Abstract
Background
Fabry disease (FD) is a lysosomal storage disorder resulting in systemic accumulation of globotriaosylceramide (Gb3) causing multi-organ dysfunction. The audiologic involvement in FD has been neglected in previous studies; while not a lethal aspect of the disease, hearing loss can have a significantly negative impact on quality of life.
Objective
To investigate hearing loss from baseline through 16 years follow-up of the Danish FD cohort and to compare audiometric data to other clinical variables.
Methods
Data was collected prospectively and assessed retrospectively during a period of 16 years from 83 patients (age: 9–72 years; sex: 29 males and 54 females). 55 patients underwent treatment. Air conduction thresholds was assessed at six frequencies between 0.25 and 8 kHz bilaterally. Data was analyzed using multilinear models.
Results
Mean follow-up period for patients undergoing a FD specific treatment was 7.8 years (0–12.8 years, SD 3.8 years, n = 55). Hearing thresholds for FD patients deviated from healthy individuals at all frequencies for both sexes (p < 0.001). Males had more profound hearing loss than females at high frequencies (4,8 kHz) (p = 0.025). There was no improvement in hearing with treatment (p = 0.343♂, p = 0.256♀). No associations between hearing loss and measured glomerular filtration rate, left ventricular wall thickness or cerebral white matter lesions were found. Lower plasma Gb3 concentration correlated with better hearing (p = 0.046) in males.
Conclusion
Our findings demonstrated significant hearing loss in FD patients compared to audiologically healthy individuals at all frequencies, and no change in hearing during treatment. Lower plasma Gb3 concentrations correlated with better hearing in males.
Keywords: Fabry disease, Hearing loss, Genetic disorder, Albuminuria, Chronic kidney disease, Lysosomal storage disorder, X-linked disorder, Cardiac dysfunction, White matter lesions
Abbreviations: FD, Fabry Disease; Gb3, globotriaosylceramide; mGFR, measured glomerular filtration rate; dB, Decibel; PTA, pure tone average; LVMi, Left ventricular mass index; ERT, Enzyme replacement therapy
Synopsis
Fabry specific treatment does not improve hearing levels in patients with Fabry disease despite baseline hearing loss among these patients.
1. Introduction
Anderson-Fabry disease (FD) is an X-linked lysosomal storage disorder that affects glyco- sphingolipid catabolism due to alfa galactosidase deficiency. This results in systemic accumulation of predominantly globotriaosylceramide (Gb3) in the plasma and lysosomes, which accounts for a range of clinical complications [1], including but not restricted to angiokeratomas, acroparesthesia, abdominal pain, diarrhea, neuropathy, hypohidrosis, cerebral and ocular affection [1], [2]. Furthermore, severe cardiac and renal effects can ultimately lead to cardiac and renal failure [3]. Males are typically more affected than females due to hemizygosity. Conversely, females present as mosaics with varying degrees of symptoms and signs depending on the X chromosome inactivation pattern in the individual [4]. Current specific treatment options consist of intravenous enzyme replacement therapy (ERT) with infusions every second week or oral chaperone treatment.
The audiologic involvement in FD has not been the subject of much research. Although audiologic dysfunction is not lethal, it impacts quality of life, and with increased organ/patient survival of renal (dialysis and renal transplants) and cardiac (implantable cardioverter defibrillator) failure, quality of life becomes more important for survivors. Sakurai and colleagues, demonstrated hearing loss of PTA4 (= mean hearing threshold in decibels (dB) at 0.5, 1, 2 and 4 kHz) in 9/15 males and 3/12 females with FD when compared to the 90th percentile of a healthy Japanese population [5]. Likewise, in a Portuguese study, 122 patients with FD were found to have worse hearing levels at all frequencies compared to a healthy population [6].
Previously, we presented the hearing status of the nationwide Danish Fabry cohort prior to treatment [7]. In the present study, we investigated the hearing through 16 years of follow-up of the Danish nationwide cohort. Moreover, hearing levels were compared to markers of FD severity such as renal function variables, cardiac ventricular mass, brain magnetic resonance imaging (MRI) and biochemical data. We hypothesized that ERT and chaperone treatment stabilized or, at best, improved the hearing ability.
2. Materials and methods
2.1. Study design and population
The study was a nationwide longitudinal retrospective analysis of prospectively collected data. All genetically verified Danish Fabry probands and their family members who underwent comprehensive family screening since 2001 were eligible for this study (n = 88). Diagnostic criteria for FD included the presence of a FD causing mutation regardless of the alpha-galactosidase A activity in leukocytes. Once a proband was diagnosed with FD, all biological family members were offered genetic testing. If an FD causing mutation was identified, screening for organ manifestations followed. All Danish patients were referred to Copenhagen University Hospital (Rigshospitalet), Department of Endocrinology and Metabolism for single-center diagnosis, treatment plan and follow-up in collaboration with a multidisciplinary Fabry team including many other departments such as Otolaryngology, Nephrology, Neuroradiology and Cardiology.
Only patients with FD and at least one baseline audiogram were included (n = 83) (Fig. 1)). All audiograms were collected between 2001 and 2017. Patients were either never treated (n = 28) or treated (n = 55) with ERT (agalsidase-beta or agalsidase-alfa) or an oral chaperone (Migalastat). Seven patients switched to chaperone treatment, however this was towards the end of their follow-up, and due to limited data no attempts have been made to distinguish treatment types. For the never treated patients, the baseline audiogram was defined as the oldest available audiogram. For the treated patients, baseline audiogram was defined as the most recent audiogram available prior to initiation of Fabry specific treatment. Due to limited data in the non-treated group, longitudinal analysis was only performed in the treated group which has in part been described before concerning other organ assessments [7], [8], [9], [10], [11], [12].
Fig. 1.
Characterization of the cohort of patients with Fabry disease.
The study was approved by the Danish Health and Medicine Authority (3–3013-667/1/), the Regional Health Research Ethics Committee (H-3-2014-FSP8) and the Danish Data Protection Agency (2014–641-0055 and 2013-41-1949).
2.2. Audiometric definitions and Z-scores
This study incorporated pure tone average (PTA) values, which are defined as the mean hearing levels in decibels (dB) of several frequencies ranging from 250 Hz to 8 kHz. PTA4 was defined as mean hearing levels in dB of 500 Hz, 1 kHz, 2 kHz and 4 kHz. High frequency PTA48 is defined as mean hearing levels in dB of 4 kHz and 8 kHz. All frequency PTA6 is likewise defined as the mean hearing levels in dB of all measured frequencies, 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz and 8 kHz and lastly, PTA3 is defined as the mean hearing levels in dB of 500 Hz, 1 kHz, and 2 kHz. When appropriate, results are standardized as Z-scores (ie. zPTA4, zPTA48, zPTA6), in which sex and age expected hearing loss has been corrected based on data from the International Organization of Standardization ISO 7029 [13]. Z-scores indicate the number of standard deviations the values deviate from the mean of an age and sex matched healthy population (See supplementary material for Z-score calculations, available in supplementary data).
An otologically normal person (i.e. an individual with normal hearing) is defined as a person in a normal state of health who at the time of testing is free from excess wax in the ear canals, is without known ear pathology and who has no history of undue exposure to noise.
2.3. Audiometric data
All tests were carried out using pure tone audiometry. Air conduction was in all cases measured in both ears. When the air conduction threshold was poorer than 20 dB hearing level, bone conduction was also performed. Additionally, when appropriate the contralateral ear would be masked. All audiological tests were performed by an audio technician. Tympanometry was not performed on a regular basis. The absence of excess ear wax was ensured through otoscopy prior to all measurements. Pure tone audiometry at frequencies 0.25, 0.5, 1, 2, 4 and 8 kHz was carried out in accordance with ISO 8253–1. The modified Hughson-Westlake technique (−10/+5 dB) was employed using a Madsen Astera Audiometer, Madsen Orbiter OB 922 Clinical Audiometer or Interacoustics AC40 Clinical Audiometer, with Sennheiser HDA 200 circumaural earphones. The equipment was calibrated in accordance with IEC 60318–2, ISO 389–5, and ISO 389–8 using a Brüel and Kjaer 2610 measuring amplifier with a 4144 microphone in a 4152 coupler [14], [15], [16], [17].
Reports on hearing impairment were based on WHOs grading of hearing loss [18], which was in turn based on PTA4 values of the better ear. Moreover, unilateral hearing difference was defined as a hearing difference in PTA4 of more than 15 dB between the left and right ear. Additionally, speech discrimination scores were determined by presenting each patient with 25 nationally validated Danish words from a Compact Disc. The score was calculated based on the percentage of words a patient could successfully repeat to the audio technician. Each ear was tested individually. In general, a speech discrimination score above 90% was considered normal. All patients could speak Danish.
2.4. Other clinical variables
Measurements of glomerular filtration rate (mGFR) values were performed within three months of the auditory assessment. The method used for mGFR was one sample 51Cr-ethylenendia-minetetraacetic acid (EDTA) clearance technique, previously described in detail [19], [20]. All mGFR values have been standardized based on work from Grewal & Blake as Z-scores to correct for the age expected decay in filtration [21], [22]. Albuminuria was calculated based on collection of urine through 24 h and analyzed for contents of albumin (see supplementary data for in-depth description) [23].
Cardiac left ventricular mass (LVM) values included were collected within six months of the auditory assessment. All echo cardiograms were completed using a Phillips IE 33 transducer. Measurements of left ventricular thickness and chamber dimensions were performed by two dimensional parasternal images. LVM was calculated based on the American Association of Echocardiography's equation and indexed to body surface area (LVMi) [8].
Gb3 measurements have been performed at Sahlgrenska institute up to 2006 by densitometric evaluation after high performance thin layer chromatography and orcinol detection [24] and afterwards in Genzyme's laboratories, using a rapid LC/MS/MS method for quantification of total plasma Gb3 [25]. Given the discrepancy in units between the two laboratories all values have been corrected using the molar mass of Gb3 (786 g/mol), for coherent statistical analysis (see supplementary table).
Leukoaraiosis and cerebral focal white matter lesions (WML) were quantified both numerically and using Fazekas scale within six months of the most recent audiologic follow-up data of all available patents (n = 67). Cerebral MRI images were analyzed by a board certified neuroradiologist (VA) using both T2 and FLAIR sequences [26].
2.5. Statistical analysis
Data was analyzed using multiple linear modelling with zPTA4, zPTA48 and zPTA6 as dependent variables with age and other clinical/biochemical measurements as independent variables. Where appropriate a two-sample t-test or ANOVA was used. A p-value of less than 5% was considered significant (p < 0.05). All data was compiled and analyzed in STATA 14.1® (StataCorp, Texas USA).
3. Results
3.1. Study population
This study comprised 83 patients, 29 males and 54 females. The mean age at baseline was 35 years (yr) (range 9-73 yr, standard deviations (SD) 16.6 yr) for all patients with females being older in average (p = 0.012). All but one male and 41 out of 54 females were of the classic phenotype (supplementary table 1 for genotypes). In total 55 (24 males, 31 females) patients underwent treatment and 28 (5 males, 23 females) did not (Fig. 1).
A total of 365 audiograms were included. Mean number of audiometries through the follow-up period per patient was 4.4 for all patients (SD 3.0, range 1–11), and 5.7 for treated patients (SD 2.6, range 1–11). Mean otologic follow-up period among treated patients with follow-up audiograms was 7.8 yr (range 0.9–12.8 yr, SD 3.7 yr) (males 6.9 yr, SD 4.0 yr, range 1.1–12.8 yr); (Females 8.3 yr, SD 3.4 yr, range 0.9–11.7 yr) (males vs females p = 0.158). Three male patients under treatment did not have any follow-up audiograms. Only one never treated patients had otologic follow-up data over a period of 12.9 years. All baseline and follow-up data for treated patients is presented in Table 1. Baseline data for both treated and untreated patients are available in a former publication [7].
Table 1.
Baseline and 16 years follow-up data of 55 treated patients with Fabry disease.
| Baseline |
Final
follow-up visit |
|||
|---|---|---|---|---|
| Females (n = 31) | Males (n = 24) | Females (n = 31) | Males (n = 21) | |
| zPTA4 (SD) | −0.66 (−2.51–0.20) | −0.97 (−3.2–0.43) | −0.51 (−2.97–0.96) | −0.75 (−3.05–0) |
| zPTA48 (SD) | −0.85 (−2.10–0.43)⁎ | −1.56 (−6.34–0.38)⁎ | −0.79 (−2.48–0.28)⁎ | −1.45 (−5.49 to −0.02)⁎ |
| zPTA6 (SD) | −0.79 (−2.27–0.22) | −1.09 (−3.36 to −0.11) | −0.56 (−2.22–0.60) | −0.89 (−3.04 to −0.09) |
| Age (year) | 36.4 (10–66.4)⁎ | 27.1 (9.4–47.4)⁎ | 44.8 (16.4–71.8)⁎ | 32.6 (15.0–50.5)⁎ |
| zmGFR (SD) | −0.46 (−2.73–1.56) | −0.98 (−5.70–1.77) | −0.82 (−3.42–1.15) | −0.96 (−6.12–1.91) |
| Albuminuria (mg/day) | 180 (4–1003)⁎ | 811 (4–3787)⁎ | 86 (2–390)⁎ | 479 (3–2007)⁎ |
| LVMi (g/m2) | 108 (58–187) | 120 (95–197) | 87 (47–188)⁎ | 124 (83–306)⁎ |
| Corrected p Gb3 (μmol/L) | 4.57 (2.27–7.76)⁎ | 7.62 (3.56–15.14)⁎ | 6.08 (2.93–11.07) | 5.48 (2.93–8.65) |
| A-gal (nmol/h/mg) | 15.8 (1.6–39)⁎ | 1.9 (0.4–3.1)⁎ | – | – |
Baseline and 16 years follow-up data of 55 treated patients with Fabry disease out of a nationwide cohort of 83. First column shows median parenthesis indicates range.
SD=Standard Deviation, z = Z-score, mGFR = measured glomerular filtration rate, LVMi = left ventricular mass index, p Gb3 = Plasma Globotriaosylceramide, A-gal = Alfa-galactosidase activity.
PTA6 = Pure tone average (mean values of 0.25 kHz 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8Khz).
PTA48 = Pure tone average (mean values of 4 kHz and 8 kHz).
PTA4 = Pure tone average (mean values of 0.5 kHz, 1 kHz, 2 kHz and 4 kHz).
Significant difference between males and females at baseline/final follow-up (p < 0.05).
3.2. Individual patient hearing levels and discrimination scores
The following section applies WHO's definition of hearing loss [18]. At any given point from baseline to final follow-up, slight hearing impairment was present in two male patients (6.9%). One female (1.9%) had slight hearing loss and one (1.9%) had moderate hearing impairment. WHO's definition is based on the better hearing ear. When considering the most affected ear, four male patients (13.8%) had slight hearing loss and one male (3.4%) had moderate hearing impairment. A total of five females (9.3%) had slight hearing loss and one (1.9%) had moderate hearing impairment. Two male patients (6.9%) exhibited a unilateral hearing difference at baseline. At follow-up one male (3.4%) and one female (1.9%) still had a unilateral hearing difference.
Mean speech discrimination scores at baseline were 98.7% for males and 99.6% for females with no difference at baseline between treated and never treated patients (p = 0.661). At follow-up speech discrimination scores for treated patients were 99.8% and 99.6% for males and females, respectively, and no change from baseline to follow-up of treated males (p = 0.423) or females (p = 0.083).
3.3. Hearing levels
There was no difference in hearing thresholds between left and right ears of all patients (males p = 0.880, females p = 0.787), thus all data presented will be the mean values of both ears. Additionally, no patients exhibited any significant air bone gap when comparing air-conduction with bone conduction, hence all described hearing loss will be considered sensorineural (cochlear/retro-cochlear).
All patients had the same hearing Z-scores at baseline regardless of them being at different ages at baseline (p = 0.277).
Hearing thresholds at all frequencies (zPTA4, zPTA48, zPTA6) deviated from the expected levels of an otologically healthy population (p < 0.001). zPTA48 was different between males and females for both treated (p = 0.025) and never treated (p = 0.018) patients.
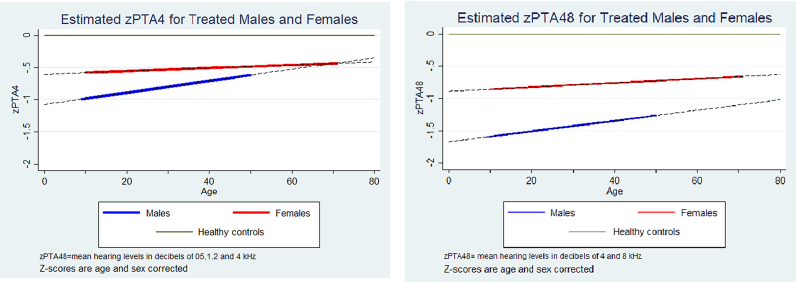
Among treated patients, time in treatment did not change hearing levels for either sex at the tested zPTAs (zPTA4, zPTA48, zPTA6). Hence, hearing levels did neither improve nor worsen in both sexes over the course of treatment compared to the background population. This modelled data is presented in Table 2 and Fig. 2.
Table 2.
Time in treatment does not improve nor worsen hearing levels.
| Females |
Males |
M vs F |
|||||
|---|---|---|---|---|---|---|---|
| P-value | Beta | CI95 | P-value | Beta | CI95 | P-value | |
| zPTA4 | 0.700 | 0.003 | −0.010–0.015 | 0.383 | 0.009 | −0.011–0.030 | 0.238 |
| zPTA48 | 0.579 | 0.003 | −0.008–0.015 | 0.637 | 0.008 | −0.026–0.042 | 0.025* |
| zPTA6 | 0.256 | 0.007 | −0.005–0.019 | 0.343 | 0.011 | −0.011–0.032 | 0.068 |
PTA4 = Pure tone average (mean values of 0.5 kHz, 1 kHz, 2 kHz and 4 kHz).
PTA48 = Pure tone average (mean values of 4 kHz and 8 kHz).
PTA6 = Pure tone average (mean values of 0.25 kHz 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8Khz).
Fig. 2.
Development of hearing levels in patients with Fabry disease.
3.4. Hearing loss, renal function, cardiac mass and biomarkers
In treated patients, mGFR was not associated with zPTA48 when correcting for age (males p = 0.807, females p = 0.331). Likewise, albuminuria was not associated with zPTA48 of females undergoing treatment when correcting for age, (p = 0.842). However, in treated males, higher albuminuria correlated with better zPTA48 (beta = 0.00019, p = 0.015), zPTA4 (beta = 0.00017, p = 0.019) and zPTA6 (beta = 0.00019, p = 0.014).
There was no relationship between LVMi and zPTA48 when correcting for age (males p = 0.177, females p = 0.469).
Corrected Gb3 values could not predict zPTA48 when adjusting for age in females (p = 0.391). However, there was a strong trend of association for increase in corrected plasma Gb3 values predicting a worse zPTA48 in males when adjusting for age (beta = −0.045 p = 0.065), and there was a significant association to both in zPTA4 (beta = −0.05, p = 0.017) and zPTA6 (beta = −0.04, p = 0.046).
There was no association between the amount of WML and Z-scores for high frequency hearing levels (males: p = 0.953; females: p = 0.463). Likewise, no association between high frequency hearing levels and the different Fazekas scale groups (males: p = 0.561; females: p = 0.741).
4. Discussion
The present longitudinal analysis of 55 treated patients with FD revealed a presence of hearing loss, compared to the background population, not only before treatment, but also during follow-up, regardless of sex. Hearing loss was more profound at higher frequencies. In addition, males had more profound hearing loss compared to females at all frequencies. No change in hearing levels could be demonstrated over time in treated patients of both sexes in comparison to the background population (z = 0). Additionally, increasing Gb3 levels correlated with worse hearing levels.
Few studies have investigated longitudinal hearing status in patients with FD. A Dutch study described a baseline hearing loss in 16.8% of patients upon using 1980 WHO classification criteria wherein hearing loss was predominantly observed in males (67%) [27]. Their longitudinal analysis revealed that all baseline frequencies deviated significantly from the expected values from healthy individuals, which was in accordance with our findings. In a more recent study by Rodrigues and colleagues, follow-up analysis of 19 patients one-year post ERT, revealed no difference in hearing thresholds in PTA3 in comparison to baseline values [6]. Likewise, a study by Sergi and colleagues could not demonstrate any difference in hearing levels (PTA4, PTA48) in 20 patients, 4.5 years after initiating ERT treatment [28]. In contrast, a study from the UK used data from the Fabry Outcome Survey (FOS), to follow 26 patients for an average of 1 year after starting agalsidase-alfa, and observed a significant improvement from baseline to follow-up in most frequencies in patients who initially had moderate degrees of hearing impairment [29]. No improvement was observed in patients with normal hearing or profound impairment [29].
However, there are limitations when only using the FOS registry and agalsidase-alfa treated patients, such as obtaining results from a pre-selected population only. Even more relevant for most abovementioned studies is the relatively small number of patients with heterogenous cohort compositions and short follow-up periods.
Our study showed no significant change in hearing over the course of the study, even if the data in Fig. 2 suggests that the hearing slope among male subjects improved over the course of treatment. The lack of significance was potentially due to a small sample size of n = 21.
The lack of hearing decline over time in patients treated with ERT found in this study, could indicate that ERT stabilizes hearing, or that hearing loss had already occurred early in life before commencement of treatment. The latter explanation was in agreement with a recent study by Suntjens and colleagues investigating the degree of hearing loss in 47 Fabry children [30]. They found that hearing thresholds of children deviated significantly from values of healthy children at all frequencies and most prominently at ultra-high frequencies (>8 kHz). However, this longitudinal follow-up of Fabry patients could not be related to non-treated patients, since only one non-treated patient had audiological follow-up data. All other non-treated patients had only baseline audiological evaluation which we have previously described [7].
We could not establish any correlation between change in hearing levels and mGFR or LVMi in keeping with some studies [5], [6]. However these relationships have been observed in cross sectional studies [31], [32]. Germain and colleagues observed an association between mGFR and hearing loss in 8/10 men who had mGFR <40 ml/min/1.73m2 and mean bilateral PTA3 > 25 dB hearing level [31]. We observed an association between an improvement in hearing and higher albuminuria, which contrasts with other findings and logic [6]. This outcome may be a sporadic correlation or an indication that albuminuria can progress in males at an accelerated level despite halted hearing loss by treatment of FD.
No relationship between hearing levels and left ventricular thickness (>13 mm) was observed [30]. Köping and colleagues presented an association between hearing levels (PTA6) for both ‘Kidney Disease Global Outcome’ categories and ‘New York Heart Association’ class (0 vs 3) in 68 patients [32].
A study from 2007 demonstrated that the load of WML correlated to hearing loss [33], which could not be established in our cohort in keeping with our own baseline data [7]. However, the former study included a majority of male subjects (N = 86), while our nationwide cohort based on family screening consisted mostly of female patients. It is possible that a larger sample size of patients or more male patients are required in order to demonstrate a possible correlation.
A study from 1989 conducted histopathological examination of the temporal bones in two Fabry males, with bilateral hearing loss, sloping towards high frequencies [34]. Atrophy of the stria vascularis and spiral ligaments, with additional loss of outer hair cells was found in the organ of Corti. The authors concluded that both direct and indirect accumulations of Gb3 resulted in the dysfunction. A weak but significant association between lower Gb3 levels and better hearing was found in the current study in treated males, which could indicate that reduced Gb3 concentrations correlated with better hearing overall. Thus, it may be essential to start treatment in early childhood in males with the classical form of Fabry in order to mitigate baseline hearing loss. In females with late onset Fabry, hearing does not seem to be compromised early.
4.1. Strengths and limitations
To our knowledge, we present one of the longest (if not the longest) audiological related follow-up studies of treated patients with FD. Another merit of the study was the inclusion of a nationwide cohort. All Danish patients with FD are followed in a single center, which ensured consistency across all examinations. More importantly all family members were offered genetic testing once a Fabry positive diagnosis was established and complete organ assessment was performed in all patients irrespective of symptoms. Consequently, we have twice the number of females in our cohort compared to males. This allows presentation of a comprehensive unbiased picture of the disease in a national cohort. Presenting sex and age corrected data allowed us to compare function across both sexes and all ages. Nonetheless, the latter can also be considered a limitation as it is highly reliant on the standardization material.
Due to compliance issues, some patients lacked audiometric evaluation and the absence of consistent questioning about vertigo, sudden deafness and self-reported hearing loss limited our otological data. Moreover, we have not performed advanced audiometric evaluation such as auditory brain stem response and vestibular evoked myogenic potential examinations, which could have contributed to further understanding the otologic/cerebral involvement. Given that our Gb3 results were obtained from two laboratories, it is difficult to accurately compare these results. Hence, it is possible for these assessments to be over- or under-correcting. Also, since a substantial part of our data was collected prior to the lyso-Gb3 era we could not include this data. Additionally, we have not used a correction for multiple PTA calculations in our statistical analysis. We could also not look at differences in hearing between ERT and Chaperone treated patients due to missing data. Finally, examining 83 patients and smaller subgroups gives a high risk of type two statistical errors, which is a given limitation when studying rare disorders. This is also more challenging when many patients have mild symptoms and mild organ involvement, which can potentially blur any correlation.
5. Conclusion
In conclusion, we observed that our patients with FD had hearing loss compared to the background population from baseline throughout follow-up. Hearing loss was more profound at higher frequencies (4 & 8 kHz) particularly in males. There was no significant improvement in hearing levels over time in treated patients of both sexes. Finally, a lower plasma Gb3 level in males correlated with better hearing levels. It is possible that ERT and chaperone treatment halts progression of hearing loss in some individuals, but this could not be confirmed.
Declaration of Competing Interest
U·F-R. has received Advisory Board honoraria from Amicus Therapeutics, Freeline Therapeutics, Sanofi Genzyme, and Takeda, speaker honoraria from Amicus Therapeutics, Sanofi Genzyme, and Takeda, grant support from Shire and Sanofi Genzyme, and is a member of the European Advisory Board of the Fabry and FollwMe Registries.
Acknowledgements
Ira Hagen Pedersen (Nurse), Anne Marie Jensen (Audiological technical officer), Erik Finn Kjærbøl (M.Sc. E. E.) and Casper Kok (laboratory technician) are thanked for excellent assistance. Members of the Fabry Team at Rigshospitalet are thanked for continuously supplying data. UFR's research salary was supported by the Kirsten and Freddy Johansen's Fund. The project was financially supported by Sanofi-Genzyme.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2022.100841.
Appendix A. Supplementary data
Supplementary material
References
- 1.Desnick R.J., Ioannou Y.A., Eng C.M. OMMBID Online Metab. Mol. Bases Inherit. Dis. 8th ed. 2013. α-Galactosidase A deficiency: fabry disease. p. α-Galactosidase A Deficiency: Fabry Disease. [DOI] [Google Scholar]
- 2.Keshav S. NCBI Bookshelf. Oxford; 2006. Gastrointestinal manifestations of Fabry disease. [PubMed] [Google Scholar]
- 3.Macdermot K.D., Holmes A., Miners A.H. Anderson-fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J. Med. Genet. 2001;38:750–760. doi: 10.1136/jmg.38.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echevarria L., Benistan K., Toussaint A., Dubourg O., Hagege A.A., Eladari D., Jabbour F., Beldjord C., De Mazancourt P., Germain D.P. X-chromosome inactivation in female patients with fabry disease. Clin. Genet. 2016;89:44–54. doi: 10.1111/cge.12613. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai Y., Kojima H., Shiwa M., Ohashi T., Eto Y., Moriyama H. The hearing status in 12 female and 15 male japanese fabry patients. Auris Nasus Larynx. 2009;36:627–632. doi: 10.1016/j.anl.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues J., Azevedo O., Sousa N., Cunha D., Mexedo A., Fonseca R. Inner ear involvement in fabry disease: clinical and audiometric evaluation of a large cohort of patients followed in a reference Centre. Eur. J. Med. Genet. 2018:1. doi: 10.1016/j.ejmg.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Yazdanfard P.D., Madsen C.V., Nielsen L.H., Rasmussen Å.K., Petersen J.H., Seth A., Sørensen S.S., Køber L., Feldt-Rasmussen U. Significant hearing loss in fabry disease: study of the danish nationwide cohort prior to treatment. PLoS One. 2019;14:2–13. doi: 10.1371/journal.pone.0225071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen C.V., Bundgaard H., Rasmussen Å.K., Sørensen S.S., Petersen J.H., Køber L., Feldt-Rasmussen U., Petri H. Echocardiographic and clinical findings in patients with fabry disease during long-term enzyme replacement therapy: a nationwide danish cohort study. Scand. Cardiovasc. J. 2017;51:207–216. doi: 10.1080/14017431.2017.1332383. [DOI] [PubMed] [Google Scholar]
- 9.Fledelius H.C., Sandfeld L., Rasmussen Å.K., Madsen C.V., Feldt-Rasmussen U. Ophthalmic experience over 10 years in an observational nationwide danish cohort of fabry patients with access to enzyme replacement. Acta Ophthalmol. 2015;93:258–264. doi: 10.1111/aos.12588. [DOI] [PubMed] [Google Scholar]
- 10.Mersebach H., Johansson J.O., Rasmussen Å.K., Bengtsson B.Å., Rosenberg K., Hasholt L., Sørensen S.A., Sørensen S.S., Feldt-Rasmussen U. Osteopenia: a common aspect of fabry disease. Predictors of bone mineral density. Genet. Med. 2007;9:812–818. doi: 10.1097/GIM.0b013e31815cb197. [DOI] [PubMed] [Google Scholar]
- 11.Madsen C.V., Granqvist H., Petersen J.H., Rasmussen Å.K., Lund A.M., Oturai P., Sørensen S.S., Feldt-Rasmussen U. Age-related renal function decline in fabry disease patients on enzyme replacement therapy: a longitudinal cohort study. Nephrol. Dial. Transplant. 2018:1–9. doi: 10.1093/ndt/gfy357. [DOI] [PubMed] [Google Scholar]
- 12.Borgwardt L., Uf R., Ak R., Ba M., Lund A. Fabry Disease in Children: Agalsidase-Beta Enzyme Replacement Therapy. 2012. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 13.International Standard . 2000. ISO 7029-2000 Acoustics - Statistical Distribution of Hearing Thresholds as a Function of Age.https://www.iso.org/standard/26314.html [Google Scholar]
- 14.ISO 389-8 . 2004. Acoustics – Reference Zero for the Calibration of Audiometric Equipment - Part 8: Reference Equivalent Threshold Sound Pressure Levels for Pure Tones and Circumaural Earphones. doi:ISO 389-2. [Google Scholar]
- 15.Institution British Standards. 1998. BS EN ISO 8253-1: 1998 - Acoustics — Audiometric Test Methods - Part 1: Basic Pure Tone Air and Bone Conduction Threshold Audiometry. [Google Scholar]
- 16.ISO . 2006. Standard ISO 389-5:2006. Acoustics - Reference Zero for the Calibration of Audiometric Equipment - Part 5: Reference Equivalent Threshold Sound Pressure Levels for Pure Tones in the Frequency Range 8 kHz to 16 kHz. [Google Scholar]
- 17.I. 60318-2 . 1998. Electroacoustics-Simulators of Human Head and Ear-Part 2: An Interimacoustic Coupler for the Calibration of Audiometric Earphones in the Extended High- [Google Scholar]
- 18.WHO - World Health Organization . Prevention of Blindness and Deafness - Grades of Hearing Impairment. Who; 2013. p. 4000.http://www.who.int/pbd/deafness/hearing_impairment_grades/en/ [Google Scholar]
- 19.Groth S., Aasted M. 51Cr-EDTA clearance determined by one plasma sample. Clin. Physiol. 1981;1:417–425. doi: 10.1111/j.1475-097X.1981.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 20.Kereiakes D.J., Parmley W.W. Myocarditis and cardiomyopathy. Am. Heart J. 1984;108:1318–1326. doi: 10.1016/0002-8703(84)90760-9. [DOI] [PubMed] [Google Scholar]
- 21.Blake G.M., Gardiner N., Gnanasegaran G., Dizdarevic S. Reference ranges for 51Cr-EDTA measurements of glomerular filtration rate in children. Nucl. Med. Commun. 2005;26:983–987. doi: 10.1097/01.mnm.0000179294.16339.4a. [DOI] [PubMed] [Google Scholar]
- 22.Grewal G.S., Blake G.M. Reference data for 51Cr-EDTA measurements of the glomerular filtration rate derived from live kidney donors. Nucl. Med. Commun. 2005;26:61–65. doi: 10.1097/00006231-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Region H. 2018. Rigshospitalets Labportal, Labportal RH.https://labportal.rh.dk/Metodeliste.asp?Mode=Display&Id=5289 [Google Scholar]
- 24.Apelland T., Gude E., Strøm E.H., Gullestad L., Eiklid K.L., Månsson J.E., Reinholt F.P., Houge G., Dahl C.P., Almaas V.M., Heiberg A. Familial globotriaosylceramide-associated cardiomyopathy mimicking fabry disease. Heart. 2014;100:1793–1798. doi: 10.1136/heartjnl-2014-305616. [DOI] [PubMed] [Google Scholar]
- 25.Roddy T.P., Nelson B.C., Sung C.C., Araghi S., Wilkens D., Zhang X.K., Thomas J.J., Richards S.M. Liquid chromatography-tandem mass spectrometry quantification of globotriaosylceramide in plasma for long-term monitoring of fabry patients treated with enzyme replacement therapy. Clin. Chem. 2005;51:237–240. doi: 10.1373/clinchem.2004.038323. [DOI] [PubMed] [Google Scholar]
- 26.Korsholm K., Feldt-Rasmussen U., Granqvist H., Højgaard L., Bollinger B., Rasmussen A.K., Law I. Positron emission tomography and magnetic resonance imaging of the brain in fabry disease: a Nationwide, long-time, prospective follow-up. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suntjens E.B., Smid B.E., Biegstraaten M., Dreschler W.A., Hollak C.E.M., Linthorst G.E. Hearing loss in adult patients with fabry disease treated with enzyme replacement therapy. J. Inherit. Metab. Dis. 2015;38:351–358. doi: 10.1007/s10545-014-9783-7. [DOI] [PubMed] [Google Scholar]
- 28.Sergi B., Conti G., Paludetti G. Interdisciplinary study group on fabry disease, inner ear involvement in Anderson-fabry disease: long-term follow-up during enzyme replacement therapy. Acta Otorhinolaryngol. Ital. 2010;30:87–93. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2882147&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 29.Hajioff D., Hegemannn S., Conti G., Beck M., Sunder-Plassmann G., Widmer U., Mehta A., Keilmann A. Agalsidase alpha and hearing in fabry disease: data from the fabry outcome survey. Eur. J. Clin. Investig. 2006;36:663–667. doi: 10.1111/j.1365-2362.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 30.Suntjens E., Dreschler W.A., Hess-Erga J., Skrunes R., Wijburg F.A., Linthorst G.E., Tøndel C., Biegstraaten M. Hearing loss in children with fabry disease. J. Inherit. Metab. Dis. 2017;40:725–731. doi: 10.1007/s10545-017-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germain B.P., Chassaing A. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: a study of twenty-two hemizygous male patients. BMC Med. Genet. 2002;10:1–10. doi: 10.1186/1471-2350-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köping M., Shehata-Dieler W., Cebulla M., Rak K., Oder D., Müntze J., Nordbeck P., Wanner C., Hagen R., Schraven S. Cardiac and renal dysfunction is associated with progressive hearing loss in patients with fabry disease. PLoS One. 2017;12:1–12. doi: 10.1371/journal.pone.0188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ries M., Kim H.J., Zalewski C.K., Mastroianni M.A., Moore D.F., Brady R.O., Dambrosia J.M., Schiffmann R., Brewer C.C. Neuropathic and cerebrovascular correlates of hearing loss in fabry disease. Brain. 2007;130:143–150. doi: 10.1093/brain/awl310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schachern P.A., Paparella M.M., Shea D.A., Yoon T.H. Otologic histopathology of Fabry’s disease. Ann. Otol. Rhinol. Laryngol. 1989;98 doi: 10.1177/000348948909800509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material