ABSTRACT
Background
Diagnosing sepsis early is important for its successful management. Various biomarkers are being used currently, but mostly they are either expensive or not readily available. This study aims to evaluate usefulness of automated immature granulocyte count (IG#) and immature granulocyte percentage (IG%) as early diagnostic markers of sepsis and compares it to other established predictive markers.
Patients and methods
In this prospective observational study, 137 eligible, critically ill, nonseptic intensive care unit patients were analyzed for automated IG#, IG%, serum procalcitonin (PCT), and blood lactate (Lac), daily for 7 days after recruitment. Patients were followed for the development of sepsis, defined by the new Sepsis-3 criteria. The study was divided into four time periods of 24 hours each with respect to the day of developing organ dysfunction. Using area under receiver operator characteristic and diagnostic odds ratio (DOR) methods, the best biomarker for the prediction of sepsis in each time period was calculated.
Results
IG# and IG% were the earliest biomarkers to have a significant discriminating value with area under the curve of 0.81 and 0.82, respectively, as early as 24 hours before clinical sepsis is diagnosed by Sepsis-3 criteria. Both IG# and IG% have a high DOR of 34.91 and 18.11, respectively, when compared to others like PCT and Lac having a DOR of 27.06 and 4.78, respectively.
Conclusion
IG# and IG% are easily available, rapid, and inexpensive tools to differentiate between septic and nonseptic patients with high specificity and sensitivity. It is the earliest biomarker to show a significant rise in patients developing sepsis.
How to cite this article
Bhansaly P, Mehta S, Sharma N, Gupta E, Mehta S, Gupta S. Evaluation of Immature Granulocyte Count as the Earliest Biomarker for Sepsis. Indian J Crit Care Med 2022;26(2):216–223.
Keywords: Biomarker, Early sepsis, Immature granulocyte, Procalcitonin, Sepsis, Sysmex
INTRODUCTION
Sepsis is a serious condition and is a major cause of mortality around the world. The mortality of severe sepsis in India reaches up to 65.2%.1 Early diagnosis has always been the key for successful management, but sepsis continues to escape a precise diagnostic definition. Microbiological blood cultures are used to identify pathogens but have low specificity and are poor early markers because of the time needed to obtain results. Furthermore, 40% of sepsis patients remain culture negative.2 It is also important to differentiate patients with sepsis from those with noninfectious inflammation, as these disease conditions require different therapeutic regimens. The Surviving Sepsis Campaign recommends that antibiotics should be administered within 1 hour of the onset of sepsis.3 Every hour of delay in antibiotic administration has been shown to increase the mortality of septic shock by 7.6%.4 Hence, diagnosing an infection rapidly and accurately differentiating inflammatory response from sepsis are both challenging and vital for early induction of appropriate therapy. This has necessitated studies to address the usefulness of various other parameters to predict sepsis earlier. A biomarker with high sensitivity, specificity, speed, and accuracy would be revolutionary for differentiating sepsis from noninfectious systemic inflammatory response syndrome (SIRS), given the limitations and time required for microbial verification of pathogens.
Biomarkers such as serum procalcitonin (PCT), C-reactive protein (CRP), lactic acid (Lac), interleukin-6 (IL-6), and others have been found helpful to predict sepsis, but are not widely used due to limited availability and high costs. Functional and morphological evaluation of leukocytes is considered a more affordable and practical strategy for monitoring the inflammatory response in sepsis. Parameters like manual band count, left shift, and immature-to-total neutrophils (I/T) ratio are widely used in pediatric patients to predict bacterial infection but have poor accuracy and reliability in adult populations.5 Recently, there has been a shift from tedious manual band counts to automated systems that analyze leukocytes accurately and categorize them according to their sizes and cytoplasmic and nuclear characteristics. In a Sysmex hematology analyzer, the “left shift” is represented by immature granulocyte count (IG#). The IG# includes the promyelocytes, myelocytes, and metamyelocytes fraction of the neutrophils. This fraction is believed to be the first reaction of the bone marrow in an infection.6 Quantification of this fraction can be used as a predictive marker for sepsis. The automated IG# is not only easily available but also simple, inexpensive, and reproducible.
Patients in the intensive care units (ICUs) are commonly administered broad-spectrum antibiotics starting from the day of admission. The INDICAP study done in 120 ICUs in India revealed that 67.1% of the patients were overtreated with antibiotics.7 Judicious use of antibiotics is of prime importance because of increasing antimicrobial resistance and development of multidrug-resistant strains of microbes. The need of the hour is a sound system of early and accurate diagnosis of sepsis to optimize antimicrobial treatment and to prevent overtreatment and misuse.
The aim of this study is to evaluate the diagnostic usefulness of IG# as an early marker of sepsis and also to compare it with other established predictive markers of sepsis. A biomarker, which can easily, rapidly, and accurately differentiate sepsis from other inflammatory conditions in the ICUs, will help in better management of sepsis and will also decrease the overall antibiotic use.
MATERIALS AND METHODS
In this prospective observational study, done in Sawai Man Singh Hospital, Jaipur, a tertiary care hospital, we enrolled 150 consecutive eligible critically ill patients ≥15 years of age, admitted to the medical ICU of this institution from July 2018 to January 2019. The patients were either admitted or referred for a cause other than sepsis. Patients with sepsis at the time of admission and those who developed signs of sepsis within 48 hours of ICU admission were excluded based on baseline investigations. Patients with blood malignancy, immunodeficiency diseases, and those being treated with immunosuppressive medications were excluded from the study. Patients who developed sepsis and expired within 24 hours of developing sepsis or those who did not develop sepsis but expired within 7 days of ICU admission were also excluded from the study.
The study was initiated after due approval from the Institutional Ethics Committee. All information regarding the study was explained to the patient or the patient's attendant, and a written consent was obtained. This study was conducted in accordance with the Declaration of Helsinki and the standard of good clinical practice.
Blood samples were collected from all patients within 24 hours of ICU admission for automated complete blood count), IG# and IG%, serum creatinine, bilirubin, PCT, Lac, and arterial blood gas analysis. For supportive diagnosis and to identify the suspected source of infection based on clinical suspicion, appropriate blood/pus/urine/body fluid cultures and imaging studies were done. PCT was quantified using Chemi E411 cobas immunoassay kits. Automated hematology analyzer used was Sysmex XN-1000 (Kobe, Japan). Blood and pus cultures were done by CLSI standards. All the patients received treatment as per standard of practice and were visited by the principal investigator at least once daily.
Sepsis-3 criteria define organ dysfunction organ dysfunction as change in sequential organ failure assessment (SOFA) ≥2.8 All the investigations including SOFA scoring were done daily up to a maximum period of 7 days or until the patient either developed sepsis or died. Patients who developed sepsis as per the Sepsis-3 criteria within 7 days of admission were grouped under the sepsis group, and others were grouped under the no-sepsis group.
Statistical Analysis
For statistical analysis, the study was divided into four time periods based on the day of developing sepsis or organ dysfunction. Time period 1 was 48 hours before to 24 hours before developing sepsis or organ dysfunction, Time period 2 was 24 hours before to the hour of developing sepsis or organ dysfunction, Time period 3 lasted from hour of development of sepsis or organ dysfunction till 24 hours after, and Time period 4 was 24 to 48 hours after developing sepsis or organ dysfunction. For the patients who did not develop sepsis or organ dysfunction, the first 96 hours of ICU admission was divided into four time periods of 24 hours each.
Data were analyzed using IBM SPSS v21 and MedCalc. Descriptive statistics were done for appropriate population characteristics, and categorical variables were presented in numbers and percentages. Normally distributed variables were summarized in the form of mean and standard deviation (SD). The biomarkers in this study do not follow a normal distribution, so the median and interquartile range were used. The difference in mean was analyzed using Student's t-test, and the difference in median was analyzed using Mann–Whitney U-test. A receiver operator characteristic (ROC) curve was plotted for each of the markers in different time periods, and area under the curve (AUC) was calculated for each biomarker to evaluate the validity and accuracy of the tests. The Youden's index was used to determine the best cutoff values for each biomarker, and the best sensitivity and specificity was calculated. Diagnostic odds ratio (DOR) was calculated to find the best predictive biomarker for each study period. A p value less than 0.05 was considered as statistically significant.
RESULTS
A total of 150 critically ill ICU patients were recruited, out of which 137 patients were finally analyzed. Our study included patients 15 to 85 years old with a median age of 45 years and a sex ratio of 0.9. All patients were followed up for the next 7 days for the development of sepsis, and subsequently, they were divided into two outcome groups: sepsis and no-sepsis. Out of 137, 79 (57.6%) patients developed sepsis and the rest 58 patients (42.4%) did not.
Sepsis group consisted of both blood culture-positive and blood culture-negative sepsis patients. Of 79 patients, 37 (46.8%) were blood culture positive, and the rest were blood culture negative. The spectrum of organisms isolated in blood culture-positive patients is enumerated in Table 1. Among all the patients in sepsis group, the most common source of infection was chest (48 of 79, 61%) followed by urogenital tract (13 of 79, 16%). Mortality was 81% (30 of 37) in blood culture-positive sepsis group and 47.6% (20 of 42) in blood culture-negative sepsis group. Overall mortality in sepsis group patients was 63.3% (50 of 79). Mortality was 32.7% (19 of 58) in no-sepsis group.
Table 1.
Spectrum of organism isolated in patients developing blood culture-positive sepsis
| 1 | Klebsiella sp. | 6 |
| 2 | Acinetobacter sp. | 5 |
| 3 | Enterobacter sp. | 5 |
| 4 | Coagulase-negative Staphylococcuss | 4 |
| 5 | Enterococcus sp. | 4 |
| 6 | Staphylococcus aureus | 3 |
| 7 | Pseudomonas sp. | 2 |
| 8 | E. coli | 2 |
| 9 | Proteus sp. | 2 |
| 10 | Streptococcus sp. | 2 |
| 11 | Citrobacter sp. | 1 |
| 13 | Meningococcus sp. | 1 |
| Total | 37 |
Baseline study characteristics that were done at the time of ICU admission are summarized in Table 2. Except for the TLC (p = 0.01), comparison between the two groups showed no significant differences among the baseline parameters.
Table 2.
Comparison of baseline (at the time of ICU admission) parameters among sepsis and no-sepsis group
| Sepsis | No-sepsis | p value | |
|---|---|---|---|
| Age (years) | 49.1 ± 19.2 | 45.0 ± 18.2 | 0.210* |
| Sex (% male) | 49.4% | 56.9% | |
| Platelets (lakh/µL) | 2.61 ± 0.87 | 2.49 ± 1.13 | 0.49* |
| SOFA score | 5.47 ± 2.91 | 4.74 ± 2.94 | 0.15* |
| TLC (103/µL) | 9.91 ± 3.48 | 8.40 ± 3.05 | 0.01* |
| IG# (103/µL) | 0.03 (0.01–0.06) | 0.03 (0.01–0.08) | 0.82# |
| IG% | 0.3 (0.1–0.6) | 0.3 (0.1–0.8) | 0.34# |
| Procalcitonin (ng/mL) | 0.54 (0.32–1.06) | 0.67 (0.34–1.54) | 0.34# |
| Lactate (mmol/L) | 0.4 (0.3–0.7) | 0.5 (0.4–1.1) | 0.07# |
Age, platelet count, SOFA score, total leukocyte count (TLC) in mean ± S.D, and comparison by
Independent t-test. IG#, IG%, procalcitonin, lactate in median (interquartile range), and comparison by
Mann–Whitney U-test. Sepsis group included all patients by Sepsis-3 definition. No-sepsis group includes noninfectious organ dysfunction and controls
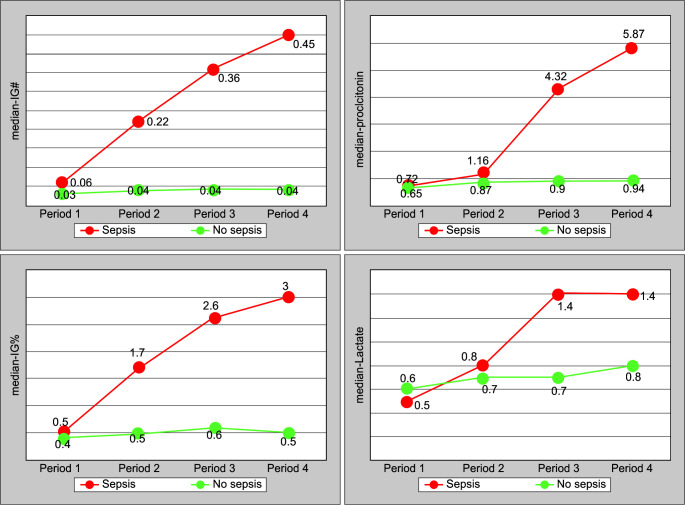
When tabulated as mean change from the baseline values and mean change percentage in Table 3, a higher drop (41 vs 23.3%) in mean platelet count was seen in the sepsis group when compared to no-sepsis groups in Time period 3. There was a higher rise of IG# (666 vs 33%) and IG% (500 vs 33%) from the baseline value starting from Time period 2 in the sepsis group when compared to the no-sepsis group. Median values of the other biomarkers also show an increase from the baseline in the sepsis group when compared to no-sepsis group, but only in later time periods, that is, Time period 3 and Time period 4. An early rise of IG# and IG% in sepsis can also be appreciated graphically in Figure 1, while the rising trends of other biomarkers—PCT and Lac, start relatively late. This suggests that IG# and IG% could be an early marker of sepsis.
Table 3.
Summary of means/medians of various study parameters and calculation of mean/median change and percentage change from the baseline value across time periods among the sepsis and no-sepsis group
| Baseline | Period 1 | Period 2 | Period 3 | Period 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean/median | Mean/median | Difference (%change) | Mean/median | Difference (%change) | Mean/median | Difference (%change) | Mean/median | Difference (%change) | |
| Platelet counts (lakhs/µL) | Sepsis | 2.61 ± 0.87 | 2.18 ± 0.93 | −0.43 (16.5%) | 1.92 ± 0.88 | −0.69 (26.4%) | 1.54 ± 0.76 | −1.07 (41%) | 1.44 ± 1.01 | −1.17 (44.8%) |
| No-sepsis | 2.49 ± 1.13 | 2.24 ± 1.14 | −0.25 (10%) | 2.24 ± 0.93 | −0.25 (10%) | 2.03 ± 0.87 | −0.58 (23.3%) | 2.06 ± 0.88 | −0.55 (22.1%) | |
| SOFA score | Sepsis | 5.47 ± 2.91 | 6.33 ± 3.16 | 0.86 (15.7%) | 6.24 ± 3.06 | 0.77 (14.1%) | 9.49 ± 3.18 | 4.02 (73.5%) | 10.52 ± 3.53 | 5.05 (92.3%) |
| No-sepsis | 4.74 ± 2.94 | 4.95 ± 3.01 | 0.21 (4.1%) | 4.45 ± 2.25 | −0.29 (6.1%) | 6.00 ± 3.26 | 1.26 (26.6%) | 5.62 ± 3.83 | 0.88 (18.5%) | |
| TLC (103/µL) | Sepsis | 9.91 ± 3.48 | 11.02 ± 4.21 | 1.11 (11.2%) | 11.72 ± 4.97 | 1.81 (18.3%) | 14.09 ± 7.05 | 4.18 (42.2%) | 14.80 ± 7.57 | 4.89 (49.3%) |
| No-sepsis | 8.40 ± 3.05 | 8.54 ± 3.40 | 0.14 (1.7%) | 9.11 ± 4.09 | 0.71 (8.4%) | 8.48 ± 3.39 | 0.08 (1%) | 8.53 ± 3.20 | 0.13 (1.5%) | |
| IG# (103/µL) | Sepsis | 0.03 (0.01–0.06) | 0.06 (0.02–0.12) | 0 (0%) | 0.22 (0.11–0.43) | 0.2 (666%) | 0.36 (0.19–0.55) | 0.31 (1033%) | 0.45 (0.24–0.68) | 0.39 (1300%) |
| No-sepsis | 0.03 (0.01–0.08) | 0.03 (0.01–0.10) | 0 (0%) | 0.04 (0.03–0.11) | 0.01 (33%) | 0.04 (0.02–0.15) | 0.02 (66%) | 0.04 (0.02–0.10) | 0.01 (33%) | |
| IG% | Sepsis | 0.3 (0.1–0.6) | 0.5 (0.2–0.9) | 0.1 (33%) | 1.7 (1.1–2.9) | 1.5 (500%) | 2.6 (1.6–4.4) | 2.2 (733%) | 3.0 (2.0–4.4) | 2.5 (833%) |
| No-sepsis | 0.3 (0.1–0.8) | 0.4 (0.2–1.2) | 0.1 (33%) | 0.5 (0.3–1.0) | 0.1 (33%) | 0.6 (0.3–1.6) | 0.2 (67%) | 0.5 (0.3–1.2) | 0.2 (67%) | |
| Procalcitonin (ng/mL) | Sepsis | 0.54 (0.32–1.06) | 0.67 (0.34–1.67) | 0.1 (18.5%) | 1.16 (0.77–2.55) | 0.6 (111%) | 4.32 (2.56–9.44) | 3.9 (722%) | 5.87 (3.60–12.67) | 5.1 (944%) |
| No-sepsis | 0.67 (0.34–1.54) | 0.65 (0.31–1.76) | 0 (0%) | 0.87 (0.40–1.86) | 0.1 (14.9%) | 0.90 (0.34–3.87) | 0.2 (29.8%) | 0.94 (0.66–2.01) | 0.3 (44.7%) | |
| Lactate (mmol/L) | Sepsis | 0.4 (0.3–0.7) | 0.5 (0.3–1.0) | 0 (0%) | 0.8 (0.5–1.2) | 0.2 (50%) | 1.4 (0.7–1.9) | 0.7 (175%) | 1.4 (1.0–2.6) | 1 (250%) |
| No-sepsis | 0.5 (0.4–1.1) | 0.6 (0.4–1.2) | 0 (0%) | 0.7 (0.5–1.3) | 0.1 (20%) | 0.7 (0.4–1.2) | 0.1 (20%) | 0.8 (0.5–1.4) | 0.2 (40%) | |
Platelet count, SOFA score, TLC in mean ± S.D; IG#, IG%, procalcitonin, and lactate in median (interquartile range). Difference is calculated by subtracting the mean/median baseline value from the particular time period mean/median value. Percentage change (% change) denotes the differences of mean/median value as a per cent change from the baseline mean/median value; (−) sign denotes the drop in mean/median values from the baseline mean/median values
Fig. 1.
Comparison of serial median values of various biomarkers in sepsis and no-sepsis group
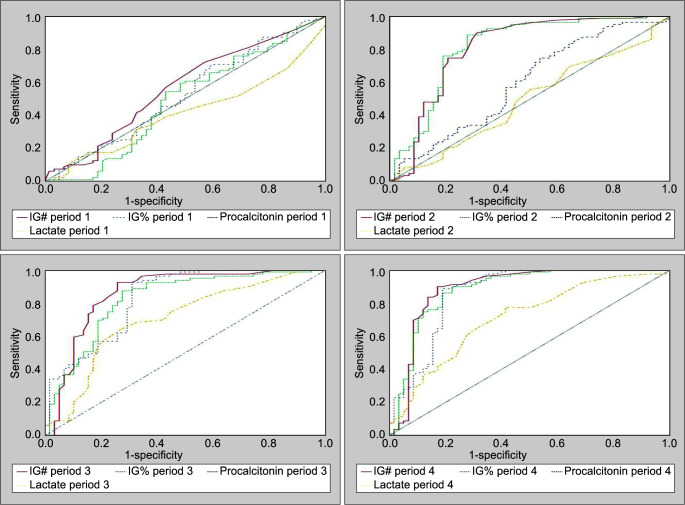
The ROC curve and AUC for all biomarkers across all time periods are tabulated in Table 4 and Figure 2. In Period 1, the ROC curve reveals that none of the four biomarkers under study, that is, IG#, IG%, PCT, and Lac, have any significant predictive value for sepsis. In Period 2, IG# and IG% have a significant predictive value with AUC reaching up to 0.809 for IG# (p <0.001) and 0.818 for IG% (p <0.001), while PCT and Lac were not significantly predictive (p >0.05) in this time period. In Period 3, all the biomarkers under study were significantly predictive for sepsis with highest AUC for IG# (0.859, p <0.001) and PCT (0.828, p <0.001). Again in Period 4, all the biomarkers under study were significantly predictive for sepsis (p <0.001). Figure 3 compares AUC of all biomarkers in all the time periods.
Table 4.
ROC analysis and AUC for various diagnostic tests across time period
| Period 1 | Period 2 | Period 3 | Period 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | AUC | Sig | AUC | Sig | AUC | Sig | AUC | Sig |
| IG# | 0.565 (0.466–0.664) | 0.195 | 0.809 (0.727–0.892) | 0.000 (S) | 0.859 (0.788–0.931) | 0.000 (S) | 0.887 (0.820–0.954) | 0.000 (S) |
| IG% | 0.490 (0.388–0.592) | 0.838 | 0.818 (0.739–0.896) | 0.000 (S) | 0.823 (0.749–0.897) | 0.000 (S) | 0.882 (0.818–0.945) | 0.000 (S) |
| Procalcitonin | 0.525 (0.425–0.624) | 0.619 | 0.597 (0.498–0.695) | 0.054 | 0.828 (0.756–0.900) | 0.000 (S) | 0.864 (0.794–0.933) | 0.000 (S) |
| Lactate | 0.416 (0.321–0.511) | 0.093 | 0.493 (0.395–0.592) | 0.896 | 0.704 (0.614–0.794) | 0.000 (S) | 0.709 (0.621–0.797) | 0.000 (S) |
(S) denotes significant (p <0.001) p values
Fig. 2.
ROC curves for the biomarkers and their respective performances across various time periods
Fig. 3.
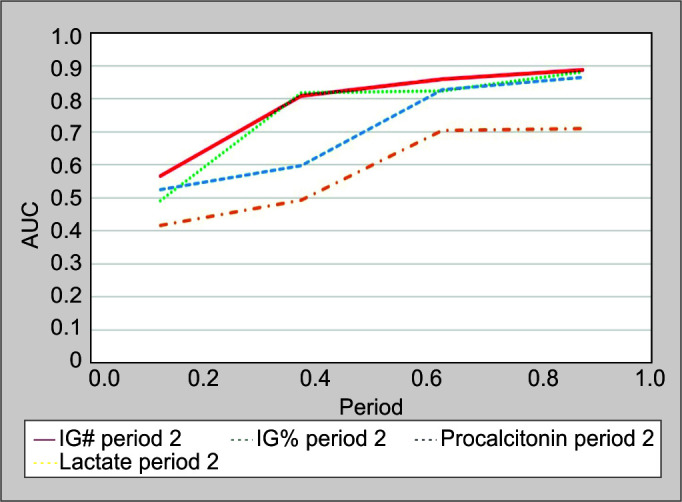
AUC for biomarkers across time periods. Predictive value of biomarkers in the study were computed using ROC curve and AUC
The diagnostic power of every biomarker was calculated against each time period, which is shown in Table 5. Among all the biomarkers across all time periods, best DOR is seen for IG# (42.6) in Period 4, followed by IG# (34.9) in Period 3. In Time period 2, IG% and IG# had the best DOR of 20.42 and 19.72, respectively. Thus, we conclude that IG% and IG# are the first biomarker to have a significant diagnostic value in patients with sepsis. Youden's index gave the best cutoff values for each biomarker. Based on the findings of this study, the cutoff values for diagnosis of sepsis should be as follows: IG#-0.12 thousand/µL, IG%-1.22%, PCT-1.78 ng/mL, and Lac-1.1 mmol/L.
Table 5.
Sensitivity, specificity, Youden's index, and DOR
| Biomarkers | Sensitivity | Specificity | Youden's index | Best criteria | DOR | |
|---|---|---|---|---|---|---|
| Period 1 | IG# | 72.15 | 43.10 | 0.15 | 0.03 × 103/µL | 1.96 |
| IG% | 97.47 | 18.97 | 0.16 | 2.03% | 9.02 | |
| Procalcitonin | 69.62 | 41.38 | 0.11 | 0.42 ng/mL | 1.62 | |
| Lactate | 31.96 | 86.21 | 0.18 | 0.3 mmol/L | 2.94 | |
| Period 2 | IG# | 89.87 | 68.97 | 0.59 | 0.06 × 103/µL | 19.72 |
| IG% | 88.61 | 72.41 | 0.61 | 0.7% | 20.42 | |
| Procalcitonin | 74.68 | 46.55 | 0.21 | 0.77 ng/mL | 2.57 | |
| Lactate | 13.92 | 93.10 | 0.07 | 0.3 mmol/L | 2.18 | |
| Period 3 | IG# | 92.41 | 74.14 | 0.67 | 0.12 × 103/µL | 34.91 |
| IG% | 87.34 | 72.41 | 0.60 | 1.22% | 18.11 | |
| Procalcitonin | 92.41 | 68.97 | 0.61 | 1.78 ng/mL | 27.06 | |
| Lactate | 64.56 | 72.41 | 0.37 | 1.1 mmol/L | 4.78 | |
| Period 4 | IG# | 89.87 | 82.76 | 0.73 | 0.13 × 103/µL | 42.59 |
| IG% | 89.87 | 77.59 | 0.67 | 1.22% | 30.72 | |
| Procalcitonin | 88.61 | 81.03 | 0.69 | 2.22 ng/mL | 33.23 | |
| Lactate | 72.22 | 58.62 | 0.36 | 0.9 mmol/L | 3.68 |
Sensitivity, specificity, and best criteria or cutoff value are given for each biomarker in every time period. DOR compares the diagnostic power of biomarkers
DISCUSSION
Neutrophils are crucial components of the immune response, releasing important regulatory cytokines, chemokines, and leukotrienes.9 The granulocytic shift to left characterized by the presence of immature granulocytes in the peripheral blood reflects active bone marrow response to bacterial infection. Formally, these are classified on the basis of cell morphology by the microscopical examination of blood films into promyelocytes, myelocytes, metamyelocytes, and band forms.10 But such measurements have been difficult to obtain. With advances in technology, automated hematology analyzers accurately identify and count immature granulocytes and are cheap and simple to use. White blood cells (WBCs) have been an integral part of the definition of Sepsis given by Bone et al.11 in 1992. The most recent definition of sepsis, the Sepsis-3 criteria,8 has excluded WBCs and neutrophils from its definition. Nonetheless, neutrophils in the form of left shift remain an important part of sepsis pathobiology and the body's response to infection, and are considered one of the earliest indicators of sepsis. Studies done by Buoro et al.,12 Bourne et al.,13 MacQueen et al.14, and others have validated immature granulocyte count by Sysmex as an indicator of the left shift and thus sepsis.
Studies done in the past on different study populations reveal IG# as a novel marker of sepsis. Nierhaus et al.6 suggested that IG# significantly differentiates septic from nonseptic patients with a DOR of 26.7 within the first 48 hours of sepsis, and IG# was more indicative of sepsis than other markers like CRP, lipopolysaccharide-binding protein (LBP), and IL-6. Ha et al.15 reported that IG% could differentiate between severe sepsis and uncomplicated sepsis with an odds ratio of 2.530, p = 0.004. Senthilnayagam et al.16 in their study population of febrile patients reported that IG# and IG% are diagnostic of sepsis with an AUC of 0.69 and 0.66, respectively. Van der Geest et al.17 suggested that IG% is a better predictor of sepsis with area under receiver operator characteristic (AUROC) of 0.73 to 0.78 when compared to CRP and WBC count. Recent study done by Ayres et al.18 also reports a high AUC of 0.75 for culture-positive sepsis. Most of the studies done so far have considered IG# or IG% values after the development of clinical signs or symptoms of inflammation or SIRS alert in the patients and have either differentiated sepsis from noninfectious SIRS or compared septic patients to healthy volunteers. Our study was a prospective study, where ICU patients without sepsis at the time of admission were followed for 7 days and were divided into sepsis and no-sepsis groups based on Sepsis-3 criteria. With the serial data for the biomarkers, we were able to calculate the earliest as well as the best biomarker to predict sepsis. This study suggests that IG# is the earliest biomarker which can predict sepsis, and its diagnostic value is better than other markers used currently.
Median IG# (0.36 vs 0.04 thousand/µL) and IG% (2.6 vs 0.6%) in Period 3 were significantly higher in the sepsis group compared to the no-sepsis group. Study done by Nierhaus et al.6 documented a mean IG# of 0.63 ± 1.04 thousand/µL in blood culture-positive patients and 0.42 ± 1.38 thousand/µL in blood culture-negative patients with colonization. An Indian study done by Senthilnayagam et al.16 reported a mean IG# (0.62 vs 0.17 thousand/µL, p <0.0001) and IG% (3.68 vs 1.46%) in culture-positive sepsis patients compared to culture-negative patients. The higher means of IG# and IG% in these studies compared to our observations were probably because only culture-positive patients were considered as having sepsis in these studies while we included all the patients fitting the Sepsis-3 criteria in the sepsis group. Another reason for the difference in observations could be that the mean values were compared in these studies while we calculated the median values as our data for biomarkers were not normally distributed. A study with similar findings as ours was done by van der Geest et al.17, where median IG% was found to be 1.8% in infected blood culture-positive patients and 0.3% in the no-infection group. Buoro et al.19 documented median IG# of 0.23 thousand/µL in sepsis and 0.14 thousand/µL in nonseptic patients.
To know the accuracy and validity of biomarkers, a ROC and AUC analysis was done. In our study, none of the biomarkers considered had a significant diagnostic AUC in Period 1. In Period 2 of the study, IG# and IG% had significant AUROC of 0.809 (p <0.001) and 0.818 (p <0.001), thus confirming our previous findings that in Period 2 raised IG# and IG% can predict sepsis. In Period 3, AUROC of IG#, IG%, and PCT was 0.859, 0.823, and 0.828, respectively, implying that the three biomarkers are almost equally predictive of sepsis in this time period. Our observations are similar to study by Nierhaus et al.6, which reported AUROC of IG# to be 0.861, which was the best among other markers in their study like CRP, LBP, and IL-6. Similar to their study, diagnostic AUROC for Lac was less than 0.7 in our study. Senthilnayagam et al.16 had similar AUROC findings for the IG# and IG% (0.73 and 0.71) in adult sepsis patients. In our study, Lac consistently showed poor diagnostic performance at all time periods (AUROC between 0.55 and 0.71).
Performance characteristics of different biomarkers were calculated using sensitivity, specificity, DOR, and Youden's index, shown in Table 5. IG# and IG% both had a high DOR of 19.72 and 20.42, respectively, in Period 2. IG# had a high DOR of 34.91 and 42.59 in Period 3 and Period 4, respectively. Among the other biomarkers, PCT performed well as a diagnostic test in Period 3 with DOR of 27.06. In our study, the best cutoff value of IG# and IG% to predict sepsis in Time period 2 was 0.06 thousand/µL and 0.7% with a sensitivity of 89.9 and 88.6%, respectively, and a specificity of 69.0 and 72.4%, respectively, while the best cutoff value for IG# and IG% in Time period 3 was 0.12 thousand/µL and 1.22%, respectively, with a sensitivity of 92.4 and 87.3%, respectively, and a specificity of 74.1 and 72.4%, respectively. The best cutoff value for PCT was 1.78 ng/mL in Time period 3. Study by Roehrl et al.20 recommends 0.07 thousand/µL and 0.9% for IG# and IG%, respectively, to predict infection. A study by Ansari-Lari et al.21 reported that the elevation of IG% to more than 2.0–3.0% is specific for the detection of sepsis. Ha et al.15 gives an optimal cutoff value for diagnosis of sepsis to be 0.5%, which is close to our study's cutoff value of 0.7%.
There were several limitations to this study. First, there is no gold standard for sepsis so far, and we used Sepsis-3 criteria and supportive investigation to diagnose sepsis. Second, only patients admitted in medical ICUs were enrolled in the study excluding patients in surgical and trauma ICUs. Other confounders like comorbidities and prior hospitalizations/sepsis, which could affect the development of sepsis and change in biomarkers, were not accounted for. Underlying undiagnosed conditions in these patients could also affect the biomarker levels. Finally, a small sample was analyzed, and all patients were recruited from a single center. Our findings should be validated in a multicenter study recruiting a larger sample before the suggested biomarker is used on a larger scale.
CONTRIBUTION
(I) Conception and design: P Bhansaly, S Mehta2 (II) Provision of study materials or patients: all authors; (III) Collection and assembly of data: all authors; (IV) Data analysis and interpretation: P Bhansaly, E Gupta (V) Manuscript writing: all authors; (VI) Final approval of manuscript: all authors.
HIGHLIGHTS
ICU patients are empirically treated with broad-spectrum antibiotics, which compounds the problem of antibiotic resistance and multidrug-resistant bugs. Predicting sepsis accurately can decrease overall antibiotic use.
IG# and IG% are a readily available, easy-to-use, cheap, and rapid alternative to predict sepsis early. Predicting sepsis early could have many implications in management protocols and could decrease the overall high mortality in ICU patients.
Since biomarkers like serum PCT and blood lactate levels are costly tests, their use is limited by the clinicians and is only ordered when there is a high suspicion of sepsis. IG% and IG# can be obtained at no-added cost, and monitoring the serial levels can predict sepsis early, allowing the caregivers to initiate treatment early, optimize antibiotic use, and reduce overall mortality.
Footnotes
Source of support: Nil
Conflict of interest: None
ORCID
Prabhav Bhansaly https://orcid.org/0000-0002-1164-5432
Sudhir Mehta https://orcid.org/0000-0003-2506-3174
Nidhi Sharma https://orcid.org/0000-0002-2282-3185
Esha Gupta https://orcid.org/0000-0003-3691-3752
Shourya Mehta https://orcid.org/0000-0002-5609-8719
Sweta Gupta https://orcid.org/0000-0001-9451-3162
REFERENCES
- 1.Todi S, Chatterjee S, Sahu S, Bhattacharyya M. Epidemiology of severe sepsis in India: an update. Crit Care. 2010;14(Suppl. 1):382. doi: 10.1186/cc8614. [DOI] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the soap study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Meer W, Van Gelder W, De Keijzer R, Willems H. Does the band cell survive the 21st century? Eur J Haematol. 2006;76(3):251–254. doi: 10.1111/j.1600-0609.2005.00597.x. [DOI] [PubMed] [Google Scholar]
- 6.Nierhaus A, Klatte S, Linssen J, Eismann NM, Wichmann D, Hedke J, et al. Revisiting the white blood cell count: immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis-a prospective, observational study. BMC Immunol. 2013;14:8. doi: 10.1186/1471-2172-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, et al. Intensive care in India: The Indian intensive care case mix and practice patterns study. Indian J Crit Care Med. 2016;20(4):216–225. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Journal of the American Medical Association. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25(3):321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 10.Shen BJ, Ekert H, Tauro GP, Balderas A. Left shift in the peripheral blood count at diagnosis in acute lymphocytic leukemia is significantly correlated with duration of complete remission. Blood. 1984;63(1):216–218. doi: 10.1182/blood.V63.1.216.216. [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, Knaus WA, Schein RMH, Sibbald WJ, et al. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 12.Buoro S, Mecca T, Vavassori M, Azza G, Espos SA, Dominoni P, et al. Immature granulocyte count on the new Sysmex XN-9000: performance and diagnosis of sepsis in the intensive care unit. Signa Vitae. 2015;10(2):54–64. doi: 10.22514/SV102.122015.4. [DOI] [Google Scholar]
- 13.Bourne S, Ma N, Gulati G, Gong J. Evaluation of automated versus manual immature granulocyte counts. Lab Med. 2013;44(3):282–287. doi: 10.1309/LMF0OBUBPMWPOKS1. [DOI] [Google Scholar]
- 14.MacQueen BC, Christensen RD, Yoder BA, Henry E, Baer VL, Bennett ST, et al. Comparing automated vs manual leukocyte differential counts for quantifying the ‘left shift’ in the blood of neonates. J Perinatol. 2016;36(10):843–848. doi: 10.1038/jp.2016.92. [DOI] [PubMed] [Google Scholar]
- 15.Ha SO, Park SH, Park SH, Park JS, Huh JW, Lim CM, et al. Fraction of immature granulocytes reflects severity but not mortality in sepsis. Scand J Clin Lab Invest. 2015;75(1):36–43. doi: 10.3109/00365513.2014.965736. [DOI] [PubMed] [Google Scholar]
- 16.Senthilnayagam B, Kumar T, Sukumaran J, Jeya M. Automated measurement of immature granulocytes. Performance characteristics and utility in routine clinical practice. Pathol Res Int. 2012;2012:483670. doi: 10.1155/2012/483670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Geest PJ, Mohseni M, Brouwer R, van der Hoven B, Steyerberg EW, Groeneveld AB. Immature granulocytes predict microbial infection and its adverse sequelae in the intensive care unit. J Crit Care. 2014;29(4):523–527. doi: 10.1016/j.jcrc.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Ayres LS, Sgnaolin V, Munhoz TP. Immature granulocytes index as early marker of sepsis. Int J Lab Hematol. 2019;41(3):392–396. doi: 10.1111/ijlh.12990. [DOI] [PubMed] [Google Scholar]
- 19.Buoro S, Mecca T, Azzarà G, Apassiti Esposito S, Seghezzi M, Vavassori M, et al. Extended leukocyte differential count and C-reactive protein in septic patients with liver impairment: diagnostic approach to evaluate sepsis in intensive care unit. Ann Transl Med. 2015;3(17):244. doi: 10.3978/j.issn.2305-5839.2015.09.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehrl MHA, Lantz D, Sylvester C, Wang JY. Age dependent reference ranges for automated assessment of immature granulocytes and clinical significance in an outpatient setting. Arch Pathol Lab Med. 2011;135(4):471–477. doi: 10.1043/2010-0258-OA.1. [DOI] [PubMed] [Google Scholar]
- 21.Ansari-Lari MA, Kickler TS, Borowitz MJ. Immature granulocyte measurement using the Sysmex XE-2100 – relationship to infection and sepsis. Am J Clin Pathol. 2003;120(5):795–799. doi: 10.1309/LT30BV9UJJV9CFHQ. [DOI] [PubMed] [Google Scholar]