Abstract
The passive mechanical properties of the vertebrate heart are controlled in part by the composition of the extracellular matrix (ECM). Changes in the ECM, caused by increased blood pressure, injury or disease can affect the capacity of the heart to fill with blood during diastole. In mammalian species, cardiac fibrosis caused by an increase in collagen in the ECM, leads to a loss of heart function and these changes in composition are considered to be permanent. Recent work has demonstrated that the cardiac ventricle of some fish species have the capacity to both increase and decrease collagen content in response to thermal acclimation. It is thought that these changes in collagen content help maintain ventricle function over seasonal changes in environmental temperatures. This current work reviews the cellular mechanisms responsible for regulating collagen deposition in the mammalian heart and proposes a cellular pathway by which a change in temperature can affect the collagen content of the fish ventricle through mechanotransduction. This work specifically focuses on the role of transforming growth factor β1, MAPK signaling pathways, and biomechanical stretch in regulating collagen content in the fish ventricle. It is hoped that this work increases the appreciation of the use of comparative models to gain insight into phenomenon with biomedical relevance.
keywords: Extracellular matrix, Cardiac fibroblasts, Mechanotransduction, Cardiac fibrosis, miR-29b, TGF-β
Glossary
- Concentric left ventricular hypertrophy
Condition where changes in heart morphology have resulted in a relative increase in ventricle wall thickness, but a decrease in the chamber volume
- Eccentric left ventricular hypertrophy
Condition where changes in heart morphology have resulted in a relative increase in ventricle wall thickness and chamber volume
- ECM
Extracellular Matrix: Dynamic extracellular scaffold, comprised primarily of collagen, that provides biomechanical stability to the cell and structural integrity to the tissue that the cell is part of
- Fibroblast
A cell that creates the extracellular matrices specific to the requirements of the organ or tissue with which they are associated
- FAK
Intracellular focal adhesion kinase: Signaling molecule that regulates cell adhesion, migration, and survival
- Integrins
Protein receptors used by animal cells to bind to the extracellular matrix
- MAPK
Mitogen-activated protein kinase, a family of protein kinases, that target serine and threonine residues for phosphorylation and are involved in multitier cell signalling cascades
- Mechanotransduction
Translation of a physical force, experienced by a cell surface receptor, into a chemical signal passed through cellular proteins
- miR
micro mRNA: Small non-coding RNAs that bind to the 3′ untranslated seed region of messenger RNA (mRNA) to promote degradation of the mRNA or prevent it from binding to ribosomes
- MMPs
Matrix metalloproteinases: Family of zinc-dependent endopeptidases that degrade collagen in the ECM
- SMAD2
Small mothers against decapentaplegic 2, family of proteins that comprise a signalling cascade activated by TGF-β1 involved in the regulation of cell development and growth
- TIMPs
Tissue inhibitor of matrix metalloproteinases, Endogenous protein regulator of matrix metalloproteinases
- TGF-β1
Transforming growth factor-β1, Ubiquitously expressed cytokine involved in the activation of cell signalling pathways including those involving SMAD2
1. Introduction
Cardiac output is the product of heart rate and stroke volume, and is influenced by the size and shape of the heart. In vertebrates, cardiac output is tightly matched to the aerobic requirements of the animal. This is possible due to a variety of mechanisms that span levels of biological organization and time scales. For example, almost instantaneous changes to the phosphorylation state of regulatory proteins, associated with Ca2+ handling and myofilament function, result in modification to the rate and strength of cardiac contraction (Shaffer and Gillis, 2010). These changes, activated through α-adrenergic receptors, are rapid but temporary, and allow regulation of cardiac output on a beat to beat basis (Shaffer and Gillis, 2010). Associated with changes to the Ca2+ sensitivity of the myofilament with stimulation of α-adrenergic pathways are adjustments of heart rate, stroke volume as well as hematocrit caused by splenic contraction (Evans, 1985). These work together to increase the oxygen carrying capacity of the cardiovascular system (Evans, 1985). Appropriately, as the duration of higher oxygen requirements of the animal increases, the response of the heart shifts to initiate more permanent changes to its structure and function though the activation of specific patterns of gene and protein expression. For example, endurance training of human athletes initiates an increase in blood volume requirements and this can result in eccentric left ventricular hypertrophy (LVH), where the chamber volume and thickness of the ventricle wall are increased (Weiner and Baggish, 2012). This growth of the heart muscle is due to the addition of sarcomeres in series, and occurs over a period of weeks (Weiner and Baggish, 2012). It is also important to note that contrary to what will be described below for concentric LVH, cardiac fibrosis does not typically develop with eccentric LVH (Weiner and Baggish, 2012; Richey and Brown, 1998). The end result of eccentric LVH is improved systolic function and an increase in stroke volume (Weiner and Baggish, 2012). This remodeling is considered a physiological response that increases cardiac output while maintaining the mechanical properties of the heart (Richey and Brown, 1998). As with exercise induced changes to skeletal muscle, such changes to the heart are reversible (Maron et al., 1993; Olah et al., 2017; Waring et al., 2015).
While the response of the mammalian heart to a physiological stressor, such as endurance training, is generally considered beneficial, cardiac remodeling also occurs following injury, or in response to a chronic condition such as hypertension. For example, chronic hypertension in mammals can induce concentric LVH and interstitial fibrosis (Richey and Brown, 1998; Breisch et al., 1984; Yildiz et al., 2020). With concentric LVH, there is an increase in ventricle wall thickness and a concomitant decrease in chamber volume (Richey and Brown, 1998; Breisch et al., 1984; Yildiz et al., 2020). This growth of the muscle is caused by an increase in the width of cardiac myocytes resulting from the addition of sarcomeres in parallel (Lorell and Carabello, 2000). While this result does cause an increase in systolic function, the changes in ventricle structure and associated interstitial fibrosis leads to an increase in myocardial stiffness and impaired left ventricular relaxation (Richey and Brown, 1998; Breisch et al., 1984; Yildiz et al., 2020; Lorell and Carabello, 2000). The end result is diastolic dysfunction causing a decrease in the amount of blood pumped per cardiac cycle, and an increased incidence of heart failure (Breisch et al., 1984; Lorell and Carabello, 2000; Messerli et al., 2017). For example, Levy et al. (1996) reported that hypertension, a cause of concentric LVH, had been identified in 91% of patients before the development of heart failure. Together, these changes to the heart resulting from increased blood pressure represent a significant challenge to the survival of the affected individual.
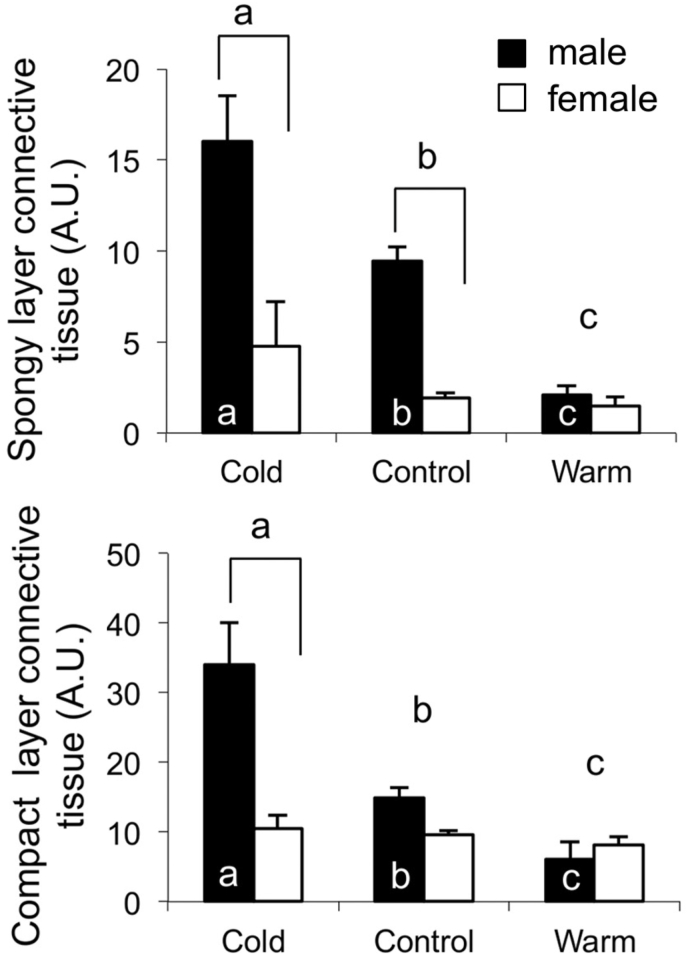
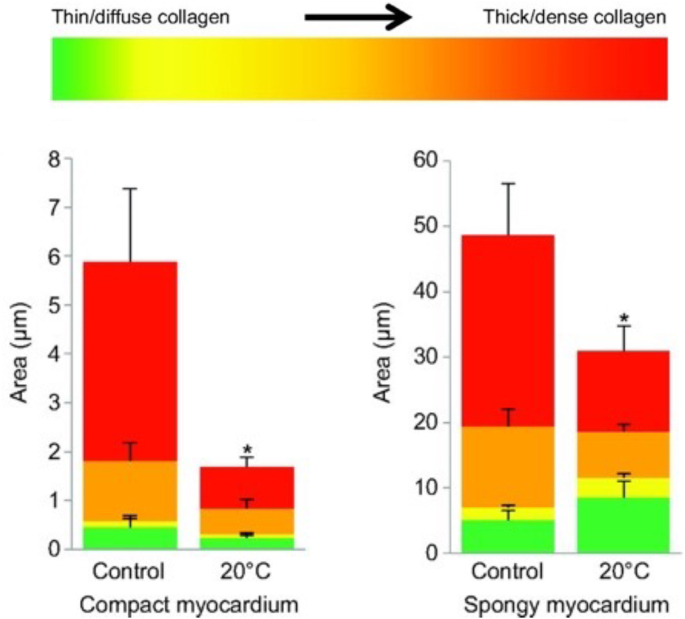
Recent work using comparative models, including zebrafish and rainbow trout, have been providing novel insight into how an increase in deposited collagen, permanent in the mammalian heart, may be reversible (Klaiman et al., 2011; Johnson et al., 2014). This work has been examining the response of the fish heart, primarily the single cardiac ventricle, to thermal acclimation (Klaiman et al., 2011, 2014; Johnson et al., 2014; Keen et al., 2016a, 2017, 2018; Ding et al., 2021). Fish are ectothermic and their physiological temperature is determined by that of their environment. For some fish species, seasonal changes in temperature can lead to physiological temperature in the winter being 10 °C less than that in the summer. While there are some fish that significantly reduce physical activity with such a decrease in physiological temperature, salmonids, including rainbow trout, as well as zebrafish do not (Klaiman et al., 2011; Anttila et al., 2014; Elliott and Elliott, 2010; Rodnick et al., 2004; Cortemeglia and Beitinger, 2005; Sundin et al., 2019). Previous work has demonstrated that the heart of these fish can undergo changes in form, composition and function to help compensate for this change in physiological temperature so as to maintain contractility, and therefore activity of the animal (Klaiman et al., 2011, 2014; Gamperl and Farrell, 2004; Aho and Vornanen, 1999, 2001; Keen et al., 2016b; Driedzic et al., 1996). Cold acclimation of rainbow trout has also been found to cause an increase in the collagen content of the myocardium while warm acclimation reduces collagen content (Fig. 1) (Klaiman et al., 2011). In a mammalian heart, an increase in collagen content would lead to an increase in myocardial stiffness. Work by Keen et al. (2017) has demonstrated that the increase in collagen content of the trout ventricle, caused by cold acclimation, results in an increase in tissue stiffness. Furthermore, Johnson et al. (2014) have demonstrated that cold acclimation of zebrafish causes a decrease in total collagen content as well as in the amount of thick collagen fibers in the ventricle (Fig. 2). Together these studies illustrate that the collagen composition of the fish heart is quite responsive to changes in physiological conditions. The current work reviews the cellular mechanisms responsible for regulating collagen deposition in the mammalian heart and uses this as the basis to propose a cellular pathway by which a change in temperature can affect the collagen content of the fish ventricle. It is hoped that this approach helps to underscore the relevance of studying comparative models to gaining insight into physiological phenomena.
Fig. 1.
Influence of thermal acclimation on the connective tissue content of trout ventricular myocardium quantified using Masson's trichrome staining. (A) The hearts of cold acclimated male trout had significantly more connective tissue in the spongy layer than that of either control or warm acclimated male fish. (B) Cold acclimation of male trout caused an increase in connective tissue content in the compact layer compared to controls while warm acclimation of male trout caused a decrease in connective tissue content, compared to controls. The amount of connective tissue is presented as arbitrary units (A.U.) representing the ratio of connective tissue present in the compartment in relation to muscle. Values are mean ± SEM. Brackets, if present indicate a significant difference between sexes within an acclimation group. Different letters above the bars indicate a significant difference between acclimation groups. Different letters within the bars indicate significant differences between acclimation temperatures when each sex is analyzed separately (p < 0.05). Figure modified from (Klaiman et al., 2011).
Fig. 2.
Influence of cold acclimation on collagen composition in the compact and spongy myocardium of zebrafish, Danio rerio. (A) Area, calculated as μm2, occupied by each of the four collagen fiber types in the compact myocardium and spongy myocardium within the middle cross-section of hearts from control (27 °C) and cold-acclimated (20 °C) zebrafish. Red denotes the thickest/densest fibers, while green denotes the thinnest. *Values of the same fiber type in the same myocardial layer are significantly different between treatment groups (P < 0.05); n = 9 for all measurements. Figure modified from (Johnson et al., 2014). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. The extracellular matrix
The extracellular matrix (ECM), composed almost entirely of collagen, provides passive biomechanical structure to the myocardium, and plays a role in relaying physical and chemical signals through the tissue. The passive biomechanical properties of the ventricle, including its stiffness, directly impact the functional capacity of the heart. For example, as discussed above, if a ventricle is too stiff its ability to fill with blood during diastole will be reduced. In the myocardium, the ECM is synthesized and regulated by cardiac fibroblasts, and under normal homeostatic conditions, these maintain the integrity and passive properties of the tissue, by facilitating collagen turnover. The increase in collagen deposition that occurs with the onset of concentric LVH in the mammalian heart is the result of the balance between deposition and degradation shifting to favor deposition. This is caused by an increase in collagen synthesis by the fibroblasts as well as a decrease in the activity of matrix metalloproteinases (MMPs), proteases that target collagen (Fomovsky et al., 2010). This shift in fibroblast activity is due to changes in the pattern of cell signaling pathways that regulate the expression of relevant gene transcripts and is caused by changes in biophysical forces experienced by the myocardium as well as in the levels of specific cytokines, such as transforming growth factor-β1 (TGF-β1) (Fomovsky et al., 2010; Siwik et al., 2001). For further insight of the role of the ECM in regulating the mechanical properties of the mammalian heart see: (Fomovsky et al., 2010).
3. Cardiac fibroblasts as regulators of the extracellular matrix
Fibroblasts make up approximately two-thirds of the total myocardial cell population, and among the cardiac interstitial cells, fibroblasts are the predominant cell type (Chang et al., 2016). However, due to their relatively small size in comparison to myocytes, fibroblasts comprise approximately one-third of the total heart volume (Chang et al., 2016). Collagen turnover in the mammalian heart, carried out by these cells, is under the influence of multiple factors. This includes TGF-β1 and biomechanical stress (Chiquet et al., 2009; Creemers et al., 2001; Murphy, 2011; Border and Noble, 1994; Lal et al., 2007; Verma et al., 2011). TGF-β1 is ubiquitously expressed and binds to most cell types initiating intracellular signaling cascades involved in cellular proliferation, differentiation and migration (Overall et al., 1991; Van Obberghen-Schilling et al., 1988). In the mammalian heart, TGF-β1 production and release from cardiac myocytes increases during pathological remodeling such as that which occurs after myocardial infarction (Bujak et al., 2007) or with the onset of hypertension (Lijnen et al., 2003). These pathologies are associated with an increase in blood pressure and blood viscosity (Devereux et al., 1984), leading to an increase in cardiac workload, and as a result increased cellular deformation caused by biomechanical stretch and/or shear stress on the endocardium (Husse et al., 2007; Reed et al., 2014; Waring et al., 2014). This increased deformation of the myocardium leads to a corresponding increase in TGF-β1 production (Katsumi et al., 2004; MacKenna et al., 1998). An increase in TGF-β1 has been associated with the induction of collagen synthesis in fibroblasts as a result of phosphorylation events through protein signaling cascades. The biological activity of TGF-β1 is facilitated by a 112–114 amino acid sequence in the C-terminal domain (Khalil, 1999; Verrecchia and Mauviel, 2007). In mammalian cells, cell surface receptors, specific to this region of the cytokine (Verrecchia and Mauviel, 2007), initiate a signaling cascade via protein conformation changes and phosphorylation events that translocates the signal to transcriptional factors located in the nucleus, influencing gene expression. One critical protein involved in this signaling cascade is small mothers against decapentaplegic 2 (SMAD2) whose activation leads to an increase in the transcription of timp-2 and collagen type I (col1); resulting in an increase in connective tissue in the ECM (Visse and Nagase, 2003; Kolosova et al., 2011; Reed et al., 1994).
Exposure of mammalian cardiac fibroblasts to increased biomechanical stretch also leads to an increase in collagen production. As with TGF-β1, biomechanical activation of stretch sensitive proteins located in the ECM, leads to the activation of cell signalling cascades that regulate gene transcription and translation (Lammerding et al., 2004). More specifically, biomechanical stimulation of integrins or heterotrimeric guanine nucleotide binding proteins (G-proteins) leads to the subsequent activation of multiple, mitogen activated protein kinases (MAPKs) that affect the expression of collagen monomers, TIMPs and MMPs (Lammerding et al., 2004; Herum et al., 2017; MacKenna et al., 2000; Manso et al., 2009). The MAPKs involved include p38, c-Jun N-terminal kinase (JNK) and extracellular regulated kinase (ERK), and these form the p38-JNK-ERK pathway, associated with cardiac remodeling (Katsumi et al., 2004; Pramod and Shivakumar, 2014; Sinfield et al., 2013; Johnston and Gillis, 2020). Integrin subunits are also associated with intracellular focal adhesion kinase, the phosphorylation of which is thought necessary for ERK and p38 signaling (Katsumi et al., 2004). The end result of the activation of the p38-JNK-ERK pathway is an increase in deposited collagen (Fan et al., 2012). Such change in cardiac collagen has been reported in patients suffering from cardiac hypertension, dilated cardiomyopathy and chronic congestive heart failure (Jalil et al., 1988, 1989; Marijianowski et al., 1995; Pauschinger et al., 1999). In mammals, altered ECM composition is also associated with pathological conditions such as post-infarct remodeling. In addition, changes in the collagen composition can also have a functional consequence (Marijianowski et al., 1995; Junqueira et al., 1978). For example, an increase in the ratio of type I collagen (thick) to type III collagen (thin) has been reported in the hearts of patients suffering from dilated cardiomyopathy (Marijianowski et al., 1995; Pauschinger et al., 1999; Junqueira et al., 1978). In addition, work by Keen at al., (Keen et al., 2018), has demonstrated that the increase in collagen content measured in the trout ventricle with cold acclimation translated into an increase in tissue stiffness (Klaiman et al., 2014; Keen et al., 2018). It should be noted here that collagen type I is the predominant collagen found in vertebrate tissues. The difference between collagen type I in mammals and that in fish is that the mammalian molecule is composed of two strands translated from the col1a1 gene and one from the col1a2 gene (Poschl et al., 1988) while fish collagen type I is composed of three monomers, transcribed from three different transcripts (col1a1, col1a2 and col1a3), with col1a3 being unique to fish (Keen et al., 2018).
The changes to the mammalian myocardium caused by endurance training and chronic hypertension are initiated, at least in part, by increased biomechanical stimulation of the muscle. For example, endurance training of mammals causes an increase in heart rate and stroke volume and as a result, an increase in the rate and level of cellular deformation as the left ventricle pushes blood through the systemic circulation. Importantly, with endurance training, there is no associated increase in vascular resistance (Cornelissen and Fagard, 2005). With chronic hypertension, hemodynamic overload leads to an increase in left ventricular wall stress. As a result, the myocytes, fibroblasts and other cell types that compose the ventricular wall, experience chronically elevated levels of biomechanical strain. How such an increase in biomechanical stimulation is transformed into a biological response is via stretch sensitive elements in the cellular membranes and ECM (Komuro and Yazaki, 1993). One example of such an element are integrin proteins that anchor the cell cytoskeleton to the ECM, and also transmit extracellular information through cells via mechanotransduction as described above. The end result of the activation of these MAPK pathways in the mammalian heart is changes in the expression of specific gene transcripts, including those that transcribe MMPs, TIMPS, as well as collagen monomers (Komuro and Yazaki, 1993).
4. The role of matrix metalloproteinases and tissue inhibitor of metalloproteinase in collagen deposition and turnover
MMPs are a large family of zinc-dependent enzymes that share structural domains but differ in their targeted substrates, cellular sources, and activation requirements (Creemers et al., 2001). Together these proteases, can digest all types of connective tissue (Visse and Nagase, 2003). Of particular importance to the ECM of mammalian hearts as well as fish hearts are MMP-13, MMP-2 and MMP-9 (Meschiari et al., 2017; Riaz et al., 2017; Austin et al., 2013; Dreger et al., 2002). MMP-13 digests native collagen type I into hydrolyzed collagen, otherwise known as gelatin, while MMP-2 and MMP-9 are gelatinases that convert the gelatin into waste that is removed from the cells (Hillegass et al., 2007; Pedersen et al., 2015; Kubota MK et al., 2003; Li et al., 2002). While gelatinases typically do not cleave the collagen triple helix directly, their activity is indicative of matrix degradation during the remodeling process (Riley et al., 2002).
Due to their capacity to digest the ECM, the activity of MMPs is tightly controlled. This is through regulation of their transcription as a latent proenzyme, by the activation of the proenzyme via proteolysis, and by the expression of endogenous regulators called tissue inhibitors of metalloproteinase (TIMP) (Creemers et al., 2001; Husse et al., 2007; Lee et al., 2002). MMPs, and TIMPs are produced by cardiac fibroblasts and then excreted into the ECM. Changes in the expression levels, and regulation, of MMPs and TIMPs have been identified as contributing to the development of cardiac fibrosis (Roldan et al., 2008) and dilated cardiomyopathy (Matsumoto et al., 2009; Givvimani et al., 2010) in mammalian models. Importantly, as described below, changes in the transcripts for MMPs and TIMPs have also been characterized in fish ventricles is response to thermal acclimation (Johnson et al., 2014; Keen et al., 2018; Ding et al., 2021) as well as in trout cardiac fibroblasts with TGF-β1 treatment (Johnston and Gillis, 2017, 2018). This suggests that there are similarities in the cellular pathways that alter collagen content in the fish heart in response to thermal acclimation and in the mammalian heart in response to changes in blood pressure.
5. Proposed cellular mechanisms for regulating collagen deposition in the fish ventricle during thermal acclimation
An acute decrease in physiological temperature causes a reduction in the rate and level of force development by the vertebrate heart (Klaiman et al., 2014; Churcott et al., 1994; Harrison and Bers, 1990; Stephenson and Williams, 1985). This effect of temperature change is due, at least in part, to a decrease in the Ca2+ sensitivity of the myofilament and to a decrease in enzymatic activity including that of actin-myosin ATPase (Gillis et al., 2000; Gillis and Tibbits, 2002). It is such challenges that temperate fish, which remain active in winter, must overcome. One response to a decrease in cardiac contractility in fish, caused by a decrease in physiological temperature, is cardiac hypertrophy (Klaiman et al., 2011; Gamperl and Farrell, 2004; Driedzic et al., 1996; Graham and Farrell, 1989; Aho and Vornanen, 1998). It is thought that the added muscle helps to compensate for the loss of contractile power (Klaiman et al., 2011; Graham and Farrell, 1989). More recent work by Klaiman et al. (2014), has demonstrated that cold acclimation of trout increases the Ca2+ sensitivity of the myofilament and that this translates into an increase in the level and rate of force generation by the ventricle. Such a change in function would help compensate for the influence of a decrease in temperature on the active properties of the ventricle. To learn more of the impact of thermal acclimation on the function of the fish heart please see the reviews by Gamperl and Farrell (2004) and Keen et al. (2017).
Thermal acclimation has been demonstrated to affect the collagen content in the trout and zebrafish ventricle and this response is thought to result from changes in the biomechanical forces experienced by the myocardium and to help maintain the passive properties of the heart (Klaiman et al., 2011; Keen et al., 2017; Graham and Farrell, 1989; Farrell, 1984). In fish, heart rate is affected by temperature, increasing with an increase in temperature and decreasing with a decrease in temperature (Farrell, 1984). For fish undergoing cold acclimation, the decrease in heart rate increases the ventricle filling time and as a result, more blood enters the heart between contractions (Farrell, 1984). This causes greater expansion of the ventricle and greater stretch of the myocardium, resulting in the activation of mechanically sensitive pathways as discussed previously. The second proposed source of a change in biomechanical force with temperature change, is a change in blood viscosity resulting from modifications to the fluidity of erythrocyte membranes (Keen et al., 2017; Farrell, 1984; Graham et al., 1985). Such changes to the membranes of all cell types result from the influence of temperature on the biophysical properties of the membrane phospholipids (Cameron et al., 1980; Meraldi and Schlitter, 1981). For example, a decrease in temperature decreases the kinetic energy of the phospholipids and this causes them to pack closer together (Cameron et al., 1980; Meraldi and Schlitter, 1981). As a result, the membrane is more stiff and resistant to deformation (Hazel, 1995). Such a change in the erythrocyte membranes helps to explain the increase in blood viscosity detected with an acute decrease in temperature (Graham and Fletcher, 1983, 1984). As mentioned above, such a change in hemodynamic load in mammalian models leads to an increase in the release of TGF-β1 and in the activity of fibroblasts. The increase in blood viscosity, caused by a decrease in environmental temperature would be temporary, as active modification of membrane composition would increase membrane fluidity and normalize viscosity (Hazel, 1995). Such modification to the cellular membranes of multiple tissues from trout, in response to cold acclimation, have been characterized after three weeks of thermal acclimation (Hazel, 1983; Zehmer and Hazel, 2005).
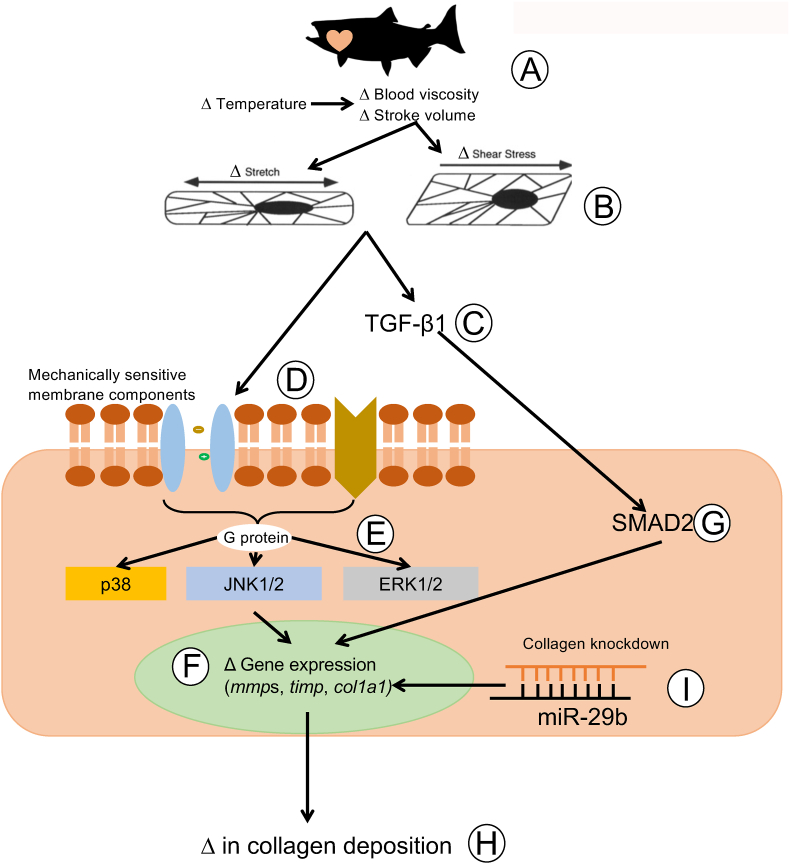
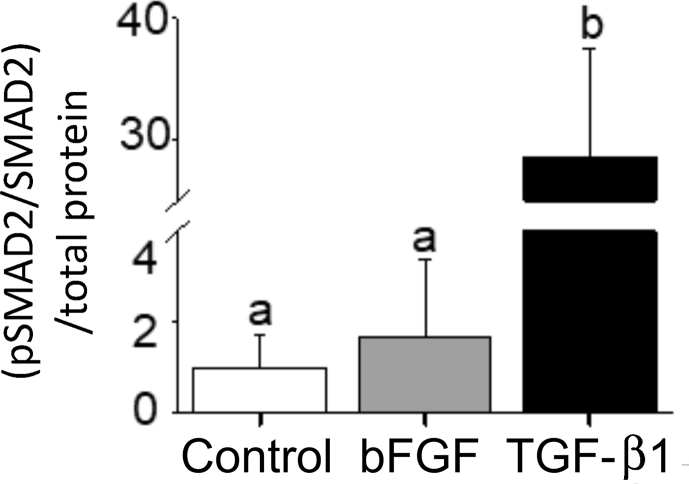
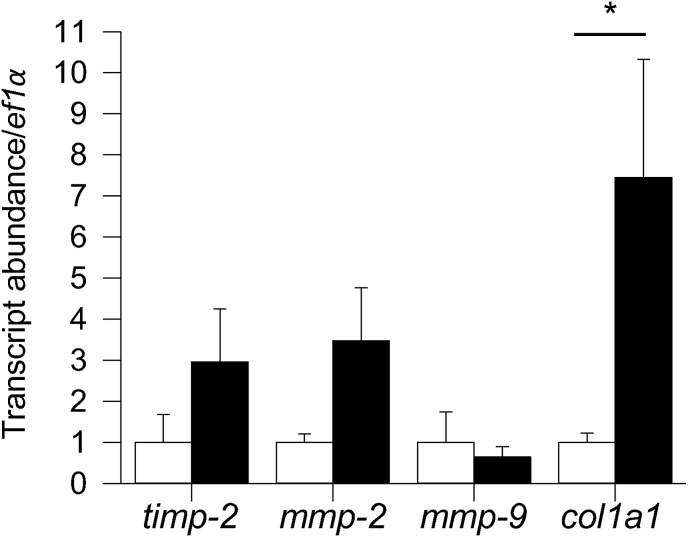
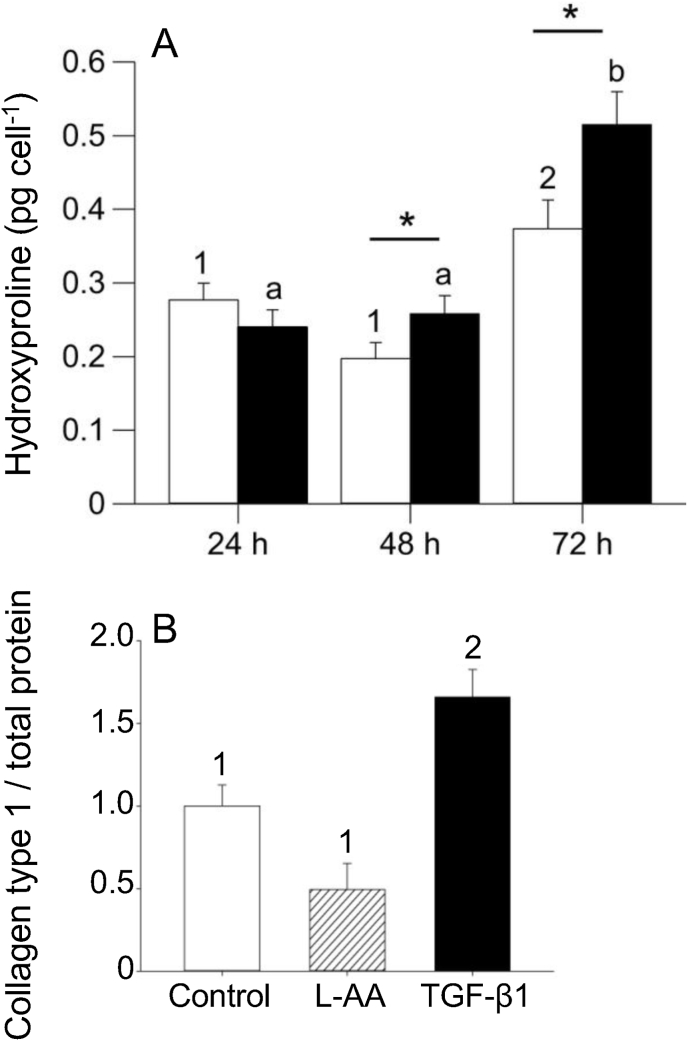
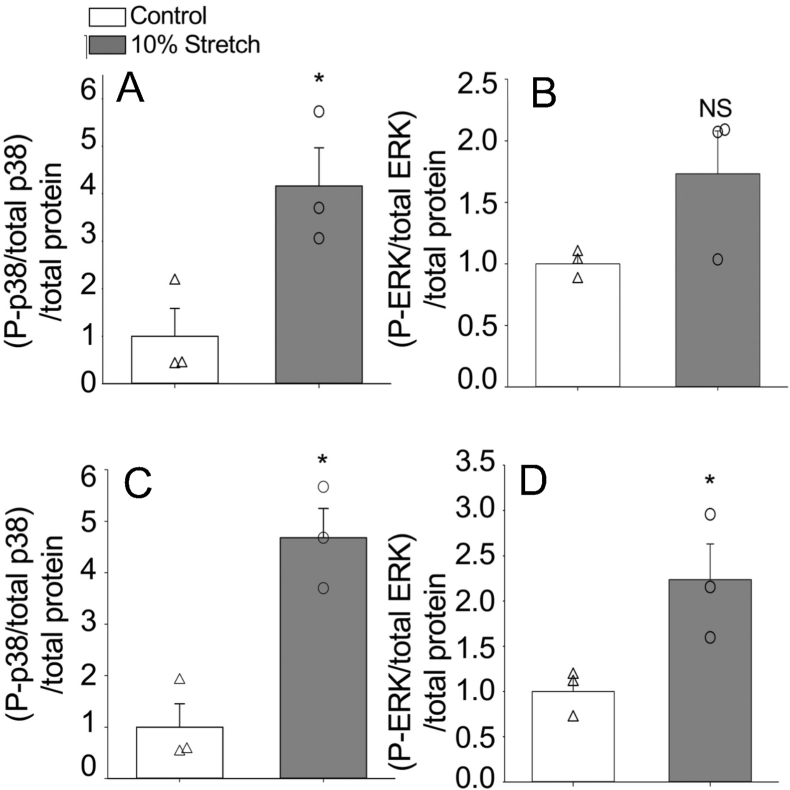
Fig. 3 illustrates a proposed pathway by which a decrease in physiological temperature could lead to an increase in the deposition of collagen in the trout heart. This figure has been generated by integrating the results of thermal acclimation studies of trout (Keen et al., 2017; Farrell, 1984; Graham et al., 1985) and experiments completed on cultured trout cardiac fibroblasts (Johnston and Gillis, 2017, 2018, 2020; Johnston et al., 2019) with what is known of the effects of biomechanical forces on mammalian cardiac fibroblasts (Katsumi et al., 2004; MacKenna et al., 1998, 2000; Visse and Nagase, 2003; Kolosova et al., 2011; Reed et al., 1994; Herum et al., 2017; Manso et al., 2009; Pramod and Shivakumar, 2014; Sinfield et al., 2013). It is proposed that it is an increase in biomechanical forces, experienced by component cells of the myocardium (myocytes and fibroblasts) that activate cellular signaling pathways that lead to changes in the collagen composition of the trout ventricle (Fig. 3). More specifically, stretch activation of integrins and stretch sensitive ion channels are proposed to initiate cellular signaling pathways that result in changes in gene transcription and translation. Within myocytes this results in an increased release of TGF-β1, and in cardiac fibroblasts in the activation of the MAPK pathway. This pathway is an example of the phenomenon called mechanotransduction where mechanical stimuli experienced by a cell, is translated into a biological process. Support for this hypothesis results from a series of studies from Johnston and Gillis (Johnston and Gillis, 2017, 2018, 2020; Johnston et al., 2019); that utilized cultured trout cardiac fibroblasts. These authors demonstrated that exposure of these cells to physiological levels of TGF-β1 caused a 30-fold increase in the phosphorylation of SMAD2 (Johnston and Gillis, 2018) (Fig. 4), changes in the expression of gene transcripts that would support an increase in collagen deposition, specifically an 8-fold increase in col1a1 (Fig. 5), and an increase in total collagen and collagen type 1 (Johnston and Gillis, 2017) (Fig. 6). Importantly, the changes in gene expression seen with treatment of the fibroblasts with TGF-β1 are similar to those seen in the ventricle of cold acclimated trout (Keen et al., 2016c). Johnston and Gillis (2020) have also demonstrated that exposure of cardiac fibroblasts to increased levels of biomechanical stretch activates the MAPK pathway, as indicated by an increase in the phosphorylation of p38 and ERK with the increase in p38 phosphorylation occurring by 20 min of the stretch treatment (Fig. 7). At the molecular level, the activation of p38 and ERK would translate the stretch stimuli to a change in gene expression and subsequent modification of collagen deposition. This means that the trout fibroblasts are highly reactive to biomechanical stimuli. In addition, recent work by Ding et al. (2021) has demonstrated that thermal acclimation caused an increase in p38 MAPK phosphorylation in the ventricle of female fish and in the phosphorylation of ERK in the ventricle of male trout. This work demonstrates that MAPK signaling pathways are responsive to thermal acclimation.
Fig. 3.
Proposed pathway by which a change in environmental temperature leads to changes in collagen deposition in the trout heart. The change in cardiac composition that occurs with thermal acclimation is proposed to result from changes in the biomechanical forces experienced by the heart (A). For example, heart rate decreases at low temperature resulting in an increase in ventricle filling time, and as a result, an increase in stroke volume and a greater extension of the myocardium (Keen et al., 2017; Farrell, 1984). In addition, an acute decrease in environmental temperature causes an increase in blood viscosity, which in turn increases cardiac workload, and deformation of the myocardium cells (Farrell, 1984; Graham et al., 1985). Combined, these effects of low temperature are proposed to lead to an increase in the biomechanical stretch and shear stress experienced by the cardiomyocytes and fibroblasts that compose the myocardium (Keen et al., 2017) (B). Increased stretch of myocytes leads to the release of transforming growth factor β (TGF-β) (Katsumi et al., 2004; MacKenna et al., 1998) (C), while increased stretch of cardiac fibroblasts lead to the activation of mechanically sensitive proteins, and integrin proteins in the membrane (D) (Herum et al., 2017; MacKenna et al., 2000; Manso et al., 2009). This leads to the activation of G-coupled proteins and associated MAPK signaling proteins in the fibroblast (E) (Katsumi et al., 2004; Johnston and Gillis, 2020). Activated component proteins of the MAPK pathway in turn activate transcriptional factors that influence the expression of gene transcripts associated with the regulation of cardiac collagen (F) (Pramod and Shivakumar, 2014; Sinfield et al., 2013). Exposure of cardiac fibroblasts to TGF-β also causes increased activation (phosphorylation) of SMAD2 (G) (Visse and Nagase, 2003; Kolosova et al., 2011; Reed et al., 1994; Johnston and Gillis, 2018), another regulator of gene transcripts associated with collagen deposition, including col1A1. These changes in gene expression result in an increase in the expression and deposition of collagen in the extra cellular matrix (H) (Johnston and Gillis, 2017). Recent work also demonstrates that exposure of trout cardiac fibroblasts to microRNA 29b (miR-29b) leads to a decrease in the expression of col1A3 and in collagen type 1 content of the ECM (Johnston et al., 2019). These results suggest that regulation of collagen turnover by miRs could be involved in the removal of collagen from the heart (Johnston et al., 2019). It is not clear how the expression of miRs may be regulated by a temperature change.
Fig. 4.
The effect of bFGF and TGF-β1 on SMAD2 phosphorylation in cultured trout cardiac fibroblasts. Cardiac fibroblast cultures were treated with medium alone or medium containing 15 ng ml−1 bFGF or 15 ng ml−1 TGF-β1 repetitively for 7 days and total SMAD2 and phosphorylated SMAD2 were quantified using Western blot and densitometry. (B) Mean pSMAD2 and SMAD2 levels measured by densitometry and standardized to total protein. Different letters denote an effect of treatment on phosphorylation level of SMAD2, relative to control (P < 0.05). Note the large break on the y-axis. The n for each experiment is 3, with each n being a protein sample extracted from cultured cells derived from a different trout ventricle. Figure modified from (Johnston and Gillis, 2018).
Fig. 5.
The effect of TGF-β1 treatment on the expression of genes involved in ECM regulation in cultured trout cardiac fibroblasts 72 h post-treatment. The amount of transcript in each TGF-β1 group is given relative to the control group, which is set to 1 in each panel. *Significant effect of TGF-β1 treatment on gene expression (P < 0.05). n = 3–5. Figure modified from (Johnston and Gillis, 2018).
Fig. 6.
The effect of TGF-β1 on collagen production by cultured trout cardiac fibroblasts. A) Average amount of hydroxyproline, used as a proxy for collagen, produced per cell after 24, 48 and 72 h of TGF-β1 treatment in cell pellets. B) The effect of TGF-β1 or l-ascorbic acid (l-AA) treatment on collagen type 1 deposition in cultured cardiac fibroblasts measured 7 days after treatment using Western blot. In panel A different numbers indicate a significant effect of time on the amount of hydroxyproline produced within control cells (P < 0.05) and different letters indicate a significant effect of time on the amount of hydroxyproline produced per cell in the TGF-β1-treated group (P < 0.05). *Significant effect of TGF-β1 on hydroxyproline concentration between control and TGF-β1-treated cells (P < 0.05). In panel B different numbers indicate a significant differences between values. n = 5 for extracellular matrix (ECM) data, where each n represents a separate cell line established from a single heart from a different fish, and each n contains 8–15 technical replicates. Figure modified from (Johnston and Gillis, 2018).
Fig. 7.
Activation of p38 and ERK1/2 pathways in response to stretch. Phosphorylation levels of p38 proteins in stretched and control (unstretched) cells measured after 20 min of stretch (A) and 24 h of stretch (C). Phosphorylation levels of ERK proteins in stretched and control cells measured at 20 min (B) and 24 h (D). Asterisks (*) indicate a significant effect of stretch on MAPK phosphorylation (P < 0.05). Open triangles (Δ) signify individual control (unstretched) data points, and open circles (○) are individual data points from stretched cells. Points with similar numerical values were staggered for better readability. Error bars represent standard error of the mean of each group. N = 3 for each group. The n-value represents a fibroblast line from the same individual fish maintained in separate passages, cryopreserved on a different passage number and day, and thawed for experiments on different days. Figure modified from (Johnston and Gillis, 2020).
6. Removal of collagen from the ventricle
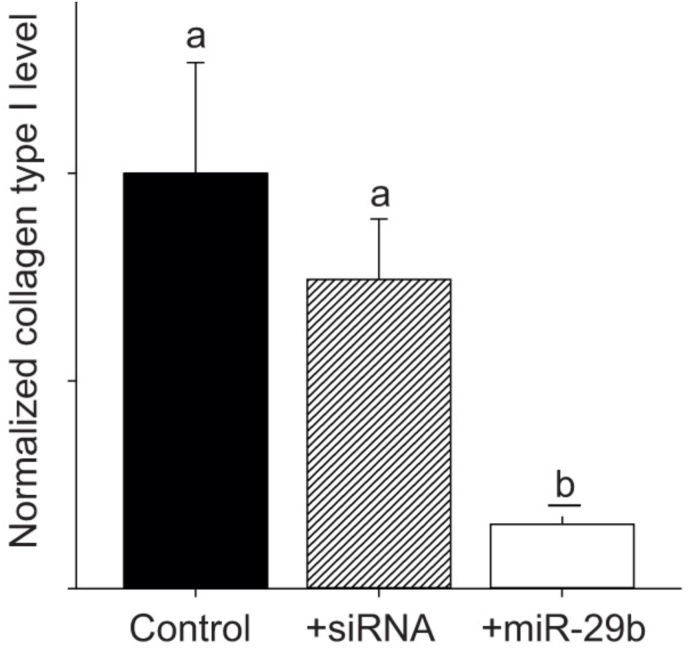
The removal of collagen from the ventricle would logically involve a process by which there is a decrease in the production of collagen monomers, and an increase in MMP activity caused by an increase in the expression of MMPs and/or a decrease in expression of TIMPs. Work by Keen et al. (2018), that demonstrated that warm acclimation caused a decrease in the collagen content of the trout ventricle, as well as a decrease in the stiffness of the myocardium, found a significant decrease in the expression of three collagen transcripts (col1a1, col1a2, and col1a3) as well as an increase in the expression of mmp-9. These changes in gene expression would support the observed decrease in collagen content. In addition, the decrease in collagen content characterized in the zebrafish ventricle with cold acclimation was accompanied by a 12-fold increase in mmp-2 transcript level and a 2.5-fold increase in mmp-9 transcript level (Johnson et al., 2014). Again, such changes in gene expression would support the measured decrease in collagen content. One obvious question related to the removal of collagen from the trout ventricle is what triggers it. A current hypothesis is that microRNA-29b (miR-29b) is involved in the decrease in collagen in the fish ventricle observed in response to thermal acclimation. MicroRNAs (miRs) are small non-coding RNAs that bind to the 3′ untranslated seed region of messenger RNA (mRNA) to promote degradation of the mRNA or prevent it from binding to ribosomes (Bartel, 2004; Griffiths-Jones et al., 2006). Such targeting leads to a decrease in the translation of the gene transcript and in the synthesis of the associated protein. In mammalian cardiac fibroblasts, miR-29b has been identified as a regulator of the expression of collagen gene transcripts and consequently collagen protein deposition (van Rooij et al., 2008). For example, transfection of mouse cardiac fibroblasts with miR-29b affected the expression of multiple collagen monomers including col1A1, col3A1 col4A2 as well as MMP2 and TIMP2, all proteins involved in the fibrotic response (Abonnenc et al., 2013). In addition, the levels of miR-29b have been found to be comparatively lower at the site of an infarction in hearts from mice and humans where collagen levels increase, and to also decrease following TGF-β1 treatment (van Rooij et al., 2008). Relevant to the proposed hypothesis under discussion, is that Johnston et al. (2019) reported that the nucleotide sequence of human miR-29b is 100% identical to trout miR-29b, and that seed sequences for miR-29b are present in the 3’ prime untranslated region of both trout col1a1 and trout col1a3. This indicates that the expression of both of these genes can be affected by miR-29b in the trout ventricle. Johnston et al. (2019), also demonstrated that trout cardiac fibroblasts transfected with miR-29b expressed 54% less col1a3 then control cells, and 84% less collagen type 1 protein (Fig. 8). While no work to date has examined the influence of thermal acclimation of trout or zebrafish on the expression of miR-29b in the ventricle, this is a logical next step in determining how fish are able to reduce collagen content in the ventricle in response to temperature change.
Fig. 8.
The effect of miR-29b transfection on collagen type I protein levels. Cardiac fibroblast cultures were transfected with 10 nmol l−1 mature zebrafish miR-29b mimic or a nonsense small interfering RNA (siRNA), and re-transfected after 3 days, with sampling occurring at 7 days. Mean collagen type I levels measured by densitometry and standardized to total protein on the same membrane, which was cut in half and blotted separately. Letters represent a significant change relative to control (P < 0.05). n = 3 for each experiment, with each n being a protein sample extracted from cultured cells derived from the same trout ventricle maintained in separate passages and cryovials, and thawed on different days, as previously described (Johnston and Gillis, 2017). Figure modified from (Johnston et al., 2019).
7. Perspective
The capacity of some fish, including trout and zebrafish, to regulate the collagen content in their ventricle in response to a change in environmental temperature is reflective of how plastic the heart of these animals is. While this plasticity enables the maintenance of contractile function with changes in physiological temperature, this capacity may also be related, at least in part, to the ability of the zebrafish ventricle to regenerate following injury (Poss et al., 2002; Chablais et al., 2011). This repair involves the formation of a fibrin clot at the site of injury and then the appearance of a collagen scar (Gamba et al., 2017; Jopling et al., 2010). However, unlike what occurs in mammalian hearts following injury, this scar is degraded and replaced with myocytes. As a result, there is no evidence of the repair by 60 days post injury (Gamba et al., 2017; Jopling et al., 2010). The common feature between cardiac regeneration and temperature induced cardiac remodeling is the process of collagen removal from the heart. In both cardiac regeneration and cardiac remodeling, this relies on the expression of TGF-β1 and miRs, the activity of MMPs, and the production of TIMPS and collagen monomers (Gamba et al., 2017; Jopling et al., 2010; Yin et al., 2012; Chablais and Jazwinska, 2012; Xu et al., 2018). Recent work in this area suggests that the ECM plays a significant role in regulating the remodeling response and that differences in its composition, between species, may determine regenerative competency (Chen et al., 2016). Going forward, focusing on the role of the ECM in regulating cardiac remodeling of the fish ventricle, and in controlling collagen deposition and removal may prove a useful avenue to understand how fish are able to remove collagen from their ventricle. Understanding how these processes are regulated in the fish heart may prove useful in characterizing the cellular triggers of the repair response and what limits such a response in other animal groups including mammals.
Editorial disclosure statement
Todd Gillis reports financial support was provided by Natural Sciences and Engineering Research Council of Canada.
CRediT authorship contribution statement
Elizabeth F. Johnston: Conceptualization, Writing – original draft, Writing – review & editing, First draft authorship, Editing and revision. Todd E. Gillis: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, First draft authorship, Editing and revision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abonnenc M., Nabeebaccus A.A., Mayr U., Barallobre-Barreiro J., Dong X., Cuello F., Sur S., Drozdov I., Langley S.R., Lu R., et al. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ. Res. 2013;113:1138–1147. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- Aho E., Vornanen M. Ca2+-ATPase activity and Ca2+ uptake by sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J. Exp. Biol. 1998;201:525–532. doi: 10.1242/jeb.201.4.525. [DOI] [PubMed] [Google Scholar]
- Aho E., Vornanen M. Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout oncorhynchus mykiss: effects of thermal acclimation. J. Exp. Biol. 1999;202:2663–2677. doi: 10.1242/jeb.202.19.2663. [DOI] [PubMed] [Google Scholar]
- Aho E., Vornanen M. Cold acclimation increases basal heart rate but decreases its thermal tolerance in rainbow trout (Oncorhynchus mykiss) J. Comp. Physiol. [B] 2001;171:173–179. doi: 10.1007/s003600000171. [DOI] [PubMed] [Google Scholar]
- Anttila K., Couturier C.S., Overli O., Johnsen A., Marthinsen G., Nilsson G.E., Farrell A.P. Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nat. Commun. 2014;5:4252. doi: 10.1038/ncomms5252. [DOI] [PubMed] [Google Scholar]
- Austin K.M., Covic L., Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121:431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Border W.A., Noble N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Breisch E.A., White F.C., Bloor C.M. Myocardial characteristics of pressure overload hypertrophy. A structural and functional study. Lab. Invest. 1984;51:333–342. [PubMed] [Google Scholar]
- Bujak M., Ren G., Kweon H.J., Dobaczewski M., Reddy A., Taffet G., Wang X.F., Frangogiannis N.G. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- Cameron D.G., Casal H.L., Mantsch H.H. Characterization of the pretransition in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine by Fourier transform infrared spectroscopy. Biochemistry. 1980;19:3665–3672. doi: 10.1021/bi00557a005. [DOI] [PubMed] [Google Scholar]
- Chablais F., Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development. 2012;139:1921–1930. doi: 10.1242/dev.078543. [DOI] [PubMed] [Google Scholar]
- Chablais F., Veit J., Rainer G., Jazwinska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.W., Dalgliesh A.J., Lopez J.E., Griffiths L.G. Cardiac extracellular matrix proteomics: challenges, techniques, and clinical implications. Proteonomics Clin. Appl. 2016;10:39–50. doi: 10.1002/prca.201500030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.C., Wang Z., Missinato M.A., Park D.W., Long D.W., Liu H.J., Zeng X., Yates N.A., Kim K., Wang Y. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Gelman L., Lutz R., Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Churcott C.S., Moyes C.D., Bressler B.H., Baldwin K.M., Tibbits G.F. Temperature and pH effects on Ca2+ sensitivity of cardiac myofibrils: a comparison of trout with mammals. Am. J. Physiol. 1994;267:R62–R70. doi: 10.1152/ajpregu.1994.267.1.R62. [DOI] [PubMed] [Google Scholar]
- Cornelissen V.A., Fagard R.H. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- Cortemeglia C., Beitinger T.L. Temperature tolerances of wild-type and red transgenic zebra danios. Trans. Am. Fish. Soc. 2005;134:1431–1437. [Google Scholar]
- Creemers E.E., Cleutjens J.P., Smits J.F., Daemen M.J. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ. Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- Devereux R.B., Drayer J.I., Chien S., Pickering T.G., Letcher R.L., DeYoung J.L., Sealey J.E., Laragh J.H. Whole blood viscosity as a determinant of cardiac hypertrophy in systemic hypertension. Am. J. Cardiol. 1984;54:592–595. doi: 10.1016/0002-9149(84)90255-8. [DOI] [PubMed] [Google Scholar]
- Ding Y., Johnston E.F., Gillis T.E. Mitogen-activated protein kinases contribute to temperature-induced cardiac remodelling in rainbow trout (Oncorhynchus mykiss) J. Comp. Physiol. B. 2021;192:61–76. doi: 10.1007/s00360-021-01406-5. [DOI] [PubMed] [Google Scholar]
- Dreger S.A., Taylor P.M., Allen S.P., Yacoub M.H. Profile and localization of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in human heart valves. J. Heart Valve Dis. 2002;11:875–880. discussion 880. [PubMed] [Google Scholar]
- Driedzic W.R., Bailey J.R., Sephton D.H. Cardiac adaptations to low temperature non-polar teleost fish. J. Exp. Zool. 1996;275:186–195. [Google Scholar]
- Elliott J.M., Elliott J.A. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. J. Fish. Biol. 2010;77:1793–1817. doi: 10.1111/j.1095-8649.2010.02762.x. [DOI] [PubMed] [Google Scholar]
- Evans D.L. Cardiovascular adaptations to exercise and training. Vet. Clin. N. Am. Equine Pract. 1985;1:513–531. doi: 10.1016/s0749-0739(17)30748-4. [DOI] [PubMed] [Google Scholar]
- Fan D., Takawale A., Lee J., Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A.P. A review of cardiac-performance in the teleost heart - intrinsic and humoral regulation. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1984;62:523–536. [Google Scholar]
- Fomovsky G.M., Thomopoulos S., Holmes J.W. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell. Cardiol. 2010;48:490–496. doi: 10.1016/j.yjmcc.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba L., Amin-Javaheri A., Kim J., Warburton D., Lien C.L. Collagenolytic activity is associated with scar resolution in zebrafish hearts after cryoinjury. J. Cardiovasc. Dev. Dis. 2017;4 doi: 10.3390/jcdd4010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamperl A.K., Farrell A.P. Cardiac plasticity in fishes: environmental influences and intraspecific differences. J. Exp. Biol. 2004;207:2539–2550. doi: 10.1242/jeb.01057. [DOI] [PubMed] [Google Scholar]
- Gillis T.E., Tibbits G.F. Beating the cold: the functional evolution of troponin C in teleost fish. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2002;132:763–772. doi: 10.1016/s1095-6433(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Gillis T.E., Marshall C.R., Xue X.H., Borgford T.J., Tibbits G.F. Ca(2+) binding to cardiac troponin C: effects of temperature and pH on mammalian and salmonid isoforms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1707–R1715. doi: 10.1152/ajpregu.2000.279.5.R1707. [DOI] [PubMed] [Google Scholar]
- Givvimani S., Tyagi N., Sen U., Mishra P.K., Qipshidze N., Munjal C., Vacek J.C., Abe O.A., Tyagi S.C. MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch. Physiol. Biochem. 2010;116:63–72. doi: 10.3109/13813451003652997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.S., Farrell A.P. The effect of temperature-acclimation and adrenaline on the performance of a perfused trout heart. Physiol. Zool. 1989;62:38–61. [Google Scholar]
- Graham M.S., Fletcher G.L. Blood and plasma viscosity of wonter flounder: influence of temperature, red cell concentration, and shear rate. Can. J. Zool. 1983;62:2164–2170. [Google Scholar]
- Graham M.S., Fletcher G.L. On the blood viscosity of a cold-water, marine sculpin: a comparison with the winter flounder. J. Comp. Physiol. B. 1984;155:455–459. [Google Scholar]
- Graham M.S., Fletcher G.L., Haedrich R.L. Blood viscosity in arctic fishes. J. Exp. Zool. 1985;234:157–160. doi: 10.1002/jez.1402340118. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.M., Bers D.M. Temperature dependence of myofilament Ca sensitivity of rat, Guinea pig, and frog ventricular muscle. Am. J. Physiol. 1990;258:C274–C281. doi: 10.1152/ajpcell.1990.258.2.C274. [DOI] [PubMed] [Google Scholar]
- Hazel J.R. The incorporation of unsaturated fatty acids of the n-9, n-6, and n-3 families into individual phospholipids by isolated hepatocytes of thermally-acclimated rainbow trout, Salmo gairdneri. J. Exp. Zool. 1983;227:167–176. doi: 10.1002/jez.1402270202. [DOI] [PubMed] [Google Scholar]
- Hazel J.R. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Herum K.M., Choppe J., Kumar A., Engler A.J., McCulloch A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell. 2017;28:1871–1882. doi: 10.1091/mbc.E17-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegass J.M., Villano C.M., Cooper K.R., White L.A. Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 2007;100:168–179. doi: 10.1093/toxsci/kfm192. [DOI] [PubMed] [Google Scholar]
- Husse B., Briest W., Homagk L., Isenberg G., Gekle M. Cyclical mechanical stretch modulates expression of collagen I and collagen III by PKC and tyrosine kinase in cardiac fibroblasts. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1898–R1907. doi: 10.1152/ajpregu.00804.2006. [DOI] [PubMed] [Google Scholar]
- Jalil J.E., Doering C.W., Janicki J.S., Pick R., Clark W.A., Abrahams C., Weber K.T. Structural vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricle. J. Mol. Cell. Cardiol. 1988;20:1179–1187. doi: 10.1016/0022-2828(88)90597-4. [DOI] [PubMed] [Google Scholar]
- Jalil J.E., Doering C.W., Janicki J.S., Pick R., Shroff S.G., Weber K.T. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ. Res. 1989;64:1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- Johnson A.C., Turko A.J., Klaiman J.M., Johnston E.F., Gillis T.E. Cold acclimation alters the connective tissue content of the zebrafish (Danio rerio) heart. J. Exp. Biol. 2014;217:1868–1875. doi: 10.1242/jeb.101196. [DOI] [PubMed] [Google Scholar]
- Johnston E.F., Gillis T.E. Transforming growth factor beta-1 (TGF-beta1) stimulates collagen synthesis in cultured rainbow trout cardiac fibroblasts. J. Exp. Biol. 2017;220:2645–2653. doi: 10.1242/jeb.160093. [DOI] [PubMed] [Google Scholar]
- Johnston E.F., Gillis T.E. Transforming growth factor-beta1 induces differentiation of rainbow trout (Oncorhynchus mykiss) cardiac fibroblasts into myofibroblasts. J. Exp. Biol. 2018;221 doi: 10.1242/jeb.189167. [DOI] [PubMed] [Google Scholar]
- Johnston E.F., Gillis T.E. Short-term cyclical stretch phosphorylates p38 and ERK1/2 MAPKs in cultured fibroblasts from the hearts of rainbow trout, Oncorhynchus mykiss. Biol Open. 2020;9 doi: 10.1242/bio.049296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston E.F., Cadonic I.G., Craig P.M., Gillis T.E. microRNA-29b knocks down collagen type I production in cultured rainbow trout (Oncorhynchus mykiss) cardiac fibroblasts. J. Exp. Biol. 2019;222 doi: 10.1242/jeb.202788. [DOI] [PubMed] [Google Scholar]
- Jopling C., Sleep E., Raya M., Marti M., Raya A., Izpisua Belmonte J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira L.C.U., Cossermelli W., Brentani R. Differential staining of collagens type-I, type-ii and type-iii by Sirius red and polarization microscopy. Arch. Histol. Jpn. 1978;41:267–274. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]
- Katsumi A., Orr A.W., Tzima E., Schwartz M.A. Integrins in mechanotransduction. J. Biol. Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Keen A.N., Shiels H.A., Crossley D.A. 2nd: cardiovascular function, compliance, and connective tissue remodeling in the turtle, Trachemys scripta, following thermal acclimation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;311:R133–R143. doi: 10.1152/ajpregu.00510.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen A.N., Shiels H.A., Crossley D.A. 2nd: cardiovascular function, compliance and connective tissue remodeling in the turtle, Trachemys scripta, following thermal acclimation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;311:R133–R143. doi: 10.1152/ajpregu.00510.2015. ajpregu 00510 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen A.N., Fenna A.J., McConnell J.C., Sherratt M.J., Gardner P., Shiels H.A. The dynamic nature of hypertrophic and fibrotic remodeling of the fish ventricle. Front. Physiol. 2016;6:427. doi: 10.3389/fphys.2015.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen A.N., Klaiman J.M., Shiels H.A., Gillis T.E. Temperature-induced cardiac remodelling in fish. J. Exp. Biol. 2017;220:147–160. doi: 10.1242/jeb.128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen A.N., Fenna A.J., McConnell J.C., Sherratt M.J., Gardner P., Shiels H.A. Macro- and micromechanical remodelling in the fish atrium is associated with regulation of collagen 1 alpha 3 chain expression. Pflügers Archiv. 2018;470:1205–1219. doi: 10.1007/s00424-018-2140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N. TGF-beta: from latent to active. Microb. Infect. 1999;1:1255–1263. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Klaiman J.M., Fenna A.J., Shiels H.A., Macri J., Gillis T.E. Cardiac remodeling in fish: strategies to maintain heart function during temperature change. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiman J.M., Pyle W.G., Gillis T.E. Cold acclimation increases cardiac myofilament function and ventricular pressure generation in trout. J. Exp. Biol. 2014;217:4132–4140. doi: 10.1242/jeb.109041. [DOI] [PubMed] [Google Scholar]
- Kolosova I., Nethery D., Kern J.A. Role of Smad2/3 and p38 MAP kinase in TGF-beta1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. J. Cell. Physiol. 2011;226:1248–1254. doi: 10.1002/jcp.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I., Yazaki Y. Control of cardiac gene expression by mechanical stress. Annu. Rev. Physiol. 1993;55:55–75. doi: 10.1146/annurev.ph.55.030193.000415. [DOI] [PubMed] [Google Scholar]
- Kubota Mk M., Takeuchi K., Kubota S., Toyohara H., Sakaguchi M. Solubilization of type I collagen from fish muscle connective tissue by matrix metalloproteinase-9 at chilled temperature. Fish. Sci. 2003;69:1053–1059. [Google Scholar]
- Lal H., Verma S.K., Smith M., Guleria R.S., Lu G., Foster D.M., Dostal D.E. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J. Mol. Cell. Cardiol. 2007;43:137–147. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Kamm R.D., Lee R.T. Mechanotransduction in cardiac myocytes. Ann. N. Y. Acad. Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Hong S.H., Bae S.C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- Levy D., Larson M.G., Vasan R.S., Kannel W.B., Ho K.K. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- Li J., Schwimmbeck P.L., Tschope C., Leschka S., Husmann L., Rutschow S., Reichenbach F., Noutsias M., Kobalz U., Poller W., et al. Collagen degradation in a murine myocarditis model: relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc. Res. 2002;56:235–247. doi: 10.1016/s0008-6363(02)00546-1. [DOI] [PubMed] [Google Scholar]
- Lijnen P.J., Petrov V.V., Fagard R.H. Association between transforming growth factor-beta and hypertension. Am. J. Hypertens. 2003;16:604–611. doi: 10.1016/s0895-7061(03)00847-1. [DOI] [PubMed] [Google Scholar]
- Lorell B.H., Carabello B.A. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- MacKenna D.A., Dolfi F., Vuori K., Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J. Clin. Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenna D., Summerour S.R., Villarreal F.J. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc. Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- Manso A.M., Kang S.M., Ross R.S. Integrins, focal adhesions, and cardiac fibroblasts. J. Invest. Med. 2009;57:856–860. doi: 10.231/JIM.0b013e3181c5e61f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijianowski M.M., Teeling P., Mann J., Becker A.E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J. Am. Coll. Cardiol. 1995;25:1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- Maron B.J., Pelliccia A., Spataro A., Granata M. Reduction in left ventricular wall thickness after deconditioning in highly trained Olympic athletes. Br. Heart J. 1993;69:125–128. doi: 10.1136/hrt.69.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Park I.K., Kohyama K. Matrix metalloproteinase (MMP)-9, but not MMP-2, is involved in the development and progression of C protein-induced myocarditis and subsequent dilated cardiomyopathy. J. Immunol. 2009;183:4773–4781. doi: 10.4049/jimmunol.0900871. [DOI] [PubMed] [Google Scholar]
- Meraldi J.P., Schlitter J. A statistical mechanical treatment of fatty acyl chain order in phospholipid bilayers and correlation with experimental data. A. Theory. Biochim. Biophys. Acta. 1981;645:183–192. doi: 10.1016/0005-2736(81)90189-9. [DOI] [PubMed] [Google Scholar]
- Meschiari C.A., Ero O.K., Pan H., Finkel T., Lindsey M.L. The impact of aging on cardiac extracellular matrix. Geroscience. 2017;39:7–18. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli F.H., Rimoldi S.F., Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5:543–551. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12:233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah A., Kellermayer D., Matyas C., Nemeth B.T., Lux A., Szabo L., Torok M., Ruppert M., Meltzer A., Sayour A.A., et al. Complete reversion of cardiac functional adaptation induced by exercise training. Med. Sci. Sports Exerc. 2017;49:420–429. doi: 10.1249/MSS.0000000000001127. [DOI] [PubMed] [Google Scholar]
- Overall C.M., Wrana J.L., Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J. Biol. Chem. 1991;266:14064–14071. [PubMed] [Google Scholar]
- Pauschinger M., Knopf D., Petschauer S., Doerner A., Poller W., Schwimmbeck P.L., Kuhl U., Schultheiss H.P. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- Pedersen M.E., Vuong T.T., Ronning S.B., Kolset S.O. Matrix metalloproteinases in fish biology and matrix turnover. Matrix Biol. 2015;44–46:86–93. doi: 10.1016/j.matbio.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Poschl E., Pollner R., Kuhn K. The genes for the alpha 1(IV) and alpha 2(IV) chains of human basement membrane collagen type IV are arranged head-to-head and separated by a bidirectional promoter of unique structure. EMBO J. 1988;7:2687–2695. doi: 10.1002/j.1460-2075.1988.tb03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K.D., Wilson L.G., Keating M.T. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Pramod S., Shivakumar K. Mechanisms in cardiac fibroblast growth: an obligate role for Skp2 and FOXO3a in ERK1/2 MAPK-dependent regulation of p27kip1. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H844–H855. doi: 10.1152/ajpheart.00933.2013. [DOI] [PubMed] [Google Scholar]
- Reed M.J., Vernon R.B., Abrass I.B., Sage E.H. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J. Cell. Physiol. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- Reed A., Kohl P., Peyronnet R. Molecular candidates for cardiac stretch-activated ion channels. Glob. Cardiol. Sci. Pract. 2014;2014:9–25. doi: 10.5339/gcsp.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz S., Zeidan A., Mraiche F. Myocardial proteases and cardiac remodeling. J. Cell. Physiol. 2017;232:3244–3250. doi: 10.1002/jcp.25884. [DOI] [PubMed] [Google Scholar]
- Richey P.A., Brown S.P. Pathological versus physiological left ventricular hypertrophy: a review. J. Sports Sci. 1998;16:129–141. doi: 10.1080/026404198366849. [DOI] [PubMed] [Google Scholar]
- Riley G.P., Curry V., DeGroot J., van El B., Verzijl N., Hazleman B.L., Bank R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- Rodnick K.J., Gamperl A.K., Lizars K.R., Bennett M.T., Rausch R.N., Keeley E.R. Thermal tolerance and metabolic physiology among redband trout populations in south-eastern Oregon. J. Fish. Biol. 2004;64:310–335. [Google Scholar]
- Roldan V., Marin F., Gimeno J.R., Ruiz-Espejo F., Gonzalez J., Feliu E., Garcia-Honrubia A., Saura D., de la Morena G., Valdes M., et al. Matrix metalloproteinases and tissue remodeling in hypertrophic cardiomyopathy. Am. Heart J. 2008;156:85–91. doi: 10.1016/j.ahj.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Shaffer J.F., Gillis T.E. Evolution of the regulatory control of vertebrate striated muscle: the roles of troponin I and myosin binding protein-C. Physiol. Genom. 2010;42:406–419. doi: 10.1152/physiolgenomics.00055.2010. [DOI] [PubMed] [Google Scholar]
- Sinfield J.K., Das A., O'Regan D.J., Ball S.G., Porter K.E., Turner N.A. p38 MAPK alpha mediates cytokine-induced IL-6 and MMP-3 expression in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 2013;430:419–424. doi: 10.1016/j.bbrc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwik D.A., Pagano P.J., Colucci W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- Stephenson D.G., Williams D.A. Temperature-dependent calcium sensitivity changes in skinned muscle fibres of rat and toad. J. Physiol. 1985;360:1–12. doi: 10.1113/jphysiol.1985.sp015600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin J., Morgan R., Finnoen M.H., Dey A., Sarkar K., Jutfelt F. On the observation of wild zebrafish (Danio rerio) in India. Zebrafish. 2019;16:546–553. doi: 10.1089/zeb.2019.1778. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N.S., Flanders K.C., Sporn M.B., Roberts A.B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J. Biol. Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- van Rooij E., Sutherland L.B., Thatcher J.E., DiMaio J.M., Naseem R.H., Marshall W.S., Hill J.A., Olson E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.K., Lal H., Golden H.B., Gerilechaogetu F., Smith M., Guleria R.S., Foster D.M., Lu G., Dostal D.E. Rac1 and RhoA differentially regulate angiotensinogen gene expression in stretched cardiac fibroblasts. Cardiovasc. Res. 2011;90:88–96. doi: 10.1093/cvr/cvq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F., Mauviel A. Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 2007;13:3056–3062. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Waring C.D., Vicinanza C., Papalamprou A., Smith A.J., Purushothaman S., Goldspink D.F., Nadal-Ginard B., Torella D., Ellison G.M. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur. Heart J. 2014;35:2722–2731. doi: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring C.D., Henning B.J., Smith A.J., Nadal-Ginard B., Torella D., Ellison G.M. Cardiac adaptations from 4 weeks of intensity-controlled vigorous exercise are lost after a similar period of detraining. Phys. Rep. 2015;3 doi: 10.14814/phy2.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner R.B., Baggish A.L. Exercise-induced cardiac remodeling. Prog. Cardiovasc. Dis. 2012;54:380–386. doi: 10.1016/j.pcad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Xu S., Webb S.E., Lau T.C.K., Cheng S.H. Matrix metalloproteinases (MMPs) mediate leukocyte recruitment during the inflammatory phase of zebrafish heart regeneration. Sci. Rep. 2018;8:7199. doi: 10.1038/s41598-018-25490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz M., Oktay A.A., Stewart M.H., Milani R.V., Ventura H.O., Lavie C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020;63:10–21. doi: 10.1016/j.pcad.2019.11.009. [DOI] [PubMed] [Google Scholar]
- Yin V.P., Lepilina A., Smith A., Poss K.D. Regulation of zebrafish heart regeneration by miR-133. Dev. Biol. 2012;365:319–327. doi: 10.1016/j.ydbio.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehmer J.K., Hazel J.R. Thermally induced changes in lipid composition of raft and non-raft regions of hepatocyte plasma membranes of rainbow trout. J. Exp. Biol. 2005;208:4283–4290. doi: 10.1242/jeb.01899. [DOI] [PubMed] [Google Scholar]