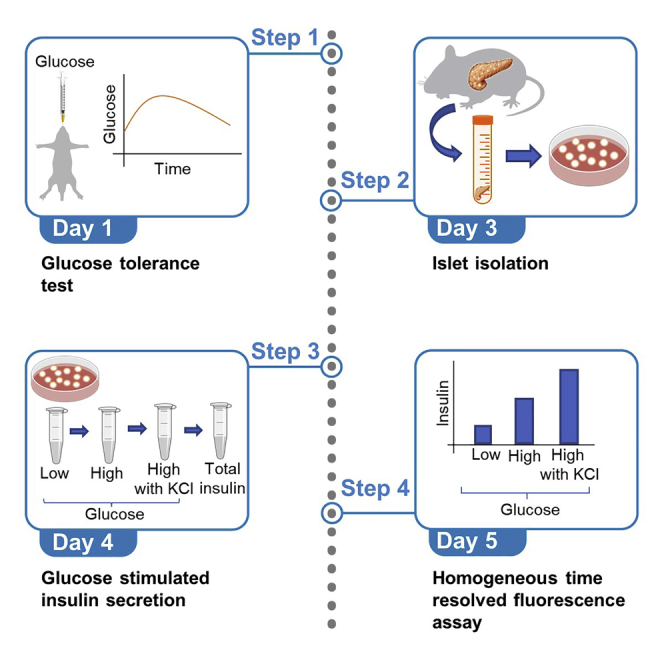
Summary
Glucose tolerance test and glucose stimulated insulin secretion are important measures to determine glucose homeostasis and islet function and assess diabetes. Here, we provide a protocol where glucose tolerance test is used to study glucose homeostasis and insulin secretion in vivo, followed by islet isolation and glucose stimulated insulin secretion to determine islet function ex vivo. This protocol enables evaluation of glucose homeostasis and islets in mice which can also be applied to rat, beta cell lines and human studies.
For complete details on the use and execution of this profile, please refer to Al Rijjal et al. (2021).
Subject areas: Cell Biology, Cell isolation, Metabolism, Model Organisms
Graphical abstract
Highlights
-
•
Glucose tolerance test is an important measure for studying diabetes
-
•
Islet isolation allows for the specific direct study of islets and beta cells
-
•
Glucose stimulated insulin secretion can determine islet function
-
•
Techniques to study islet function can be applied to other species
Glucose tolerance test and glucose stimulated insulin secretion are important measures to determine glucose homeostasis and islet function and assess diabetes. Here, we provide a protocol where a glucose tolerance test is used to study glucose homeostasis and insulin secretion in vivo, followed by islet isolation and glucose stimulated insulin secretion to determine islet function ex vivo. This protocol enables evaluation of glucose homeostasis and islets in mice which can also be applied to rat, beta cell lines and human studies.
Before you begin
This protocol is designed to provide precise details of testing glucose homeostasis and islet function in mice. For glucose homeostasis, we provide a step-by-step methodology on performing an oral and intraperitoneal glucose tolerance test and monitoring insulin secretion during the test. We have also provided specific steps to isolate islets from mice, perform glucose stimulated insulin secretion in vitro and read insulin measurements using a homogeneous time resolved fluorescence assay. We have previously used glucose stimulated insulin secretion to test effects of substances on mouse islets of different backgrounds, human islets and MIN6 cells in vitro with minor optimization based on the cell line or organism used. The University of Toronto animal care committee approved all experiments and methods, and the Canadian Council of Animal Care guidelines and standard were followed. The human study design and all procedures were approved by the Office of Research Ethics at the University of Toronto.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human: Islets from males aged 30–53 years old | Islet Core and Clinical Islet Laboratory (Alberta Islet Distribution Program, University of Alberta) and Multi-Organ Transplant/University Health Network Islet Isolation Program (University Health Network, Toronto, Ontario, Canada) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| D-Glucose | Sigma-Aldrich | Cat# 50-99-7 |
| Emla cream | Aspen | 900394 |
| 50% dextrose solution | Hospira | 06648 |
| Roswell park memorial institute (RPMI) 1640 media | Sigma-Aldrich | R8758 |
| Fetal bovine serum (FBS) | Sigma-Aldrich | F1051 |
| Penicillin-Streptomycin (PS) | Sigma-Aldrich | P4333 |
| Collagenase type V | Sigma-Aldrich | C9263 |
| Bovine serum albumin (BSA) | Sigma-Aldrich | A7906 |
| Isoflurane | Fresenius Kabi | CP0406V2 |
| Dulbecco's phosphate-buffered saline (DPBS) | Gibco | 14190-144 |
| Dithizone | Sigma-Aldrich | D5130 |
| Dimethyl sulfoxide | Sigma-Aldrich | 276855 |
| Sodium chloride (NaCl) | BioShop | CAS# 7647-14-5 |
| Potassium chloride (KCl) | BioShop | CAS# 7447-40-7 |
| Potassium phosphate monobasic (KH2PO4) | BioShop | CAS# 7778-77-0 |
| Magnesium sulfate (MgSO4) | BioShop | CAS# 7487-88-9 |
| Calcium chloride dihydrate (CaCl2) | BioShop | CAS# 10035-04-8 |
| Sodium bicarbonate (NaHCO3) | BioShop | CAS# 144-55-8 |
| HEPES | BioShop | CAS# 7365-45-9 |
| Bovine serum albumin (BSA) | Sigma-Aldrich | A7906 |
| Dulbecco's Modified Eagle’s Medium (D-MEM) media, high glucose | Sigma-Aldrich | D5796 |
| DMEM media, low glucose | Gibco | 11885-084 |
| Critical commercial assays | ||
| Mouse ultrasensitive insulin enzyme-linked immunosorbent assay (ELISA) kit | Alpco | 80-INSMSU-E01 |
| Insulin ultra-sensitive assay kit | Cisbio | 62IN2PEG |
| Experimental models: Cell lines | ||
| Mouse: MIN6 K8 | Prof. S. Seino (Kobe University, Japan) and Prof. J. Miyazaki (Osaka University, Japan) | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: Male C57BL/6 at 8–18 weeks old | Charles River Laboratories | 027 |
| Mouse: Male CD-1 at 8–12 weeks old | Charles River Laboratories | 022 |
| Mouse: Male FVB at 8–12 weeks old | Charles River Laboratories | 207 |
| Software and algorithms | ||
| GraphPad Prism | GraphPad Software | www.graphpad.com |
| Other | ||
| Blood collection tubes | SARSTEDT | Microvette® CB 300 K2E |
| Gavage plastic feeding tubes, 20 ga × 30 mm | Instech | FTP-20-30 |
| Millex GP filter (0.22 μm polyethersulfone membrane) | Sigma-Aldrich | SLGPR33RS |
| Glucometer | Ascensia Diabetes Care | Contour® NEXT |
| CONTOUR® NEXT blood glucose test strips | Ascensia Diabetes Care | 7322 |
| 1 mL syringe | BD | 309659 |
| 3 mL syringe | BD | 303061 |
| 0.5 mL insulin syringe | BD | 324703 |
| 30G PrecisionGlide needle | BD | 305106 |
| Surgical microscope | OLYMPUS | SZ51 |
| Standard dissecting scissors | Fisher Scientific | 08-951-20 |
| Surgical clamps | Fisher Scientific | 16-100-117 |
| Blunted forceps | Fisher Scientific | 10-285 |
| Pointed forceps | Fisher Scientific | 12-000-131 |
| Surgical thread | Fine Science Tools | 18020-00 |
| 384-well plate | Greiner Bio | 784075 |
| Pherastar machine | BMG – Labtech | n/a |
Materials and equipment
Oral glucose tolerance test (OGTT) materials
-
•
Glucose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| D-Glucose | 30% | 6 g |
| Drinking water for mice | n/a | 20 mL |
| Total | n/a | 20 mL |
Store at room temperature (18°C–25°C) for 12 h
Pancreas perfusion and islet isolation
-
•
Roswell park memorial institute (RPMI) 1640 media with Penicillin-Streptomycin (PS)
| Reagent | Final concentration | Amount |
|---|---|---|
| PS | 1% | 5 mL |
| RPMI 1640 media | n/a | 500 mL |
| Total | n/a | 505 mL |
Store at 4°C for 6 months
-
•
RPMI 1640 media with PS and fetal bovine serum (FBS)
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 10% | 50 mL |
| PS | 1% | 5 mL |
| RPMI 1640 media | n/a | 500 mL |
| Total | n/a | 555 mL |
Store at 4°C for 6 months
-
•
Collagenase preparation
| Reagent | Final concentration | Amount |
|---|---|---|
| Collagenase type V | 0.6 mg/mL | 0.03 g |
| Bovine serum albumin (BSA) | 0.02 g/mL | 1 g |
| RPMI media with PS | n/a | 50 mL |
| Total | n/a | 50 mL |
Collagenase should be stored at −20°C for 24 months. BSA should be stored at 4°C for 24 months. RPMI media should be stored at 4°C for 6 months. Collagenase solution prepared should be stored at −20°C for 6 months.
Glucose stimulated insulin secretion
-
•
Krebs-ringer buffer (KRB)
| Reagent | Final concentration | Stock concentration | Amount |
|---|---|---|---|
| NaCl | 128.8 mM | 2M (5.84 g/50 mL) | 3.22 mL |
| KCl | 4.8 mM | 1M (3.73 g/50 mL) | 0.24 mL |
| KH2PO4 | 1.2 mM | 1M (6.8 g/50 mL) | 0.06 mL |
| MgSO4 | 1.2 mM | 1M (6 g/50 mL) | 0.06 mL |
| CaCl2 | 2.5 mM | 1M (7.35 g/50 mL) | 0.125 mL |
| NaHCO3 | 5 mM | 0.5M (1.96 g/50 mL) | 0.25 mL |
| HEPES | 10 mM | 0.5M (5.96 g/50 mL) | 1 mL |
| BSA | 0.1% | 1% = 0.01 g/10 mL | 50 mg |
| Double distilled water | n/a | n/a | 45.045 mL |
| Total | n/a | n/a | 50 mL |
All the reagents except BSA can be stored for 24 months at room temperature (18°C–25°C). BSA powder should be stored at 4°C for 24 months. All the stock solutions prepared for KRB including BSA should be stored at 4°C for 6 months. NAHCO3 stock solution should be prepared and used on the same day. KRB solution should be stored at 4°C to be used the following day.
CRITICAL: CaCl2 may cause irritation and may be harmful if swallowed. Avoid contact of CaCl2 and MgSO4 with skin, eyes and clothing. Wash thoroughly after handling.
-
•
Low glucose solution
| Reagent | Final concentration | Stock concentration | Amount |
|---|---|---|---|
| D-Glucose | 2.8 mM | 1M (9 g/50 mL) | 28 μL |
| KRB | n/a | n/a | 10 mL |
| Total | n/a | n/a | 10 mL |
Store at 4°C for 2 days
-
•
High glucose solution
| Reagent | Final concentration | Stock concentration | Amount |
|---|---|---|---|
| D-Glucose | 16.7 mM | 1M (9 g/50 mL) | 167 μL |
| KRB | n/a | n/a | 10 mL |
| Total | n/a | n/a | 10 mL |
Store at 4°C for 2 days
-
•
High glucose and KCl solution
| Reagent | Final concentration | Stock concentration | Amount |
|---|---|---|---|
| D-Glucose | 16.7 mM | 1M (9 g/50 mL) | 167 μL |
| KCl | 30 mM | 1M (3.73 g/50 mL) | 250 μL |
| KRB | n/a | n/a | 10 mL |
| Total | n/a | n/a | 10 mL |
Store at 4°C for 2 days
Note: D-Glucose should be stored at room temperature. D-glucose stock solution and KRB solution should be stored at 4°C.
Step-by-step method details
Glucose tolerance test and insulin secretion
Timing: 2 days
This section will provide specific details on how to perform a glucose tolerance test (GTT) and measure insulin secretion during the test in order to assess glucose homeostasis and determine the beta cell insulin secretory capacity in mice in vivo. There are two different types of glucose tolerance test: oral GTT (OGTT) and intraperitoneal GTT (IPGTT). Both OGTT and IPGTT are important measures to determine glucose homeostasis and measure glucose responsiveness. The difference between the two methods is that the OGTT takes into account the effect of incretin hormones and gastric emptying time whereas IPGTT excludes these effects. We provide here the protocol for both OGTT and IPGTT. Although the protocols are very similar, they differ in the method of glucose administration (oral vs intraperitoneal) and the glucose solution and volume used.
-
1.Preparation for OGTT and IPGTT
-
a.Prepare glucose solution based on the type of GTT performed.
-
i.For OGTT, prepare 30% of glucose solution (see materials and equipment) and filter glucose solution using a 0.22 μm polyethersulfone membrane filter (Millex GP filter).
-
ii.For IPGTT, use 50% dextrose solution directly.
-
i.
-
b.Weigh mice and calculate the glucose dosage based on the type of GTT performed.
-
i.For OGTT, calculate the glucose dosage to gavage 2 g/kg of 30% glucose per mouse, use the following equation: . For example, the volume for a mouse that weighs 30 g should be 200 μL of glucose solution.
-
ii.For IPGTT, calculate the glucose dosage to inject 2 g/kg using 50% dextrose solution, use the following equation: . The volume for a mouse that weighs 30 g should be 120 μL of dextrose.
-
i.
-
c.Transfer the mice to new cages without food and fast mice overnight (length of time depends on animal care facility) to perform experiment next day. Make sure that mice have continuous access to water.
-
d.Prepare sheets to record glucose measurement, label blood collection tubes with the mouse number and time point and keep the glucometer, glucose strips, 1 mL syringes and plastic feeding tubes for OGTT or 0.5 mL insulin syringes for IPGTT, and timer ready for the experiment next day.
-
e.The following day, prepare glucose syringes for each mouse based on the type of GTT performed.
-
i.For OGTT, aspirate around 500 μL of glucose in a 1 mL syringe, add the yellow plastic feeding gavage tube to the syringe, and push out the excess glucose till the right volume is reached based on the weight of the mice. Make sure any excess bubbles are removed.
-
ii.For IPGTT, aspirate 300 μL of dextrose solution in the 0.5 mL insulin syringe. Make sure any excess bubbles are removed from the syringe and push out the dextrose solution till the right volume is reached based on the weight of the mice.
-
i.
-
f.Add numbing cream (emla cream) to the tail of the mouse for 15 min.
-
g.Snip the end of the mouse tail to allow for bleeding.
-
a.
-
2.OGTT
-
a.Measure the fasting blood glucose level using the glucometer. Gently squeeze the tail of the mouse from the base of the tail to the tip. A drop of blood will appear at the tip of the tail. Wipe away the first drop of blood with a tissue and measure the second drop of blood. Record the measurement.
-
b.Collect 20 μL of fasting blood (0 min) to be used for insulin measurement using EDTA coated blood collection tubes. Keep the tubes on ice.
-
c.Repeat the 0 min blood glucose measurement and blood collection for all mice.
-
d.Gavage first mouse with glucose and start timer. Gavage second mouse after 2 min with glucose and so on. Keep 1.5–2 min between each mouse to allow time to measure glucose and collect blood.
-
e.In addition to the 0 min time point, measure glucose at time points 10, 20, 30, 60, 90 and 120 min. Collect 20 μL of blood at time points 10 and 30 min. Collection of 120 min time point is optional.
-
f.Once all the time points are collected, feed mice and allow to rest for two to three days before performing any other experiments.
-
a.
-
3.IPGTT
-
a.Measure the fasting blood glucose levels and collect 20 μL of fasting blood for all mice for the 0 min time point as explained in the OGTT protocol (Steps 2a–2c).
-
b.Intraperitoneally inject the first mouse with dextrose solution and start timer. Inject second mouse after 2 min with dextrose solution and so on.
-
c.In addition to the 0 min time point, measure blood glucose at time points 10, 20, 30, 60, 90 and 120 min and collect 20 μL of blood at time points 10 and 30 min.
-
d.Once the time points are collected, feed mice and allow mice to rest for two to three days before performing any other experiments.
-
a.
-
4.Insulin secretion
-
a.To measure insulin secretion, centrifuge blood collection tubes at 3381×g for 10 min at 4°C.
 Pause Point: If measurements of insulin secretion will be done on another day, transfer serum to Eppendorf tubes and store at −80°C.
Pause Point: If measurements of insulin secretion will be done on another day, transfer serum to Eppendorf tubes and store at −80°C. -
b.To directly measure insulin secretion, use the mouse ultrasensitive insulin ELISA kit and follow the manual’s instructions. The mouse ultrasensitive insulin ELISA kit has superior sensitivity which can reach 0.019 ng/mL based on the volume of serum provided and thus is useful for hypoinsulinemic samples, however alternative mouse insulin ELISA kits can also be used for insulin estimation. We recommend using 10 μL of serum instead of 5 μL of serum as stated in the mouse ultrasensitive insulin ELISA kit protocol.
-
c.Absorbance should be read at 450 nm on a plate reader (Example: Pherastar machine).Note: We recommend only involving a maximum of 6–7 mice per person. If 2 people are performing the experiment, we recommend that one person should be responsible for the oral gavage of all mice to minimize variances. We also recommend that each set of mice per person should contain both control and treated mice.Alternatives: If the glucose volume to gavage is too high to be given to mice with increased weight, 50% dextrose solution could be used as an alternative.
-
a.
Pancreas perfusion and islet isolation
Timing: 3 h
In this section, we provide specific steps on how to perfuse the pancreas and isolate islets to study their function or assess their morphology. This is a terminal experiment. Previous protocols have reported methods for pancreas perfusion and islet isolation (Zhu et al., 2021). Here, we utilize a slightly different approach where the steps for isolating islets are reduced from previous protocols and no sieve is used to achieve optimal islet yield.
-
5.Pancreas perfusion troubleshooting 1
-
a.Prepare collagenase solution as stated in the materials and equipment section and keep on ice. Collagenase solution should be prepared with RPMI 1640 media containing PS only as FBS deactivates collagenase.
-
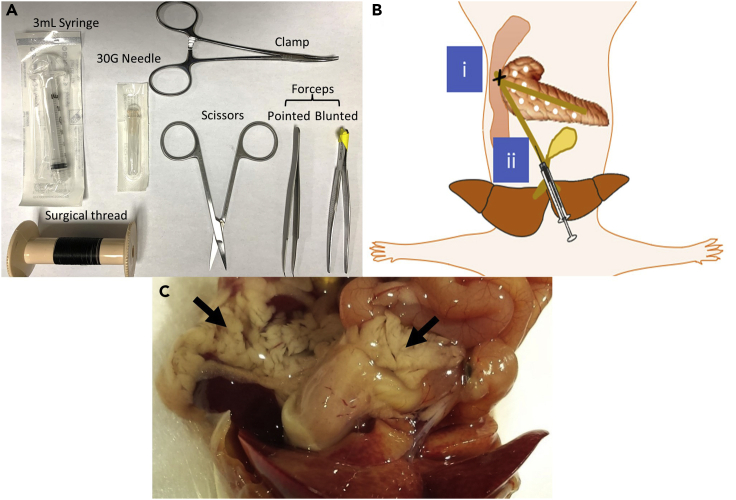
b.Fill a 50 mL tube with 5 mL of the collagenase solution. Fill a 3 mL syringe with 3 mL of collagenase and connect to a 30G needle. Refer to Figure 1A for surgical tools used to perfuse the pancreas. Keep tube and syringe on ice.
-
c.Anesthetize the mouse using isoflurane. To do that, inside a fume hood, add a few drops of isoflurane to a cotton pad. Add the cotton pad into a box or jar and place a wire mesh on top of the cotton pad to act as barrier between the isoflurane and the mouse. Put the mouse on the wire mesh inside the box so that the mouse does not come in contact with the isoflurane and close the lid. Assess that the mouse is anesthetized by firmly pinching its toes.
-
d.Use a conical tube with a cotton pad containing few drops of isoflurane added at the end of tube and place the nose of the mouse close to the terminal end of the tube inside the fume hood to keep mice under anesthesia. Disinfect the skin by spraying ethanol.
-
e.Cut through the skin vertically and through the rib cage. Cut the heart out and remove all blood using a 1 mL syringe. Cervical dislocation is another option that can be used followed by cutting the skin vertically to access the bile duct and pancreas.
-
f.Gently, push the intestinal organs covering the bile duct to the left until the bile duct is clearly visible.
-
g.Under a surgical microscope, suture the common bile duct before it connects with the ampulla of vater. This is preferably done by using forceps and a surgical thread. Use the forceps with a curved pointed end to hold on to the end of the surgical thread and go under the bile duct before it connects with the ampulla of vater. Pull the thread from the other side using tissue forceps with a blunted end and make a knot. Alternatively, a clamp could be used to seal the common bile duct before it connects with the ampulla of vater. A schematic diagram illustrates the place of the suture or the clamp (Figure 1B). Refer to pancreas perfusion part (step 3) in Zhu et al. (2021).
-
h.Once a knot is made, just under the gallbladder, use tissue forceps with a blunted end (to not puncture the bile duct) and hold under the bile duct to provide a leverage for the needle to penetrate.
-
i.Insert the needle into the common bile duct with the needle pointing in the direction of the small intestine without rupturing the bile duct as shown in Figure 1B. Troubleshooting 2
-
j.Press on the syringe to fully perfuse and fill up the pancreas with 3 mL of collagenase. A correct perfusion is demonstrated by a blown-up pancreas as shown in Figure 1C.
-
k.Extract the pancreas from the mouse body using scissors by starting to cut the pancreas at the spleen, through the intestines and to the visceral fat. Use tissue forceps with a blunted end to hold on to the pancreas. Be careful to not cut parts of other organs as they may contain digestive enzymes which can break the pancreas and digest the islets.
-
l.Place the pancreas in the 50 mL tube filled with 5 mL of collagenase solution and keep on ice.
-
a.
Pause Point: The pancreas could be kept on ice until all mice have been fully perfused for just a few hours.
-
6.Islet isolation troubleshooting 3
-
a.Incubate the pancreas added to the 50 mL tube in a hot bath at 37°C for 11 min.
-
b.After the incubation, shake the pancreas gently 4–5 times. Pancreas should be dissolved and the solution should appear cloudy.
-
c.To stop the digestion, add 45 mL of RPMI media with 10% FBS and 1% PS. The FBS will deactivate the collagenase and stop the digestion.
-
d.Pour a bit of the digestion solution onto a petri dish. Keep the rest of the 50 mL tube containing the digestion solution on ice.
-
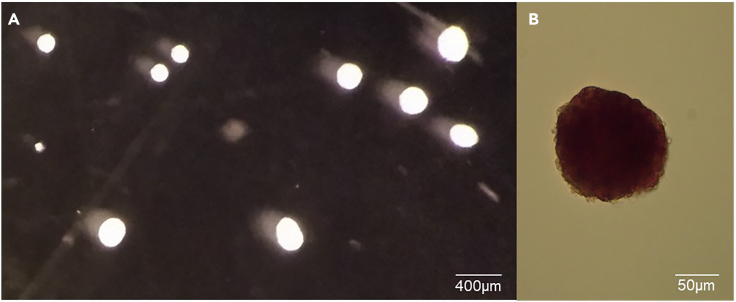
e.Under a dissection scope, using a 10 μL pipette, pick the islets from the digestion solution to the first plate. Islets are identified by having a round bright white shape as shown in Figure 2A.
-
f.Transfer the islets onto a second plate with fresh RPMI media with 10% FBS and 1% PS.
-
g.From the second plate, transfer the islets again to a third plate with fresh RPMI media to separate the islets from the exocrine tissue.
-
h.Put the dish containing pure islets in the incubator at 37°C and 5% CO2 to recover until the next day.
-
a.
Optional: To ensure that islets have been picked up from the pancreatic digest solution, dithizone staining could be performed on a few islets. This is only a confirmation step to make sure that the user can differentiate and pick the islets from the pancreatic digest. The islets stained with dithizone cannot be used for further steps. First, to prepare dithizone solution, add 40 mg of dithizone to 20 mL of dimethyl sulfoxide and vortex for 3–4 min. Add 5 mL of the solution prepared to 45 mL of magnesium and calcium free PBS. Filter solution and dilute 1:10 for staining. Add the mixture to a few islets in a dish, incubate for 3 min and view under a microscope. Islets will be stained dark red as shown in Figure 2B.
Figure 1.
Steps of pancreas perfusion
(A) Surgical tools used for pancreas perfusion.
(B) Schematic diagram showing locations to i) clamp bile duct before it connects with ampulla of vater and ii) inject collagenase into the bile duct.
(C) Image of a fully perfused pancreas under the naked eye.
Figure 2.
Appearance of islets under a microscope and after staining
(A) Picture of islets under a microscope after third pick at a magnification of 2× displaying a bright white shape. Scale bar, 400 μm.
(B) Dithizone staining on an islet showing dark red color. Scale bar, 50μm.
Glucose stimulated insulin secretion
Timing: 3 h
After isolating islets, we provide specific details on how to perform glucose stimulated insulin secretion to determine islet function and their capacity to secrete insulin. For ex vivo studies, use the islets isolated from mice directly to this step. For in vitro studies, islets are first treated with the substance of interest for 24 or 48 h and then glucose stimulated insulin secretion is performed. Here, we report the use of 30 islets to provide a more reliable and robust insulin response compared to the use of fewer islets (around 10 islets) in previous protocols (Zhu et al., 2021).
-
7.Preparation for glucose stimulated insulin secretion
-
a.Prepare low glucose, high glucose and high glucose with KCl solution as stated in the materials and equipment section by using the KRB solution prepared. KRB should be leveled to a pH of 7.4 before making the glucose solutions. Keep the solutions in a water bath at 37°C.
-
b.Label a 60 mm petri dish (60 mm × 15 mm) for each treatment and add 4 mL of low glucose solution per petri dish. Keep the dishes in an incubator at 37°C and 5% CO2.
-
c.In a 24 well plate, for each treatment, add 500 µL of low glucose in one well, 500 µL of high glucose in a second well, and 500 µL of high glucose with KCl in a third well. Keep the plate in an incubator at 37°C and 5% CO2.
-
a.
-
8.Pre-incubation
-
a.Under a surgical microscope, transfer 30 islets from the islets isolated in the previous step to the labeled 60 mm petri dish containing low glucose solution. Islets from different treatments or mice should be separated in different dishes. It is important to choose equal sized islets between the different treatment conditions as islets of different size have different insulin secretion responses to glucose. Since 30 islets are used for each treatment or mice, we recommend picking 10 big islets, 10 medium islets and 10 small islets by eye for a total of 30 islets for each different treatment.
-
b.Incubate islets in low glucose solution for 1 h at 37°C.
-
a.
-
9.Low glucose
-
a.Pick islets from the dish into the 24 well plate containing low glucose KRB solution. Repeat for separate treatments or mice. Islets should be picked up slowly with the 10 μL pipette and dispensed very gently to minimize any mechanical stimulation that could occur.
-
b.Incubate for 20 min at 37°C.
-
a.
-
10.High glucose
-
a.Pick islets from the low glucose well into well containing the high glucose KRB solution. Repeat for separate treatments.
-
b.Incubate for 20 min at 37°C.
-
a.
-
11.High glucose with KCl
-
a.Pick islets from the high glucose well into well containing the high glucose with KCl KRB solution. Repeat for separate treatments.
-
b.Incubate for 20 min at 37°C.
-
a.
-
12.Total insulin content
-
a.Pick all islets from the high glucose with KCl solution into a 1.5 mL microfuge tube containing 70 uL of acid ethanol solution that is prepared by mixing ethanol, ultrapure water and HCl in the ratio of 150:47:3.
-
b.Keep samples at 4°C overnight (12 h).
-
c.The next day, evaporate the acid ethanol from the microfuge tubes using a speed vacuum.
-
d.Add 30 μL of ultra pure water to each sample and vortex.
-
e.Heat the sample at 65°C for 10 min.
-
f.Spin all samples at 9391×g for 10 min and keep supernatant.
-
g.Measure DNA concentration using a nanodrop spectrophotometer. DNA concentration will be used to normalize insulin measurements.
-
a.
Pause Point: Save the low glucose, high glucose and high glucose with KCl solutions for analysis by transferring them into 1.5 mL microfuge tubes. The solutions should be stored at −20°C.
Optional: We have outlined here how to perform these steps using a 24 well plate. Another way to perform glucose stimulated insulin secretion is to use 1.5 mL microfuge tubes instead of 24 well plates to reduce mechanical stimulation of islets. In this case, the islets are added in microfuge tubes containing 500 μL of low glucose solution for pre-incubation. The same protocol and incubation times are followed. The difference is instead of moving the islets from one well to the next well in a 24 well plate, the islets are kept in the microfuge tube and 300 μL of the solution is taken out and stored in a new microfuge tube and 300 μL of the next solution is added.
Note: We have only demonstrated here the steps for mouse islets. Human islets and MIN6K8 cells were tested using the same procedure with minor optimizations. The media used for human islets is DMEM (1 g/L glucose with 1% PS and 10% FBS). For MIN6 cells, the media used is DMEM (4.5 g/L glucose with 1.75 uL beta-Mercaptoethanol, 1% PS and 10% FBS). It is preferred to use a low glucose concentration of 0 mM and a high glucose concentration of 11 mM for MIN6 cells. Since MIN6 cells are adherent cells, the glucose solutions should be added to the wells containing the cells. First, in a 24 well plate seeded with MIN6 cells, pipette out the media and wash the cells with 500 μL of KRB. Aspirate the KRB and add 500 μL of 0 mM LG solution for pre-incubation. Pipette out the solution and add 500 μL LG solution. Pipette out the LG solution and store for insulin measurements. Repeat process for 11 mM HG solution and 11 mM HG + 30 mM KCl solution. After pipetting out the HG+KCl solution, wash cells with 500 μL KRB. The total insulin content for MIN6 cells can be obtained by adding 200 μL of ultra pure water then freezing and thawing the cells three times. Scratch surface and transfer contents to microfuge tube. Spin at 9391×g for 10 min at 4°C and collect the supernatant. Measure DNA concentration using nanospectrometer.
Homogeneous time resolved fluorescence (HTRF) assay
Timing: 6 h
This experiment is used to measure insulin concentration in glucose stimulated insulin secretion and total insulin samples using homogeneous time resolved fluorescence (HTRF) assay. This technology is based on Forster resonance energy transfer (FRET) in which two antibodies 1) Cryptate (CR), the antibody donor, and 2) XL655 (XL), the antibody acceptor, are used. When these antibodies bind to insulin and the antibodies are in proximity, the donor excites the acceptor and a fluorescence signal is generated that is read by the machine. We have used the Cisbio HTRF assay kit with a few optimizations. The HTRF assay allows us to measure the insulin concentration for the different glucose concentration and thus determine whether there are any defects in glucose stimulated insulin secretion between different treatments. Alternative methods could be used for measurement of insulin secretion such as insulin ELISA kits, however they are more expensive and include fewer samples to run per plate.
-
13.Standard preparation
-
a.Label 1.5 mL microfuge tubes with negative control (containing only KRB), positive control, and standards from 1 to 8 (S1-S8).
-
b.To prepare the standard, dilute the stock of the standard at 1:50 by adding 10 μL of the stock to 490 μL of KRB to achieve a concentration of 10 ng/mL (S8). Perform serial dilutions by taking 200 μL from S8 and mix into 200 μL KRB (S7). Repeat serial dilutions till you reach S1.
-
c.Dilute the stock of positive control at 1:10 by adding 10 μL of positive control to 90 μL of KRB.
-
a.
-
14.Sample dilution
-
a.For each sample, label 1.5 mL microfuge tubes for the dilutions.
-
b.Pipette a volume of KRB into each tube to dilute the samples from the GSIS and mix by pipetting the solution up and down. Use the following dilutions:
-
i.No dilutions for low glucose
-
ii.1:10 dilution for high glucose (10 μL sample in 90 μL KRB)
-
iii.1:20 dilution for high glucose with KCl (10 μL sample in 190 μL KRB)
-
iv.1:4000 dilution for total insulin content (10 μL sample in 390 μL KRB and then 10 uL of first dilution into 990 μL KRB)
-
i.
-
a.
-
15.Antibody preparation
-
a.Make 1:20 dilutions (10 μL of 190 μL KRB) of each of CR and XL separately and then combine to achieve a 1:40 dilution.
-
a.
-
16.Reading plate
-
a.Using a 384-well plate, load 10 μL of your negative control, standards (S1–S8), positive control and samples.
-
b.Load 10 μL of antibody (CR and XL solution) to the negative control, standards, positive control and samples.
-
c.Spin plate for 2 min at 376×g.
-
d.Incubate at room temperature for 4–24 h in a dark drawer.
-
e.Read data using BMG Labtech Pherastar or any alternative HTRF compatible machine. Troubleshooting 4
-
a.
CRITICAL: The dilutions should be adjusted accordingly to make sure the insulin measurements are within the range of the standard. Therefore, a test run is recommended where the dilutions for different glucose concentrations are tested before performing the actual run.
Expected outcomes
Glucose tolerance test and insulin secretion
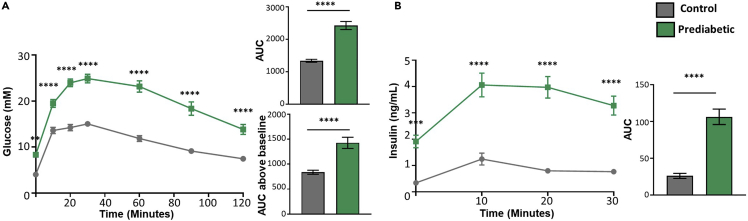
GTT provides information on glucose homeostasis in mice and their capacity to secrete insulin during the test. Fasting glucose and fasting insulin measurements should be plotted as a bar graph as higher glucose or insulin can give an indication of glucose dysmetabolism. Plasma glucose measurements obtained from GTT should be plotted as glucose vs time. Additional calculations such as total area under curve (AUC) and AUC above baseline can be calculated as previously reported (Heikkinen et al., 2007). AUC above baseline is a normalized calculation used to get some indication of how basal conditions affect the overall metabolic response. Mice that have normal glucose tolerance will have a typical shaped curve as illustrated in Figure 3A. In contrast, mice that are glucose intolerant will have an exaggerated curve compared to controls and their glucose will not fall as easily as demonstrated by a higher total AUC and AUC above baseline (Figure 3A). Glucose and insulin over the course of a glucose tolerance test are dynamic and influenced by variety of peripheral and central factors. Therefore, the three different ways of representation should be considered when making conclusions about the results.
Figure 3.
Results from glucose tolerance test in mice
(A) Glucose tolerance test plotted using i) glucose vs time, ii) total area under curve (AUC) and iii) AUC above baseline.
(B) Insulin secretion during glucose tolerance test plotted using i) insulin vs time and ii) total AUC (n=19–21/group).
Data are presented as mean ± SEM. ∗∗P<0.01 ∗∗∗P<0.001, ∗∗∗∗P<0.0001. Two-way Anova with Bonferroni post-hoc analysis was used for glucose vs time and insulin vs time plots. Student’s T-test was used for the AUC bar graphs. Permission has been obtained to re-use results from Al Rijjal et al. (2021).
Insulin secretion measured during the GTT should be plotted as insulin vs time and the total AUC can be calculated (Figure 3B). Insulin secretion measurements will differ based on the capacity of beta cells to secrete insulin in response to glucose. Mice could be hyperinsulinemic and secrete high levels of insulin that fail to reduce glucose (insulin resistance) (Figure 3B). In this case, doing a test that measures insulin resistance, like an insulin tolerance test, will reveal whether mice are also insulin resistant. In other cases, mice could have a failed capacity at secreting insulin and will have lower insulin secretion compared to control. Therefore, looking at glucose stimulated insulin secretion will provide a direct look at the islets.
Glucose stimulated insulin secretion (troubleshooting 5)
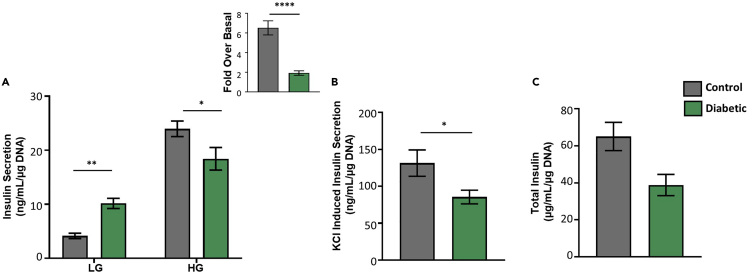
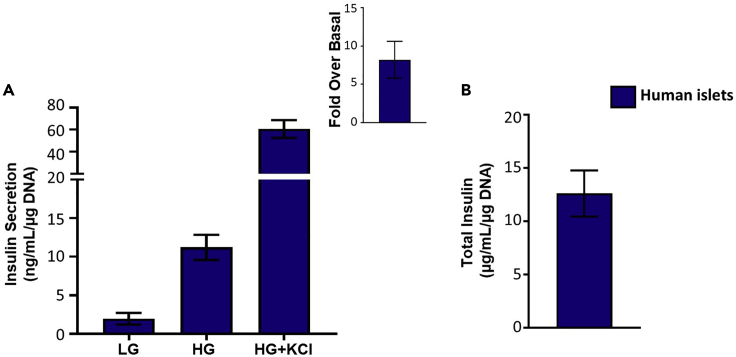
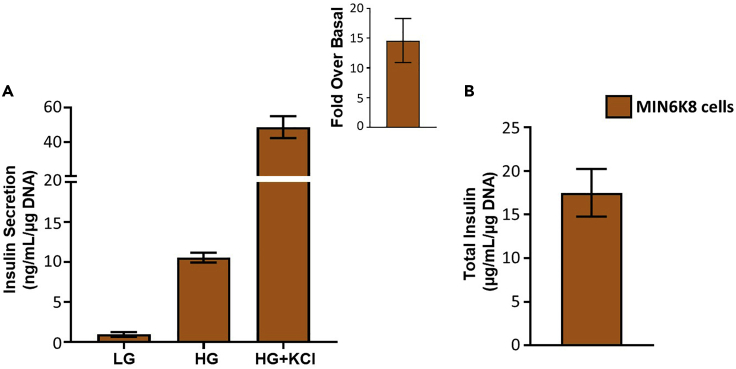
Glucose stimulated insulin secretion provides a look at how the islets behave under different glucose conditions. The results obtained from the HTRF assay are calculated by taking into account the glucose dilution and the DNA concentration detected. A good response is generated by a low basal insulin secretion followed by increased insulin secretion at high glucose (Figure 4A). Defects in glucose stimulated insulin secretion appear as higher basal insulin secretion or lower stimulation at high glucose which will present as a lower fold over basal (Figure 4A). Defects in the maximum insulin secretory capacity are detectable by looking at KCl induced insulin secretion which depolarizes the cell membrane and stimulates insulin release (Figure 4B). Defects in the insulin biosynthesis process are determined by looking at the total insulin content (Figure 4C). One or more of these defects can be shown in islets based on the pathways affected. The results for the glucose stimulated insulin secretion for human islets are obtained and calculated the same way as for mouse islets (Figure 5). In MIN6 cells, the calculation method is the same but the dilution factor changes due to changed volumes (Figure 6).
Figure 4.
Glucose stimulated insulin secretion in mouse islets
(A) Glucose stimulated insulin secretion showing insulin secretion at low glucose and high glucose with calculated fold over basal.
(B) KCl-induced insulin secretion.
(C) Total insulin content (n=11 per group).
Data are presented as mean ± SEM. ∗P<0.05, ∗∗P<0.01, ∗∗∗∗P<0.0001. Two-way Anova with Bonferroni post-hoc analysis was carried out for Figure 4A. Student’s T-test was used for fold over basal in Figure 4A and for Figures 4B and 4C.
Figure 5.
Glucose stimulated insulin secretion in human islets
(A) Glucose stimulated insulin secretion showing insulin secretion at low glucose and high glucose with calculated fold over basal and KCl-induced insulin secretion.
(B) Total insulin content (n=4).
Data are presented as mean ± SEM.
Figure 6.
Glucose stimulated insulin secretion in MIN6K8 cells
A) Glucose stimulated insulin secretion showing insulin secretion at low glucose and high glucose with calculated fold over basal and KCl induced insulin secretion.
(B) Total insulin content (n=4).
Data are presented as mean ± SEM.
Quantification and statistical analysis
Total AUC for GTT and insulin secretion is calculated using the equation where x is the total number of time points, n is the time point number, T is time and G is the blood glucose level. AUC above baseline is calculated using the equation (Heikkinen et al., 2007). The AUC and AUC above baseline can also be alternatively calculated separately for each mice using the AUC analysis option in GraphPad prism. AUC above baseline can be calculated in GraphPad prism by setting the baseline as the mean of the first row (0 time point).
The results obtained from the HTRF assay for the glucose stimulated insulin secretion experiment are calculated using the following formula: . Fold over basal is calculated by .
Student’s T-test should be used when comparing two groups for bar graphs. One-way Anova should be used for bar graphs comparing three or more groups followed by a post-hoc analysis. Two-way Anova should be used for the glucose vs time and insulin vs time graphs followed by a post-hoc analysis. GraphPad prism is used for the statistical analysis of the results. P<0.05 is considered significant.
Limitations
Although this protocol determines important measures to study diabetes such as GTT and glucose stimulated insulin secretion, further tests such as an insulin tolerance test, hyperglycemic clamps and evaluating steps in the pathway of glucose stimulated insulin secretion such as glycolysis and mitochondrial function could be required to gain further understanding based on the initial results obtained.
Troubleshooting
Problem 1
The pancreas is not fully perfused (step 5).
Potential solution
Problems in closing the bile duct at the ampulla of vater or leakage during injecting collagenase can lead to an unsuccessful perfusion. To close the bile duct, a clamp can be used instead of a thread to shut the bile duct. To aid in a successful injection, first, make sure that no collagenase is leaking from the needle tip before injecting the needle. Provide a leverage under the bile duct with a blunted forcep (making sure not to tear apart the bile duct) for the needle to then penetrate the bile duct. Use the dominant hand to hold the syringe that is connected to the needle and poke the bile duct. Once the needle is inside the bile duct, remove the forcep from under the bile duct and with the less dominant hand, press on the syringe to let the collagenase flow into the bile duct while holding onto the syringe using the dominant hand. Make sure to let the collagenase flow slowly to prevent the duct from rupturing. The pancreas should begin to be filled with collagenase. If this does not happen, that means the bile duct is ruptured, the bile duct was not shut properly where it connects with the ampulla of vater or the site of injection was incorrect.
Problem 2
Bile duct is ruptured when collagenase is injected (step 5).
Potential solution
The bile duct can be ruptured when trying to insert the needle tip to inject collagenase or while making an injection at the wrong site and releasing collagenase as a result. To avoid this, first make sure that no collagenase is leaking from the needle tip before insertion into the bile duct. When inserting the needle tip into the bile duct, make sure that the needle tip and injection site is made at a higher point closer to the gallbladder. This creates an opportunity to re-insert the needle tip into the bile duct closer to the site of clamp or suture in the event that the bile duct was ruptured. Next, if the injection was made at the wrong site, immediately pull back the needle when no pancreas inflation is noticed. Assess for any damage to the bile duct at a level closer to the ampulla of vater. Re-insert the needle tip closer to the ampulla of vater and inject collagenase.
Problem 3
The islet yield is low (step 6).
Potential solution
Based on the background of the mice, sometimes the collagenase concentration, the incubation time or the amount of shaking need to be tweaked to give the highest islet number possible. The collagenase concentration will differ based on the mice background. For FVB or CD1 mice, use 40% collagenase instead. Shaking should be more vigorous for FVB or CD1 mice. For C57BL/6 mice, use 30% collagenase with gentle shaking. We found that the best incubation time is 11 min, however if the islets are not separated properly from the pancreatic digest, increase shaking and incubation time to 12–14 min. If the islets look over digested and broken, try 9–10 min instead with gentler shaking. A good yield of islets for C57BL/6 should be 80–120. For CD1 and FVB mice, around 150–200 islets should be expected.
Problem 4
HTRF values fall too low on the standard or are outside the acceptable standard range (step 16).
Potential solution
HTRF values for glucose stimulated insulin secretion need to remain within the standard range. Values that are in the lower limit of the standard can result in unreliable results due to insufficient standard separation. Values that are outside the limit will result in exaggerated insulin responses. Therefore, the optimal range for values to fall in is from 3–10 ng/mL. To do that, a test that includes different dilutions for each glucose concentration should be evaluated to determine the best dilution to use. Once the dilutions are optimized, a run containing all the different glucose concentrations and the different treatments should be performed. Increased replicates for each sample will give better accuracy for the insulin values and is recommended.
Problem 5
The glucose stimulated insulin secretion response for a normal islet is not optimal (expected outcomes).
Potential solution
A good islet response has at least a 4-fold over basal response. If the fold change is lower for control islets, there could be a few reasons why this occurs. First, the user must make sure that the islets used are actual islets and not exocrine tissues. Dithizone staining can help ensure that the user knows how to differentiate islets from exocrine tissue especially when picking human islets as they can have more irregular shape. Next, the user should make sure that the islets used are not broken during the islet isolation process. Round dense white islets should be picked that do not have an irregular shape. Islets that have any exocrine tissue attached to them should not be used in the experiment. In addition, duplicates or triplicates should be used if enough islets are obtained to make sure that the islet response is similar between the different runs. Finally, any problems with the values obtained from the HTRF assay being too high or too low need to be corrected as troubleshooted in problem 4.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Michael B. Wheeler (michael.wheeler@utoronto.ca).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This study was funded by a Canadian Institutes of Health Research operating grant (FDN-143219) to M.B.W. D.A.R. was supported by Banting & Best Diabetes Center Studentship and Ontario Graduate Scholarship.
Author contributions
D.A.R. wrote and edited the manuscript and conducted the experiments. M.B.W. reviewed and edited the manuscript.
Declaration of interests
The authors declare that they have no competing interests.
Contributor Information
Dana Al Rijjal, Email: dana.alrijjal@mail.utoronto.ca.
Michael B. Wheeler, Email: michael.wheeler@utoronto.ca.
Data and code availability
This study did not generate/analyze any datasets/code.
References
- Al Rijjal D., Liu Y., Lai M., Song Y., Danaei Z., Wu A., Mohan H., Wei L., Schopfer F.J., Dai F.F., et al. Vascepa protects against high-fat diet-induced glucose intolerance, insulin resistance, and impaired β-cell function. iScience. 2021;24:102909. doi: 10.1016/J.ISCI.2021.102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen S., Argmann C.A., Champy M.-F., Auwerx J. Evaluation of glucose homeostasis. Curr. Protoc. Mol. Biol. 2007;77:29B.3.1–29B.3.22. doi: 10.1002/0471142727.MB29B03S77. [DOI] [PubMed] [Google Scholar]
- Zhu Y.X., Zhou Y.C., Zhang Y., Sun P., Chang X.A., Han X. Protocol for in vivo and ex vivo assessments of glucose-stimulated insulin secretion in mouse islet β cells. STAR Protoc. 2021;2 doi: 10.1016/J.XPRO.2021.100728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze any datasets/code.