Abstract
Introduction and importance
Body and tail pancreatic cancers account for one third of pancreatic ductal adenocarcinomas (PDACs). Dysphagia is an extremely rare manifestation of pancreatic cancer that may follow direct invasion of primary pancreatic cancer to esophagus. Pancreatic cancer can be confused with either pancreatic or peripancreatic lesions like gastrointestinal stromal tumors (GISTs) on diagnostic computed tomography (CT) scans. Undifferentiated pancreatic cancer, which is a rare variant of pancreatic ductal adenocarcinoma rarely, present with palpable abdominal mass. The aim of this case report is to show the rare presentation of this deadly malignancy with dysphagia and palpable abdominal mass which was mistaken on CT scan for gastric gastrointestinal stromal tumor.
Presentation of the case
A 60 years old male farmer presented with progressive dysphagia to solid food of 3 months duration. He has no history of smoking, diabetes or alcohol intake. Physical examination showed hard epigastric mass with poorly defined borders. Imaging suggested gastrointestinal stromal tumor found to be primary pancreatic body cancer at laparotomy. Biopsy later confirmed undifferentiated pancreatic cancer.
Discussion
The presentation of pancreatic ductal adenocarcinoma is nonspecific. Presence of clinical symptoms indicates advanced disease. Pancreatic body cancer has poor prognosis due to late presentation of the disease as compared to its counter pancreatic head cancer. CT scan has 85% diagnostic accuracy.
Conclusion
Both surgeons and radiologists should be familiar with common and uncommon CT scan findings of pancreatic ductal adenocarcinoma as this can avoid unnecessary invasive investigation or treatment.
Keywords: Pancreatic cancer, Palpable abdominal mass, Undifferentiated carcinoma, Dysphagia, GIST, ‘Case report’
Highlights
-
•
Dysphagia is a rare presentation of pancreatic cancer.
-
•
Undifferentiated pancreatic body cancer rarely presents with palpable mass.
-
•
Pancreatic cancer can be mistaken for gastric GISTs.
-
•
Preoperative tissue diagnosis can avoid unnecessary interventions in advanced pancreatic cancers.
1. Introduction
Pancreatic ductal adenocanocarcinoma is the commonest solid malignant tumor of the pancreas. It represents 85%–95% of all solid tumors of the pancreas [1]. Sixty to 70% of this tumor occurs in the pancreatic head while the remaining occurs in the body and tail. Undifferentiated pancreatic cancer is a rare variant of pancreatic adenocarcinoma which was reported to be 5.6% in a study of 6212 patients with pancreatic ductal adenocarcinoma [2].
PDACs have nonspecific manifestations and often start insidiously, invade locally and have distant spread before development of any clinical symptoms or signs [3]. Patients may present with jaundice, pruiritus, epigastric pain, nausea, anorexia and weight loss depending on the site of the tumor and/or stage of the tumor [3]. Undifferentiated carcinomas have similar clinical presentation with conventional PDACs [4]. However, patients with undifferentiated carcinoma have larger tumor at presentation and low median overall survival as compared to conventional PDAC (3 months vs. 11 months) [2].
Dysphagia is an extremely rare manifestation of PDAC. It occurs from either direct invasion of primary tumor, metastatic disease to the esophagus or lymph node adjacent to esophagus either causing mechanical obstruction or neural invasion of lower esophagus causing pseudoachalasia [5], [6], [7], [8], [9].
Presence of palpable mass points to diagnosis of mucinous cystadenoma/cystadenocarcinoma [10] or islet cell tumors as adenocarcinomas are rarely palpable prior to patients death [11]. Diagnostic CT scans may confuse PDAC with either pancreatic lesions like chronic pancreatitis, solid pseudopapillary tumor and endocrine tumors or peripancreatic lesions like gastrointestinal stromal tumors [12]. This work is reported in line with the SCARE 2020 criteria [13].
2. Presentation of the case
A 60 years old male farmer from Arsi, Ethiopia presented with progressive dysphagia to solid food of 3 months duration. In association with the above complaint, he has also dull epigastric pain radiating to the back, malaise, and fatigue. He has no history of smoking, diabetes or alcohol intake. Physical examination revealed a hard poorly defined epigastric mass.
Complete blood count (CBC), liver function tests and renal function tests were normal. Abdominal ultrasound was done with description of 6.6 × 5 cm gastric fundal mass with index of gastric carcinoma. Upper GI endoscopy done with report of multiple erosions and ulcerations seen in distal esophagus, gross bulge in the gastric fundus with normal mucosa and conclusion was gastric fundal sub mucosal mass secondary to ?gastrointestinal stromal tumor and erosive esophagitis. CT scan of abdomen was performed and commented by two different radiologists – revealed a 7.3 × 6.7 cm solid enhancing mass in gastro hepatic space with central cystic change with the impression of gastro hepatic space mass r/o GIST r/o neuroendocrine tumor(see Fig. 1, Fig. 2). Chest x-ray, ECG, and echocardiography were normal.
Fig. 1.

Solid enhancing mass with central cystic change pushing stomach anteriorly (arrow and arrowhead).
Fig. 2.

Solid enhancing mass with central cystic change pushing stomach to the left and against the diaphragm seen on coronal CECT (arrow and arrow head) respectively.
Exploratory laparotomy revealed a large retroperitoneal mass arising from body of pancreas. The mass extends to gastro-hepatic space invading gastric body partly, fundus, cardia and gastro-esophageal junction pushing the stomach and gastro-esophageal junction up wards and to the left (see Fig. 3, Fig. 4). There were lymph-node enlargements mainly near the mass and splenic hilum. Other areas were free. FNAC was done intra-operatively which revealed adenocarcinoma. Lymph-node biopsy showed undifferentiated carcinoma. Chemotherapy differed as the patient was not fit. Finally, patient died after two months.
Fig. 3.
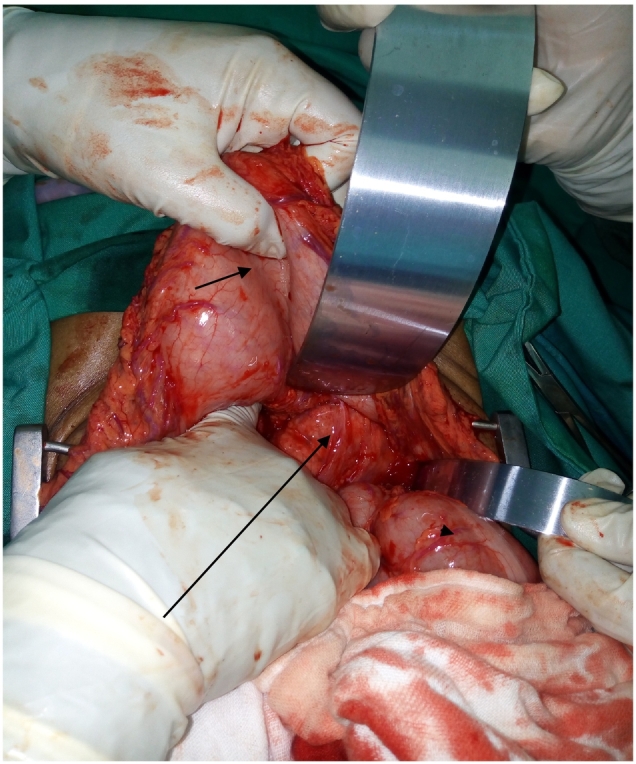
Intraoperative photo showing pancreatic body mass (long arrow) invading stomach (short arrow) posteriorly and transverse colon (arrowhead) retracted downward.
Fig. 4.
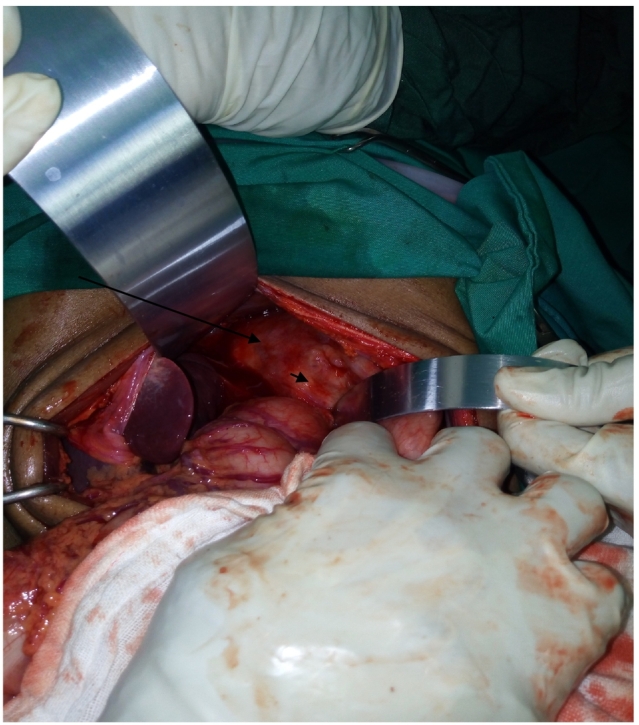
Intraoperative photo of the pancreatic body mass invading the cardia (short arrow), and extending all the way to diaphragm (long arrow).
3. Discussion
Pancreatic ductal adenocanocarcinoma is the commonest solid malignant tumor of the pancreas. Because of its poor prognosis pancreatic cancer is the seventh, fourth, and third leading cause of cancer death globally [14], in America [15], and in European countries [16] respectively. It is anticipated as the second leading cause of death by 2030 in America [17]. Overall 5 year survival rate is 8% with the current available treatment [15]. Undifferentiated pancreatic cancer is a rare variant of PDACs with low overall survival rate [2], and similar clinical presentation to the conventional PDACs [4].
Dysphagia is an extremely rare manifestation of PDAC. The mechanism could be from direct invasion of primary tumor which is possible in the case presented here; metastatic disease to the esophagus or lymph node adjacent to esophagus either causing mechanical obstruction or neural invasion of lower esophagus causing pseudoachalasia [5], [6], [7], [8], [9]. There is a report of dysphagia from pancreatic cancer metastasis to esophagus prior to diagnosis of the primary lesion [18].
In 2011, there was a case report 74-year-old Caucasian man who presented with dysphagia of several months. The final diagnosis was made by endoscopic ultrasound guided fine needle aspiration cytology to be adenocarcinoma of pancreatic tail invading the gastric wall causing a hemorrhagic cyst that compressed the esophagus leading to dysphagia. EUS is a well-known diagnostic modality to evaluate gastrointestinal submucosal lesions [8]. In 1954, there were three cases of dysphagia secondary to pancreatic cancer in the body in two patients and in the head of pancreas in one patient. Two of them had palpable abdominal mass as the case presented in here. Distant metastasis was seen in all the cases, in contrast to the case presented here [7].
Presence of palpable abdominal mass in patients with pancreatic cancer is a rare scenario these days because of wide spread diagnostic modalities and raised awareness for the disease as compared to earlier times. In 1959 out of 100 patients with pancreatic cancer, palpable mass seen in 36% of carcinoma of the body and tail of the pancreas and 15.6% in that of the head [19].
Multiphase contrast-enhanced CT scan is the first investigation of choice for the diagnosis of pancreatic cancer [20]. But there are multitude of both pancreatic and peripancreatic lesions that can cause diagnostic confusions on CT scans, of these being GISTs are one of them [12]. Therefore, radiologists should be familiar with the typical and unusual radiologic manifestations of pancreatic cancer. In the case presented here even though CT scan was commented by two different radiologists living in different cities both of them put gastric GIST as their first impression for which unnecessary laparotomy was done which later confirmed inoperable undifferentiated pancreatic body cancer.
Therefore, this case is of interest in many aspects as the patient presented with dysphagia secondary to a palpable primary pancreatic body cancer. Both dysphagia and palpable mass as a clinical presentation for less common undifferentiated pancreatic body adenocarcinoma is extremely rare. The other thing worth mentioning in this patient is there was no evidence of distant metastasis seen both on imaging and intra-operatively.
4. Conclusion
-
1.
Primary pancreatic body ductal adenocarcinoma can present with dysphagia and palpable mass even though extremely rare.
-
2.
Both surgeons and radiologists, especially those practicing in resource limited areas should be familiar with common and uncommon CT scan findings of PDAC as this can avoid unnecessary invasive investigation or treatment.
-
3.
Tissue diagnosis prior to surgical exploration decision is important with endoscopic ultrasound guided FNAC for sub mucosal tumors if available, if not image guided FNAC especially in large tumors as this may avoid unnecessary morbidity from surgery.
-
4.
Finally, absence of metastasis in the presence of large masses on imaging may not rule out PDAC.
Provenance and peer review
Not commissioned, externally peer reviewed.
Consent
Written informed consent was obtained from the patient's next of kin for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
The study is exempt from ethical approval in our setup.
Funding
This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Guarantor
Gosa Bejiga.
Research registration number
N/a.
CRediT authorship contribution statement
One author did all the work.
Declaration of competing interest
The author has no conflict of interest to declare.
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016 Mar 1;7(2):418–419. doi: 10.3945/an.116.012211. https://academic.oup.com/advances/article/7/2/418/4558126 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark C.J., Graham R.P., Arun J.S., Harmsen W.S., Reid-Lombardo K.M. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J. Am. Coll. Surg. 2012 Nov;215(5):627–634. doi: 10.1016/j.jamcollsurg.2012.06.418. https://linkinghub.elsevier.com/retrieve/pii/S1072751512009519 Available from: [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin W.R., Allen P.J., Chapman W.C., D’Angelica M.I., RP DeMatteo, Do R.K.G. Sixth edition. Elsevier; Philadelphia, PA: 2017. Blumgart’s Surgery of the Liver, Biliary Tract, and Pancreas. [Google Scholar]
- 4.Strobel O., Hartwig W., Bergmann F., Hinz U., Hackert T., Grenacher L. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery. 2011 Feb;149(2):200–208. doi: 10.1016/j.surg.2010.04.026. https://linkinghub.elsevier.com/retrieve/pii/S0039606010002278 Available from: [DOI] [PubMed] [Google Scholar]
- 5.Pesce A., Scilletta R., Branca A., Portale T.R., Puleo S. A rare case of pancreatic cancer presenting as pseudoachalasia. Ann. Ital. Chir. 2012 Sep;10:2012. [PubMed] [Google Scholar]
- 6.Moschoutis P., Soule J.-C. Dysphagia as a unique presenting symptom of pancreatic cancer. Dig. Dis. Sci. 1983 Jan;28(1) http://link.springer.com/10.1007/BF01393367 94–94. Available from: [Google Scholar]
- 7.Langton L., Laws J.W. Dysphagia in carcinoma of the pancreas. J. Fac. Radiol. 1954 Oct;6(2):134–138. doi: 10.1016/s0368-2242(54)80065-0. https://linkinghub.elsevier.com/retrieve/pii/S0368224254800650 Available from: [DOI] [PubMed] [Google Scholar]
- 8.Walia S., Sharif O., Moonka D., Olds G., Pompa R., Keller Christopher, DO Silvio Melo. Dysphagia secondary to pancreatic adenocarcinoma: 1026. Am. J. Gastroenterol. 2011 Oct;106 http://journals.lww.com/00000434-201110002-01024 Available from: [Google Scholar]
- 9.López Pardo P. Cause of dysphagia in elderly with dementia: pancreatic tail neoplasm. Digit. Syst. 2017;1(1) http://www.oatext.com/cause-of-dysphagia-in-elderly-with-dementia-pancreatic-tail-neoplasm.php Available from: [Google Scholar]
- 10.de Calan L., Levard H., Hennet H., Fingerhut A. Pancreatic cystadenoma and cystadenocarcinoma: diagnostic value of preoperative morphological investigations. Eur. J. Surg. 1995 Jan;161(1):35–40. [PubMed] [Google Scholar]
- 11.Brennan M.F. Blumgart’s Surgery of Liver, Biliary Tracts and Pancreas. third. W.B Saunders; 2000. Blumgart’s surgery of liver, biliary tracts and pancreas; pp. 1059–1071. [Google Scholar]
- 12.Yang M.-J., Li S., Liu Y.-G., Jiao N., Gong J.-S. Common and unusual CT and MRI manifestations of pancreatic adenocarcinoma: a pictorial review. Quant. Imaging Med. Surg. 2013;3(2):8. doi: 10.3978/j.issn.2223-4292.2013.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. https://linkinghub.elsevier.com/retrieve/pii/S1743919120307718 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021 Feb 4;71(3):209–249. doi: 10.3322/caac.21660. doi: 10.3322/caac.21660. https://onlinelibrary.wiley.com/doi/10.3322/caac.21660 Available from: caac.21660. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018: cancer statistics, 2018. CA Cancer J. Clin. 2018 Jan;68(1):7–30. doi: 10.3322/caac.21442. http://doi.wiley.com/10.3322/caac.21442 Available from: [DOI] [PubMed] [Google Scholar]
- 16.Ferlay J., Partensky C., Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016 Oct 2;55(9–10):1158–1160. doi: 10.1080/0284186X.2016.1197419. https://www.tandfonline.com/doi/full/10.1080/0284186X.2016.1197419 Available from: [DOI] [PubMed] [Google Scholar]
- 17.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014 Jun 1;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. http://cancerres.aacrjournals.org/lookup/doi/10.1158/0008-5472.CAN-14-0155 Available from: [DOI] [PubMed] [Google Scholar]
- 18.Burns E.A., Kasparian S., Khan U., Abdelrahim M. Pancreatic adenocarcinoma with early esophageal metastasis: a case report and review of literature. WJCO. 2020 Feb 24;11(2):83–90. doi: 10.5306/wjco.v11.i2.83. https://www.wjgnet.com/2218-4333/full/v11/i2/83.htm Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gullick H.D. Carcinoma of the pancreas. a review and ciritcal study of 100 cases. Medicine. 1959 Feb;38(1):47. doi: 10.1097/00005792-195902000-00003. http://journals.lww.com/00005792-195902000-00003 Available from: [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Bai X., Bian D., Cai S., Chen R., Cao F. Guidelines for the diagnosis and treatment of pancreatic cancer in China (2021) J. Pancreatol. 2021 Jun;4(2):49–66. https://journals.lww.com/10.1097/JP9.0000000000000072 94–94. Available from: [Google Scholar]


