Abstract
There is an increasing interest in non-pharmacological treatments for children with attention-deficit/hyperactivity disorder (AD/HD), especially digital techniques that can be remotely delivered, such as neurofeedback (NFT) and computerized cognitive training (CCT). In this study, a randomized controlled design was used to compare training outcomes between remotely delivered NFT, CCT, and combined NFT/CCT training approaches. A total of 121 children with AD/HD were randomly assigned to the NFT, CCT, or NFT/CCT training groups, with 80 children completing the training program. Pre- and post-training symptoms (primary outcome), executive and daily functions were measured using questionnaires as well as resting EEG during eyes-closed (EC) and eyes-open (EO) conditions. After 3 months of training, the inattentive and hyperactive/impulsive symptoms, inhibition, working memory, learning and life skills of the three groups of children were significantly improved. The objective EEG activity showed a consistent increase in the relative alpha power in the EO condition among the three training groups. Training differences were not observed between groups. There was a positive correlation between pre-training EO relative alpha power and symptom improvement scores of inattention and hyperactivity/impulsivity, as well as a negative correlation between pre-training inattention scores and change in EO relative alpha. This study verified the training effects of NFT, CCT, and combined NFT/CCT training in children with AD/HD and revealed an objective therapeutic role for individual relative alpha activity. The verified feasibility and effectiveness of home-based digital training support promotion and application of digital remote training.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00787-022-01956-1.
Keywords: AD/HD, Non-pharmacological treatments, Neurofeedback, Computerized cognitive training, Electroencephalogram
Introduction
Attention-deficit/hyperactivity disorder (AD/HD) is a childhood-onset neurodevelopmental disorder characterized by cognitive deficits associated with attention [1, 2]. Although pharmacotherapy is considered the first-line option in the treatment of AD/HD, side effects and partial drug responses have limited clinical applications [3, 4]. As a result, non-pharmacological treatments have garnered increasing attention, especially digital approaches such as remotely available neurofeedback and cognitive training—an aspect of increased importance for remote/rural populations and during the coronavirus disease 2019 (COVID-19) pandemic.
EEG neurofeedback training (NFT) is a well-researched classical non-pharmaceutical treatment for AD/HD. Through real-time visualization of specific aspects of brain electrical activity (e.g., alpha power), participants can learn to self-regulate their brain activity to increase or reduce specific frequency components known to underpin functional/behavioral abnormalities [4]. Abnormal EEG activity is common in children with AD/HD, generally manifesting as increased slow-wave activity and decreased fast-wave activity [5–7], and is associated with state-regulation dysfunction and executive function deficits [8–11]. Targeted self-regulating training can provide neural functional recovery [12] and improved symptoms [13–15] with medium to large intervention effects [16].
Computerized cognitive training (CCT) is designed to enhance and maintain cognitive performance through structured, repetitive practice that targets specific cognitive processes [17]. For children with AD/HD, CCT typically targets improvements in inhibitory control and working memory, both identified as core deficits [18–20]. Through step-by-step adaptive training, usually involving adjustments to the level of difficulty based on performance, children’s specific cognitive functions are developed, and functional physiological indicators can be significantly improved [21, 22]. Recent studies have also shown that game-based training programs have a therapeutic effect in children with AD/HD, with some of which are certified by the FDA [23, 24].
With the popularization and application of digital network technology and the demand for remote home-based therapy during the COVID-19 pandemic, more attention has been paid to non-contact, online, interactive interventions [25–27]. Synthesizing the online and computerized characteristics of NFT and CCT, a game-based training program, Focus Pocus, was developed by Johnstone et al. with NFT and CCT modules integrated into one themed training environment [28–30]. Using a validated portable Bluetooth EEG acquisition device [31, 32], effective NFT and CCT training can be carried out at home for children with AD/HD with some assistance from their parents. Studies have confirmed the beneficial effects of this type of training on AD/HD symptoms and academic performance [29, 30, 33]. These studies have examined the effect of combined NFT/CCT training; to date, no study has examined the efficacy of NFT and CCT as independent components compared with combined NFT/CCT training. In this study, we configured three different training types (i.e., NFT alone, CCT alone, and combined NFT/CCT), first, to allow a direct comparison of the effects of the training types controlling for training duration, game look/feel, and training environment, and second, to verify the intervention effect under remote home-based training conditions. We predicted that the effectiveness could be verified for NFT and CCT, with better training effects for combined NFT/CCT training. Furthermore, verification of the feasibility and efficacy of home-based training will be beneficial in the promotion of digital interventions for remote/rural families and other conditions that require physical distance (e.g., during COVID-19 restrictions).
In addition to symptoms and functional improvement, cognitive training has been reported to reshape physiological brain function; for example, video game training could remediate age-related deficits in neural signatures measured with electroencephalography [34]. As a result, in the current study, resting EEG was collected at pre- and post-training sessions as an objective neural indicator. EEG was recorded during eyes-closed (EC) and eyes-open (EO) conditions, as different levels of arousal were reflected in the corresponding neural oscillations [35, 36]. It is predicted that objective neural functions will be optimized along with the symptoms and behavior improvement.
Methods
Participants
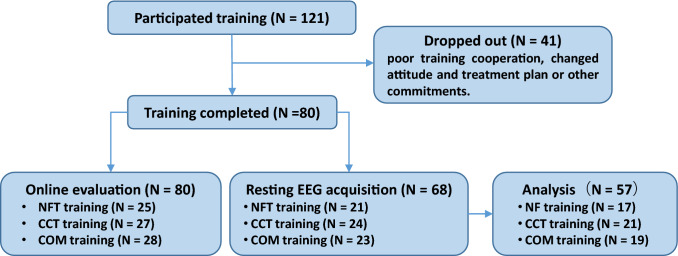
The children who participated were outpatients in a clinic at Peking University Sixth Hospital and were interviewed and underwent a diagnostic process for AD/HD using DSM-IV criteria by a qualified psychiatrist. All participants were excluded from other serious neuropsychiatric diseases with an IQ > 80. Please refer to the Supplementary Material S1 for specific inclusion and exclusion criteria. One hundred and twenty-one families indicated their intention to participate in the study. Forty-one families dropped out due to poor training cooperation (16 families), changed attitudes and treatment plans (22 families), or other commitments (3 families). The remaining 80 children (66 males, M = 8.94 years; range 7.1–12.3 years) completed the training and their parents completed the follow-up evaluation (see Fig. 1).
Fig. 1.
The consort flow. The flowchart shows the participants from enrollment to analysis
Children who were diagnosed with AD/HD and whose parents agreed to participate in the study were randomly assigned to one of the three training programs (i.e., NFT, CCT, or the combined NFT/CCT program) and joined an online training group to track the training situation and progress. The location of the family (country, suburbs and city) and parents’ educational level were measured to evaluate the socioeconomic status of the families. The parents’ educational level of the parents was divided into low (primary school to junior high school), middle (high school to bachelor’s degree), and high (master’s and doctorate degrees). Among the completed training families, eight did not provide information on the residential location, and six did not provide information on the educational level. The composition of different levels were counted (e.g., missing:country:suburb:city for family location). Four children were taking medication with the dose maintained during the training program, two in the NFT group (one Strattera; one Concerta), one in the CCT group (Concerta), and one in the COM group (Strattera).
Among the 80 children with AD/HD who completed the training, 68 completed the pre- and post-training EEG recordings, with 11 excluded due to the poor data quality; therefore, data from 57 children were used in the final EEG analysis.
The study was approved by the Ethics Committee of the Peking University Institute of Mental Health (02/2017) and registered in the Chinese Clinical Trial Registry (ChiCTR1900021891). Written consent was obtained from all children and their parents.
Materials
Behavior rating scale
The AD/HD Rating Scale IV (AD/HD-RS) was used to assess the severity of AD/HD symptoms [37], which is a 4-point severity scale (0, never; 1, sometimes; 2, usually and 3, always) and includes two dimensions of inattention and hyperactivity/impulsivity.
The Behavior Rating Inventory of Executive Function (BRIEF) was used for an ecological assessment of executive functions [38], which is a 3-point rating scale (1, never; 2, sometimes and 3, often) and includes eight factors of executive functions: inhibition, shifting, emotional control, monitoring, initiating, working memory, planning, and organization. Given the training objectives of our training program, inhibition and working memory were the focus of the analysis.
The Weiss Functional Impairment Scale-Parent Report (WFIRS-P) was used to assess daily functional impairment [39], with a 4-point rating scale (0, never; 1, sometimes; 2, usually and 3, always) and an additional option “not applicable” scored as 0. Six domains of daily functions were evaluated; family, school and learning, life skills, children’s self-concept, social activities, and risky activities.
The scales were evaluated online by parents at pre- and post-training sessions. Four participants only completed AD/HD-RS evaluations (one in the NFT group, one in the CCT group, and two in the COM group).
EEG recording and analysis
EEG was recorded under each condition for approximately 6 min using 128 channels (HydroCel Geodesic Sensor Net; Electrical Geodesics, Inc., Eugene, OR) with Net Station EEG Software, with 1000 Hz sample rate, 0.01–400 Hz bandpass filter, Cz referenced and below 50 kΩ impedance. Off-line EEG analysis was performed by using EEGLAB toolbox in the MATLAB environment [40]. Thirty-eight lateral electrodes were excluded due to their susceptibility to movement interference (see Supplementary Material S3), with the remaining EEG data on 91 electrodes resampled to 250 Hz and the bandpass filtered to 1–45 Hz. The filtered data were manually checked to interpolate the bad electrodes and reject excessive artifacts before independent component analysis (ICA). ICA components associated with vertical and horizontal eye movements were visually identified and removed. The EEG was segmented into contiguous 2-s windows, and any segments with voltages exceeding ± 100 µV were rejected. Non-contiguous data were not concatenated during this process. For each child, the first 60 epochs (2 min) of the EC and EO data were extracted for spectral analysis.
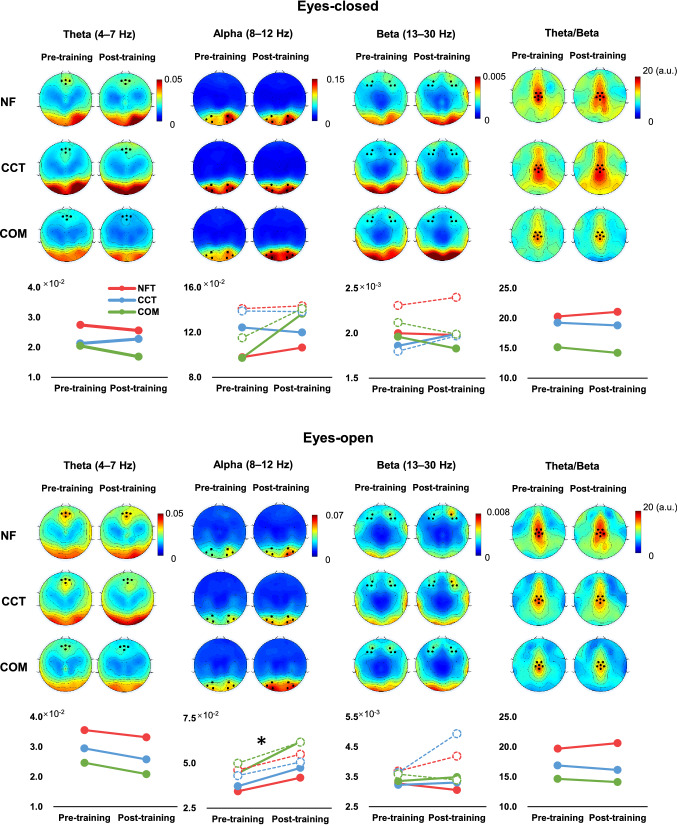
Spectral analysis was carried out using the pwelch function, and the relative power of the frequency bands theta (4–7 Hz), alpha (8–12 Hz), and beta (13–30 Hz) was calculated by dividing the power (μV2) of the total frequency (1–45 Hz). The relative power of theta, alpha, and beta was extracted from the peak distribution of topographic maps, with theta around Fz (E11, E16), beta around F3 (E23, E24, E27) and F4 (E3, E123, E124), alpha around O1 (E65, E66, E70) and O2 (E83, E84, E90), and theta/beta ratio around Cz (E7, E31, E55, E80, E106, Cz, see Fig. 3). The average relative power of the selected electrodes was extracted and used in the statistical analysis.
Fig. 3.
Pre- and post-training EEG topographic map and line charts. Topographic maps of relative EEG power, with selected electrodes marked by black dots. The line chart below illustrates the changes pre- and post-training, with solid lines representing the left electrodes and the dotted lines representing the right electrodes for alpha and beta. *p < 0.05
Training design and procedure
The Focus Pocus training program was developed by Neurocognitive Solutions Pty Ltd. (Australia) using intellectual property licensed from the University of Wollongong. Focus Pocus includes NFT and CCT games with a unified theme and graphics. Three versions of the training program were created for this study: NFT (only neurofeedback games), CCT (only cognitive training games), and COM (both neurofeedback and cognitive training games). Each training session consisted of 14 randomly ordered mini-games, and, as each mini-game took approximately 1 min to complete, the total time per session was approximately 15 min. The NFT games aimed to promote awareness and control of brain activity with EEG recorded via a portable Bluetooth device that provided the participant with real-time feedback. The CCT games were designed to train and improve inhibitory control and working memory abilities. The inhibitory control games were based on the Go/No-Go paradigm, and the working memory games involved memory recall tasks.
Each grouped child would receive an Android smart pad with the appropriate training program installed (e.g., children assigned to the NFT group trained with the NFT training program). It is important to note that all children used the portable Bluetooth EEG device in each training session, and the device was used to monitor attention level and was part of the reward point calculation for each CCT game.
Each group was trained for 3 months at home, including three to five training sessions per week, and came to the hospital for the post-training evaluation after the training was completed. The research team monitored the training progress weekly and contacted parents if the children did not meet the training plan. The average number of completed training sessions for the three groups was 34 ± 14 (NFT), 40 ± 11 (CCT), and 36 ± 14 (COM), with no significant differences between the three groups (F = 1.630, p = 0.203).
Statistical analyses
AD/HD-RS scores were used as the primary outcome, while executive and daily function scores as well as EEG activity were secondary outcomes. For normally distributed data, confirmed by Shapiro–Wilk test, repeated measures analysis of variance (ANOVA) were used to analyze training effects on symptoms and executive functions with Training (pre-training, post-training) as a within-subjects factor and Group (NFT, CCT, COM) as a between-subjects factor. For non-normally distributed data, repeated measures ANOVA were first used for universal effectiveness, and nonparametric analysis was used to verify the reliability of the results. For EEG data with two peak regions, alpha (O1, O2) and beta (F3, F4), an additional within-subject factor was included. For the ANOVA analysis, if the interaction effect was significant, a simple effect analysis was performed and adjusted using the Bonferroni method. The alpha level was 0.05.
Spearman correlation analyses were performed between the change value in EEG relative power (post-training–pre-training) and symptom improvement scores (SIS = (initial symptom score–final symptom score)/(initial symptom score); SIS-I, symptom improvement scores of inattention; SIS-HI, symptom improvement scores of hyperactivity/impulsivity) to investigate the association between brain functional evolution and symptom changes. Further correlation analyses were also performed on baseline symptoms and EEG components to investigate the potential effects of pre-training indicators.
Results
Symptoms and daily performance
Before training, there were no significant differences in demographic data, symptom severity, executive function, daily function, and socioeconomic status (see Table 1). In addition, we also compared the basic information of families who completed the training with those who dropped out, and no significant differences were found (see Supplementary Material S4).
Table 1.
Demographic data of groups
| NFT | CCT | COM | F/χ2 | p | |
|---|---|---|---|---|---|
| Symptoms | |||||
| Number | 25 | 27 | 28 | – | – |
| Age | 8.8 ± 1.5 | 8.8 ± 1.2 | 9.1 ± 1.0 | 0.40 | 0.67 |
| Sex (M:F) | 19: 6 | 26: 1 | 22: 6 | 5.18 | 0.07 |
| IQ | 100.8 ± 25.2 | 106.7 ± 14.4 | 104.1 ± 17.6 | 0.74 | 0.48 |
| Family location (missing:country:suburb:city) | 3: 0: 0: 22 | 2: 0: 1: 24 | 3: 0: 3: 22 | 3.20 | 0.56 |
| Parents' educational level (missing:low:middle:high) | 2: 2: 18: 3 | 2: 0: 21: 4 | 2: 2: 19: 5 | 2.91 | 0.87 |
| Inattention | 18.1 ± 4.6 | 17.7 ± 4.2 | 17.0 ± 3.5 | 0.55 | 0.58 |
| Hyperactivity/impulsivity | 13.1 ± 7.1 | 12.5 ± 5.2 | 11.4 ± 5.4 | 0.58 | 0.56 |
| Inhibition | 18.8 ± 5.0 | 19.5 ± 5.3 | 18.9 ± 5.1 | 0.17 | 0.84 |
| Working memory | 22.3 ± 4.4 | 22.6 ± 3.4 | 22.7 ± 3.6 | 0.11 | 0.90 |
| Family | 6.9 ± 5.0 | 8.7 ± 5.3 | 6.7 ± 5.3 | 1.12 | 0.33 |
| School and learning | 7.0 ± 5.3 | 8.7 ± 5.0 | 7.5 ± 4.0 | 0.82 | 0.45 |
| Life skills | 10.3 ± 3.1 | 10.7 ± 4.0 | 9.1 ± 2.7 | 1.77 | 0.18 |
| Child’s self-concept | 2.5 ± 2.0 | 2.4 ± 2.1 | 2.8 ± 1.8 | 0.30 | 0.74 |
| Social activities | 5.7 ± 2.7 | 7.0 ± 3.7 | 5.9 ± 3.8 | 1.10 | 0.34 |
| Risky activities | 2.8 ± 2.1 | 3.5 ± 2.9 | 2.4 ± 2.0 | 1.46 | 0.24 |
| Number | 17 | 21 | 19 | - | - |
| Age | 8.9 ± 1.5 | 8.7 ± 1.2 | 9.3 ± 0.9 | 1.19 | 0.31 |
| EEG recording | |||||
| Sex (M:F) | 11: 6 | 20: 1 | 14: 5 | 6.05 | 0.04 |
| IQ | 104.7 ± 13.3 | 106.4 ± 16.2 | 105.0 ± 11.6 | 0.08 | 0.92 |
| EC theta Fz (× 10–2) | 2.8 ± 1.6 | 2.1 ± 1.3 | 2.1 ± 0.7 | 1.74 | 0.19 |
| EC alpha O1 (× 10–2) | 9.8 ± 4.4 | 12.4 ± 7.4 | 9.7 ± 5.9 | 1.26 | 0.29 |
| EC alpha O2 (× 10–2) | 14.1 ± 7.8 | 13.9 ± 8.2 | 11.5 ± 7.3 | 0.65 | 0.53 |
| EC beta F3 (× 10–3) | 2.0 ± 1.6 | 1.9 ± 0.9 | 2.0 ± 0.8 | 0.08 | 0.92 |
| EC beta F4 (× 10–3) | 2.3 ± 2.0 | 1.8 ± 0.7 | 2.1 ± 0.8 | 0.84 | 0.44 |
| EC theta/beta | 20.3 ± 11.3 | 19.3 ± 12.0 | 15.1 ± 4.8 | 1.38 | 0.26 |
| EO theta Fz (× 10–2) | 3.6 ± 2.3 | 2.9 ± 2.2 | 2.5 ± 0.7 | 1.52 | 0.23 |
| EO alpha O1 (× 10–2) | 3.4 ± 1.6 | 3.7 ± 2.3 | 4.4 ± 3.1 | 0.81 | 0.45 |
| EO alpha O2 (× 10–2) | 4.6 ± 2.9 | 4.3 ± 2.7 | 5.0 ± 2.9 | 0.30 | 0.74 |
| EO beta F3 (× 10–3) | 3.3 ± 2.0 | 3.2 ± 1.4 | 3.4 ± 2.4 | 0.02 | 0.98 |
| EO beta F4 (× 10–3) | 3.7 ± 2.5 | 3.6 ± 1.5 | 3.6 ± 1.9 | 0.01 | 0.99 |
| EO theta/beta | 19.7 ± 11.0 | 16.9 ± 11.3 | 14.6 ± 3.9 | 1.30 | 0.28 |
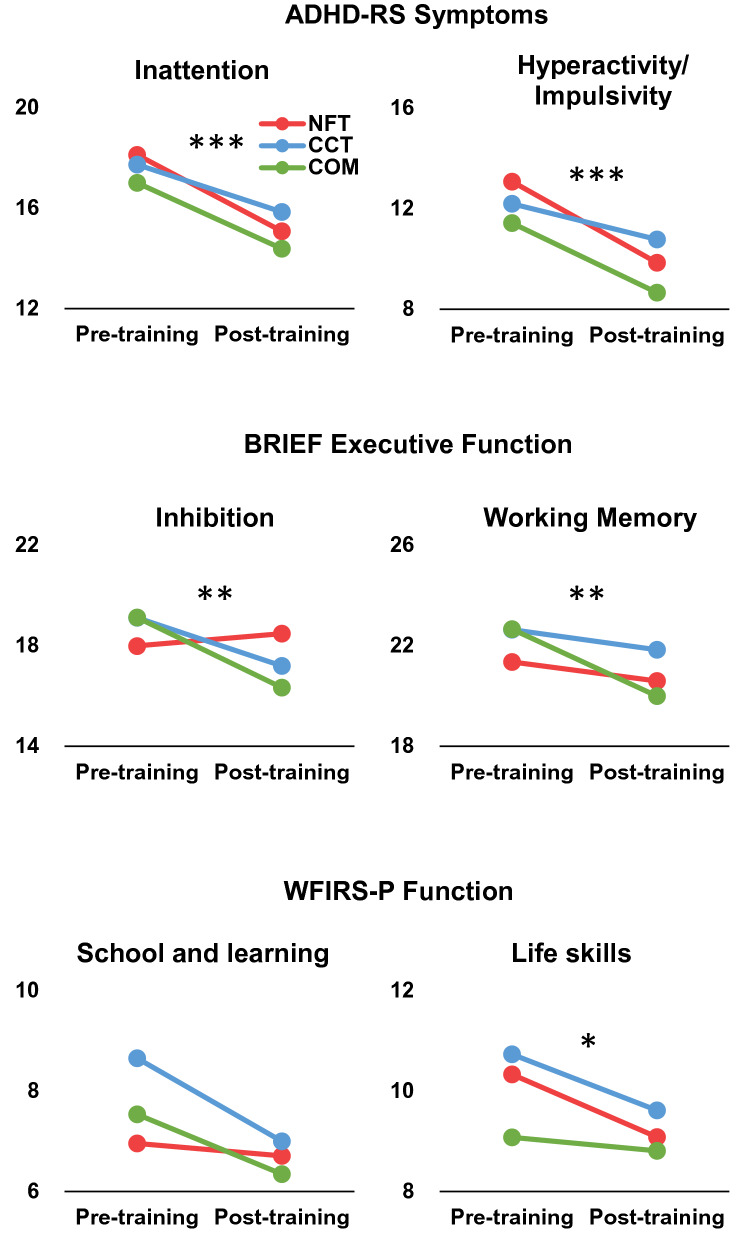
For AD/HD-RS, the pre- and post-training comparison showed a significantly decreased inattention severity scores after training (F = 16.157, p < 0.001, η2 = 0.173, see Fig. 2), no significant Group effect (F = 0.402, p = 0.670, η2 = 0.010), and no Training × Group interaction (F = 0.244, p = 0.784, η2 = 0.006). Similar effects were shown for hyperactivity/impulsivity symptom severity, with a significant main effect of Training (F = 28.586, p < 0.001, η2 = 0.271), and no Group main effect (F = 0.855, p = 0.429, η2 = 0.022) or Training × Group interaction (F = 1.017, p = 0.367, η2 = 0.026).
Fig. 2.
Behavioral and EF outcomes. Training effects on behavior performance evaluated by parents. Red for NFT, blue for CCT, and green for COM. *p < 0.05; **p < 0.01; ***p < 0.001
For the executive function evaluated using BRIEF, the main effect of Training on inhibition and working memory was significant (inhibition: F = 12.900, p = 0.001, η2 = 0.150; working memory: F = 12.926, p = 0.001, η2 = 0.150), and inhibition and working memory scores decreased significantly after training. The Group main effect and Training × Group interaction were not significant for inhibition and working memory. Further nonparametric analysis verified that the training effect was significant for inhibition (Z = −3.450, p = 0.001) and working memory (Z = −3.268, p = 0.001).
For daily functions, as evaluated by WFIRS-P, there was a significant post-training improvement in the life skills domain (F = 5.448, p = 0.022, η2 = 0.069), and improvements in the school and learning domains, which were not significant (F = 3.782, p = 0.056, η2 = 0.049). The nonparametric analysis verified the training effect on life skills (Z = −2.836, p = 0.005) and school and learning (Z = −2.415, p = 0.016). No other training effects were observed.
EEG performance
Pre-training tests showed significant differences in the sex distribution analyzed with Fisher’s exact test (p = 0.04), with fewer girls in the CCT group. Therefore, we reanalyzed all results in boys and obtained similar results (see Supplementary Material S6).
Theta
In the EC condition, the frontal relative theta did not show a significant main effect of Training (F = 1.289, p = 0.261, η2 = 0.023), Group main effect, or Training × Group interaction. The non-significant training effect was also shown in the nonparametric analysis (Z = −1.212, p = 0.226).
In the EO condition, the frontal relative theta decreased after training, but did not reach significance (Training main effect: F = 3.234, p = 0.078, η2 = 0.056). The Group main effect and Training × Group interaction were not significant. The training effect was significant in the nonparametric analysis (Z = −2.022, p = 0.043; Fig. 3).
Alpha
In the EC condition, the posterior alpha showed a significant Hemisphere main effect (F = 14.735, p < 0.001, η2 = 0.214) with increased power in O2 compared to O1. There was an increase in relative alpha after training, which did not reach significance (training main effect: F = 3.147, p = 0.082, η2 = 0.055). No other significant effect was observed. A non-significant training effect was also shown in the nonparametric analysis (O1, Z = −1.577, p = 0.115; O2, Z = −1.529, p = 0.126).
In the EO condition, the relative alpha increased significantly after training (F = 5.198, p = 0.027, η2 = 0.088). A significant Hemisphere main effect (F = 11.750, p = 0.001, η2 = 0.179) indicated that the relative alpha was larger for O2 than for O1. No other significant effect was observed. Furthermore, nonparametric analysis showed a significant training effect on O1 (Z = −2.451, p = 0.014), but not on O2 (Z = −1.371, p = 0.171).
Beta
In the EC and EO conditions, the frontal relative beta analysis showed larger power at F4 than at F3 (EC: F = 4.156, p = 0.046, η2 = 0.710; EO: F = 6.676, p = 0.013, η2 = 0.110). No other significant effect was observed.
Theta/beta ratio
In both the EC and EO conditions, the central theta/beta ratio did not show any significant effects.
Correlation analysis
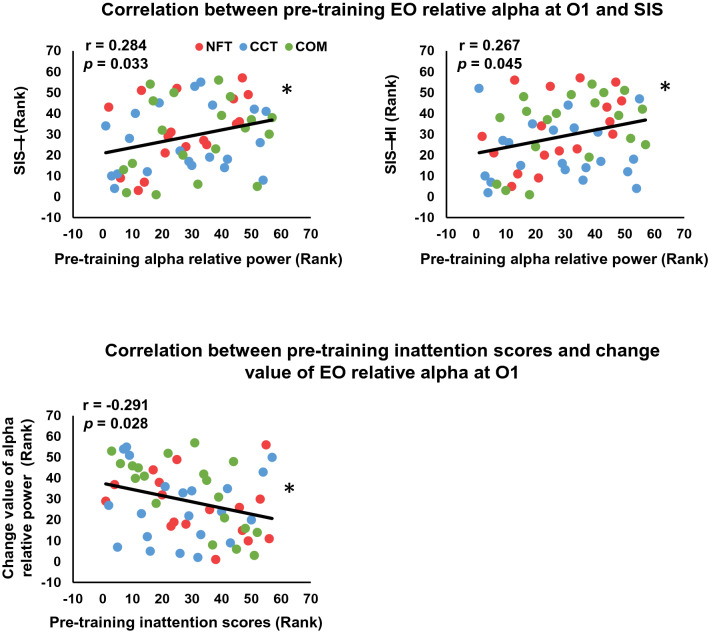
No significant correlation was observed between the EO relative alpha change value and SIS-I (O1, r = −0.237, p = 0.076; O2, r = −0.172, p = 0.202) or SIS-HI (O1, r = −0.057, p = 0.672; O2, r = −0.008, p = 0.952). There was a significant positive correlation between pre-training EO alpha relative power on O1 and SIS-I (r = 0.284, p = 0.033, Fig. 4) and SIS-HI (r = 0.267, p = 0.045), but not for O2 (SIS-I, r = 0.136, p = 0.313; SIS-HI, r = 0.078, p = 0.567). A significant negative correlation was observed between pre-training inattention scores and the change value of EO relative alpha on O1 (r = −0.291, p = 0.028), but not for O2 (r = −0.214, p = 0.111).
Fig. 4.
Scatter plots. A Scatter plots for pre-training EO relative alpha at O1 and SIS-I and SIS-HI. B Scatter plots for pre-training inattention scores and change value of EO relative alpha at O1. *p < 0.05
Discussion
This study extended previous work by examining the outcomes of exclusive NFT and exclusive CCT training programs compared to the combined NFT/CCT program. The results showed significantly improved AD/HD symptoms, executive and daily functions in children with AD/HD after 3 months of training, as well as significantly altered neural activities that are associated with symptoms. No differences were observed in the training effects between the NFT, CCT and combined NFT/CCT training conditions.
Training effects on behavior and EEG
The primary outcome of AD/HD-RS evaluated by parents showed a significant improvement after 3 months of training, as well as executive and daily functions, verifying the training effect of the Focus Pocus program [28–30, 33]. Compared to traditional laboratory-based tasks, inhibition and working memory assessed by the BRIEF questionnaire are believed to have more ecological validity [41], and improved functional ability, indicating that NFT and CCT could train core defects in children with AD/HD, which is consistent with previous findings [42, 43]. In addition to functional defects, daily function improvements were also shown in school learning and life skills, verifying the transfer effect of the study and living with Focus Pocus training [30]. Although the training effects were not significant in other daily dimensions, this likely results from the training design, which targeted attentional ability but not social functional dimensions [44, 45].
Notably, in addition to subjective evaluation, the objective EEG also changed after training. A significant increase in alpha relative power was observed after training in all three groups, which might reflect the reshaping of brain function after training. As neural activity is not easily affected by parental expectations, the evolution of neural indicators is more likely to result from training for children. Cognitive training can increase and normalize theta activity in elderly individuals [34]. The increase in alpha activity in children with AD/HD is also a normalization of their weakened fast-wave activity and an improvement in neural function [46–48]. Furthermore, a more prominent increase in alpha was observed in the EO condition, which might be because the training consisted mainly of visual tasks that trained the ability of anti-interference to visual stimuli. However, when the eyes are closed, there is little to no visual information being processed [36], suggesting the need for multidimensional attentional training in different perceptual states.
Correlation between EEG and behavior performance
Although there was no correlation between symptom improvement and changes in EEG, pre-training alpha activity was found to be positively correlated with SIS-I and SIS-HI, indicating a better symptom improvement for children with higher relative alpha-A higher relative alpha suggests milder arousal defects, and children with AD/HD may benefit more from cognition enhancing training. More importantly, since pre-training alpha is associated with training improvement, individual relative alpha power could be used as a pre-treating index to assist clinical decisions around treatment planning, a finding of significance for clinical interventions for AD/HD.
Another interesting finding was the negative association between the pre-training inattention score and pre-to post-training change in relative alpha, indicating that the training increase in relative alpha is smaller for children with higher pre-training inattention scores. Considering that increased alpha reflects the training effect on physiological brain function, this suggests that children with more severe symptoms have less cognitive improvement after NFT/CCT training. Indeed, it seems reasonable that serious inattention may limit training participation and result in a smaller training effect on cognition. This result may also suggest that non-pharmacological training might not be suitable for children with serious symptoms, suggesting a possible range of clinical applications for non-pharmacological training.
Independent and combined training effects
Previous studies have suggested medium training effects for NFT and CCT [49], while the outcomes of NFT may be better than those of CCT [43, 49]. However, in this study, when controlling for training duration, game look/feel, and training environment, the training effects of NFT and CCT were very similar. It may be the case that engaging context effectively promotes motivation and participation in training and achieves better training results. Recent video game training studies have also reported positive training effects [23, 24], some of which are equivalent to pharmacological therapy [50]. Monitoring EEG during training also encourages children to maintain a stable training state and limits the extent and intensity of movement, which may also contribute to the training effect [13]. Similar training effects were also obtained for the COM group, which engaged in a wider variety of training types. This consistent benefit allows a choice in training selection that can be applied to the interests of children and parents in practical clinical applications.
Limitations
Although this study verified the effects of NFT/CCT training on symptoms, executive/daily function, and objective EEG indicators in children with AD/HD, some limitations must be considered. First, the effective sample size of the study was quite small as the COVID-19 pandemic hindered some families who had completed training from coming to the hospital for post-training measurements; this was especially true for EEG indicators and might have caused more variability in the EEG findings. However, we believe that the observation of objective neural markers is necessary to validate the curative effect of cognitive training and needs to be replicated in future research. Second, the reasons for dropouts should be considered, although our analysis showed that they were not related to symptom severity or socioeconomic status. Since most of the included families were of middle-and high-level status, they were unable to fully represent the whole socioeconomic impact. Informal feedback indicates that technical concerns, such as connectivity of the EEG headset via bluetooth and internet connectivity may have contributed to dropouts. The stability of these connections must be optimized during remote interventions. Third, considering the treatment needs and research ethics of the patients, the study design did not include a non-training control group, which may have resulted in an overestimation of training outcomes. However, combined with the moderate training effect and compared with a previous RCT study of Focus Pocus [29, 49]], the symptom improvements in our study most likely resulted from the training intervention, but not subjective bias.
Conclusion
Through the designed NFT, CCT and combined NFT/CCT training programs, we verified their similar training effects on AD/HD symptoms, executive and daily functions. Non-pharmacological training further reshaped the objective neural activity reflected in the increased relative alpha, which was also associated with the training improvements. The results confirmed the feasibility and effectiveness of home-based digital training and revealed the potential therapeutic value of relative alpha activity for cognitive rehabilitation, which is of great significance for the promotion and application of digital remote intervention for AD/HD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (L.S., 81771479, 81971284); the Beijing Municipal Science and Technology Program (L.S., Z171100001017089), the Key scientific research projects of capital health development (L.S., 2020-1-4111). The authors wish to thank the children and their parents for participating.
Author contribution
XL contributed to investigation, data curation, formal analysis, writing (original draft), and writing (review and editing); XG contributed to investigation, supervision, data acquisition and follow, formal analysis, writing (original draft); QZ contributed to investigation, data acquisition and follow, and writing (review and editing); YZ contributed to investigation, data acquisition, supervision, and writing (review and editing); YC contributed to investigation, data acquisition, supervision and writing (review and editing); DZ contributed to methodology, software, writing (review and editing); HJ contributed to methodology, software, and writing (review and editing); YW contributed conception, design, methodology, and writing (review and editing); SJ contributed to conception, design, methodology, software, and writing (review and editing); LS contributed to conception, design, recruitment, and writing (review and editing).
Declarations
Conflicts of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stuart Johnstone was a co-inventor of intellectual property licensed by the University of Wollongong to Neurocognitive Solutions Pty Ltd. and was entitled to a small portion of royalties received by UOW in relation to the sale of any product that used the UOW intellectual property between 2013 and 2017. This intellectual property makes up a proportion of the intellectual property used in Focus Pocus. Other authors reported no conflicts of interest.
Footnotes
Xiangsheng Luo and Xiaojie Guo contributed equally to this work.
Contributor Information
Stuart Johnstone, Email: sjohnsto@uow.edu.au.
Li Sun, Email: sunlioh@bjmu.edu.cn.
References
- 1.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Sonuga-Barke E. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2014;35:448–457. doi: 10.1097/DBP.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez-Geppert S, Smit D, Pimenta MG, Arns M. Neurofeedback as a treatment intervention in ADHD: current evidence and practice. Curr Psychiatry Rep. 2019;21:46. doi: 10.1007/s11920-019-1021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 6.Byeon J, Choi TY, Won GH, Lee J, Kim JW. A novel quantitative electroencephalography subtype with high alpha power in ADHD: ADHD or misdiagnosed ADHD? PLoS ONE. 2020;15:e0242566. doi: 10.1371/journal.pone.0242566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buyck I, Wiersema JR. Resting electroencephalogram in attention deficit hyperactivity disorder: developmental course and diagnostic value. Psychiatry Res. 2014;216:391–397. doi: 10.1016/j.psychres.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 8.Loo SK, Cho A, Hale TS, McGough J, McCracken J, Smalley SL. Characterization of the theta to beta ratio in ADHD: identifying potential sources of heterogeneity. J Atten Disord. 2013;17:384–392. doi: 10.1177/1087054712468050. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DW, Li H, Wu Z, Zhao Q, Song Y, Liu L, et al. Electroencephalogram theta/beta ratio and spectral power correlates of executive functions in children and adolescents with AD/HD. J Atten Disord. 2019;23:721–732. doi: 10.1177/1087054717718263. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DW, Johnstone SJ, Roodenrys S, Luo X, Li H, Wang E, et al. The role of resting-state EEG localized activation and central nervous system arousal in executive function performance in children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2018;129:1192–1200. doi: 10.1016/j.clinph.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DW, Roodenrys S, Li H, Barry RJ, Clarke AR, Wu Z, et al. Atypical interference control in children with AD/HD with elevated theta/beta ratio. Biol Psychol. 2017;128:82–88. doi: 10.1016/j.biopsycho.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht B, Uebel-von Sandersleben H, Gevensleben H, Rothenberger A. Pathophysiology of ADHD and associated problems—starting points for NF interventions? Front Hum Neurosci. 2015;9:359. doi: 10.3389/fnhum.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold LE, Arns M, Barterian J, Bergman R, Black S, Conners CK, et al. Double-blind placebo-controlled randomized clinical trial of neurofeedback for attention-deficit/hyperactivity disorder with 13-month follow-up. J Am Acad Child Adolesc Psychiatry. 2021;60:841–855. doi: 10.1016/j.jaac.2020.07.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurigova BG, Gerdes MR, Anguera JA, Marco EJ. Sustained benefits of cognitive training in children with inattention, three-year follow-up. PLoS ONE. 2021;16:e0246449. doi: 10.1371/journal.pone.0246449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Doren J, Arns M, Heinrich H, Vollebregt MA, Strehl U, Loo K, S, Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2019;28:293–305. doi: 10.1007/s00787-018-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arns M, Clark CR, Trullinger M, DeBeus R, Mack M, Aniftos M. Neurofeedback and attention-deficit/hyperactivity-disorder (ADHD) in children: rating the evidence and proposed guidelines. Appl Psychophysiol Biofeedback. 2020;45:39–48. doi: 10.1007/s10484-020-09455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171:510–522. doi: 10.1176/appi.ajp.2013.13081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirabella G. Inhibitory control and impulsive responses in neurodevelopmental disorders. Dev Med Child Neurol. 2021;63:520–526. doi: 10.1111/dmcn.14778. [DOI] [PubMed] [Google Scholar]
- 19.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonuga-Barke E, Brandeis D, Holtmann M, Cortese S. Computer-based cognitive training for ADHD. A review of current evidence. Child Adolesc Psychiatr Clin N Am. 2014;23:807–824. doi: 10.1016/j.chc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Hoekzema E, Carmona S, Tremols V, Gispert JD, Guitart M, Fauquet J, et al. Enhanced neural activity in frontal and cerebellar circuits after cognitive training in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2010;31:1942–1950. doi: 10.1002/hbm.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Covey TJ. Neurophysiological indices of the transfer of cognitive training gains to untrained tasks. Neurobiol Learn Mem. 2020;171:107205. doi: 10.1016/j.nlm.2020.107205. [DOI] [PubMed] [Google Scholar]
- 23.Kollins SH, DeLoss DJ, Cañadas E, Lutz J, Findling RL, Keefe RSE, et al. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial. Lancet Digit Health. 2020;2:e168–e178. doi: 10.1016/S2589-7500(20)30017-0. [DOI] [PubMed] [Google Scholar]
- 24.Pandian GSB, Jain A, Raza Q, Sahu KK. Digital health interventions (DHI) for the treatment of attention deficit hyperactivity disorder (ADHD) in children—a comparative review of literature among various treatment and DHI. Psychiatry Res. 2021;297:113742. doi: 10.1016/j.psychres.2021.113742. [DOI] [PubMed] [Google Scholar]
- 25.Jeon JY, Kim H, Yu KS. The impact of COVID-19 on the conduct of clinical trials for medical products in Korea. J Korean Med Sci. 2020;35:e329. doi: 10.3346/jkms.2020.35.e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camargo CP, Tempski PZ, Busnardo FF, Martins MA, Gemperli R. Online learning and COVID-19: a meta-synthesis analysis. Clin (Sao Paulo) 2020;75:e2286. doi: 10.6061/clinics/2020/e2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Sun W, Huang X, Yu S, Wang H, Bi X, et al. Online antenatal care during the COVID-19 pandemic: opportunities and challenges. J Med Internet Res. 2020;22:e19916. doi: 10.2196/19916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnstone SJ. Computer gaming and ADHD: potential positive influences on behavior [opinion] IEEE Technol Soc Mag. 2013;32:20–22. [Google Scholar]
- 29.Johnstone SJ, Roodenrys SJ, Johnson K, Bonfield R, Bennett SJ. Game-based combined cognitive and neurofeedback training using Focus Pocus reduces symptom severity in children with diagnosed AD/HD and subclinical AD/HD. Int J Psychophysiol. 2017;116:32–44. doi: 10.1016/j.ijpsycho.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Johnstone SJ, Sun L, Zhang DW. Effect of neurocognitive training for children with ADHD at improving academic engagement in two learning settings. J Atten Disord. 2021;25:414–431. doi: 10.1177/1087054718799931. [DOI] [PubMed] [Google Scholar]
- 31.Rogers JM, Johnstone SJ, Aminov A, Donnelly J, Wilson PH. Test-retest reliability of a single-channel, wireless EEG system. Int J Psychophysiol. 2016;106:87–96. doi: 10.1016/j.ijpsycho.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone SJ, Blackman R, Bruggemann JM. EEG from a single-channel dry-sensor recording device. Clin EEG Neurosci. 2012;43:112–120. doi: 10.1177/1550059411435857. [DOI] [PubMed] [Google Scholar]
- 33.Zhang DW, Johnstone SJ, Li H, Luo X, Sun L. Comparing the transfer effects of three neurocognitive training protocols in children with attention-deficit/hyperactivity disorder: a single-case experimental design. Behav Change. 2021 doi: 10.1017/bec.2021.26. [DOI] [Google Scholar]
- 34.Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan L, Huang H, Schwab N, Tanner J, Rajan A, Lam NB, et al. From eyes-closed to eyes-open: role of cholinergic projections in EC-to-EO alpha reactivity revealed by combining EEG and MRI. Hum Brain Mapp. 2019;40:566–577. doi: 10.1002/hbm.24395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woltering S, Jung J, Liu Z, Tannock R. Resting state EEG oscillatory power differences in ADHD college students and their peers. Behav Brain Funct. 2012;8:60. doi: 10.1186/1744-9081-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale-IV (for children and adolescents) checklists, norms, and clinical interpretation. New York: Guilford Publications; 1998. [Google Scholar]
- 38.Gioia GA, Isquith PK, Guy SC, Kenworthy L, Baron IS. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 39.Thompson T, Lloyd A, Joseph A, Weiss M. The Weiss functional impairment rating scale-parent form for assessing ADHD: evaluating diagnostic accuracy and determining optimal thresholds using ROC analysis. Qual Life Res. 2017;26:1879–1885. doi: 10.1007/s11136-017-1514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Shimoni M, Engel-Yeger B, Tirosh E. Executive dysfunctions among boys with attention deficit hyperactivity disorder (ADHD): performance-based test and parents report. Res Dev Disabil. 2012;33:858–865. doi: 10.1016/j.ridd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Spawton-Rice JH, Walker Z. Do cognitive training applications improve executive function in children with adverse childhood experiences? A pilot study. Appl Neuropsychol Child. 2020 doi: 10.1080/21622965.2020.1854094. [DOI] [PubMed] [Google Scholar]
- 43.Steiner NJ, Frenette EC, Rene KM, Brennan RT, Perrin EC. Neurofeedback and cognitive attention training for children with attention-deficit hyperactivity disorder in schools. J Dev Behav Pediatr. 2014;35:18–27. doi: 10.1097/DBP.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka JW, Wolf JM, Klaiman C, Koenig K, Cockburn J, Herlihy L, et al. Using computerized games to teach face recognition skills to children with autism spectrum disorder: The Let’s Face It! program. J Child Psychol Psychiatry. 2010;51:944–952. doi: 10.1111/j.1469-7610.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- 45.Berggren S, Fletcher-Watson S, Milenkovic N, Marschik PB, Bölte S, Jonsson U. Emotion recognition training in autism spectrum disorder: a systematic review of challenges related to generalizability. Dev Neurorehabil. 2018;21:141–154. doi: 10.1080/17518423.2017.1305004. [DOI] [PubMed] [Google Scholar]
- 46.Barry RJ, De Blasio FM, Fogarty JS, Clarke AR. Natural alpha frequency components in resting EEG and their relation to arousal. Clin Neurophysiol. 2020;131:205–212. doi: 10.1016/j.clinph.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Shephard E, Tye C, Ashwood KL, Azadi B, Asherson P, Bolton PF, McLoughlin G. Resting-state neurophysiological activity patterns in young people with ASD, ADHD, and ASD + ADHD. J Autism Dev Disord. 2018;48:110–122. doi: 10.1007/s10803-017-3300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambez B, Harwood-Gross A, Golumbic EZ, Rassovsky Y. Non-pharmacological interventions for cognitive difficulties in ADHD: a systematic review and meta-analysis. J Psychiatr Res. 2020;120:40–55. doi: 10.1016/j.jpsychires.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Kollins SH, Childress A, Heusser AC, Lutz J. Effectiveness of a digital therapeutic as adjunct to treatment with medication in pediatric ADHD. NPJ Digit Med. 2021;4:58. doi: 10.1038/s41746-021-00429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.