empagliflozin did not change serum calcium and 25(OH)D concentrations. This is consistent with previous studies using dapagliflozin (de Jong et al., 2019; List et al., 2009), but contrasts to studies using canagliflozin reporting moderate increase of serum calcium and 25(OH)D (Weir et al., 2014). Moreover, markers of bone resorption (β-CTX) and formation (osteocalcin) were unaffected by empagliflozin in our study after 3 days and 3 months, while β-CTX and osteocalcin were significantly increased under treatment with canagliflozin after 52 weeks of treatment in comparison to placebo (Bilezikian et al., 2016). This might reflect compound specific characteristics or reflect differential time points of investigation which will require further investigations.
Kohan et al. described increased bone fractures with dapagliflozin treatment in 252 patients with T2D and chronic kidney disease (Kohan et al., 2014). In contrast, no difference in fracture risk was reported in DECLARE-TIMI 58 study randomizing 17,160 patients with T2D to dapagliflozin or placebo for a period of 4.2 years (Cahn et al., 2020). In EMPA-REG OUTCOME upper extremity fractures were numerically higher under treatment with empagliflozin, but no significant differences in bone markers were found in a pooled analysis of 8500 patients with T2D treated with empagliflozin or placebo (FDA, 2016). In the CANVAS trial canagliflozin was associated with increased incidence of fractures in patients with T2D (Watts et al., 2016) while decreasing total hip bone mineral density in a randomized, placebo-controlled study of 716 patients with T2D (Bilezikian et al., 2016).
A recent meta-analysis suggested only canagliflozin but none of the other SGLT2 inhibitors to increase fracture risk (Lou et al., 2020) – suggesting compound specific differences.
The transient effect of empagliflozin on phosphate homeostasis with a normalization after 3 months of treatment as well as the lack of an effect of empagliflozin on markers of bone absorption or formation in our study suggest empagliflozin to have minimal effects on bone and mineral homeostasis. These findings might explain why empagliflozin treatment is not associated with bone fractures in long-term treatment.
This study has certain limitations. Our study is a secondary analysis of a randomized, placebo-controlled study and included only a limited number of patients. Changes in biomarker levels of serum phosphate homeostasis were not predefined endpoints. As such, the effect on phosphate homeostasis is an exploratory finding and warrants confirmation in a larger study with phosphate homeostasis being the primary outcome. Still, the present study was randomized, blinded and placebo-controlled.
5. Conclusion
Taken together, our data suggest that empagliflozin treatment of patients with TD2 and ASCVD/high CV risk leads to an increase of serum phosphate, PTH and FGF23, and in parallel to a decrease in 1,25(OH)2D after 3 days of treatment which does not persist after 3 months of treatment. Moreover serum calcium, 25(OH)D, urine excretion of calcium and phosphate, and markers of bone resorption (β-CTX) and formation (osteocalcin) were unaffected by empagliflozin treatment. Our finding suggests a transient effect of empagliflozin on phosphate homeostasis and may explain why empagliflozin treatment is not associated with bone fractures in long-term treatment.
The following are the supplementary data related to this article.
Baseline characteristics of the study population.
Funding
This investigator-initiated trial was supported by a research grant provided by Boehringer Ingelheim Pharma GmbH & Co. KG. NM and ML are supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, TRR 219, Project-ID 322900939 [M03, M05]). MH is supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, TRR 219, Project-ID 322900939 [M02/S02]).
CRediT authorship contribution statement
Matthias Rau: Writing – original draft, Investigation, Validation, Visualization. Kirsten Thiele: Writing – original draft, Investigation, Validation, Visualization. Niels-Ulrik Korbinian Hartmann: Investigation. Julia Möllmann: Investigation, Validation. Stephanie Wied: Formal analysis. Mathias Hohl: Writing – review & editing. Nikolaus Marx: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Michael Lehrke: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
MR, KT, NUKH, JM, SW and MH report no potential conflict of interest. NM has received support for clinical trial leadership from Boehringer Ingelheim, Novo Nordisk, served as a consultant to Boehringer Ingelheim, Merck, Novo Nordisk, AstraZeneca, BMS, received grant support from Boehringer Ingelheim, Merck, Novo Nordisk, and served as a speaker for Boehringer Ingelheim, Merck, Novo Nordisk, Lilly, BMS, and Astra Zeneca. ML received grants and personal fees from Boehringer Ingelheim, MSD and Novo Nordisk, personal fees from Amgen, Sanofi, Astra Zeneca, Bayer and Lilly.
Acknowledgements
The authors gratefully acknowledge the expert technical assistance of Gabriele Heuer, Hedwig Reichardt, Zakiya Coenen-Basmadjie, Mareike Wienands, Irmgard Diepolder and continuous support by Prof. Müller-Wieland, employees of the University Hospital Aachen, Department of Internal Medicine I.
References
- Berndt T.J., Knox F.G. In: The Kidney Physiology and Pathophysiology. 2nd edn. Seldin D.W., Giebisch G., editors. Raven; New York: 1992. Renal regulation of phosphate excretion; pp. 2511–2532. [Google Scholar]
- Bilezikian J.P., Watts N.B., Usiskin K., et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J. Clin. Endocrinol. Metab. 2016;101:44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau J.E., Bauman V., Conway E.M., et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn A., Raz I., Bonaca M., et al. Safety of dapagliflozin in a broad population of patients with type 2 diabetes: analyses from the DECLARE-TIMI 58 study. Diabetes Obes Metab. 2020;22:1357–1368. doi: 10.1111/dom.14041. [DOI] [PubMed] [Google Scholar]
- Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- FDA . Food and Drug Administration; 2016. Briefing Document: Endocrine and Metabolic Drug Advisory Committee Meeting Empagliflozin.https://www.fda.gov/media/98910/download Published June 28. [Google Scholar]
- Ferrannini E., Baldi S., Frascerra S., et al. Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40:771–776. doi: 10.2337/dc16-2724. [DOI] [PubMed] [Google Scholar]
- Gallo L.A., Wright E.M., Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab. Vasc. Dis. Res. 2015;12:78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M.A., Petrykiv S.I., Laverman G.D., et al. Effects of dapagliflozin on circulating markers of phosphate homeostasis. Clin. J. Am. Soc. Nephrol. 2019;14:66–73. doi: 10.2215/CJN.04530418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan D.E., Fioretto P., Tang W., List J.F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List J.F., Woo V., Morales E., Tang W., Fiedorek F.T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y., Yu Y., Duan J., et al. Sodium-glucose cotransporter 2 inhibitors and fracture risk in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Ther. Adv. Chronic Dis. 2020;11 doi: 10.1177/2040622320961599. 2040622320961599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- Perwad F., Azam N., Zhang M.Y., Yamashita T., Tenenhouse H.S., Portale A.A. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- Rau M., Thiele K., Hartmann N.K., et al. Empagliflozin does not change cardiac index nor systemic vascular resistance but rapidly improves left ventricular filling pressure in patients with type 2 diabetes: a randomized controlled study. Cardiovasc. Diabetol. 2021;20:6. doi: 10.1186/s12933-020-01175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha S., Polidori D., Heise T., et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- Taylor S.I., Blau J.E., Rother K.I. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3:8–10. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervloet M.G., van Ittersum F.J., Buttler R.M., Heijboer A.C., Blankenstein M.A., ter Wee P.M. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin. J. Am. Soc. Nephrol. 2011;6:383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N.B., Bilezikian J.P., Usiskin K., et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2016;101:157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M.R., Kline I., Xie J., Edwards R., Usiskin K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR) Curr. Med. Res. Opin. 2014;30:1759–1768. doi: 10.1185/03007995.2014.919907. [DOI] [PubMed] [Google Scholar]
- Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of the study population.