Introduction
Posttraumatic stress disorder (PTSD) is a psychiatric disorder or mental illness that can develop upon exposure to or witnessing of a traumatic event. PTSD was first officially recognized in the Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.(DSM III) in 1980 as an anxiety disorder (American Psychiatric Association, 1980). Since then, a wealth of knowledge has assembled regarding symptoms, epidemiology, assessment, subtypes, and treatment of this disorder. This has led to the transition in the fifth edition of the DSM (DSM-5), of PTSD to a trauma- and stressor-related disorder (American Psychiatric Association, 2013). The revised criteria reflect four symptom clusters of intrusive reexperiencing (i.e., flashbacks, unwanted memories), avoidance (i.e., avoiding reminders of the trauma), negative alterations in cognition and mood (i.e., thoughts of self-blame, difficulty experiencing positive emotion) and arousal (i.e., hyperarousal, difficulty sleeping)(American Psychiatric Association, 2013). The individual must experience all of these symptom clusters for duration of at least one month following direct or indirect exposure to actual or threatened death, serious injury, or sexual violence and be associated with distress or impairment in one or more areas of functioning to meet diagnostic criteria for PTSD. For Veterans, deployment for an extended period of time itself is a stressor and, coupled with high rates of combat exposure that are likely to occur, is a high-risk factor for PTSD. This disorder is frequently observed with other psychiatric diagnoses and traumatic brain injury (TBI) along with other comorbid symptoms like depression, particularly in military populations (Jaffee and Meyer, 2009; Tanev et al., 2014; Moore et al., 2014). Underscoring a need for future study in military and Veteran populations, servicemembers from the most recent conflicts show a prevalence of PTSD 2.5x greater than the general population (Fulton et al., 2015) and are less likely to benefit from first line treatments (Straud et al., 2019).
PTSD prevalence rates have been reported to be 13.8% among Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) Veterans (Tanielian and Jaycox, 2008), 12.1% in Gulf War Veterans (Kang, 2003) and 15.2 % in male Vietnam War Veterans (Kulka et al 1990), which is greater than the 12-month prevalence in the US of 4.7% (Kilpatrick et al., 2013). Importantly, it is noted that the symptoms may not appear for several months to years after trauma exposure (Seal et al., 2008; O’Toole and Catts, 2017). Moreover, PTSD is associated with a host of other comorbidities including hypertension (Howard et al., 2018), cardiovascular disease (Dyball et al., 2019), cardiometabolic disease (Levine et al., 2014), suicidal thoughts, etc. (Millner et al., 2019) in addition to chronic pain (Toblin et al., 2014). Further elucidation of these comorbidities suggests poor long‐term outcomes through several interacting pathways, including alteration in mental health, sociodemographic adjustments, health behavior, etc. (Ramsey et al., 2017). Consequences of PTSD for Veterans include increased healthcare utilization, decreases in functioning and increased risk for suicide (Marshall et al., 2000; Asnaani et al., 2014; Lutwak, and Dill, 2017), underscoring a need to understand the mechanisms of PTSD and inform effective interventions. Numerous predictors of PTSD have been identified, with peritraumatic factors (i.e., dissociation, life stress) being stronger than pre-trauma factors (e.g., education, prior trauma; Ozer et al., 2003, Brewin et al., 2000). More recently, biological factors have been associated with PTSD onset and treatment response. As examples, the inflammatory protein C-Reactive protein has been shown to prospectively predict PTSD among recently deployed marines (Early et al., 2014), and cortisol levels in response to waking predicted PTSD treatment response (Rauch et al., 2020). Thus, investigation of novel biomarkers is warranted to stretch our knowledge in PTSD and provide novel insights into possible biological mechanisms.
Pathophysiology of PTSD
The pathophysiology of PTSD is complex as it covers vast functional aspects including noradrenergic, serotonergic, opioid, cannabinoid and hypothalamic-pituitary-adrenal (HPA) axis. PTSD is a neuropsychiatric condition derived from maladaptive alterations in neural plasticity including synaptic connection, dendritic remodeling, and neuronal growth which impacts neurocircuitry function and behaviors (Apfel et al., 2011). Functionally, the amygdala is the nodal point of fear regulation, and PTSD may evolve from hyperactivity of neurons in impaired amygdala (Helmuth L, 2003). In this review, we will briefly touch base of each aspect of pathophysiology in PTSD.
The adrenoreceptor (AR) system is important in PTSD as it influences amygdala functioning (Strawn and Geracioti., 2008). The AR system primarily activates CNS activity and simulates sympathetic autonomic response in prefrontal cortex (PFC) and limbic systems resulting in the fear response (O’Donnell et al., 2004). Similarly, serotonergic receptors like 5-HT1A, 5-HT1B, 5-HT3, a group of G-protein-coupled receptors, are engaged in emotional and behavioral modulation (Cools et al., 2008). The opioid receptors (δ, κ an μ), a superfamily of G protein-coupled receptors, are implicated in etiology of PTSD (Dhawan et al., 1996). The κ-opioid receptor is particularly relevant to PTSD due to its expression in the PFC and cortex- hippocampal-limbic regions as well as associations with fear or anxiety related behavior (Bruchas et al., 2009). The endogenous cannabinoid receptors (CB1 and CB2) play a pivotal role in development of PTSD (Sbarski B., Akirav L., 2020). Among the two receptors, CB1 is well-studied at preclinical level and is of interest in PTSD. as it is distributed throughout the forebrain limbic structure (Leo and Abood, 2021) and modulates various behavioral issues including fear. Physiologically, PTSD affects multiple systems, including the HPA-axis, cortical function, and the immune system (Parsons and Ressler, 2013; MacNamara et al., 2016). A recent genome-wide association study of PTSD underscored the likelihood of genetic risk with schizophrenia, depressive-disorder, or bipolar depression (Duncan et al., 2018). It is suggested that three specific regions of the brain - amygdala, hippocampus, and PFC - are linked to fear memory or responsiveness in preclinical models and play a major role in the development of PTSD symptomatology ( Haubensak et al.,2010; Dieter, Engel, 2019; Andrewes et al., 2019; Henigsberg et al., 2019). The brain consists of several interrelated neural systems that activate and deactivate in response to different stimuli in a closely regulated way. Imaging studies showed patterns of dysregulation of both hippocampus and the medial PFC in patients with PTSD (Andrewes et al., 2019; Henigsberg et al., 2019; Bremner, 2007). Therefore, the neurobiology of PTSD is a complex process as exposure to traumatic events change neuronal morphology, function and neurochemistry (Cacciaglia et al.; Weiss, 2007).
In pursuit of biomarkers and a deeper understanding of PTSD pathophysiology, the role of micro ribonucleic acid (miRNAs), a class of non-coding RNAs, are emerging in psychiatric and neurological disorders, including schizophrenia, PTSD, anxiety, and major depressive disorder (Bartel, 2004). Deregulation of miRNA can impact the expression of multiple genes and their associated biological networks. Therefore, if it is supported that this is a novel molecular mechanism underling the pathogenesis of PTSD, the study of miRNA opens up a new area of investigation for novel therapeutic targets in PTSD. Therefore, investigating the role of miRNAs in the pathophysiology of PTSD using blood may provide a quick and an easy novel insight correlating the presence of disease. In this review article, we will describe miRNA biogenesis, miRNAs signature in Veterans with PTSD, HPA axis-FKBP5-miRNA-PTSD, epigenetic modification in PTSD, and conclude with future directions for study of miRNAs in PTSD.
miRNA Biogenesis
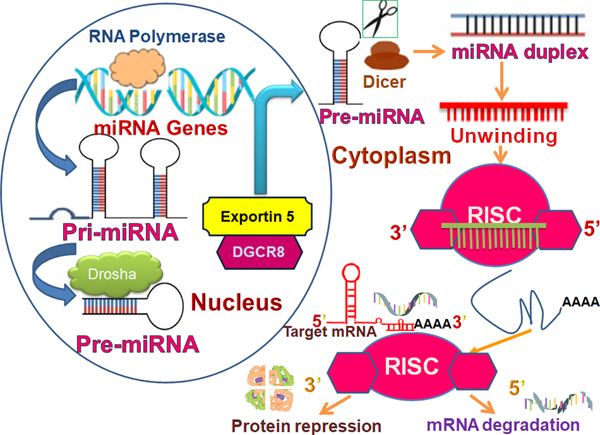
Biogenesis of miRNA is an endogenous cellular process to generate mature and functional miRNA destined to target a specific messenger RNA (mRNA) for modulation. The miRNAs are a class of short noncoding regulatory RNAs, 21 to 23 nucleotides in length, that negatively regulate gene transcription through binding to the 3′ untranslated region (UTR) of target mRNAs (Michlewski and Caceres, 2019; Alural B, Genc, 2017). The miRNA biogenesis pathway generates hundreds of unique miRNAs in mammalian cells. Each miRNA is capable of targeting hundreds of genes, thus simultaneously controlling multiple biological processes. It is thought that miRNAs regulate up to 60% of the protein‐coding genome (Ruegger and Grosshans, 2012; Gregory et al., 2004). The biogenesis of miRNA starts in the nucleus. The miRNA is transcribed as a precursor molecule called primary transcript (pri-miRNA) by RNA polymerase III which turns it into a hairpin-like structure (Han et al, 2006). The nuclear event is catalyzed by a microprocessor complex that include a RNase III enzyme, Drosha, cofactors such as DGCR8 (DiGeorge syndrome critical region 8 gene) and associated proteins (Han et al, 2006; Ha M, Kim; Bohnsack et al., 2004). The microprocessor complex cleaves the pri‐miRNA into a 70‐nucleotide‐stem-loop precursor miRNA (pre‐miRNA), which is subsequently exported to the cytoplasm by exportin‐5 (Bohnsack et al., 2004; Hutvágner et al., 2001). Once in the cytoplasm, pre-miRNAs undergo a final processing by another RNase type III enzyme, DICER, to give rise to miRNA duplexes (Kobayashi and Tomari, 2006; Kwak and Tomari, 2012). Next, with another RNA binding protein like Argonaute 2(Ago2), the pre-miRNA is incorporated into the miRNA‐induced silencing complex (miRISC), while the “passenger” strand is degraded with the formation of a mature, single-stranded ~21-nt-long miRNA (Han et al., 2006; (Kobayashi and Tomari, 2006; Michopoulos et al., 2017). The strand recognition event is followed where the guide strand is recognized by “seed” sequence in the mature miRNA. The guide strand binds target gene and initiate mRNA degradation and translational repression. The mRNA degradation can be achieved by many mechanisms like binding to the 3′UTRs or the open reading frames (ORFs) of target genes leading to the degradation of target mRNAs or repression of mRNA translation (Fig. 1).
Figure 1.
Schematic of miRNA biogenesis. Pri-miRNA, Primary miRNA; Pre-miRNA, Precursor miRNA; RISC, RNA-induced silencing complex; AGO, Argonaute protein; DGCR8, DiGeorge syndrome critical region 8
miRNAs Signature in PTSD among Veterans
Since the discovery of miRNA, it has been established that the tiny molecule is highly conserved and suggested to be a key regulator in diverse physiological processes, including functioning of the nervous system. A large body of evidence elucidate the critical role of miRNA in psychiatric diseases (Issler and Chen, 2015), however, evaluation of the specific role of miRNA in Veterans with PTSD has more recently started emerging. On July 19 of 2021, a literature search using key words; miRNA, posttraumatic stress disorder, veterans (https://pubmed.ncbi.nlm.nih.gov/?term=mirna+posttraumatic+stress+disorder+veterans&sort=date&size=50) resulted in only 13 articles relevant to miRNA. Of these, 7 studies were original data articles examining miRNA in a sample of Veterans with PTSD. Currently, with the limited amount of scientific information available, we will illuminate the recent progress in miRNA dysregulation in Veterans with PTSD.
The first non-coding RNA snapshot was revealed in the peripheral blood mononuclear cells (PBMC) of OIF and OEF Veterans with and without mild traumatic brain injury (mTBI), approximately two thirds of whom also screened positive for PTSD (Pasinetti et al., 2012). Using Affymetrix Human gene 1.0 ST Array chip, authors have identified thirteen downregulated candidate small RNA biomarkers along with one miRNA, the miR-671–5p, in PBMCs of mTBI subjects (Pasinetti et al., 2012). Unsupervised clustering analysis further narrowed down to three small nucleolar biomarker panel; HBII-289, ENSG199411 and U35A which accurately selected mTBI from non-mTBI Veterans (Pasinetti et al., 2012). However, as both groups included Veterans with PTSD, no firm conclusions can be drawn about miRNA and PTSD. Presumably, the first miRNA landscape in combat Veterans with clinically diagnosed PTSD was reported in 2014 (Zhou et al., 2014). The study used a sample of combat Veterans returning from Persian Gulf, Iraq, or Afghanistan war who also had PTSD. Previous research has suggested that immune components contributed a pivotal role in PTSD (Kawamura et al., 2001; Jiang, 2008; Breen et al., 2015; Jones KA, Thomsen C., 2013); therefore, this study was aimed to determine the role of miRNA in immune dysfunction linked with PTSD. Using high-throughput miRNA microarray hybridization analysis, the study investigated 1163 miRNAs in PBMC. Compared to control subjects, the PTSD group showed 7 upregulated miRNAs and 64 down-regulated miRNA (Zhou et al., 2014). The finding suggested significant alterations in miRNA expression corroborating with immunological changes, specifically enhanced pro-inflammatory Th1 and Th17cytokine profile and decreased the regulatory T cells (Tregs) (Zhou et al., 2014). Together, the analysis showed that there was significant association between alterations in miRNA expression and immunological changes in combat Veterans with PTSD. Further, using the same Veteran cohorts, RNA-Seq on RNA samples from PBMCs of PTSD was performed by the same group. The study revealed 326 mRNA and 40 non-coding RNAs which were significantly altered in samples of those with PTSD compared to controls (Bam et al., 2016). Furthermore, a panel of downregulated miRNAs were identified associated with DNA methylation and immune deregulation (Bam et al., 2016). The study is interesting as the authors showed evidence of association between miRNA and DNA methylation and suggested that they play a critical role in immune system modulation in PTSD. Epigenetic changes in DNA methylation is an emerging concept and is associated with PTSD (Hammamieh et al., 2017). The authors have used extensive bioinformatic tools to dissect molecular signaling or pathways involved in PTSD pathology. Although promising, these findings need to be validated experimentally and in other cohorts.
Elevated level of pro-inflammatory cytokines has been observed in war Veterans with PTSD. suggesting a link between PTSD and inflammation (Gill et al., 2009). To determine the association between miRNA-mediated inflammatory response in PTSD, Bam M et al used PBMC samples from War Veterans of either 1991 Persian Gulf war, or Iraq or Afghanistan wars with PTSD, and age matched healthy controls. The authors showed that 183 miRNAs were downregulated that target several inflammatory genes in a first set of 4 control and 5 Veterans with PTSD (Bam et al., 2017). The observation was validated in an independent sample of 7 controls and 3 Veterans with PTSD by RT-PCR analyses and showed that JAK2, STAT1, IL23A, TGFB1, TGFB2, TGFB3, T-BET and CXCL3 were the predicted target for downregulated miRNAs. Furthermore, using healthy and PTSD patients’ PBMCs, authors confirmed PTSD patients elicits more CD4+T cells that contribute to lowering miRNA expression (Bam et al., 2017). Mechanistically, authors demonstrated that inflammation in PTSD is partly due to the reduction of Argonaute 2 (AGO2) and Dicer1 (DCR1) elicits lowering the miRNA abundance and attenuation of STAT3 transcript (Bam et al., 2017). The findings suggest that inflammation in PTSD could be the result of alteration in miRNA biogenesis components (AGO2 and DCR1) and depletion of Stat3 mRNA. An extensive study is warranted for targeting miRNA biosynthesis component(s), which may in turn inform therapeutic management in PTSD and inflammation.
Another study used peripheral blood samples of 24 returned military personnel from OEF/OIF conflicts with and without PTSD, to test for miRNA alteration (Martin et al., 2017). The miRNA sequencing analysis showed four upregulated miRNAs (miR-19a-3p, miR-101–3p, miR-20a-5p, and miR-20b-5p) and four downregulated miRNAs (miR-15b-3p, miR-125b-5p, miR-128–3p and miR-486–3p) expression in PTSD samples compared to those without PTSD (Martin et al., 2017). Furthermore, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis predicted that these miRNAs are associated with axonal guidance and Wnt signaling in addition other physiological pathways at functional standpoint (Martin et al., 2017). The Wnt or Wnt/β-catenin signaling, a highly conserved biological pathway involved in cell development, proliferation and fate and recently implicated to synaptic plasticity (Moon et al., 2004; Murase, Mosser and Schuman, 2002; Maguschak and Ressler, 2011; Maguschak and Ressler, 2012). Alteration of miRNA and Wnt signaling may provide a link to neurological process in the development of PTSD, but more studies are required to validate the findings.
An association between miRNA and Wnt signaling was further demonstrated in the PBMCs of Gulf war Veterans with PTSD. The authors demonstrated using RNA-seq and miRNA array analysis that an Wnt signaling component, the Wnt10b was upregulated (Bam et al., 2020). Wnt10b, a glycoprotein of Wnt family is known to contribute in cancer development (Milovanovic et al., 2004; Kharaishvili et al., 2011; Benhaj, Akcali and Oztuk, 2006). In the study, the authors showed that the miR-7113–5p was upregulated in the PBMCs, a bonafide candidate for epigenetic modification and inflammation (Bam et al., 2020). Interestingly, Wnt10b was shown to be associated with enhancing the proinflammatory response in PBMCs. Mechanistically, miR-7113–3p was found to be a target for Wnt10b and was significantly downregulated indicated an epigenetic modification and likely contributing the inflammatory response in PTSD (Bam et al., 2020).
Cell-free miRNAs circulating in the bloodstream have been found to be enclosed into extracellular vesicle (EV), called exosome (Raposo and Stoorvogel, 2013). Exosomes are emerging as a new communicating cellular vehicle in diverse biological processes including neuroinflammation and TBI (Raposo and Stoorvogel, 2013; Brites and Fernande, 2015; Andjus et al., 2020; Harrell et al., 2021; Guedes et al., 2020). Critically, exosomes transport miRNA. There is only one study that showed alteration of miRNA in the exosome of combat Veterans in EV and EV-depleted (EVD) plasma separately. Study showed that pattern of miRNAs was different between EV and EVD plasma among male OEF/OIF combat Veterans with and without PTSD (n = 12 each group) (Lee et al., 2019). Interestingly, the report showed a concentration dependent alteration of miRNA in PTSD group (Lee et al., 2019). The concentration changes of two miRNAs from EV (miR-203a-3p) and EVD plasma (miR-339–5p) were validated in an independent cohort of 20 Veterans (Lee et al., 2019). This may suggest EV as possible biomarkers to identify PTSD in Veterans. More studies are warranted to validate the finding in diverse cohorts. The observation may highlight the benefits of EV as a treatment module.
In summary, a growing literature has started to include miRNA in the study of PTSD among Veterans. The extant literature on miRNA and PTSD shows several miRNAs that are dysregulated in Veterans with PTSD compared to no PTSD control groups. The most common medium for sample utilization has been PMBCs, with other samples using serum or plasma. Though limited by small samples in many studies, data showed a spectrum of altered miRNA, consistent with system dysregulation seen in PTSD. Further, miRNA linked to immune function and inflammation were consistently associated with PTSD. However, the studies reviewed varied on several domains, including the use Veterans of different eras, inclusion of combat related and non-combat related PTSD as well as the use of control groups (i.e., some used trauma exposed Veterans while others used civilian healthy volunteers). These differences, as well as examination of different miRNAs limit conclusions that can be drawn. However, there are several directions for future research.
Future Perspectives of miRNAs in PTSD
In this review article, we have discussed and presented evidence that miRNAs hold a key role in PTSD among Veterans. There is a prospect in future of using miRNAs as circulatory biomarker in detection of PTSD. Below we discuss directions for future research, particularly as it relates to epigenetic modification and the HPA axis, as well as treatment implications.
Epigenetic modification in PTSD
Recent research has indicated an epigenetic modification in the central nervous system that may influence alterations in neurological diseases (Provencal and Binder, 2015). The term ‘epigenetics’ signifies chemical modifications to the chromatin structure that alter gene transcription while the DNA sequence remains un-altered. The alteration includes DNA methylation, DNA hydroxy-methylation, histone modifications and the processes are designated as methylation, acetylation, and phosphorylation, respectively. Other epigenetic modulators are non-coding RNAs like miRNAs which act as translational repressors (Auger and Auger, 2013; Bam et., 2016, Roth, 2014; Martin et al., 2018). The war Veterans are no exception in this epigenetic modification. There were reports that DNA methylation contributed significant role in the pathophysiology of PTSD as the process is essentially connected with gene regulation (Mehta et al., 2017; Uddin M et al., 2010; Rusiecki et al., 2012). Using a sample of OEF/OIF Veterans, Rusiecki J et al (Rusiecki et al., 2012) showed two repetitive elements, the long-interspersed nucleotide element 1 (LINE-1) and the interspersed Alu were hypomethylated in post-deployment situation. The authors suggested their findings as highlighting potential resilience or vulnerability factors (Rusiecki et al., 2012). The study reviewed above using Gulf War Veterans showed a link between Wnt signaling pathway and miR-7113–5p in PBMCs of PTSD subjects, which suggests both miRNA dysregulation and histone modifications (Bam et al., 2020). The miRNA modulation in epigenetic modification is intriguing as miRNA can regulate a set of gene expression at post-transcriptional level.
miRNA-FKBP5-HPA axis-PTSD
A dysfunctional HPA-axis is a hallmark in PTSD (Speer et al., 2019). Further, recent research has posited that unique aspects of Veterans’ history, their deployment characteristics and their readjustment to civilian life may uniquely impact HPA axis functioning in ways that make them more vulnerable to maladaptive coping, such as alcohol use (Szabo Y et al., 2020). The HPA-axis is designed to respond to stress. Physiological stimuli and stress activate it, ultimately leading to the release of cortisol from the adrenal cortex (Olff et al., 2006; Yehuda, 2009; Carrasco and Van de Kar, 2003). Essentially, corticotropin‐releasing factor is released from the hypothalamus and stimulates the synthesis and release of ACTH from the pituitary. The ACTH binds to receptors in the adrenal cortex and promotes the release of glucocorticoids (GCs) from the adrenal cortex (de Kloet, 2003). The circulating GCs, including cortisol, are counter regulated by negative feedback mechanism within the HPA and is critical for stress response maintenance. GCs mediate their effect through glucocorticoid receptor (GR) and mineralocorticoid receptors (MR) (Castro-Vale, 2016). FK506-binding protein 5 (FKBP5), a co-chaperon, is a critical modulator in GR signaling and has been implicated in the development of PTSD (Fries, Gassen and Rein, 2017; Hawn et al., 2019). Previous research has shown an association between FKBP5 and the development of PTSD including studies of Veterans (Binder et al., 2008; Fani et al., 2016; Young et al., 2018). As miRNA emerges as a circulating biomarker in PTSD, it would be interesting to assess the role of miRNA in the regulation of FKBP5. Using both Fkbp5 knock-out (KO) mouse model and human PTSD subjects, a panel of miRNA was identified which correlated with serum marker and miRNA expression in the pathology of PTSD at molecular and behavioral levels (Kang et al., 2020). The candidate miRNA derived from mouse study was validated with human subjects with PTSD and showed exosomal FKBP-linked miRNA in the blood as a possible biomarker. The study further determined the neuronal correlate with serum biomarker depicting HPA-axis and miRNA expression, with a composite score of miRNA expression positively correlated with higher prefrontal/limbic cerebral blood flow and a higher grey matter volume ratio within the PTSD group (Kang et al., 2020). This is the first study to show a panel of differential miRNAs profiling in Fkbp5 KO mice, a critical modulator in HPA-axis. Furthermore, a follow-up study by the same group conducted RNA sequence analysis using WT (wild type) and Fkbp5 KO mice with restrain stress, a form of physical and mental stress that is induced by placing the mice in a plastic tube in order to block their movements, to determine the specific miRNA affected in medial prefrontal cortex (mPFC). The study showed that 41 miRNAs were dysregulated, of which, 23 miRNAs were reduced and, 18 miRNAs were increased. Among upregulated miRNA, miR-690 showed significantly high level of expression and was chosen for further characterization (Park et al., 2020). Using green fluorescent protein (GFP)-tagged recombinant adeno-associated virus (rAAV) and viral construct containing miR-690 (rAAV-GFP-miR-690) into the pre-limbic cortices of the mPFC of mice, the authors showed in restrain stress mouse model that overexpression of miR-690 revealed higher sucrose preference and lower immobility time compared to stressed mice (Park et al., 2020). This finding may provide novel insights into the epigenetic regulation of stress-associated biological functions like PTSD. Further research in human models and specifically Veterans are needed to verify these findings. However, identification of miRNA in FKBP5 modulation in PTSD may uncover new mechanism of PTSD development and offer possible therapeutic target.
Other directions for future research
One potential avenue for future research is focusing on enzymes that play a role in generating miRNA. One study showed offering promising results is one that focused on Dicer1, an enzyme that generates mature miRNAs, which regulate gene expression. In this study, levels of Dicer1 were associated with increased amygdala activation to fearful stimuli, a neural correlate for PTSD among civilians (Wingo et al., 2015). The finding specifically demonstrated that miR-3130–5p was significantly reduced in PTSD with depression subjects compared to controls with a history of trauma but no PTSD or depression, indicating that DICER1 and miR-3130–5p impart a critical role in the pathogenesis of PTSD (Wingo et al., 2015). This is the first human study showed DICER1 and miRNA modulation in underpinning the PTSD comorbid with depression. Given that PTSD and depression are highly comorbid in Veterans (Ikin et al., 2010) and the prevalence of both disorders is associated with greater psychological burden than PTSD alone (Nichter et al., 2019), investigation into their comorbidity may provide important information for etiology of these disorders and offer new avenues for treatment.
The studies included in this review used syndromal PTSD versus no PTSD controls. Within the control groups, they ranged from Veterans with combat exposure to healthy civilian volunteers. Future research using trauma-exposed Veterans will help inform the specificity of PTSD for changes in miRNA regulation. Finally, individuals can have significant symptoms, without meeting criteria for the PTSD syndrome. Future studies are needed to understand how the presence of symptoms compared to the severity of symptoms associated with the alteration of miRNAs. Some preliminary research with a civilian sample has found associations between ratios of miRNA expression and PTSD symptom severity (Kang et al., 2020).
Treatment Implications
At present, it may be premature to offer miRNA as psychiatric therapeutic tool but, possibilities exist. Introducing targeted miRNA into the central nervous system would be challenging and several off-target effects would have dire side effects. However, current knowledge in bioinformatics has provided powerful information regarding precise targets among multiple predicted targets. Moreover, identification and validation of miRNA and its target gene(s) would further enrich our understanding of underlying molecular mechanism of PTSD. The novel RNA-based therapeutics can be developed by taking the advantage of CRISPR/CAS9 gene editing (Dominguez et al., 2016). Furthermore, the FDA approved selective serotonin reuptake inhibitors (SSRIs) currently used for PTSD treatment may be considered for miRNA modulation. Regarding miRNA involved in SSRI, mouse model of PTSD showed that fluoxetine is associated with a significant reduction in miR-1971 expression (Schmidt et al., 2013). However, the use of SSRIs to treat PTSD in Veterans has been mixed. PTSD participants treated with SSRI (antidepressants) showed modest protective effect against relapse relative to placebo subjects (Martenyi et al., 2002; Martenyi and Soldatenkova, 2006; Cavaljuga et al., 2003). However, other studies have shown fluoxetine was not superior to placebo in a study of combat Veterans (Hertzberg et al., 2000). Furthermore, a study conducted by Copeland L et al showed there some SSRIs are associated with increased risk of long QT syndrome, a disorder of the heart’s electrical system and, there was no significant risk using two SSRI drugs, citalopram and fluoxetine, in PTSD (Stock et al., 2018). Together, these suggest that fluoxetine may have less QT prolongation risk and, may cause change in miRNA. More research is needed to observe the dynamic of circulatory miRNAs in SSRI treated patients to understand factors associated with efficacy in Veterans, as it may that there is subset of Veterans who may benefit from this treatment most. Further, venlafaxine has showed increased remission rates compared to other SSRIs in a routine clinical care study of Veterans enrolled in VA care (Shiner et al., 2010). Thus, future research examining whether venlafaxine or other SSRI agents alter miRNA may inform better treatment efficacy in Veterans.
The authors are not aware of any research suggesting psychotherapy has been associated with changes in miRNA. However, other research in DNA methylation, another epigenetic modification, in psychotherapy treatment is emerging (Wolf et al., 2019). There is research showing that pre-treatment methylation has prognostic value in that it predicts outcomes (Yehuda et al., 2013). If targeting miRNA can impact methylation, then Veterans who previously didn’t respond to psychotherapy might become responders and, thus, leading to better treatment responses. Together, studies correlating miRNA alteration with clinical outcomes may contribute in the development of biomarkers and miRNA-based therapeutics in PTSD diagnosis and prognosis.
Conclusion
Veterans are suffering from many psychiatric disorders due to exposure of concussive brain injuries resulted in TBI and large quantities of psychological trauma exposure that result in high rates of PTSD. There is a great prospect of use of miRNA as biomarkers for the diagnosis, prognosis and therapeutic opportunities for Veterans with psychiatric disorders, including PTSD. The fact that differential expression of circulatory miRNA originated from neuronal dysfunction in brain tissue, miRNA have been associated with several disease processes pertaining to brain tissues advocating the potential use of miRNA as a next generation biomarker for the treatment of neuropsychiatric conditions. Circulating miRNAs in Veterans with PTSD can be used as independent biological indexes. The insights may offer gene networks related PTSD symptomatology, improve biological mechanisms of PTSD and provide pharmacological targets avenue. The implication and ramification of miRNA in psychiatric research is at budding stage compared to the more established study in cardiovascular or cancer fields; therefore, larger studies are warranted using Veterans with appropriate control cohort. The present review summarizes the small literature on miRNA in Veterans, considers directions for future research and proposes how this field of study can be used to improve the treatment of PTSD for Veterans. The outcome will help us to understand the deeper function and, novel insight into the mechanism of miRNA and, the target genes in the pathophysiology of PTSD in war Veterans. Finally, it may lead to the clinical application of miRNAs in PTSD diagnosis and prognosis.
Acknowledgement
This material is the result of work with resources and the use of facilities at the VISN 17 Center of Excellence for Research on Returning War Veterans and the Central Texas Veterans Health Care System. Dr. Szabo is supported by Career Development Award IK1-RX003122 from the United States (U.S.) Department of Veterans Affairs, Rehabilitation Research and Development Service. The authors acknowledge Richard W. Seim, Director of the VISN 17 Center of Excellence for Research on Returning War Veterans, Waco, Texas, for his support. The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Department of Veterans Affairs or the United States Government.
Footnotes
The authors declare no conflict of interest.
References
- 1.Diagnostic and statistical manual of mental disorders, 3rd ed.1980. Washington, DC: American Psychiatric Association [Google Scholar]
- 2.American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 5th ed.2013. American Psychiatric Association, Washington, DC [Google Scholar]
- 3.Jaffee MS, Meyer KS, 2009. A brief overview of traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) within the Department of Defense. Clin Neuropsychol 23,1291–1298. [DOI] [PubMed] [Google Scholar]
- 4.Tanev KS, Pentel KZ, Kredlow MA, Charney ME, 2014. PTSD and TBI co-morbidity: Scope, clinical presentation, and treatment options. Brain Inj 28, 261–270. [DOI] [PubMed] [Google Scholar]
- 5.Moore BA, Brock MS, Brager A, Collen J, LoPresti M, Mysliwiec V., 2020. Posttraumatic Stress Disorder, Traumatic Brain Injury, Sleep, and Performance in Military Personnel. Sleep Med Clin 15(1), 87–100. [DOI] [PubMed] [Google Scholar]
- 6.Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, ... & Beckham JC (2015). The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: A meta-analysis. Journal of anxiety disorders, 31, 98–107. [DOI] [PubMed] [Google Scholar]
- 7.Straud CL, Siev J, Messer S, & Zalta AK (2019). Examining military population and trauma type as moderators of treatment outcome for first-line psychotherapies for PTSD: A meta-analysis. Journal of anxiety disorders, 67, 102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanielian T & Jaycox L (Eds.). (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation [Google Scholar]
- 9.Kang HK, Natelson BH, Mahan CM, Lee KY, & Murphy FM (2003). Post-Traumatic Stress Disorder and Chronic Fatigue Syndrome-like illness among Gulf War Veterans: A population-based survey of 30,000 Veterans. American Journal of Epidemiology, 157(2):141–148. [DOI] [PubMed] [Google Scholar]
- 10.Kulka RA, Schlenger WA, Fairbanks JA, Hough RL, Jordan BK, Marmar CR, Weiss DS, Grady DA, Cranston AS (1990). Trauma and the Vietnam War generation: Report of findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/Mazel. [Google Scholar]
- 11.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seal KH., Metzler TJ, Gima KS, Bertenthal D, Maguen S, & Marmar CR, 2009. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. Public Health, 99, 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Toole BI., & Catts SV, 2017. The course and correlates of combat‐related PTSD in Australian Vietnam Veterans in the three decades after the war. Journal of Traumatic Stress, 30(1), 27–35. [DOI] [PubMed] [Google Scholar]
- 14.Dyball D, Evans S, Boos CJ., Stevelink SAM, and Fear NT, 2019. The association between PTSD and cardiovascular disease and its risk factors in male veterans of the Iraq/Afghanistan conflicts: A systematic review. International Review of Psychiatry, 31(1), 34–48. [DOI] [PubMed] [Google Scholar]
- 15.Levine AB., and Levine LM, Levine TB, 2014. Posttraumatic stress disorder and cardiometabolic disease. Cardiology, 127(1):1–19. [DOI] [PubMed] [Google Scholar]
- 16.Millner AJ., Ursano RJ, Hwang I, J. King A, Naifeh JA, Sampson NA, Zaslavsky AM, Stein MB, Kessler RC, & Nock MK, 2019. Prior mental disorders and lifetime suicidal behaviors among US Army soldiers in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Suicide & Life‐Threatening Behavior, 49(1), 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toblin RL, Quartana PJ, Riviere LA, Walper KC, Hoge CW. Chronic pain and opioid use in US soldiers after combat deployment. JAMA Intern Med. 2014;174(8):1400–1401. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey R, Dziura J, Justice AC, Hamada HA., Bathulapalli H, Burg M, Decker D, Driscoll M, Goulet J, Haskell S, Kulas J, Wang KH, Mattocks K, and Brandt C, 2017. Incidence of Mental Health Diagnoses in Veterans of Operations Iraqi Freedom, Enduring Freedom, and New Dawn, 2001–2014, Am J Public Health, 107:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall RP, Jorm AF, Grayson DA, & O’Toole BI (2000). Medical-care costs associated with posttraumatic stress disorder in Vietnam veterans. Australian and New Zealand Journal of Psychiatry, 34(6), 954–962. [DOI] [PubMed] [Google Scholar]
- 20.Asnaani A, Reddy MK, & Shea MT (2014). The impact of PTSD symptoms on physical and mental health functioning in returning veterans. Journal of anxiety disorders, 28(3), 310–317. [DOI] [PubMed] [Google Scholar]
- 21.Lutwak N, & Dill C (2017). PTSD and Risk of Suicide. Military medicine, 182(9–10), 1684–1684. [DOI] [PubMed] [Google Scholar]
- 22.Ozer EJ, Best SR, Lipsey TL, & Weiss DS (2003). Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological bulletin, 129(1), 52. [DOI] [PubMed] [Google Scholar]
- 23.Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of consulting and clinical psychology, 68(5), 748. [DOI] [PubMed] [Google Scholar]
- 24.Early SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG (2014). Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry.71(4):423–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch SAM, King A, Kim HM, Powell C, Rajaram N, Venners M, Simon NM, Hamner M, Liberzon I(2020). Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology. 118:104714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, and Neylan TC, 2011. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol. Psychiatry 69, 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmuth L, 2003. Fear and trembling in the amygdala. Science; 300: 568–569. [DOI] [PubMed] [Google Scholar]
- 28.Strawn JR, Geracioti TD Jr. 2008. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 25(3):260–71. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell T, Hegadoren KM, Coupland NC, 2004. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology, 50(4):273–83. [DOI] [PubMed] [Google Scholar]
- 30.Cools R, Roberts AC, Robbins TW, 2008. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci.;12(1):31–40. [DOI] [PubMed] [Google Scholar]
- 31.Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradlet PB, Portoghese PS, Hamon M 1996. International Union of Pharmacology: XII. Classification of opioid receptors. Pharmacol Rev;48(4):567–92. [PubMed] [Google Scholar]
- 32.Bruchas MR, Land BB, Lemos JC, Chavkin C,.2009. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One.4(12):e8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sbarski B, Akirav I. 2020. Cannabinoids as therapeutics for PTSD. Pharmacol Ther. 211:107551. [DOI] [PubMed] [Google Scholar]
- 34.Leo LM, Abood ME, 2021. CB1 Cannabinoid Receptor Signaling and Biased Signaling. Molecules. 26(17):5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons RG, Ressler KJ, 2013. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosi 16, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, Phan KL, 2016. Emotion regulatory brain function and SSRI treatment in PTSD: Neural correlates and predictors of change. Neuropsychopharmacology 41, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, Baker DG, Beckham JC, Bierut LJ, Bisson J, Bradley B, Chen CY, Dalvie S, Farrer LA, Galea S, Garrett ME, Gelernter JE, Guffanti G, Hauser MA, Johnson EO, Kessler RC, Kimbrel NA, King A, Koen N, Kranzler HR, Logue MW, Maihofer AX, Martin AR, Miller MW, Morey RA, Nugent NR, Rice JP, Ripke S, Roberts AL, Saccone NL, Smoller JW, Stein DJ, Stein MB, Sumner JA, Uddin M, Ursano RJ, Wildman DE, Yehuda R, Zhao H, Daly MJ, Liberzon I, Ressler KJ, Nievergelt CM, Koenen KC, 2018. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry 23, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ, 2010. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dieter JN, Engel SD., 2019. Traumatic Brain Injury and Posttraumatic Stress Disorder: Comorbid Consequences of War Neurosci Insights 14, 1179069519892933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrewes DG, Jenkins LM., 2019. The Role of the Amygdala and the Ventromedial Prefrontal Cortex in Emotional Regulation: Implications for Post-traumatic Stress Disorder. Neuropsychol Rev 29, 220–243. [DOI] [PubMed] [Google Scholar]
- 41.Henigsberg N, Kalember P, Petrović ZK, Šečić A., 2019. Neuroimaging research in posttraumatic stress disorder - Focus on amygdala, hippocampus and prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 90, 37–42. [DOI] [PubMed] [Google Scholar]
- 42.Bremner JD, 2007. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin N Am 17, 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cacciaglia R, Nees F, Grimm O, Ridder S, Pohlack ST, Diener SJ, Liebscher C, Flor H., 2017.Trauma exposure relates to heightened stress, altered amygdala morphology and deficient extinction learning: implication for psychopathology. Psychoneuroendocrinology 76, 19–28. [DOI] [PubMed] [Google Scholar]
- 44.Weiss SJ., 2007. Neurobiological alterations associated with traumatic stress. Perspect Psychiatr Care 43, 114–22. [DOI] [PubMed] [Google Scholar]
- 45.Bartel DP., 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- 46.Michlewski G, Caceres JF., 2019. Post-transcriptional control of miRNA biogenesis RNA. 25, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alural B, Genc S, Haggarty SJ Diagnostic and therapeutic potential of microRNAs in neuropsychiatric disorders: Past, present, and future. Prog Neuropsychopharmacol Biol Psychiatry. 2017. Feb 6;73:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruegger S, Grosshans H., 2012. MicroRNA turnover: when, how, and why. Trends Biochem Sci. 37, 436–446. [DOI] [PubMed] [Google Scholar]
- 49.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R., 2004. The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240. [DOI] [PubMed] [Google Scholar]
- 50.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN., 2006. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125, 887–901. [DOI] [PubMed] [Google Scholar]
- 51.Ha M, Kim VN., 2014. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15, 509–524. [DOI] [PubMed] [Google Scholar]
- 52.Bohnsack MT, Czaplinski K, Gorlich D., 2004. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10, 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD., 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi H, Tomari Y., 2016. RISC assembly: coordination between small RNAs and Argonaute proteins. Biochim Biophys Acta 1859, 71–81. [DOI] [PubMed] [Google Scholar]
- 55.Kwak PB, Tomari Y., 2012. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat Struct Mol Biol 19, 145–151. [DOI] [PubMed] [Google Scholar]
- 56.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T., 2017. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42, 254–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Issler O and Chen A, 2015. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci 16, 201–212. [DOI] [PubMed] [Google Scholar]
- 58.Pasinetti GM, Ho L, Dooley C, Abbi B, Lange G., 2012. Select non-coding RNA in blood components provide novel clinically accessible biological surrogates for improved identification of traumatic brain injury in OEF/OIF Veterans. Am J Neurodegener Dis 1,88–98. [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M., 2014. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. 9, e94075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawamura N, Kim Y, Asukai N., 2001. Suppression of cellular immunity in men with a past history of post-traumatic stress disorder. Am J Psychiatry 158, 484–486 [DOI] [PubMed] [Google Scholar]
- 61.Jiang JX., 2008. Posttraumatic stress and immune dissonance. Chin J Traumatol 11, 203–8. [DOI] [PubMed] [Google Scholar]
- 62.Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, Risbrough VB, Baker DG, O’Connor DT, Nievergelt CM, Woelk CH., 2015. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. 20,1538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones KA, Thomsen C., 2013. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci 53, 52–62. [DOI] [PubMed] [Google Scholar]
- 64.Bam M, Yang X, Zumbrun EE, Zhong Y, Zhou J, Ginsberg JP, Leyden Q, Zhang J, Nagarkatti PS, Nagarkatti M., 2016. Dysregulated immune system networks in war veterans with PTSD is an outcome of altered miRNA expression and DNA methylation. Sci Rep 6, 31209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammamieh R, Chakraborty N, Gautam A, Muhie S, Yang R, Donohue D, Kumar R, Daigle BJ Jr, Zhang Y, Amara DA, Miller SA, Srinivasan S, Flory J, Yehuda R, Petzold L, Wolkowitz OM, Mellon SH, Hood L, Doyle FJ 3rd, Marmar C, Jett M. 2017. Whole-genome DNA methylation status associated with clinical PTSD measures of OIF/OEF veterans. Transl Psychiatry. 7(7):e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gill JM, Saligan L, Woods S, Page G., 2009. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care 45 262–277. [DOI] [PubMed] [Google Scholar]
- 67.Bam M, Yang X, Zumbrun EE, Ginsberg JP, Leyden Q, Zhang J, Nagarkatti PS, Nagarkatti M., 2017. Decreased AGO2 and DCR1 in PBMCs from War Veterans with PTSD leads to diminished miRNA resulting in elevated inflammation. Transl Psychiatry 7, e1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin CG, Kim H, Yun S, Livingston W, Fetta J, Mysliwiec V, Baxter T, Gill JM., 2017. Circulating miRNA associated with posttraumatic stress disorder in a cohort of military combat veterans. Psychiatry Res 251, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signaling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. [DOI] [PubMed] [Google Scholar]
- 70.Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. [DOI] [PubMed] [Google Scholar]
- 71.Maguschak KA, Ressler KJ. Wnt Signaling in Amygdala-Dependent Learning and Memory. The Journal of Neuroscience. 2011;31(37):13057–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maguschak KA, Ressler KJ. A Role for WNT/P-Catenin Signaling in the Neural Mechanisms of Behavior. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7(4):763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bam M, Yang X, Busbee BP, Aiello AE, Uddin M, Ginsberg JP, Galea S, Nagarkatti PS, Nagarkatti M., 2020. Increased H3K4me3 methylation and decreased miR-7113–5p expression lead to enhanced Wnt/beta-catenin signaling in immune cells from PTSD patients leading to inflammatory phenotype. Mol Med. 26, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milovanovic T, Planutis K, Nquyen A, Marsh JL, Lin F, Hope C and Holcombe RF, 2004. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol. 25,1337–1342. [PubMed] [Google Scholar]
- 75.Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, Bouchal J. 2011. Wnt signaling in prostate development and carcinogenesis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 155,11–18. [DOI] [PubMed] [Google Scholar]
- 76.Benhaj K, Akcali KC and Oztuk M. 2006. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 15, 701–707. [PubMed] [Google Scholar]
- 77.Raposo G, Stoorvogel W., 2013. Extracellular vesicles: exosomes, microvesicles, and friends.J Cell Biol. 200, 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brites D, Fernandes A., 2015. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci. 9, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andjus P, Kosanović M, Milićević K, Gautam M, Vainio SJ, Jagečić D, Kozlova EN, Pivoriūnas A, Chachques JC, Sakaj M, Brunello G, Mitrecic D, Zavan B., 2020. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int J Mol Sci.21,6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrell CR, Volarevic A, Djonov V, Volarevic V., 2021. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int J Mol Sci. 22, 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guedes VA, Devoto C, Leete J, Sass D, Acott JD, Mithani S Gill., 2020. Extracellular Vesicle Proteins and MicroRNAs as Biomarkers for Traumatic Brain Injury. Front Neurol.11, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee MY, Baxter D, Scherler K, Kim TK, Wu X, Abu-Amara D, Flory J, Yehuda R, Marmar C, Jett M, Lee I, Wang K, Hood L., 2019. Distinct Profiles of Cell-Free MicroRNAs in Plasma of Veterans with Post-Traumatic Stress Disorder. J Clin Med. 8, 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Provencal N & Binder EB 2015. The neurobiological effects of stress as contributors to psychiatric disorders: focus on epigenetics. Current Opinion in Neurobiology 30 31–37 [DOI] [PubMed] [Google Scholar]
- 84.Auger CJ & Auger AP., 2013. Permanent and plastic epigenesis in neuroendocrine systems. Frontiers in Neuroendocrinology 34,190–197. [DOI] [PubMed] [Google Scholar]
- 85.Roth TL., 2014. How Traumatic Experiences Leave Their Signature on the Genome: An Overview of Epigenetic Pathways in PTSD. Front Psychiatry 5, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bam M, Yang X, Zhou J, Ginsberg JP, Leyden Q, Nagarkatti PS, Nagarkatti MJ, 2016. Evidence for Epigenetic Regulation of Pro-Inflammatory Cytokines, Interleukin-12 and Interferon Gamma, in Peripheral Blood Mononuclear Cells from PTSD Patients. J Neuroimmune Pharmacol 11,168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin C, Cho YE, Kim H, Yun S, Kanefsky R, Lee H, Mysliwiec V, Cashion A, Gill J., 2018. Altered DNA Methylation Patterns Associated With Clinically Relevant Increases in PTSD Symptoms and PTSD Symptom Profiles in Military Personnel. Biol Res Nurs 20, 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mehta D, Bruenig D, Carrillo-Roa T, Lawford B, Harvey W, Morris CP, Smith AK, Binder EB, Young RM, Voisey J., 2017. Genome wide DNA methylation analysis in combat veterans reveals a novel locus for PTSD. Acta Psychiatr Scand 136, 493–505. [DOI] [PubMed] [Google Scholar]
- 89.Uddin M, Aiello A, Wildman D, Karestan C Koenen, Graham Pawelec, de Los Regina Santos, Emily Goldmann, Sandro Galea, 2010. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc. Natl Acad. Sci 107, 9470–9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rusiecki JA, Chen L, Srikantan V, Zhang L, Yan L, Polin ML, Baccarelli A., 2012. DNA methylation in repetitive elements and post-traumatic stress disorder: a case-control study of US military service members. Epigenomics 4, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Speer KE, Semple S, Naumovski N, D’Cunha NM, McKune AJ. HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiol Stress. 2019;11:100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szabo YZ, Breeding T, Hejl C, Guleria RS, Nelson SM, & Zambrano-Vazquez L 2020. Cortisol as a biomarker of alcohol use in combat veterans: A literature review and framework for future research. Journal of Dual Diagnosis, 16 (3), 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olff M, Guzelcan Y, de Vries GJ, Assies J, Gersons BP., 2006. HPA‐ and HPT‐axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology 31,1220–1230. [DOI] [PubMed] [Google Scholar]
- 94.Yehuda R, 2009. Status of glucocorticoid alterations in post‐traumatic stress disorder. Ann N Y Acad Sci 1179,56–69. [DOI] [PubMed] [Google Scholar]
- 95.Carrasco GA, Van de Kar LD., 2003. Neuroendocrine pharmacology of stress. Eur J Pharmacol 463, 235–272. [DOI] [PubMed] [Google Scholar]
- 96.de Kloet ER., 2003. Hormones, brain and stress. Endocr Regul 37,51–68. [PubMed] [Google Scholar]
- 97.Castro-Vale I, van Rossum EF, Machado JC, Mota-Cardoso R, Carvalho D, 2016. Genetics of glucocorticoid regulation and posttraumatic stress disorder--What do we know? Neurosci Biobehav Rev 63,143–157. [DOI] [PubMed] [Google Scholar]
- 98.Fries GR, Gassen NC, Rein T., 2017. The FKBP51 glucocorticoid receptor co-chaperone: regulation, function, and implications in health and disease. Int. J. Mol. Sci.18, 2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawn SE, Sheerin CM, Lind MJ, Hicks TA, Marraccini ME, Bountress K, Bacanu SA, Nugent NR, Amstadter AB, 2109. GxE effects of FKBP5 and traumatic life events on PTSD: A meta-analysis. J. Affect. Disord. 243, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Binder EB, Rebekah G Bradley, Wei Liu, Michael P Epstein, Todd C Deveau, Kristina B Mercer, Yilang Tang, Charles F Gillespie, Christine M Heim, Charles B Nemeroff, Ann C Schwartz, Joseph F Cubells, Kerry J Ressler 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, Bradley B, Ressler KJ. 2016. Structural and functional connectivity in posttraumatic stress disorder: associations with FKBP5. Depress Anxiety 33, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Young DA, Inslicht SS, Metzler TJ, Neylan TC, Ross JA., 2018. The effects of early trauma and the FKBP5 gene on PTSD and the HPA axis in a clinical sample of Gulf War veterans. Psychiatry Res 270, 961–966. [DOI] [PubMed] [Google Scholar]
- 103.Kang HJ, Yoon S, Lee S, Choi K, Seol S, Park S, Namgung E, Kim TD, Chung YA, Kim J, Han JS, Lyoo IK., 2020. FKBP5-associated miRNA signature as a putative biomarker for PTSD in recently traumatized individuals. Sci Rep 10, 3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park J, Lee J, Choi K, Kang HJ., 2021. Regulation of behavioral response to stress by microRNA-690. Mol Brain 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wingo AP, Almli LM, Stevens JJ, Klengel T, Uddin M, Li Y., Bustamante AC, Lori A, Koen N, Stein DJ, Smith AK, Aiello AE, Koenen KC, Wildman DE, Galea S, Bradley B, Binder EB, Jin P, Gibson G, Ressler KJ. 2015. DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat Commun 6, 10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ikin JF, Creamer MC, Sim MR, & McKenzie DP, 2010. Comorbidity of PTSD and depression in Korean War veterans: prevalence, predictors, and impairment. Journal of affective disorders, 125 (1–3), 279–286. [DOI] [PubMed] [Google Scholar]
- 107.Nichter B, Norman S, Haller M, & Pietrzak RH, 2019. Psychological burden of PTSD, depression, and their comorbidity in the US veteran population: suicidality, functioning, and service utilization. Journal of affective disorders, 256, 633–640 [DOI] [PubMed] [Google Scholar]
- 108.Dominguez AA, Lim WA, Qi LS, 2016. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol 17, 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt U, Herrmann L, Hagl K, Novak B, Huber C, Holsboer F, Wotjak CT, Buell DR., 2013. Therapeutic Action of Fluoxetine is Associated with a Reduction in Prefrontal Cortical miR-1971 Expression Levels in a Mouse Model of Posttraumatic Stress Disorder. Front Psychiatry 4, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martenyi F, Brown EB, Zhang H, Prakash A, Koke SC., 2002. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry 63, 199–206. [DOI] [PubMed] [Google Scholar]
- 111.Martenyi F, Soldatenkova V., 2006. Fluoxetine in the acute treatment and relapse prevention of combat-related post-traumatic stress disorder: Analysis of the veteran group of a placebo-controlled, randomized clinical trial. Eur Neuropsychopharmacol 16, 340–9. [DOI] [PubMed] [Google Scholar]
- 112.Cavaljuga S, Licanin I, Mulabegović N, Potkonjak D., 2003. Therapeutic effects of two antidepressant agents in the treatment of posttraumatic stress disorder (PTSD). Bosn J Basic Med Sci 3, 12–6. [DOI] [PubMed] [Google Scholar]
- 113.Hertzberg MA, Feldman ME, Beckham JC, Kudler HS, & Davidson JR, 2000. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Annals of Clinical Psychiatry, 12(2), 101–105. [DOI] [PubMed] [Google Scholar]
- 114.Stock EM, Zeber JE, McNeal CJ, Banchs JE, Copeland LA., 2018. Psychotropic Pharmacotherapy Associated With QT Prolongation Among Veterans With Posttraumatic Stress Disorder. Ann Pharmacother 52, 838–848. [DOI] [PubMed] [Google Scholar]
- 115.Shiner B, Leonard CE, Gui J, Cornelius SL, Schnurr PP, Hoyt JE, Young-Xu Y, Watts BV, 2020. Comparing Medications for DSM-5 PTSD in Routine VA Practice. J Clin Psychiatry. 3;81(6): 20m13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolf EJ, Logue MW, Morrison FG, Wilcox ES, Stone A, Schichman SA, McGlinchey RE, Milberg WP, Miller MW, 2019. Posttraumatic psychopathology and the pace of the epigenetic clock: a longitudinal investigation Psychol Med.; 49(5):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, Flory JD, Buxbaum JD, Meaney MJ, Bierer LMM, 2013. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Frontiers in psychiatry, 4, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]