Abstract
Carbohydrate metabolism not only functions in supplying cellular energy but also has an important role in maintaining physiological homeostasis and in preventing oxidative damage caused by reactive oxygen species. Previously, we showed that arthropod embryonic cell lines have high tolerance to H2O2 exposure. Here, we describe that Rhipicephalus microplus tick embryonic cell line (BME26) employs an adaptive glucose metabolism mechanism that confers tolerance to hydrogen peroxide at concentrations too high for other organisms. This adaptive mechanism sustained by glucose metabolism remodeling promotes cell survival and redox balance in BME26 cell line after millimolar H2O2 exposure. The present work shows that this tick cell line could tolerate high H2O2 concentrations by initiating a carbohydrate-related adaptive response. We demonstrate that gluconeogenesis was induced as a compensation strategy that involved, among other molecules, the metabolic enzymes NADP-ICDH, G6PDH, and PEPCK. We also found that this phenomenon was coupled to glycogen accumulation and glucose uptake, supporting the pentose phosphate pathway to sustain NADPH production and leading to cell survival and proliferation. Our findings suggest that the described response is not atypical, being also observed in cancer cells, which highlights the importance of this model to all proliferative cells. We propose that these results will be useful in generating basic biological information to support the development of new strategies for disease treatment and parasite control.
Keywords: glucose, metabolism, embryogenesis, ROS, arthropod
Abbreviations: ETS, electron transport system; G6P, glucose 6-phosphate; G6PDH, glucose-6-phosphate dehydrogenase; GDE, glycogen debranching enzyme; GS, glycogen synthase; GSK3β, glycogen synthase kinase 3 β; HK, hexokinase; ICDH, isocitrate dehydrogenase; IGF, insulin-like growth factor; ISP, insulin-signaling pathway; LDH, lactate dehydrogenase; OCR, oxygen consumption rate; PEPCK, phosphoenolpyruvate carboxykinase; PK, pyruvate kinase; ROS, reactive oxygen species
Embryogenesis has been typically described as an energy-consuming process (1, 2). In the context of arthropods, the egg constitutes a closed system that relies solely on yolk contents for embryo energy supply and organ development (3, 4, 5). Therefore, once laid, the egg receives no nutrients from the external environment. An important model to study embryogenesis is Drosophila melanogaster, which also has contributed to the understanding of cancer, neurological disorders, cardiovascular diseases, and several other human diseases. Genetic conservation between flies and humans is substantial, with 75% of known human disease genes having related sequences in Drosophila (6). Even under physiological conditions, fundamental metabolic processes, including energy metabolism, are well conserved among Drosophila and other animals (6). Consequently, hypotheses generated using this model are often relevant to many different areas of biological sciences. In cancer studies, Drosophila has served as a platform to develop models that recapitulate various aspects of the disease and its use has contributed to reveal different aspects of human malignant tumors (7, 8).
Research in Drosophila has served to determine how components of the insulin/insulin-like growth factor (IGF) system, including Rheb, Tsc1, and Tsc2, are organized in the insulin/Akt pathway and has contributed to define how insulin/Akt controls systemic and cellular growth (9, 10, 11). Those studies have expanded our knowledge of normal physiological roles these oncogenes and tumor suppressors play, as well as how their dysregulation promotes disease (12, 13, 14). Previous work by our group investigated the insulin-signaling pathway (ISP) and its possible role during tick embryogenesis, a controlled process of cell proliferation and differentiation, showing that those mechanisms are conserved in phylogenetically distant organisms, like insects, ticks, and mammals (15, 16). Also, determining if these aspects related to carbohydrate metabolism are relevant in the context of tumorigenesis and cancer metabolism is of great importance to support new cancer therapies.
A common feature of cancer cell metabolism is the ability to acquire necessary nutrients from a frequently nutrient-poor environment and utilize these nutrients to both maintain viability and build new biomass. Alterations in intracellular and extracellular metabolites that can accompany cancer-associated metabolic reprogramming have profound effects on gene expression, cellular differentiation, and tumor microenvironment (17). The major use of reduced carbon in proliferating cells is for the biosynthesis of a diverse array of biomolecules, among which are fatty acids and cholesterol, pentose and hexose sugar derivatives, glycerol, nucleotides, and nonessential amino acids. This realization has spurred further exploration of advantages that the uncoupling of glycolysis from oxidative phosphorylation might offer to a proliferating cell. Although glycolysis is classically depicted as a single chain of molecular events that leads to the generation of pyruvate, a number of glycolytic intermediates can be diverted into branching pathways, generating diverse biosynthetic precursors (17). The preferential conversion of glucose into lactate, an altered metabolic feature typical of cancer cells known as the Warburg effect, has also been demonstrated in genetically normal proliferating cells, as well as in cells infected by viruses (18, 19, 20). These observations suggest that, rather than being an adaptation to defective respiration, the Warburg effect is a regulated metabolic state and may in fact be beneficial under a situation of increased biosynthetic demand (17).
Interest in cancer metabolism has grown rapidly in recent years, with an emphasis on understanding how cancer metabolism is reprogrammed and regulated, its influence on disease progression, and how these factors could be exploited to improve cancer therapy (21, 22, 23). In particular, genetic alterations that target PI-3 kinase, its negative regulators PTEN and INPP4B, as well as activating mutations and gene amplifications in a variety of upstream tyrosine kinase receptors, result in constitutive glucose uptake and metabolism in diverse cancer types (7, 24, 25). Being a point of convergence of signals from tyrosine kinase receptors as well as from the extracellular matrix, PI3K/Akt signaling represents a master regulator of glucose uptake. Metabolic reprogramming can induce oxidative stress tolerance and tumor progression in cancer cells (26, 27). Curiously, arthropod cells show a plasticity that supports higher tolerance to reactive oxygen species (ROS) when compared with mammalian cells, remarkably similar to the tolerance observed in cancer cells (28).
Aided by biochemical and molecular biology tools, arthropods are emerging as valuable models for studying cancer metabolism, to expand the current understanding of the mechanisms and functional consequences of tumor-associated metabolic alterations (7, 17). Therefore, arthropods have contributed to the study of mechanistic relationships between metabolism and cancer. Metabolic changes are increasingly recognized as important drivers of malignancy, and cancer-specific metabolic alterations are becoming an important research theme. Arthropods have been broadly used to study how metabolism is orchestrated to support biological processes, including growth, proliferation, and differentiation in normal development (5, 23, 29, 30, 31). The fact that these organisms share a gene conservation with humans and use similar metabolic reprogramming strategies can make them a valuable tool for comparative analysis with cancer metabolism. In addition, some arthropods have other experimental advantages, including a short life cycle and the ease with which large numbers of individuals can be examined in well-targeted genetic screens. In this context, the tick embryonic cell line, BME26 (31, 32), showed tolerance and resistance to oxidative stress similar to many different types of cancer cells, and the present study explores its potential as a useful model of glucose metabolism in comparative and cancer research.
Results
Warburg effect and glycogen accumulation in response to oxidative challenge in arthropod embryonic cells
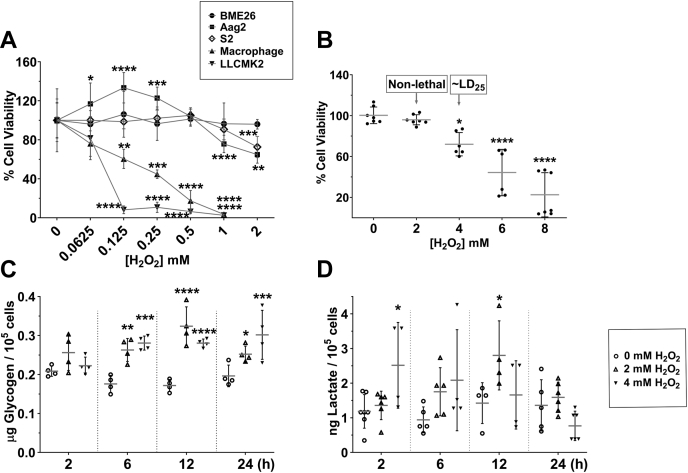
The effect of oxidative conditions on cell survival was compared among tick and other animal cells, including insect and mammals, to begin investigating the metabolic mechanisms related to H2O2 tolerance in BME26 tick embryonic cells after exposure to H2O2 (33). In general, with concentrations ranging from 62.5 to 1000 μM H2O2, arthropod embryonic cells (BME26 from Rhipicephalus microplus, Aag2 from Aedes aegypti, and S2 from D. melanogaster) were more tolerant than mammalian cells (primary culture of mouse macrophages and LLCMK2 kidney epithelial cells from Rhesus monkey) (Fig. 1A). The cell viability assay showed that S2 cells can tolerate up to 1 mM H2O2. Both mammalian cell lines presented H2O2 -induced cytotoxic effects leading to reduced cellular viability; LD50 values ranged between 125 to 250 μM H2O2 after 24 h of treatment (Fig. 1, A and B), in agreement with previous findings (30). In contrast, the mosquito embryonic cell line Aag2 exhibited cell proliferation at concentrations ranging from 125 to 250 μM H2O2, with up to 30% increase in viable cell numbers. Only at the highest tested concentration (1000 μM), H2O2 reduced by 20% the number of viable Aag2 cells (Fig. 1A). BME26 cells were unaffected by H2O2 at concentrations from 62.5 to 2000 μM.
Figure 1.
The Warburg effect and glycogen accumulation in response to oxidative challenge in arthropod embryonic cells.A, cell viability (trypan blue exclusion) was compared after challenge with H2O2 (62.5–1000 μM), in five different cell lineages, for 24 h: embryonic Rhipicephalus microplus tick cells (BME26), closed circles; embryonic Aedes aegypti cells (Aag2), closed squares; embryonic Drosophila melanogaster cells (S2), open diamond; BLACK6 mouse macrophage cells (primary culture), triangle up; renal epithelial Rhesus monkey cells (LLMCK2), triangle down. Control culture was performed at 0 μM H2O2 (100% of cells). B, BME26 cell viability upon H2O2 challenge (0–8 mM). Glycogen and lactate quantification over time (2, 6, 12, and 24 h) after H2O2 challenge in BME26 (C and D, respectively). Experiments were performed in three independent biological samples with three experimental replicates each, where ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared with the control, in Tukey’s multiple comparisons test.
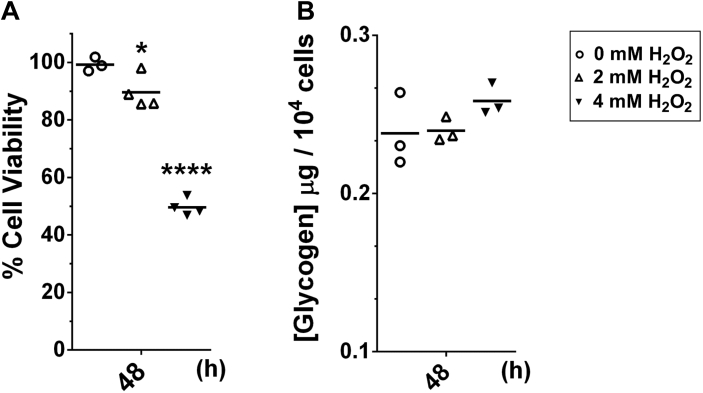
The Warburg effect is well characterized in normal as well as highly proliferative cells, with high glucose uptake and conversion to lactate. We next analyzed glycogen and lactate levels in BME26 cells exposed to 2 or 4 mM H2O2 during 2, 6, 12, or 24 h. The analysis showed that BME26 cells accumulate glycogen up to 6 h after H2O2 addition, maintaining high glycogen levels until 24 h, in both H2O2 concentrations (Fig. 1C). After 48 h, the glycogen content returned to the same level observed in the control group (Fig. S1). Lactate levels increased in BME26 cells under 4 mM H2O2 at 2 and 6 h and under 2 mM at 12 h (Fig. 1C). The Aag2 insect cell line also showed an increase in glycogen level after 6 and 12 h of exposure to 2 mM H2O2 (Fig. S2).
ROS trigger increased glycogen levels in BME26 embryonic cells
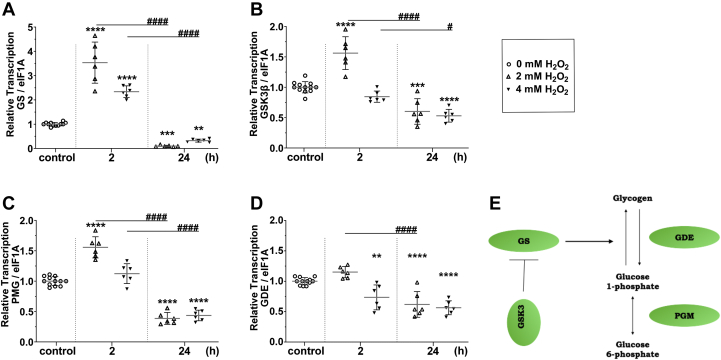
To analyze glycogen metabolism in BME26 cells after H2O2 challenge, we analyzed the transcription of selected glycogen metabolism genes. Figure 2, A–D shows the transcriptional profile of glycogen synthase (GS), glycogen synthase kinase 3 β (GSK3β), phosphoglucomutase, and glycogen debranching enzyme (GDE) after exposure to 2 or 4 mM H2O2 for 2 h and 24 h. GS, the enzyme responsible for glycogen synthesis, showed increased transcription after 2 h at both H2O2 concentrations, being highest at 2 mM (Fig. 2A). GSK3β, the enzyme that inhibits GS activity, presented a transcriptional profile similar to that of GS, except after 2 h at 4 mM, when GSK3β transcription was similar to the control (Fig. 2B). Phosphoglucomutase is an enzyme present in both glycogen synthesis (glucose 6-phosphate [G6P] to glucose 1-phosphate) and degradation (glucose 1-phosphate to G6P) pathways. Its transcription in BME26 cells after oxidative challenge followed a profile similar to that of GSK3β (Fig. 2C). In turn, GDE transfers three of the four glucose residues to other glycogen branches after the glycogen phosphorylase step. Its transcription in BME26 cells 2 h after oxidative challenge remained equal to the control condition, with a reduction in mRNA levels 24 h after H2O2 exposure (Fig. 2D). Thus, the transcription of genes related to glycogen metabolism in BME26 cells is shown to be regulated by oxidative conditions.
Figure 2.
Oxidative challenge upregulates transcriptional levels of glycogen synthesis components in BME26 cells. Transcription of (A) glycogen synthase (GS), (B) glycogen synthase kinase 3 β (GSK3β), (C) phosphoglucomutase (PGM), and (D) glycogen debranching enzyme (GDE) in Rhipicephalus microplus embryonic cell line (BME26) was measured 2 and 24 h after addition of H2O2 (2 or 4 mM). E, diagram of glycogen metabolism depicting pathways involved in glycogen synthesis and degradation. Experiments were performed in three independent biological samples with three experimental replicates each, where ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared with the control; and #p < 0.05, ####p < 0.0001, compared between the groups, in Tukey’s multiple comparisons test.
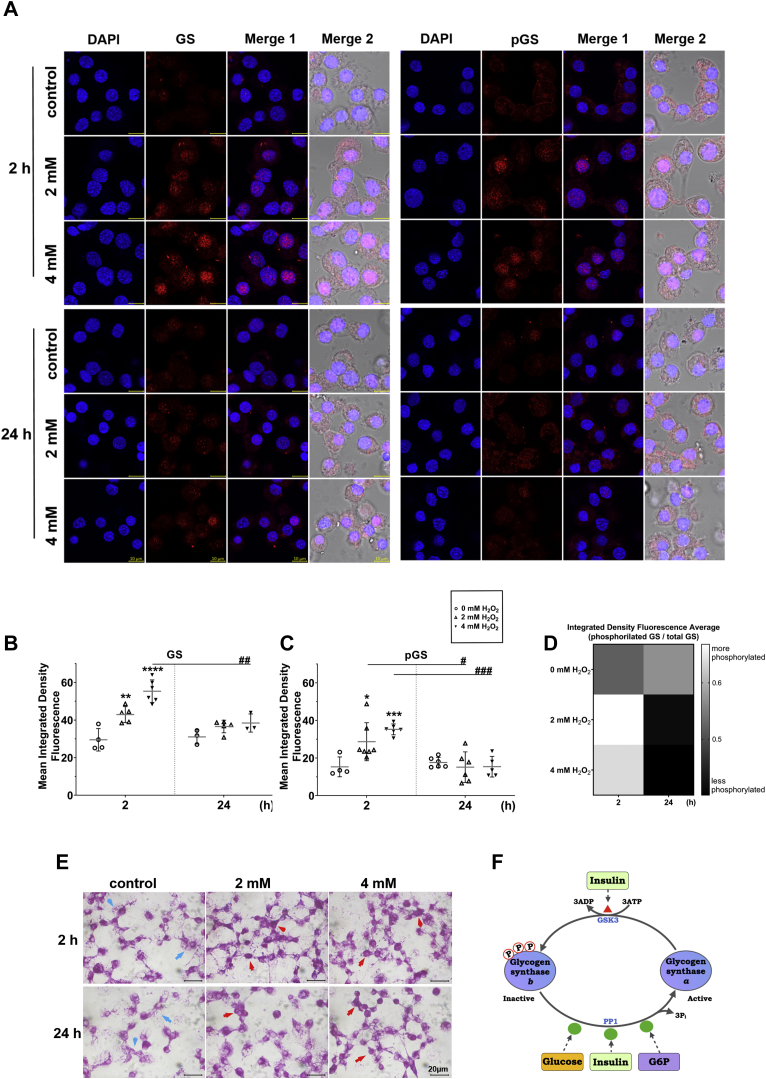
To further understand glycogen metabolism in BME26 cells treated with hydrogen peroxide, GS immunolocalization was performed, using antibodies that allow the detection of both total GS content and its inhibited (Ser641 phosphorylated) form. Cells were also treated with DAPI to stain the nuclei for imaging (Fig. 3A).
Figure 3.
Glycogen metabolism regulation in BME26 cells upon H2O2challenge. H2O2-tolerant BME26 cells increased glycogen synthase protein expression 2 h after challenge with 2 or 4 mM H2O2. A, immunolocalization (red signal) of phosphorylated Ser641 glycogen synthase (pGS) or total GS, as well as nuclei staining (blue signal), was performed after 2 or 4 mM H2O2 challenge, for 2 and 24 h. Images were captured in a confocal laser scanning microscope, LSM 710, Zeiss. The scale bar represents 10 μm. B–D, quantitative analysis of GS (B), pGS (C), and active GS (D). E, periodic acid–Schiff staining of BME26 cells after treatment with 2 and 4 mM H2O2 for 2 and 24 h. Blue arrows indicate diffuse staining and red arrows indicate perinuclear staining. Light microscope images were captured in bright field using Axio Scope.A1, Zeiss polarized light microscope through the Blue Zeiss software. The scale bar represents 20 μm. Analyses were performed on three independent biological samples with three experimental replicates. F, schematic depiction of glycogen synthase activity regulation in vertebrates, which is downregulated by phosphorylation (including at Ser641) via GSK3β (inhibited via insulin signaling), while glucose and glucose 6-phosphate upregulate GS activity in an allosteric fashion, and by PP1-mediated dephosphorylation (prompted by insulin signaling).
Images show that, under both H2O2 conditions, there was an increase in total as well as phosphorylated GS, colocalized with DAPI-stained cell nuclei (Fig. 3, B–D), in 2 h. The phosphorylated form, even with low catalytic activity, is still regulated by intracellular levels of G6P, its allosteric activator. After 24 h, the cellular distribution of the two forms of GS (active and inactive) is equivalent between the treated cells and the control condition (Fig. 3A, bottom).
The amount of GS protein also increased 2 h after H2O2 challenge, the phosphorylated form. When phosphorylated, GS is less active compared with the nonphosphorylated form. However, the phosphorylated form is also regulated allosterically by the amount of G6P, which leads to glycogen synthesis, a possible cause for the glycogen accumulation observed 6 h after H2O2 challenge (Fig. 1C). In addition, glycogen staining by Periodic acid–Schiff was performed to identify glycogen compartmentalization (Fig. 3E). We were able to see strong perinuclear staining (red arrows) after H2O2 challenge compared with a more diffuse marking in the untreated control (blue arrows). Differences are most evident 24 h after oxidative challenge is applied to BME26 cells. A representative scheme of the regulation of GS is described in Fig. 3F.
Gluconeogenesis–insulin pathways contribute to glycogen accumulation
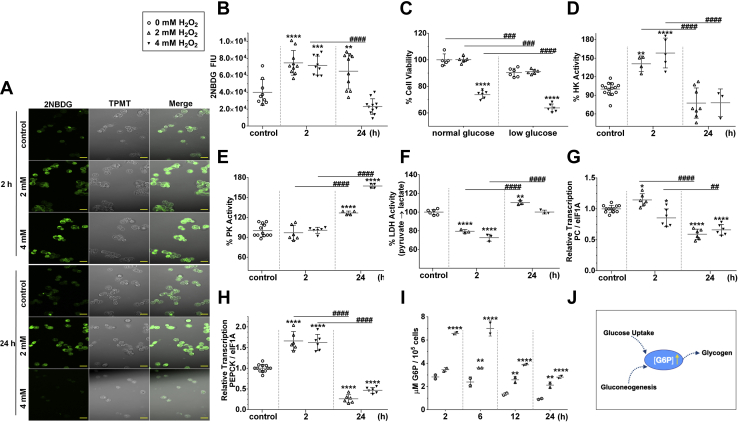
To investigate carbohydrate metabolism, particularly concerning glycogen, glucose internalization was measured, showing that the staining of 2NBDG, a glucose analogue, increased after H2O2 exposure (Fig. 4, A and B). Decrease in 2NBDG staining was observed only 24 h after exposure to 4 mM H2O2 (Fig. 4, A and B). The exposed cells were also allowed to grow in two different media, namely, low or normal glucose concentration, and a cell viability assay was performed. A decrease in cell viability was observed at the highest H2O2 challenge condition, 4 mM. However, under low-glucose conditions, this reduction in viability was more pronounced compared with the normal glucose condition (Fig. 4C). The activity of regulatory enzymes of the glycolytic pathway also changed after the administration of H2O2. Hexokinase (HK) activity increased 2 h after H2O2 challenge and decreased to levels similar to the control condition after 24 h (Fig. 4D). Pyruvate kinase (PK) activity profile was the inverse of that found for HK activity, with an increased activity 24 h after H2O2 exposure for both treatments (Fig. 4E). The activity of lactate dehydrogenase (LDH), an enzyme involved in anaerobic glycolysis, was lower within 2 h after challenge with either concentration of H2O2 compared with control conditions and increased by 24 h after H2O2 exposure (Fig. 4F). Pyruvate carboxylase transcription was also investigated, presenting an increase 2 h after challenge with 2 mM H2O2, but not 4 mM, compared with control (Fig. 4G). At 24 h, however, the transcriptional level of pyruvate carboxylase had decreased in response to both oxidative challenges (Fig. 4G). Phosphoenolpyruvate carboxykinase (PEPCK) transcription increased under both H2O2 concentrations for 2 h and decreased to levels below that of the control condition 24 h later (Fig. 4H). The levels of G6P increased after H2O2 challenge in both treatments (Fig. 4I). Figure 4J summarizes the suggested pathway leading to the production of G6P, both by glucose uptake and gluconeogenesis. Subsequently, G6P is used for glycogen synthesis.
Figure 4.
Gluconeogenesis is related to glycogen stores in H2O2-challenged BME26 cells.A, glucose analogue (2NBDG) uptake after either 2 or 4 mM H2O2 treatment for 2 or 24 h. The scale bar represents 20 μm. B, glucose uptake quantification based on A. C, BME26 cell viability (trypan blue exclusion) after 2 or 4 mM H2O2 challenge in normal or low-glucose media (see Experimental procedures for details). Enzyme activity for hexokinase (HK) (D), pyruvate kinase (PK) (E), and lactate dehydrogenase (LDH) (F) in BME26 cells in response to 2 or 4 mM H2O2 after 2- and 24-h exposure. Transcriptional analysis for pyruvate carboxykinase (PC) (G) and phosphoenolpyruvate carboxykinase (PEPCK) (H) in BME26 cells in response to 2 or 4 mM H2O2 after 2- and 24-h exposure. I, quantification of glucose 6-phosphate in BME26 cells over time after treatment with H2O2 (2, 6, 12, and 24 h). Experiments were performed with three independent biological samples in three experimental replicates each, where ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared with control; and ##p < 0.01, ###p < 0.001, ####p < 0.0001, compared between the groups, in Tukey’s multiple comparisons test. J, schematic depiction of increased G6P content (due to either increased glucose uptake or gluconeogenesis) toward glycogen formation.
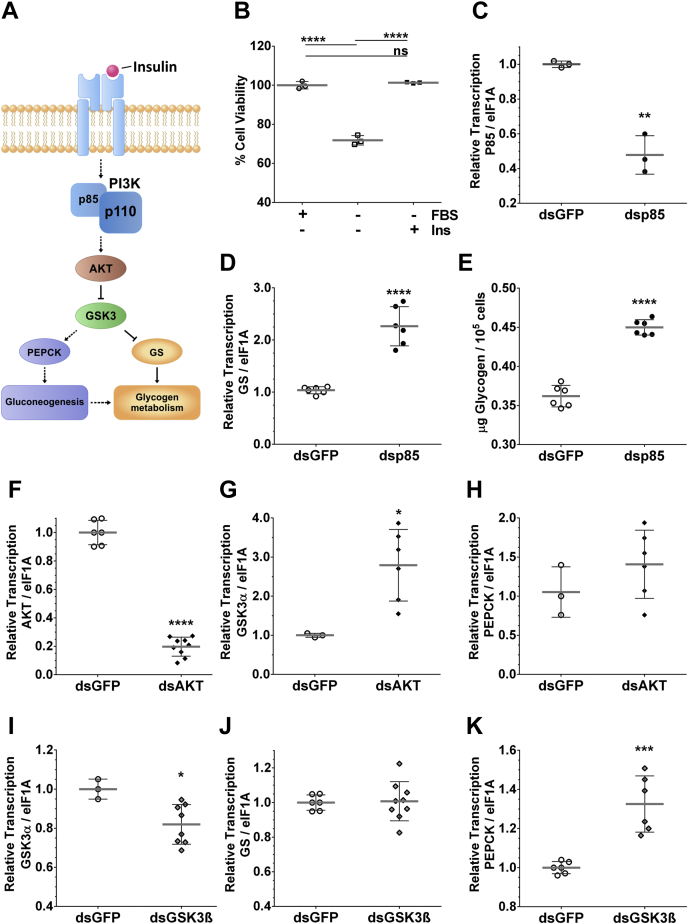
The ISP is involved in glucose uptake and glycogen metabolism. We confirmed that BME26 cells are responsive to insulin (Fig. 5B) and performed experiments using RNAi knockdown of the p85 Regulatory Subunit of PI3K (Fig. 5C). GS transcription increased after p85 knockdown (Fig. 5D), as did the glycogen levels (Fig. 5E). On the other hand, AKT knockdown (Fig. 5F) also increased GS transcription (Fig. 5G) but did not affect PEPCK transcription (Fig. 5H), whereas GSK3β knockdown (Fig. 5I) increased both GS and PEPCK transcription (Fig. 5, J and K).
Figure 5.
The conserved axis of insulin pathway is closely correlated to glycogen metabolism and gluconeogenesis in BME26 cells.A, partial schematic depiction of the insulin signaling pathway and its contribution to glycogen metabolism. Indicated components were further analyzed. Straight arrows, single-step process; dotted arrow, multistep process; arrowhead, activation; blunt arrowhead, inhibition. B, BME26 cells respond to the addition of exogenous insulin (400 nM), recovering the viability lost by removing fetal bovine serum for 24 h (C) PI3K regulatory subunit (p85) gene silencing validation and its effects on glycogen synthase (GS) transcript levels (D) and glycogen content (E). Protein kinase B (AKT) and GSK3β gene silencing validation (F and I, respectively), and its effects on GS (G and J) and PEPCK (H and K) transcript levels, respectively.
Mitochondrial oxidative control is related to the carbohydrate metabolic compensation coupled to high tolerance to oxidative stress in tick cells
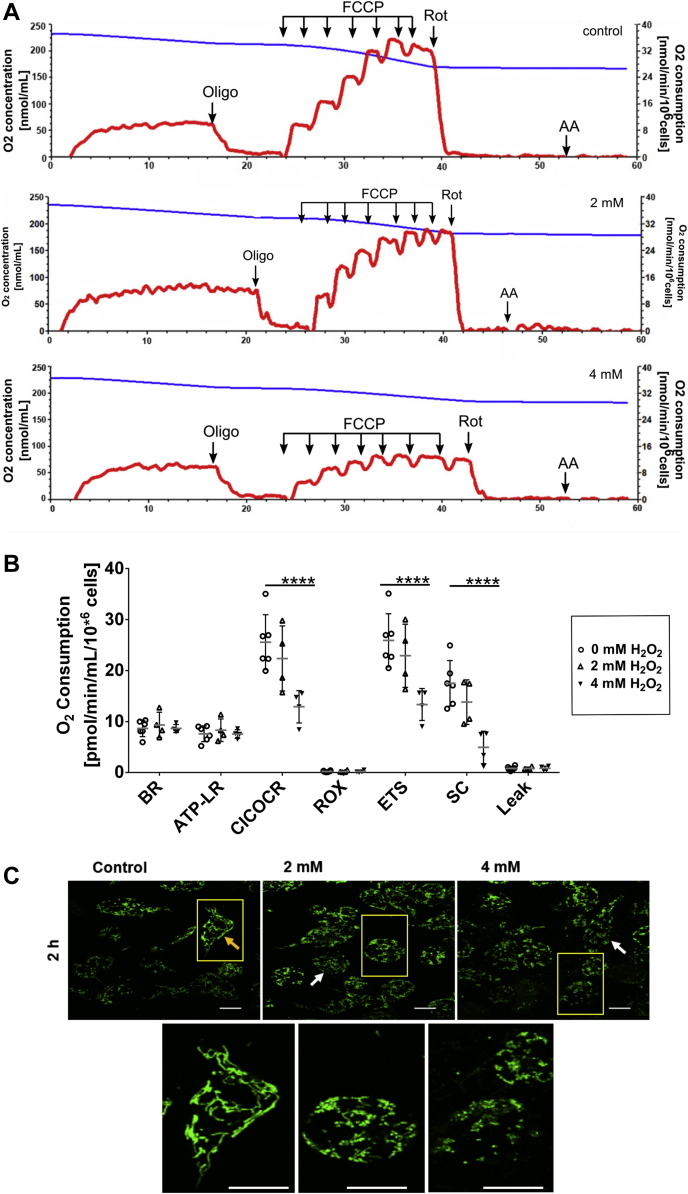
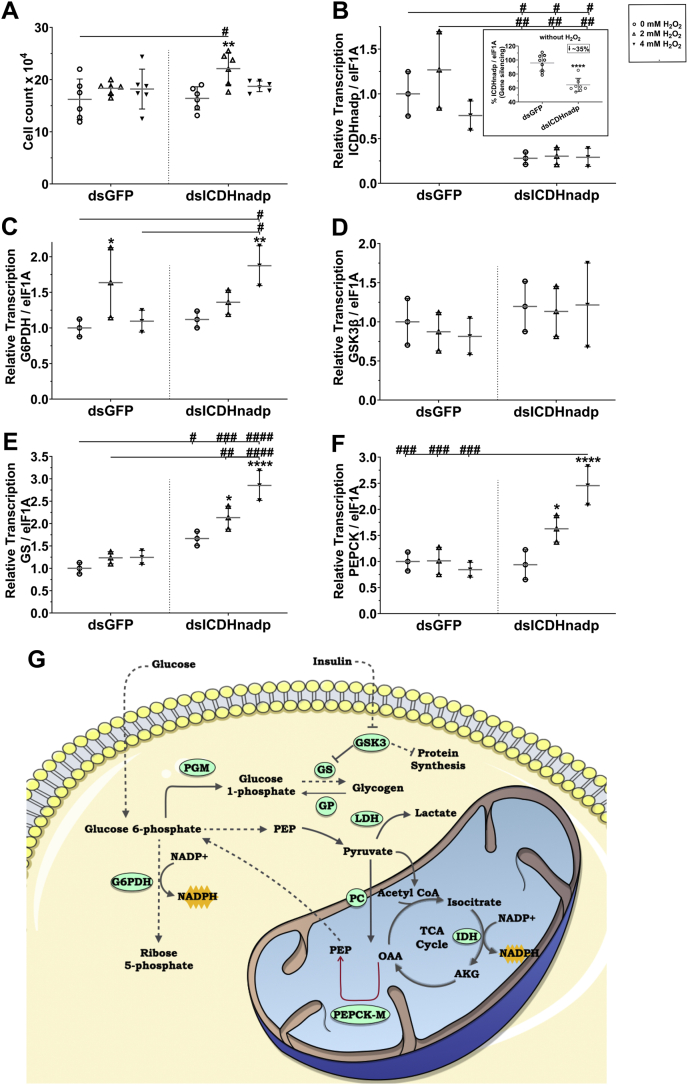
Anaerobic glycolysis is the main pathway responsible for supplying the cell with both ATP and NADH, usually associated with the Warburg effect. Therefore, we investigated a possible switch from oxidative to nonoxidative metabolism during oxidative challenge (Fig. 6). Representative traces of oxygen consumption by BME26 cells after exposure to H2O2 are shown in Figure 6A. The respiratory complex I transfers electrons from the NADPH into the electron transport chain. We measured the complex I coupled oxygen consumption rate (OCR) after inhibition of complex I with rotenone, which decreased after 2 h of H2O2 treatment at both concentrations, suggesting a reduction in the energy supply by the mitochondria (Fig. 6B). Of interest, the oxygen consumption coupled to ATP synthesis (ATP-linked respiration) was not altered by H2O2 treatment (Fig. 6B). The maximum respiratory rate of the electron transport system (ETS), induced by uncoupling of respiration, also was significantly decreased after H2O2 exposure (Fig. 6B). The spare respiratory capacity showed a similar profile, suggesting that, although ATP synthesis through oxidative phosphorylation is unchanged, the maximal capacity provided by the mitochondria is reduced, indicating a possible switch to nonoxidative metabolism during oxidative challenge (Fig. 6B). The mitochondrial morphology was accessed by MitoTracker Green staining, to observe mitochondrial change shape. H2O2 treatment indicates a dynamic morphological change (Fig. 6C), with a reduction of elongate mitochondrial shape (yellow arrow) and greater number of dotted mitochondria morphology (white arrows, yellow highlight square below). After silencing of NADPH-dependent isocitrate dehydrogenase (ICDH) (Fig. 7B), cell viability is not reduced (Fig. 7A), but the transcription of glucose-6-phosphate dehydrogenase (G6PDH), GS, and PEPCK increased (Fig. 7, C–E). The supply of glycogen via gluconeogenesis and the compensatory relationship between G6PDH and HDI are shown in the diagram (Fig. 7F).
Figure 6.
Decrease in mitochondrial respiratory rates induced by H2O2treatment on BME26 cells.A, typical traces of oxygen consumption rates (OCR, red line) and oxygen concentration (blue line) of BME26 cells after treatment with 2 and 4 mM H2O2 for 2 h. B, oxygen consumption rates from BME26 cells (open circle), and after treatment with 2 (open triangle up) or 4 mM (triangle closed down) H2O2 for 2 h. Data are expressed as mean ± standard deviation (SD) of five different experiments. Comparisons between groups were done by two-way ANOVA for repeated measurements and a posteriori Holm–Sidak test, adjusted for multiple comparisons (p < 0.001). C, fluorescence images of mitochondrial membrane of BME26 cells stained with 200 nM MitoTracker Green (λ ex = 488 nm, λ em = 500–530 nm) after 2-h oxidative challenge. Yellow boxes indicate a digital zoom of one cell. Yellow arrow shows mitochondria elongated shape, whereas white arrows show dotted mitochondria shape. The scale bar represents 100 μm.
Figure 7.
Metabolic remodeling involves simultaneous gluconeogenesis and mitochondrial activity in BME26 cells.A, viable NADP-ICDH knocked down BME26 cells after H2O2 treatment. B, NADP-ICDH gene silencing validation and its effects on the transcript levels of glucose-6-phosphate dehydrogenase (G6PDH) (C), glycogen synthase kinase 3 β (GSK3β) (D), glycogen synthase (GS) (E) and phosphoenolpyruvate carboxykinase (PEPCK) (F), as measured 2 and 24 h after 2 or 4 mM H2O2 addition, in BME26 cells. G, schematic representation of the proposed metabolic remodeling in BME26 in response to H2O2 challenge. ∗p <0.05; ∗∗p <0.01; ∗∗∗∗p < 0.0001; compared with the control without H2O2 within each H2O2 concentration of same dsRNA treatment; and #p <0.05; ##p <0.01; ###p <0.001; ####p <0.0001 compared with dsRNA nonrelated (dsGFP) within dsICDHnadp gene silencing, in Tukey’s multiple comparisons test.
Discussion
A markedly increased consumption of glucose by tumors in comparison with the nonproliferating normal tissues was first described more than 90 years ago by the German physiologist Otto Warburg (34, 35, 36). However, tumor metabolism has only recently been recognized as a hallmark of cancer, becoming a topic of a renewed interest (17, 37). More than 90 years after the proposal that the Warburg effect is responsible for maintaining cancer cell homeostasis, many authors now consider this an essential phenomenon to support the metabolic demand in these cells (38). In addition, tumorigenesis is also dependent on changes in more complex cellular metabolism, involving genetic and interaction controls (39). Cancer-promoting changes include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis, which can result in the death of the organism (37).
Lactate is the product of pyruvate degradation in the cytoplasm. For a long time, it was believed to be produced only under hypoxia. Lactate production depends on many factors and is not influenced only by the lactic anaerobic system. In addition, large concentrations of lactate are present in neoplastic cells, even at rest, a phenomenon known as the Warburg effect, as discussed above, which can occur due to the high metabolic rate of tumor cells. Interesting, BME26 cells under oxidative H2O2 challenge presented short periods of increased lactate, which happened earlier (between 2 and 6 h) after 4 mM treatment than after 2 mM treatment (12 h) (Fig. 1C). Studies in R. microplus ticks indicate that there is a mutual regulation among major enzymes of glucose metabolism at the transcriptional and enzymatic levels (40). It was shown that gluconeogenesis makes a significant contribution to maintain the energy balance in the late stages of R. microplus and A. aegypti embryo development (41, 42, 43). In this case, these arthropods use nonglycosidic compounds, such as lactate and amino acids, to resynthesize glycogen through gluconeogenesis. These findings suggest that glucose may form a wide range of substrates through this pathway, ensuring precursors are available for biosynthesis processes. The enzyme GS works primarily to regulate glycogen synthesis (Fig. 2E). An increase in GS transcription level was observed in BME26 cells challenged with 2 mM H2O2 (Fig. 2A). In addition, the transcription of GSK3β, a regulator of GS, decreased after treatment with H2O2 at 2 mM, but not 4 mM (Fig. 2B). The transcription of enzymes involved in glycogen degradation, such as phosphoglucose isomerase and GDE, also increased under 2 mM of H2O2 at 2 h, followed by a decrease at 24 h (Fig. 2, C and D). Previously published data show that R. microplus metabolism is responsive to and dependent on insulin signaling (16). Furthermore, the amount of GS (Fig. 3, A and B) and pGS (Fig. 3, A and C) increased in response to H2O2 challenge, specially at 2 h of exposure. However, the pGS/GS ratio reveals an increase in unphosphorylated GS, the active form of the enzyme, after 24 h at both H2O2 concentrations tested and as early as 2 h after challenge at the highest concentration (Fig. 3D), suggesting that glycogen metabolism is activated. The glycogen staining follows the same profile, increasing with the oxidative challenge (Fig. 3E). The increase in the phosphorylation of serine 9 on GSK3β (inhibited enzyme) after H2O2 challenge corroborates with the increased GS activity (Fig. S3). Thus, glycogen resynthesis is likely being activated in response to H2O2 in BME26 cells, suggesting that R. microplus tick cells perform a metabolic adaptation that leads to tolerance while maintaining redox balance. Moreover, BME26 cells have been previously shown to be responsive to insulin signaling (16), and we have successfully used insulin as a positive control in 2-NBDG internalization experiment (31).
Glucose supply is required to support glycogen synthesis. There is also a correlation between glucose uptake and increased glycogen content after H2O2 challenge, as demonstrated here (Fig. 4, A and B). The redox imbalance led to increased glucose uptake in BME26 cells, and the enzyme responsible for glucose phosphorylation, HK, responded by increasing its activity after 2 h of exposure to H2O2 at both concentrations (Fig. 4D). PK, a glycolysis regulator that acts downstream of HK, also increased its activity within 24 h of exposure to both H2O2 concentrations (Fig. 4D). Therefore, glucose may be the key substrate that drives this metabolic adaptation in BME26 cells during redox imbalance, since glycolysis appears to be accelerated after H2O2 challenge and glucose uptake is high under this same condition. In fact, cell viability was significantly lower at low glucose concentrations when challenged with 4 mM H2O2 (Fig. 4C), and LDH activity increases at the same time point when PK activity increases (Fig. 4E). Characteristic of the Warburg effect, an increased glucose uptake supports an anaerobic glycolysis, producing lactate as a cellular by-product (44). The lactate generated in this process can contribute to the synthesis of glucose through gluconeogenesis, as described in Exton and Park (45). Moreover, pyruvate carboxylase, which catalyzes one of the first steps of gluconeogenesis, showed increased transcription at 2 mM H2O2 after 2 h. Thus, we investigated the transcription of PEPCK, which plays a key role in energy homeostasis because it is involved in the regulation of fatty acid re-esterification, glucose synthesis, transamination, and the cataplerosis of citric acid cycle anions (46). It is well established that changes in PEPCK gene transcription regulate the total activity of this enzyme (46, 47). Further assessing the involvement of gluconeogenesis in the oxidative challenge response, we observed an increase in the relative transcription of PEPCK at 2 h after H2O2 addition for both concentrations (Fig. 4G). Concomitantly, pyruvate carboxylase transcription increased, suggesting an activation of gluconeogenesis to support the accoupling of glucose and glycogen synthesis (Fig. 4I). On the other hand, the amount of G6P did not change after H2O2 challenge (Fig. 4H), suggesting that the synthesis and mobilization of this substrate remain constant.
Such mechanisms are classically known to be regulated by insulin, especially in mammals (48). In the BME26 cells, the viability was significantly reduced in the absence of insulin or fetal bovine serum but increased in the presence of either treatment (Fig. 5B), strongly indicating the presence of an insulin-responsive system in BME26 cells, as suggested by Abreu et al. (16). To investigate whether the regulation of glycogen metabolism and gluconeogenesis in BME26 cells are also dependent on the insulin signaling cascade, we used gene silencing of the p85 protein, the regulatory subunit of PI3K. The metabolic response to insulin is mediated primarily by PI3K (49). Thereby, in the presence of insulin, the formation of the IRS complex promotes the recruitment and activation of class IA PI3K (Fig. 5A). When the insulin receptor or the IGF receptor is inactive, the p85 regulatory subunit stabilizes and maintains the p110 catalytic subunit in a state of low activity (50). Accordingly, after p85 knockdown in BME26 cells (Fig. 5C), GS transcription, as well as glycogen content, increased (Fig. 5, D and E). The same effect of increased GS transcription was observed after knockdown of another ISP component, AKT, with no effect observed on PEPCK transcription (Fig. 5, F–H). In the case of GSK3β knockdown (Fig. 5I), both GS and PEPCK transcription were induced (Fig. 5, J and K), suggesting the control of glycogen metabolism mediated by GSK3β and, surprisingly, an indirect genetic regulation of the gluconeogenesis pathway through GSK3β. Other studies suggest an indirect genetic regulation of the gluconeogenesis pathway through GSK-3 (40), with PEPCK expression control being mediated by GKS-3 in mammalian cells (51). At the enzymatic level, GSK3β is regulated by phosphorylation, and a 15% suppression may not affect the metabolism substantially overall. Effectively, the low rate in GSK3β silencing afforded analysis of the effect of GSK3β reductions on the transcription of gluconeogenesis genes. Given the conservation of these pathways of carbohydrate metabolism, and the high glycolytic activity observed after H2O2 treatment, we were prompted to investigate the mitochondrial contribution in BME26 cells responding to oxidative challenge.
Recently, we described the tolerance, survival, proliferation, and adaptability of R. microplus embryonic cell line BME26 to an oxidative challenge. In this context, a comparative analysis was performed with different cell types regarding their survival to H2O2 (31). Figure 1A presents an explanatory graph with the viability of different cell lines used for comparative tests of H2O2 susceptibility. We demonstrate that the arthropod embryonic cells tested (S2, BME26, and Aag2) display a higher H2O2 tolerance compared with mammalian cells, when subjected to treatment between 62.5 and 2000 μM H2O2. BME26 and S2 cells showed a similar pattern of cell viability, being acutely tolerant to the oxidative challenge induced by H2O2. Viability of S2 cells was affected only to about 20% at the highest tested concentration of 2 mM H2O2. However, Aag2 cells exhibited an increase of about 30% in cell numbers, at the lowest concentrations of 125 to 250 μM H2O2. Cytotoxic effects were observed at concentrations above 1000 μM (1 mM) H2O2, with about 20% lethality. Therefore, 24 h after H2O2 addition, it was possible to observe that the tested embryonic arthropod cell lines tolerate higher H2O2 concentrations than some mammalian cell lines in this experiment, as well as others reported in the literature. This surprising discovery opened up our perspective to look at the "good side" of ROS for cellular metabolism.
ROS are natural by-products of metabolism that have toxic effects associated with tissue injury and many pathological processes, including septic shock (52, 53). However, we live in an oxygen-rich environment and ROS and their chemical reactions are part of the basic chemical processes of normal metabolism (54). Accordingly, organisms have evolved sophisticated mechanisms to control these reactive molecules (55). In the last 2 decades, it has become increasingly evident that ROS also play a role in the regulation of many intracellular signaling pathways that are important for normal cell growth and inflammatory responses that are essential for host defense (55, 56, 57, 58). We hypothesize that H2O2 triggers a metabolic adaptation via gluconeogenesis switching from transamination to glycogen storage. At the same time, increasing glucose uptake maintains glycolytic pathways for energy supply, as well as pentose phosphate pathway for NADPH production. Accordingly, we observe metabolic changes leading to glycogen accumulation in arthropod embryonic cell lines after oxidative challenge. The great metabolic plasticity, also known as metabolic remodeling, generates a high energy demand. Our temporal analysis of glycogen content in BME26 cells shows a tendency to accumulate glycogen 6 h after H2O2 bolus addition (at 2 or 4 mM), keeping glycogen levels high up to 24 h (Fig. 1B) and reverting to control levels after 48 h (Fig. S1). Aag2 cells, on the other hand, which are slightly less tolerant to H2O2 than BME26 cells, showed an increase in glycogen 6 and 12 h after challenge with H2O2 at 2 mM, but not at 1 mM (Fig. S2). The similar pattern of cell viability, being acutely tolerant to the oxidative challenge induced by H2O2, involving the glucose metabolism adaptation is usually described in cancer cells.
The electron transport chain is one of the stages of cellular respiration, characterized by the transport of electrons in a compilation of molecules fixed on the inner membrane of the eukaryotic cell mitochondria, toward a final electron acceptor, in several energy-releasing steps for ATP synthesis (59). Thus, the flow of electrons in the respiratory chain is carried out through a redox spectrum from NAD+/NADH to O2/H2O, passing through four large protein complexes. Inhibitors of these complexes were used in order to investigate oxidative metabolism in BME26 cells (Fig. 6A). After treatment with 2 and 4 mM H2O2, the rates of complex I–coupled OCR, ETS, and spare capacity were significantly affected (Fig. B). Beyond this response to H2O2, change in mitochondrial shape was observed after either treatment (Fig. 6C). These results suggest a shift from oxidative to nonoxidative metabolism, corroborating with data on increased enzyme activity of PK and LDH (Fig. 4, D and E), similar to that observed in the Warburg effect. However, unlike with the Warburg effect, glycogen reserves are not being mobilized under these conditions, but increase in amount. Thus, the data suggest that glycogen synthesis is maintained by gluconeogenesis (Fig. 4, F and G) after redox challenge, which uses lactate and pyruvate to synthesize glucose that will be incorporated into glycogen.
In previous studies, after G6PDH knockdown in BME26 cells, a cellular environment was induced by oxidative stress in which NADP-ICDH seems to be a compensatory NADPH provider (31). Here, after NADP-ICDH knockdown (Fig. 7B), it is possible to observe the opposite reaction, with an increase in G6PDH transcription (Fig. 7C) without affecting cell viability (Fig. 7A). The interactions between NADPH-producing enzymes (G6PDH, NADP-ICDH) under different stress conditions, such as oxidative stress, starvation, and desiccation, has been evaluated in Drosophila. The results showed that NADPH production was mostly afforded by G6PDH and NADP-ICDH, which were more accentuated in oxidative stress (60), suggesting that both enzymes work together to maintain the oxidative balance. In the case of NADP-ICDH, GS also increased its transcriptional level, as well as PEPCK (Fig. 7, E and F). According to these results, BME26 cells treated with H2O2 induce gluconeogenesis as an adaptive compensation strategy, involving NADP-ICDH, G6PDH, and PEPCK enzymes (Fig. 7G).
A great progress has been achieved with arthropod model systems to study various human disorders including diabetes, multiple sclerosis, epilepsy, and cancer (8, 61, 62, 63). Thus, arthropod cell lines act as excellent experimental models to study physiology, gene regulatory networks, metabolic fluxes, and the regulation of energy homeostasis (15, 32, 64, 65, 66, 67, 68). The present work demonstrates that ticks are able to support high H2O2 concentrations and perform an adaptive response to H2O2 involving glucose metabolism, where tolerance is linked to metabolic control in eukaryotic cells. Moreover, this study helps to elucidate an adaptive mechanism developed by BME26 cells under H2O2 exposure to maintain cellular performance and redox balance and also contributes to a better understanding of arthropod physiology and metabolism.
Experimental procedures
Cell lines
Glucose metabolism remodeling was investigated in the BME26 cell line. In addition, other invertebrate and vertebrate cell lines were challenged with H2O2 prior to cell viability determination (S2, LLCMK2, and macrophage). Furthermore, glycogen and lactate contents in BME26 and Aag2 cell lines were compared upon H2O2 treatment. The same selection of cell lines was previously studied in our laboratory and were kept as described (31).
H2O2 treatment procedures
Hydrogen peroxide treatment was performed with a single bolus addition (33). To assess cell susceptibility, cells were incubated with H2O2 concentrations ranging from 0 to 2 mM, and cell viability was checked 24 h after treatment. Lethal dose to reduce BME26 cell viability by 25% (LD25) was estimated by treatment with H2O2 ranging from 0 to 8 mM, for 24 h. Further tests with BME26 cells used H2O2 concentrations of 2 and 4 mM based on our previous observations (31), with incubations at the indicated intervals. BME26 cells were used between passages 40 and 60.
Cell viability assays
Cell viability was determined using a Neubauer hemocytometer with 0.04% Trypan Blue (Sigma-Aldrich) exclusion technique and visual detection (69). The experimental procedure and calculations were done according to standard methodology as reported (31). The number of viable cells was counted directly on hemocytometer under a light microscope (Axio Imager 2, Zeiss) or on images captured with an Axiocam 503 color camera using the cell counter Manual Counting plugin on ImageJ software (70).
Alternatively, cell viability was measured using the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay performed on 24-well plates (16, 31). Unless otherwise stated, absorbance values (570 nm) of control condition were used for normalization (100% viability) to determine cell viability.
Glucose analogue uptake in BME26 cells
D-Glucose fluorescent analogue 2-NBDG {2-[N-(7-nitrobenz-2-oxa-1,3 diazol-4-yl)amino]-2-deoxy-d-glucose]} (Molecular probes, #N13195) was used as an indicator for direct measurement of glucose uptake (71), as reported for BME26 cells (31). Cells were allowed to adhere on a clear confocal dish (SPL Lifesciences, model 200350) for 24 h in complete medium. Cells were then washed twice with 500 μl of L15B300 medium without glucose supplementation [L15 (-) glucose]. Cells were further incubated in 500 μl of L15 (-) glucose for either 2 or 24 h with H2O2 at 2 or 4 mM. The glucose analogue, 2-NBDG, was added to the growth medium in the last 90 min of H2O2 challenge, at 100 μM final concentration, and the plate was kept at 34 °C in the dark. After two washes with 500 μl of L15 (-) glucose, live cells were observed under a confocal microscope (Zeiss LSM710). An additional positive control for 2-NBDG uptake was done with exogenous bovine insulin addition. Cells were cultured for 24 h to adhere on round glass coverslip and washed twice with L15 (-) glucose. Then, 100 μM of 2-NBDG was added to the growth medium, followed by 1 μM bovine insulin, and incubated for 1 h. Images were taken using Zeiss LSM710 confocal microscope, with a 40x magnification objective (Water Plan-Apochromat 40x/1.0 DIC) Ex. 488 nm; Em. 520 nm using the ZEN 2.3 program (black edition). Quantification of the mean fluorescence intensity per cell area in mm2 was determined with ZEN 2.3 (blue edition) software (Carl Zeiss Microscopy GmbH, 2011).
Total and Ser641-phosphorylated glycogen synthase immunolocalization
BME26 cells were challenged with H2O2 (at 2 or 4 mM, for 2 or 24 h) in 24-well plates with a round glass coverslip at the bottom of each well. After treatment, cells were washed twice with PBS pH 7.0 and fixed in 4% paraformaldehyde solution (in PBS pH 7.0) for 25 min at room temperature, then washed twice with PBS pH 7.0. Permeation was done in ice-cold acetone for 3 min, followed by five washes in PBS. Cells were kept for 1 h in blocking solution (1% bovine serum albumin in PBS), followed by a 16-h incubation at 4 to 8 °C with the respective primary antibody, anti-Glycogen Synthase (GS, total) (Cell signaling, #3893) or anti-Glycogen Synthase phosphorylated at Ser641 (pGS, inhibited) (Cell signaling, #3891), at 1:200 dilution in blocking solution. After that, cells were washed three times, incubated with secondary anti-rabbit IgG antibody conjugated to AlexaFluor 555 (Cell signaling, #4413), diluted 1:1000 in blocking solution, for 2 h, followed by incubation with DAPI (1 μg/ml) for 15 min. Round coverslips were prepared in glycerol, after two washes, and observed under microscope. Images were taken using Zeiss LSM710 confocal microscope, with a 40x magnification objective (Water Plan-Apochromat 40x/1.0 DIC) and Ex: 555 nm, Em: 565 nm.
Glycogen staining
After H2O2 treatment, cells in round glass coverslips were fixed in 4% paraformaldehyde solution, for 25 mi. Carbohydrates were oxidized in periodic acid solution for 5 min, followed by two washes in running water. Schiff reagent was added for 5 min, and washed three times with distilled water. A subsample of coverslips was used to locate cell nuclei by staining with Harris hematoxylin solution for 6 min and rinsed in running water for 5 min. For assembly, coverslips were dehydrated and clarified through 95% ethanol, absolute ethanol, and xylene, two exchanges of 2 min each, and then assembled in Entelan mounting medium. All steps were performed at room temperature. Images were captured in bright field microscopy using polarized light, 20x magnification, in an Axio Scope.A1 microscope (Zeiss) with the Blue Zeiss software.
Mitochondria staining
The green-fluorescent mitochondrial stain, MitoTracker Green (Molecular probes, Invitrogen, #M7514), was used to verify the mitochondrial location. The entire procedure was performed according to the manufacturer. After 2 or 4 mM H2O2 treatment, for 2 h, adherent cells on a clear confocal dish (SPL Lifesciences, model 200350) were washed once with 500 μl of prewarmed L15 complete medium and incubated with 500 μl of 200 μM MitoTracker Green, diluted in complete medium, for 20 min at 34 °C in the dark. After three washes with complete medium, cells were observed by confocal microscopy. Image acquisition was performed using the Zeiss LSM710 confocal laser scanning microscope, using objective 63x magnification (LD plan-neofluar 63x/1.0 DIC), through the program ZEN 2.3 (black edition).
Protein, glycogen, lactate, and glucose 6-phosphate quantification
The total protein concentration was determined using the Bicinchoninic Acid Kit (Sigma-Aldrich Product # BCA-1) according to the manufacturer’s instructions, with bovine albumin as standard (72).
The glycogen content was determined in 250 μl of extraction buffer (200 mM sodium acetate buffer, pH 4.8; 0.1% Triton-X100; protease inhibitor cocktail, Sigma Aldrich #P8340) after cells were mechanically disrupted by vortexing and 20 iterations of passing through a 26G needle in a 1-ml syringe. After centrifugation at 12,000g for 10 min, 40 μl of supernatant aliquots were incubated with 1 U of α-amyloglucosidase (Sigma-Aldrich #A7420), at 40 °C for 4 h, after which glucose concentration was measured by the glucose oxidase method using a commercial kit (Labtest Ref.133). Free glucose was measured in samples without alpha-amyloglucosidase and subtracted from the results, and a standard curve was generated to calculate glycogen levels in the samples (43). The glycogen content was normalized by the number of cells.
The lactate content was enzymatically determined with a colorimetric assay (Labtest Diagnóstica S.A. ref 138) following manufacturer instructions, using 20-μl sample aliquots (or water for blank, or standard for positive control) in a final volume adjusted to 200 μl in a 96-well microplate. Briefly, lactate oxidase generates pyruvate and H2O2, and the latter is coupled in a peroxidase reaction to generate a product with maximal absorbance at 550 nm. The assay was performed at 37 °C for 5 min. Samples were read in a spectrophotometer (Multiskan Go Thermo Scientific) to determine the lactate concentration.
The G6P content was determined using EnzyChromTM Glucose 6-Phosphate Assay Kit (EG6P-100). Cell samples (2 × 106) were homogenized in 100 μl PBS and centrifuged at 12,000g for 5 min. Cleared supernatant aliquots (20 μl) and G6P standard curve ranging from 0 to 1000 μM were diluted in a premix solution and used for assay in 80 μl of working reagent (100 μl final volume), in a 96-well plate, according to the manufacturer’s instructions. Briefly, G6P is oxidized by G6PDH and the generated NADPH is coupled to the WST-8 formazan chromogen. The intensity of the product color, measured at 460 nm, is proportional to the G6P concentration in the sample and was determined following manufacturer’s instructions.
Enzymatic activities
Hexokinase (HK) activity was determined after treatment with H2O2, using cell homogenates in 20 mM Tris-HCl, pH 7.5, containing 6 mM MgCl2, 1 mM ATP, 0.5 mM NAD+, and 10 mM NaF. The enzymatic reaction was initiated with 2 mM glucose. Newly formed G6P was indirectly measured by adding an equal volume of 1 unit/ml of G6PDH from Leuconostoc mesenteroides (Roche #10127655001) and 0.3 mM of β-NAD+, in 700 μl final volume. The production of β-NADH was determined at 340 nm, 30 °C (UVmini-1240 UV-Vis Shimadzu) using a molar extinction coefficient of 6.22 M-1, as described (40). The enzymatic activity was normalized based on the total protein quantification and expressed in U/mg of total protein. The activity was calculated as a percentage of the activity under control conditions, which was set to 100%.
For measurement of pyruvate kinase (PK) activity, 30 μl of cell homogenate was assayed in Tris-HCl buffer 20 mM pH 7.5, MgCl2 5 mM, ADP 1 mM, NADH 0.4 mM, and 1 U/ml of LDH. The reaction was initiated with phosphoenolpyruvate 1 mM, in 700 μl final volume. The consumption of β-NADH was evaluated spectrophotometrically (UVmini-1240 UV-Vis Shimadzu) at 340 nm, 25 °C, using a molar extinction coefficient of 6.22 M-1 as described above (40). The enzymatic activity was normalized based on total protein quantification and expressed in U/mg of total protein. The activity was calculated as a percentage of the activity under control conditions, which was set to 100%.
LDH activity was determined kinetically using a colorimetric kit (Labtest Diagnóstica S.A. ref 86) following manufacturer’s instructions. The activity was measured in 20-μL sample aliquots in a final volume of 200 μl in a 96-well microplate. Briefly, LDH present in samples oxidizes NADH and the reaction is monitored spectrophotometrically at 340 nm in 1-min intervals at 37 °C to evaluate reaction linearity.
Respirometry analysis on BME26 cells after H2O2 treatment
Respiratory activity was measured using a two-channel titration injection respirometer (Oxygraph-2k, Oroboros Instruments), calibrated with PBS at 28 °C. Following treatment with H2O2 (2 or 4 mM for 2 h), or control condition, cells were harvested with trypsin solution (0.25% trypsin), counted, and suspended in PBS. Treated and control cells were placed into the O2K chamber at a concentration of about 1 x 106/ml and were incubated with continuous stirring at 750 rpm for about 10 min. Assay was performed at 28 °C and 750 rpm, started by adding 1 μM oligomycin, and the oxygen consumption coupled to ATP synthesis (ATP-linked respiration) was calculated by subtracting the oxygen consumption after the addition of ATP synthase inhibitor from basal respiration rates. The maximum uncoupled respiration was assessed by stepwise titration of carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) to reach a final concentration of 50 nM. Next, the contribution of complex I to electron flow was determined by the addition of 0.3 μM of rotenone and calculated by subtracting FCCP-stimulated oxygen consumption from rotenone-resistant oxygen consumption. Finally, respiratory rates were inhibited by the injection of 1 μg/ml antimycin A. The residual oxygen consumption represents the oxygen consumed by the cells, not due to respiration. The maximum respiratory rates (ETS) were calculated by subtracting the antimycin-resistant oxygen consumption from FCCP-stimulated oxygen consumption rates. The spare capacity is the amount of extra ATP that could be produced by oxidative phosphorylation in case of increased energy demand and was calculated by subtracting FCCP-stimulated oxygen consumption rates from basal respiration rates. The leak respiratory state, representing oxygen consumption in the presence of substrates but in the absence of ATP synthesis, was calculated by subtracting the oligomycin- from the antimycin-resistant oxygen consumption. All oxygen consumption rates were normalized per 1 x 106 cells/ml.
RNA isolation, real-time quantitative PCR, and relative quantification analyses
BME26 cells were harvested after treatment from 24-well plates for total RNA isolation using Trizol reagent (Invitrogen) according to the manufacturer's instructions. One microgram of total RNA was reversely transcribed with cDNA High-Capacity cDNA Reverse transcription kit (Invitrogen). The relative transcription was analyzed with cDNA template in a StepOne Plus platform (Applied Biosciences), with previously described primers (31, 40). Serially diluted cDNA samples from controls were used to construct a calibration curve. Amplification efficiencies between 85% and 100% were determined for each set of primers in 10-μl reactions. The relative expression was determined using Cp values of each run in the Relative Expression Software Tool table (73). The R. microplus elongation factor-alpha (Elf1A) gene was used as a reference gene (74) to normalize the reactions. Relative expression of the calibrators was assigned the value of 1 unit. The statistical calculations (mean and standard deviation) were performed with data from three independent experiments in triplicates.
Double-stranded RNA synthesis and gene knockdown by RNA interference
cDNA from BME26 cells was used as template for dsRNA synthesis, using the RiboMAX Express RNAi System Kit (Promega) with previously described primers (31). dsRNA was purified according to the manufacturer's instructions, the concentration was determined spectrophotometrically at 260 nm, and an aliquot was used to check dsRNA integrity by 1% agarose gel electrophoresis stained by ethidium bromide and visualized in a transilluminator. A nonrelated dsRNA sequence coding for Escherichia coli GFP was used as a negative control for RNAi-induced gene knockdown. The size of the G6PDH dsRNA synthesized complex was 553 bp and that of the ICDHNADPH dsRNA was 565 bp (31). Specific primers were designed based on R. microplus sequences (31).
Double-stranded RNA was directly administered to BME26 cells kept on 24-well plates, as described (16). Three days after the addition of dsRNA, the cells were collected for testing. G6PDH gene silencing was confirmed by real-time quantitative PCR as well as by enzyme activity assay. To verify the persistence of G6PDH gene silencing after the first 3 days, the culture medium was fully replaced by 500 μl of complete medium and incubated for a further 72 h. The cells were then collected for G6PDH enzyme activity (extended exposure assay, 6 days after the addition of dsRNA). For the H2O2 challenge, BME26 cells had their medium replaced (500 μl) 3 days after the addition of dsRNA and were treated with a single addition of 2 or 4 mM H2O2. Cell viability was measured after 24 h of incubation.
Quantitative image analysis
Images were analyzed from three independent biological samples in three experimental repetitions (laminules). Three to five images from each replicate were recorded by confocal laser scanning microscope (LSM 710, Zeiss). For image analysis, the average fluorescence intensity per cell area in mm2 was calculated using the ZEN 2.3 software (blue edition, Carl Zeiss Microscopy GmbH, 2011), and cells and nuclei were counted using the Cell Counter plugin of ImageJ software. Percentage calculations as well as statistical and graphical analyses were performed using the GraphPad Prism v.6.0 software.
Statistical analysis
The experiments were performed in three independent biological samples with three experimental repetitions each, from which all values are expressed as mean ± SD. The data were checked for normal distribution using the Shapiro-Wilk test. When normality was confirmed, statistical significance was evaluated by unidirectional and bidirectional ANOVA to determine significant differences between groups. Tukey's test was used to compare data between groups. Significance was established and represented as follows: p < 0.05 (∗), p < 0.01 (∗∗), p < 0.0001 (∗∗∗), for comparisons with control, and p < 0.05 (#), p < 0.01 (##), p < 0.0001 (###) for comparisons between two time points within the same treatment.
Data availability
All data are contained within the article.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors are grateful to Brazilian agencies for supporting this project. This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001, CNPq-Instituto Nacional de Ciência e Tecnologia de Entomologia Molecular and FAPERJ.
Author contributions
B. D. N., C. C., and R. M. d. S. formal analysis; B. D. N., C. C., R. M. d. S., A. A., and M. V. d. C. U. investigation; I. d. S. V. Jr, S. K., K. O., and C. L. resources; R. M. d. S., I. d. S. V. Jr, S. K., and K. O. writing – original draft; L. A. d. A. and S. S. d. C. writing – review & editing; C. L. supervision; C. L. project administration.
Edited by Qi Qun Tang
Supporting information
Supplemental Figure S1.
BME26 cells exhibit different responses in cell viability and glycogen content after long exposure treatment with H2O2. Cell viability (Trypan blue exclusion) (A) and glycogen content (B) were determined 48 hours after treatment with H2O2 at 2 mM or 4 mM. Control cells were kept accordingly, but in absence of H2O2. Experiments were performed with three independent biological samples in three experimental replicates each, where ∗p < 0.05, ∗∗∗∗p < 0.0001, compared with the control, in Tukey’s multiple comparisons test.
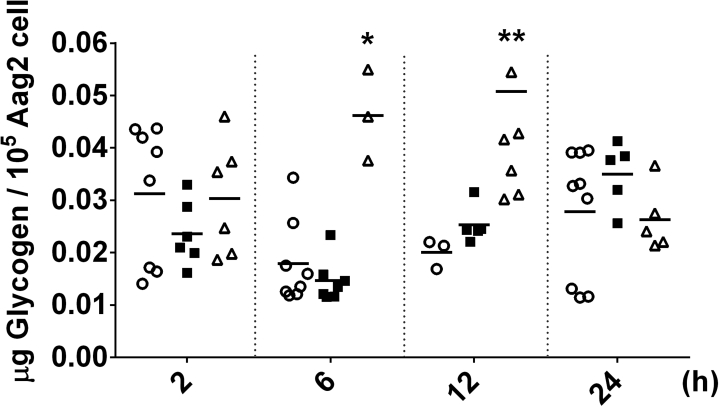
Supplemental Figure S2.
Glycogen content in Aag2 cells after H2O2 treatment. Glycogen content was determined in Aag2 cells at the indicated intervals after H2O2 treatment at 1 mM and 2 mM. Control cells were kept accordingly, but in absence of H2O2. Experiments were performed with three independent biological samples in three experimental replicates each, where ∗p < 0.05, ∗∗p < 0.01, compared with the control, in Tukey’s multiple comparisons test.
GSK3β phosphorylation at Ser9 in BME26 cells after H2O2 treatment. Immunolocalization of phosphorylated Ser9 glycogen synthase kinase (red signal) and nuclei staining (blue signal) was performed in BME26 cells after 2 mM and 4 mM H2O2 challenge. Images were captured in confocal laser scanning microscope, LSM 710, Zeiss. Scale bar: 10 μm.
References
- 1.Vleck C., Hoyt D. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge Univ. Press; Cambridge, UK: 1991. Metabolism and energetic of reptilian and avian embryos; pp. 285–306. [Google Scholar]
- 2.Thompson M.B., Stewart J.R. Embryonic metabolism and growth in lizards of the genus eumeces. Comp. Biochem. Physiol. A Physiol. 1997;118:647–654. [Google Scholar]
- 3.Yamamoto Y., Takahashi S.Y. Cysteine proteinase from Bombyx eggs: Role in programmed degradation of yolk proteins during embryogenesis. Comp. Biochem. Physiol. B. 1993;106:35–45. doi: 10.1016/0305-0491(93)90004-o. [DOI] [PubMed] [Google Scholar]
- 4.Logullo C., Da Silva Vaz I., Sorgine M.H.F., Paiva-Silva G.O., Faria F.S., Zingali R.B., De Lima M.F.R., Abreu L., Fialho Oliveira E., Alves E.W., Masuda H., Gonzales J.C., Masuda A., Oliveira P.L. Isolation of an aspartic proteinase precursor from the egg of a hard tick, Boophilus microplus. Parasitology. 1998;116:525–532. doi: 10.1017/s0031182098002698. [DOI] [PubMed] [Google Scholar]
- 5.Fagotto F. Yolk degradation in tick eggs: I. Occurrence of a cathepsin L-like acid proteinase in yolk spheres. Arch. Insect Biochem. Physiol. 1990;14:217–235. doi: 10.1002/arch.940140403. [DOI] [PubMed] [Google Scholar]
- 6.Reiter L.T., Potocki L., Chien S., Gribskov M., Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herranz H., Cohen S.M. Drosophila as a model to study the link between metabolism and cancer. J. Dev. Biol. 2017;5:15. doi: 10.3390/jdb5040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez C. Drosophila melanogaster: A model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer. 2013;13:172–183. doi: 10.1038/nrc3461. [DOI] [PubMed] [Google Scholar]
- 9.Devilliers M., Garrido D., Poidevin M., Rubin T., Le Rouzic A., Montagne J. Differential metabolic sensitivity of insulin-like-response- and mTORC1-dependent overgrowth in Drosophila fat cells. bioRxiv. 2019 doi: 10.1101/606699. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das R., Sebo Z., Pence L., Dobens L.L. Erratum: Drosophila tribbles antagonizes insulin signaling-mediated growth and metabolism via interactions with Akt kinase. PLoS One. 2015;9 doi: 10.1371/journal.pone.0109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren S., Huang Z., Jiang Y., Wang T. dTBC1D7 regulates systemic growth independently of TSC through insulin signaling. J. Cell Biol. 2018;217:517–526. doi: 10.1083/jcb.201706027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hietakangas V., Cohen S.M. Regulation of tissue growth through nutrient sensing. Annu. Rev. Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- 13.Oldham S., Hafen E. Insulin/IGF and target of rapamycin signaling: A tor de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 14.Teleman A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2010;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- 15.de Abreu L.A., Fabres A., Esteves E., Masuda A., da Silva Vaz I., Daffre S., Logullo C. Exogenous insulin stimulates glycogen accumulation in Rhipicephalus (Boophilus) microplus embryo cell line BME26 via PI3K/AKT pathway. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009;153:185–190. doi: 10.1016/j.cbpb.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 16.de Abreu L.A., Calixto C., Waltero C.F., Della Noce B.P., Githaka N.W., Seixas A., Parizi L.F., Konnai S., Vaz I. da S.J., Ohashi K., Logullo C. The conserved role of the AKT/GSK3 axis in cell survival and glycogen metabolism in Rhipicephalus (Boophilus) microplus embryo tick cell line BME26. Biochim. Biophys. Acta. 2013;1830:2574–2582. doi: 10.1016/j.bbagen.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand K., Leibold W., Luppa P., Schoerner C., Schulz A. Metabolic alterations associated with proliferation of mitogen-activated lymphocytes and of lymphoblastoid cell lines: Evaluation of glucose and glutamine metabolism. Immunobiology. 1986;173:23–34. doi: 10.1016/S0171-2985(86)80086-9. [DOI] [PubMed] [Google Scholar]
- 19.Chambers J.W., Maguire T.G., Alwine J.C. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010;84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noch E., Khalili K. Oncogenic viruses and tumor glucose metabolism: Like kids in a candy store. Mol. Cancer Ther. 2012;11:14–23. doi: 10.1158/1535-7163.MCT-11-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Berardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho-Santos Z., Cardoso-Figueiredo R., Elias A.P., Tastekin I., Baltazar C., Ribeiro C. Cellular metabolic reprogramming controls sugar appetite in Drosophila. Nat. Metab. 2020;2:958–973. doi: 10.1038/s42255-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 24.Cully M., You H., Levine A.J., Mak T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 25.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015;5 doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi N., Chen H.Y., Harris I.S., Stover D.G., Selfors L.M., Bronson R.T., Deraedt T., Cichowski K., Welm A.L., Mori Y., Mills G.B., Brugge J.S. Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell. 2018;33:985–1003.e7. doi: 10.1016/j.ccell.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 28.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutzeit H.O., Zissler D., Grau V., Liphardt M., Heinrich U.R. Glycogen stores in mature ovarian follicles and young embryos of Drosophila: Ultrastructural changes and some biochemical correlates. Eur. J. Cell Biol. 1994;63:52–60. [PubMed] [Google Scholar]
- 30.Gutzeit H.O., Zissler D., Fleig R. Oogenesis in the honeybee Apis mellifera: Cytological observations on the formation and differentiation of previtellogenic ovarian follicles. Rouxs Arch. Dev. Biol. 1993;202:181–191. doi: 10.1007/BF00365309. [DOI] [PubMed] [Google Scholar]
- 31.Della Noce B., de Carvalho Uhl M.V., Machado J., Waltero C.F., de Abreu L.A., da Silva R.M., da Fonseca R.N., de Barros C.M., Sabadin G., Konnai S., da Silva Vaz I., Ohashi K., Logullo C. Carbohydrate metabolic compensation coupled to high tolerance to oxidative stress in ticks. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-41036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esteves E., Lara F.A., Lorenzini D.M., Costa G.H.N., Fukuzawa A.H., Pressinotti L.N., Silva J.R.M.C., Ferro J.A., Kurtti T.J., Munderloh U.G., Daffre S. Cellular and molecular characterization of an embryonic cell line (BME26) from the tick Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol. 2008;38:568–580. doi: 10.1016/j.ibmb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marinho H.S., Cyrne L., Cadenas E., Antunes F. H2O2 delivery to cells: Steady-state versus bolus addition. Methods Enzymol. 2013;526:159–173. doi: 10.1016/B978-0-12-405883-5.00010-7. [DOI] [PubMed] [Google Scholar]
- 34.Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12:1131–1137. [Google Scholar]
- 35.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 36.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Liberti M.V., Locasale J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuta E., Okuda H., Kobayashi A., Watabe K. Metabolic genes in cancer: Their roles in tumor progression and clinical implications. Biochim. Biophys. Acta Rev. Cancer. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva R.M., Della Noce B., Fernanda Waltero C., Costa E.P., de Abreu L.A., Githaka N.W., Moraes J., Gomes H.F., Konnai S., da Silva Vaz I., Ohashi K., Logullo C. Non-classical gluconeogenesis-dependent glucose metabolism in Rhipicephalus microplus embryonic cell line BME26. Int. J. Mol. Sci. 2015;16:1821–1839. doi: 10.3390/ijms16011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabres A., De Andrade C.P., Guizzo M., Sorgine M.H.F., Paiva-Silva G. de O., Masuda A., Da Silva vaz I., Logullo C. Effect of GSK-3 activity, enzymatic inhibition and gene silencing by RNAi on tick oviposition and egg hatching. Parasitology. 2010;137:1537–1546. doi: 10.1017/S0031182010000284. [DOI] [PubMed] [Google Scholar]
- 42.Logullo C., Witola W.H., Andrade C., Abreu L., Gomes J., da Silva Vaz I., Imamura S., Konnai S., Ohashi K., Onuma M. Expression and activity of glycogen synthase kinase during vitellogenesis and embryogenesis of Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2009;161:261–269. doi: 10.1016/j.vetpar.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Moraes J., Galina A., Alvarenga P.H., Rezende G.L., Masuda A., da Silva Vaz I., Logullo C. Glucose metabolism during embryogenesis of the hard tick boophilus microplus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;146:528–533. doi: 10.1016/j.cbpa.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Heiden M.G.V., Cantley L.C., Thompson C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Exton J.H., Park C.R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J. Biol. Chem. 1967;242:2622–2636. [PubMed] [Google Scholar]
- 46.Yang J., Reshef L., Cassuto H., Aleman G., Hanson R.W. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 2009;284:27031–27035. doi: 10.1074/jbc.R109.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanson R.W., Reshef L. Glyceroneogenesis revisited. Biochimie. 2003;85:1199–1205. doi: 10.1016/j.biochi.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 49.Le Roith D., Zick Y. Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care. 2001;24:588–597. doi: 10.2337/diacare.24.3.588. [DOI] [PubMed] [Google Scholar]
- 50.Shekar S.C., Wu H., Fu Z., Yip S.C., Nagajyothi, Cahill S.M., Girvin M.E., Backer J.M. Mechanism of constitutive phosphoinositide 3-kinase activation by oncogenic mutants of the p85 regulatory subunit. J. Biol. Chem. 2005;280:27850–27855. doi: 10.1074/jbc.M506005200. [DOI] [PubMed] [Google Scholar]
- 51.Molinaro A., Becattini B., Solinas G. Insulin signaling and glucose metabolism in different hepatoma cell lines deviate from hepatocyte physiology toward a convergent aberrant phenotype. Sci. Rep. 2020;10:12031. doi: 10.1038/s41598-020-68721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvemini D., Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic. Biol. Med. 2002;33:1173–1185. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 53.Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Pathophysiol. Haemost. Thromb. 1993;23:118–126. doi: 10.1159/000216921. [DOI] [PubMed] [Google Scholar]
- 54.Mittler R. ROS are good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Finkel T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 56.Forman H.J., Fukuto J.M., Torres M. Redox signaling: Thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 2002;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 57.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 58.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279 doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 59.Lambers H., Szaniawski R.K., de Visser R. Respiration for growth, maintenance and ion uptake. An evaluation of concepts, methods, values and their significance. Physiol. Plant. 1983;58:556–563. [Google Scholar]
- 60.Rzezniczak T.Z., Merritt T.J.S. Interactions of NADP-reducing enzymes across varying environmental conditions: A model of biological complexity. G3 (Bethesda) 2012;2:1613–1623. doi: 10.1534/g3.112.003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King I., Tsai L.T.Y., Pflanz R., Voigt A., Lee S., Jäckle H., Lu B., Heberlein U. Drosophila tao controls mushroom body development and ethanol-stimulated behavior through par-1. J. Neurosci. 2011;31:1139–1148. doi: 10.1523/JNEUROSCI.4416-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monge-Fuentes V., Gomes F.M.M., Campos G.A.A., Silva J. de C., Biolchi A.M., dos Anjos L.C., Gonçalves J.C., Lopes K.S., Mortari M.R. Neuroactive compounds obtained from arthropod venoms as new therapeutic platforms for the treatment of neurological disorders. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:31. doi: 10.1186/s40409-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pakpour N., Camp L., Smithers H.M., Wang B., Tu Z., Nadler S.A., Luckhart S. Protein kinase C-dependent signaling controls the midgut epithelial barrier to malaria parasite infection in anopheline mosquitoes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bottino-Rojas V., Talyuli O.A.C., Jupatanakul N., Sim S., Dimopoulos G., Venancio T.M., Bahia A.C., Sorgine M.H., Oliveira P.L., Paiva-Silva G.O. Heme signaling impacts global gene expression, immunity and dengue virus infectivity in Aedes aegypti. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esteves E., Bastos C.V., Zivkovic Z., de La Fuente J., Kocan K., Blouin E., Ribeiro M.F.B., Passos L.M.F., Daffre S. Propagation of a Brazilian isolate of Anaplasma marginale with appendage in a tick cell line (BME26) derived from Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2009;161:150–153. doi: 10.1016/j.vetpar.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Hambarde S., Singh V., Chandna S. Evidence for involvement of cytosolic thioredoxin peroxidase in the excessive resistance of Sf9 Lepidopteran insect cells against radiation-induced apoptosis. PLoS One. 2013;8:58261. doi: 10.1371/journal.pone.0058261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pohl P.C., Carvalho D.D., Daffre S., da Silva Vaz I., Masuda A. In vitro establishment of ivermectin-resistant Rhipicephalus microplus cell line and the contribution of ABC transporters on the resistance mechanism. Vet. Parasitol. 2014;204:316–322. doi: 10.1016/j.vetpar.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 68.Kumar J.S., Suman S., Singh V., Chandna S. Radioresistant Sf9 insect cells display moderate resistance against cumene hydroperoxide. Mol. Cell. Biochem. 2012;367:141–151. doi: 10.1007/s11010-012-1327-6. [DOI] [PubMed] [Google Scholar]
- 69.Cadena-Herrera D., Esparza-De Lara J.E., Ramírez-Ibañez N.D., López-Morales C.A., Pérez N.O., Flores-Ortiz L.F., Medina-Rivero E. Validation of three viable-cell counting methods: Manual, semi-automated, and automated. Biotechnol. Rep. 2015;7:9–16. doi: 10.1016/j.btre.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou C., Wang Y., Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Biophys. Methods. 2005;64:207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 73.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nijhof A., Balk J.A., Postigo M., Jongejan F. Selection of reference genes for quantitative RT-PCR studies in Rhipicephalus (Boophilus) microplus and Rhipicephalus appendiculatus ticks and determination of the expression profile of Bm86. BMC Mol. Biol. 2009;10:112. doi: 10.1186/1471-2199-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GSK3β phosphorylation at Ser9 in BME26 cells after H2O2 treatment. Immunolocalization of phosphorylated Ser9 glycogen synthase kinase (red signal) and nuclei staining (blue signal) was performed in BME26 cells after 2 mM and 4 mM H2O2 challenge. Images were captured in confocal laser scanning microscope, LSM 710, Zeiss. Scale bar: 10 μm.
Data Availability Statement
All data are contained within the article.