Abstract
Crohn's disease is a chronic inflammatory disease of the gastrointestinal tract of unknown etiology. We report on the presence of cell wall-deficient Mycobacterium avium subsp. paratuberculosis in 35 of 48 paraffin-embedded tissue specimens from 33 patients with Crohn's disease by in situ hybridization with IS900 as a probe.
Mycobacterium avium subsp. paratuberculosis is the established causative agent of Johne's disease in ruminants. The hypothesis that this organism might also be involved as a cause of the chronic inflammation of the intestines of patients with Crohn's disease was first reported in 1913, but due to the extreme difficulty of culturing this organism by standard techniques, the initial hypothesis was never clearly confirmed. A survey of the United Kingdom Ministry of Agriculture, Food and Fisheries revealed the presence of M. avium subsp. paratuberculosis in 10 of 31 raw milk samples and in 6 pasteurized milk samples (1). In 1984, Chiodini (4) reported on the isolation of mycobacterial spheroplasts from patients with Crohn's disease. In 10 years, only 5% of tissue from people with Crohn's disease allowed growth of M. avium subsp. paratuberculosis when tissue samples were inoculated into the appropriate medium (15). Only recently, due to the development of molecular biology-based tools, such as the application of PCR based on the amplification of insertion sequence IS900 (IS900 PCR) to the detection of M. avium subsp. paratuberculosis directly in resected tissue samples, several studies have reported on the presence of the microorganism in a large number of people with Crohn's disease (2, 3, 6, 7, 9, 10, 13, 14). However, some investigators could not observe the presence of M. avium subsp. paratuberculosis by PCR (3, 8, 17). Discrepancies and experimental difficulties have surrounded PCR detection of M. avium subsp. paratuberculosis, primarily due to the paucibacillary form of the disease and the complexity of the cell wall of this bacterium (5, 12). Detection of M. avium subsp. paratuberculosis by in situ hybridization has been reported previously in animal tissues or by use of beef samples injected with M. avium subsp. paratuberculosis spheroplasts (11, 12). No method for the localization of this type of microorganism in tissue samples has previously been available. Recently, different investigators have reported on the detection of M. avium subsp. paratuberculosis in 40% of diseased tissues from patients with Crohn's disease by an adapted in situ hybridization technique (7, 10).
Tissue samples.
Patient's were diagnosed with Crohn's disease on the basis of clinical presentation (depending on the site of inflammation), fistula formation, transmural inflammation and deep ulceration, thickening of the bowel wall, and the presence of noncaseating granulomas (present in 40 to 60% of the patients). We analyzed 48 different paraffin-embedded intestinal tissue sections (22 ileum, 14 colon, 6 rectum, 1 stomach, and 5 duodenum samples) from 33 different patients with Crohn's disease by IS900 PCR and by in situ hybridization with a biotinylated probe of 284 bp (Table 1). Granulomas were detected in 25 (52%) samples (Table 1). As a control, 20 paraffin-embedded tissue samples from patients with another intestinal bowel disease (ulcerative colitis) and 20 paraffin-embedded tissue samples from patients without intestinal bowel disease were analyzed; moreover, other tissue controls such as Caco2 intestinal epithelial cells were hybridized with the IS900 probe. Hybridization control reactions, in which a 150-bp internal fragment of IS6110 (specific for Mycobacterium tuberculosis) was used as a probe, were also performed with all positive tissues and the controls; the probe was obtained as reported previously (16).
TABLE 1.
In situ hybridization results for patient tissuesa
| Sample | Patientb | Date (yr) | Tissue | In situ hybridization result | Presence of granulomas |
|---|---|---|---|---|---|
| 1 | B-MG* | 1998 | Rectum | + | − |
| 2 | B-MG* | 1998 | Rectum | − | − |
| 3 | C-G* | 1990 | Duodenum | − | + |
| 4 | C-G* | 1994 | Ileum | − | + |
| 5 | C-MI* | 1990 | Ileum | + | − |
| 6 | C-MI* | 1997 | Ileum | + | − |
| 7 | C-M** | 1986 | Ileum | − | + |
| 8 | C-M** | 1986 | Colon | − | + |
| 9 | C-M** | 1986 | Rectum | − | − |
| 10 | C-O* | 1986 | Colon | − | − |
| 11 | C-O* | 1986 | Ileum | − | − |
| 12 | C-PP° | 1985 | Ileum | + | − |
| 13 | C-PP° | 1997 | Ileum | + | − |
| 14 | F-S* | 1997 | Ileum | + | − |
| 15 | F-S* | 1997 | Rectum | + | − |
| 16 | M-A* | 1997 | Ileum | + | + |
| 17 | M-A* | 2000 | Ileum | + | + |
| 18 | M-S* | 1993 | Ileum | − | + |
| 19 | M-S* | 1995 | Ileum | + | + |
| 20 | M-S* | 1995 | Colon | + | + |
| 21 | O-E° | 1996 | Ileum | + | + |
| 22 | O-E° | 2000 | Rectum | − | + |
| 23 | P-P* | 1992 | Colon | + | − |
| 24 | P-P* | 1992 | Stomach | + | − |
| 25 | P-MG° | 1998 | Rectum | + | + |
| 26 | P-MG° | 1999 | Colon | + | + |
| 27 | S-PP* | 1993 | Colon | + | + |
| 28 | S-PP* | 1993 | Duodenum | + | + |
| 29 | B-DM | 1999 | Ileum | + | + |
| 30 | C-F | 1997 | Colon | − | + |
| 31 | C-A | 1987 | Ileum | + | + |
| 32 | C-M | 1995 | Colon | − | − |
| 33 | C-MC | 2000 | Colon | − | − |
| 34 | C-P | 2000 | Duodenum | + | − |
| 35 | C-G | 2000 | Colon | + | + |
| 36 | D-L | 1999 | Colon | + | − |
| 37 | F-PL | 1999 | Duodenum | + | + |
| 38 | G-TF | 2000 | Colon | + | + |
| 39 | I-A | 1987 | Ileum | + | − |
| 40 | L-F | 1998 | Ileum | + | − |
| 41 | L-P | 1999 | Ileum | + | + |
| 42 | L-C | 1993 | Duodenum | + | + |
| 43 | M-GL | 1999 | Ileum | + | + |
| 44 | M-M | 2000 | Colon | + | − |
| 45 | P-M | 1984 | Ileum | + | + |
| 46 | P-RA | 1999 | Ileum | + | − |
| 47 | P-RC | 2000 | Ileum | + | − |
| 48 | S-N | 1993 | Colon | + | − |
Thirty-five samples were positive by in situ hybridization, and 25 samples were positive for granulomas.
*, **, and ° indicate the same patient.
Probe preparation.
Briefly, the probe used was a 284-bp internal fragment of IS900 obtained by PCR amplification of M. avium subsp. paratuberculosis chromosomal DNA with primers p89 (5′-CGTCGGGTATGGCTTTCATGTGGTTGCTGTG-3′) and p92 (5′-CGTCGTTGGCCACCCGCTGCGAGAGCAAT-3′). The primers were used at concentrations of 0.2 μmol each; and the reaction was performed in a total volume of 50 μl containing 2.5 U of Taq polymerase, 20 mM Tris (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 200 μM deoxynucleoside triphosphates (Gibco BRL, Life Technology, Paisley, United Kingdom). The reaction mixtures were overlaid with 1 drop of paraffin oil and were then incubated for 2 min at 94°C, followed by 35 cycles of 94°C for 1 min, 62°C for 1 min, and 72 for 1 min, with a final extension at 70°C for 5 min. The amplification products were visualized after electrophoresis at 90 V for 90 min in a 1.8% Methaphore agarose gel (FMC Bioproducts, Rockland, Maine) and staining of the gel with ethidium bromide.
In situ hybridization.
The expected DNA fragment was purified from the gel by using the GFX DNA gel band purification kit (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) The probe was then biotinylated with the Bioprime DNA labeling system (Gibco BRL, Gaithersburg, Md.), and in situ hybridization was carried out with an in situ hybridization system (Gibco BRL) with deparaffinized tissue sections that had previously been treated with proteinase K (100 μg/ml) for 30 min at 37°C according to the manufacturer's suggestions.
Ziehl-Neelsen staining was performed as described previously (16).
DNA extraction and PCR amplification.
DNA extraction was performed as reported previously (16). Embedded paraffin tissues were deparaffinized with xylene as reported previously (14). PCR was performed with primers p89 and p92 under the same conditions described above.
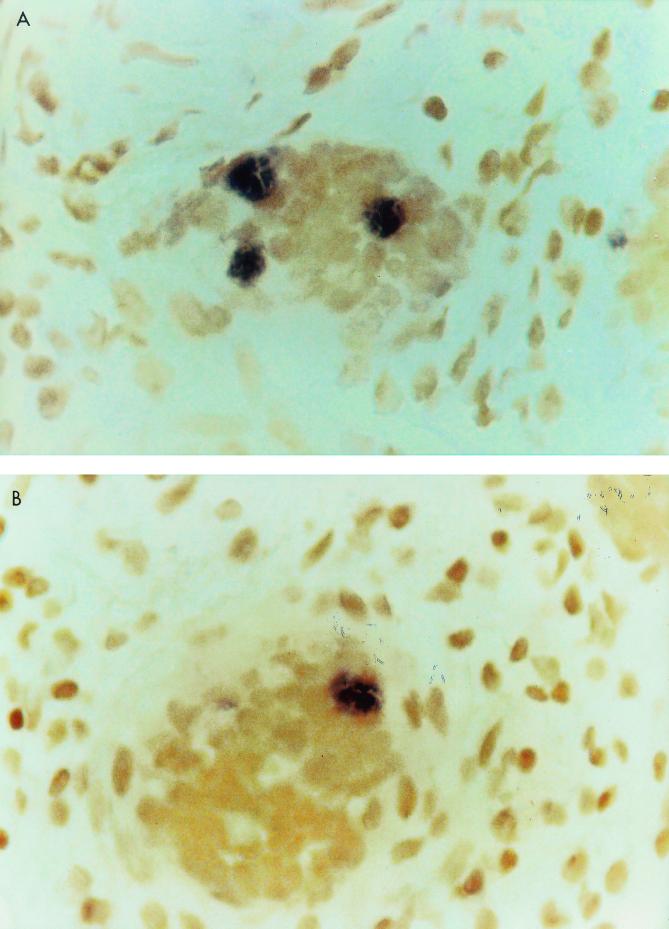
Forty-eight tissue samples from Crohn's disease patients were analyzed by the in situ hybridization technique with IS900 as a probe; 35 of them were positive. Examples of hybridizations are shown in Fig. 1A and B. As controls, 20 resected intestinal tissue specimens from patients without intestinal bowel disease, 20 tissue samples from patients with another intestinal bowel disease (ulcerative colitis), and Caco2 intestinal epithelial cells were investigated by the in situ hybridization technique. All of them were negative. To avoid possible false-positive hybridization or nonspecific phosphatase activity, the samples positive by IS900 PCR were tested with the IS6110 probe by the in situ hybridization technique; all of them were negative. Moreover, two slides were prepared for all of the tissue samples tested; one of them was stained by the Ziehl-Neelsen method. None of the tissue samples analyzed showed the presence of acid-fast bacilli. One of the causes could be the paucibacillary form of the disease and probably the presence of cell wall-deficient forms of bacteria. The IS900 PCR was performed with all 48 samples to search for the presence of M. avium subsp. paratuberculosis DNA. None of the samples generated specific amplified bands by IS900 PCR, indicating that the sensitivity of the method when it is applied to paraffin-embedded tissue is somewhat low. The negative results of the PCR experiments could also be due to the fragmentation of DNA, frequently reported in paraffin-embedded tissue, whereas in situ hybridization retains the ability to hybridize with the fragmented DNA target (8, 9).
FIG. 1.
Examples of M. avium subsp. paratuberculosis-positive granuloma biopsy specimens obtained by the in situ hybridization technique. (A) Sample 11, patient C-MI, 1997; (B) sample 31, patient M-A, 2000.
Several samples were obtained from the same patient after several years. Most of the samples from the same patient generated the same result. For instance, samples 7 (ileum), 8 (colon), and 9 (rectum) from patient C-M were all negative (Table 1). For three patients, patient B-MG (samples 1 and 2), patient M-S (samples 18, 19, and 20), and patient O-E (samples 21 and 22), discordant results were found among the samples analyzed (Table 1).
Different techniques have been used to detect M. avium subsp. paratuberculosis in patients with Crohn's disease: culture, culture followed by PCR, PCR applied to resected bowel tissue, PCR applied to tissues embedded in paraffin, and recently, in situ hybridization with paraffin-embedded tissues (7, 8, 10, 13, 14, 15).
The reduced sensitivity of PCR can result from the inefficient extraction of the mycobacteria from the sample when small numbers of organisms are involved or may be the consequence of the presence of PCR inhibitors (8, 9, 17). Moreover, DNA fragmentation may occur during the detection of DNA from paraffin-embedded tissues, causing the failure of target amplification. Our negative IS900 PCR results are in agreement with the results obtained by other researchers (17). The in situ hybridization technique was adapted to detect cell wall-defective mycobacteria in tissue specimens (10). The investigators used a digoxigenin-labeled DNA probe to detect the IS900 sequence in tissues from 40% of Crohn's disease patients with granulomas (7, 10). A similar localization was observed in tissue from animals with Johne's disease (12).
Our results showed the presence of M. avium subsp. paratuberculosis DNA in more than 70% of the diseased tissue samples from patients with Crohn's disease analyzed. Other investigators, who used the same method, reported that 40% of granulomas were positive (10). Our data support the hypothesis that infection may be caused by cell wall-defective M. avium subsp. paratuberculosis since no bacteria were detected by Ziehl-Neelsen staining (9). It is also true that the bacteria are present in small numbers, and it may be difficult for the observer to find them in clinical samples. Multiple samples from the same patients were also analyzed. The results generated were consistent; in most cases, samples from the same patient generated the same result. IS900 hybridization was detected for 72% (18 of 25) of granuloma-positive samples (Table 1), whereas IS900 hybridization was found for 73.9% (17 of 23) of nongranuloma-positive samples (Table 1). IS900 probe hybridization was localized in granuloma-like cells (as shown in Fig. 1) and around Lieberkühn crypts, whereas Hulten et al. (10) did not report positive hybridization in granulomas. At this point, we cannot establish whether the presence of these bacterial forms is the cause of tissue inflammation or whether the bacteria found a comfortable environment in the altered tissue.
In conclusion, the in situ hybridization technique rather than Zhiel-Neelsen staining or IS900 PCR may be suggested as a means of evaluating clinical samples from patients for the presence of M. avium subsp. paratuberculosis.
Acknowledgments
This work was supported by EU project “Sacrohn” N. QLK2-CT-2000-00928.
REFERENCES
- 1.Bradbury J. Need we add milk to list of worrying foods? Lancet. 1998;352:549. [Google Scholar]
- 2.Burnham W R, Lennard-Jones J E, Stanford J L, Bird R G. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet. 1978;ii:693–696. doi: 10.1016/s0140-6736(78)92699-5. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlin W, Graham D Y, Hulten K, El-Zimaity H M, Schwartz M R, Naser S, Shafran I, El-Zaatari F A. Review article: Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Aliment Pharmacol Ther. 2001;15:337–346. doi: 10.1046/j.1365-2036.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- 4.Chiodini R J. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol. 1989;2:90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocito C, Gilot P, Coene M, De Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins M T, Lisby G, Moser C, Chicks D, Christensen S, Reichelderfer M, Hoiby N, Harms B A, Thomsen O O, Skibsted U, Binder V. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J Clin Microbiol. 2000;38:4373–4381. doi: 10.1128/jcm.38.12.4373-4381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Zaatari F A, Osato M S, Graham D Y. Etiology of Crohn's disease: the role of Mycobacterium avium paratuberculosis. Trends Mol Med. 2001;7:247–252. doi: 10.1016/s1471-4914(01)01983-9. [DOI] [PubMed] [Google Scholar]
- 8.Gibson J, Riggio M, McCreary C, Lennon A, Toner M. Looking for Mycobacterium paratuberculosis DNA by polymerase chain reaction (PCR) in orofacial granulomatosis (OFG) and oral Crohn's disease tissue in an Irish population. Ir Med J. 2000;93:218. [PubMed] [Google Scholar]
- 9.Hermon-Taylor J, Bull T J, Sheridan J, Cheng J, Stellakis M L, Sumar N. Causation of Crohn's disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000;14:521–539. doi: 10.1155/2000/798305. [DOI] [PubMed] [Google Scholar]
- 10.Hulten K, El-Zimaity H M, Karttunen T J, Almashhrawi A, Schwartz M R, Graham D Y, El-Zaatari F A. Detection of Mycobacterium avium subspecies paratuberculosis in Crohn's diseased tissues by in situ hybridization. Am J Gastroenterol. 2001;96:1529–1535. doi: 10.1111/j.1572-0241.2001.03751.x. [DOI] [PubMed] [Google Scholar]
- 11.Hulten K, Karttunen T J, El-Zimaity H M, Naser S A, Almashhrawi A, Graham D Y, El-Zaatari F A. In situ hybridization method for studies of cell wall deficient M. paratuberculosis in tissue samples. Vet Microbiol. 2000;77:513–518. doi: 10.1016/s0378-1135(00)00336-9. [DOI] [PubMed] [Google Scholar]
- 12.Hulten K, Karttunen T J, El-Zimaity H M, Naser S A, Collins M T, Graham D Y, El-Zaatari F A. Identification of cell wall deficient forms of M. avium subsp. paratuberculosis in paraffin embedded tissues from animals with Johne's disease by in situ hybridization. J Microbiol Methods. 2000;42:185–195. doi: 10.1016/s0167-7012(00)00185-8. [DOI] [PubMed] [Google Scholar]
- 13.Naser S A, Schwartz D, Shafran I. Isolation of Mycobacterium avium subsp. paratuberculosis from breast milk of Crohn's disease patients. Am J Gastroenterol. 2000;95:1094–1095. doi: 10.1111/j.1572-0241.2000.01954.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson J D, Moss M T, Tizard M L V, Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut. 1992;33:890–896. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz D, Shafran I I, Romero C, Piromalli C, Biggerstaff J, Naser N, Chamberlin W, Naser S A, Romero C. Use of short-term culture for identification of Mycobacterium avium subp. paratuberculosis in tissue from Crohn's disease patients. Clin Microbiol Infect. 2000;6:303–307. doi: 10.1046/j.1469-0691.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 16.Sechi L A, Zanetti S, Duprè I, Aceti A, Sanguinetti M, Fadda G. Genotypic changes in DNA fingerprinting patterns of Mycobacterium tuberculosis strains isolated from HIV-positive patients in Sardinia. AIDS. 1998;12:2084–2086. doi: 10.1097/00002030-199815000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Van Kruiningen H J. Lack of support for a common etiology in Johne's disease of animals and Crohn's disease in humans. Inflamm Bowel Dis. 1999;5:183–191. doi: 10.1097/00054725-199908000-00007. [DOI] [PubMed] [Google Scholar]