Dear Editor,
The study by Belsky and colleagues drew attention to the impact of COVID-19 on immunosuppressed patients, especially for solid organ transplant recipients (SOTRs) given their high risk of morbidity and mortality1. Indeed, kidney transplant recipients (KTR) are poorly protected by SARS-CoV-2 mRNA vaccines2, thus prevention SOTRs from COVID 19 infection remain a difficult challenge. Owning to ineligibility in clinical trials3, the efficacy and safety of SARS-CoV-2 mRNA vaccines are currently limited in this fragile population, further decrease adherence to vaccination schemes4 , 5. Given the accumulated data available for safety and immunogenicity of mRNA vaccine6 , 7, there is an urgent need to develop recommendations and guidelines for better delivery of COVID-19 vaccines in SOTRs.
In the present study, a random-effects model meta-analysis was conducted to assess anti-SARS-CoV-2 IgG seroconversion rates, T-cell response, and side effects after mRNA vaccination. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline were followed. A comprehensive literature search from January 1, 2021 to February 10, 2022 was conducted in PubMed, Embase, Cochrane Library and ClinicalTrials without language restriction (Supplementary methods). Electronic databases search identified 1998 publications, and 98 studies involving 15,328 COVID-19 infection-naïve SOTRs (15,132 adult SOTRs, 196 young SOTRs) patients were finally included (Supplementary Figure 1). Overall, included studies were dominated by kidney transplant recipients (KTRs, 10,209, 66.6%), and included a small percentage of liver transplant recipients (LTRs, 2734, 17.8%) and thoracic organs transplant recipients (TTRs, 1842, 12.0%, Supplementary Table 1).
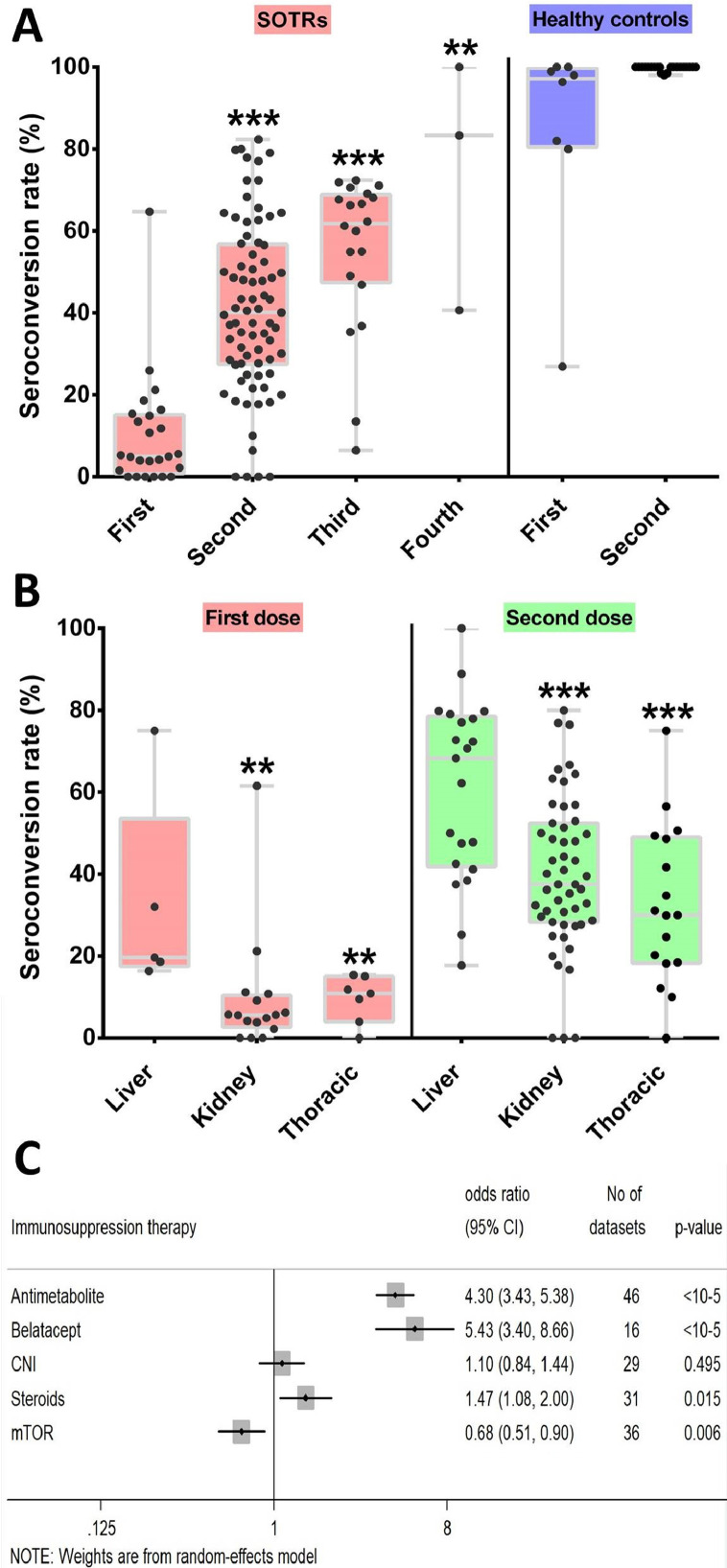
We find seroconversion rate of SOTRs patients was significantly reduced in comparison with healthy controls for the first and second dose (Fig. 1 A; P < 10−5). A lower seroconversion rate was achieved by those with incomplete vaccination regimens (7.2%; 95% CI: 4.2–10.8; Supplementary Figure 2) compared with patients who were fully immunised (40.1%; 95% CI: 35.2–45.0; Supplementary Figure 3). The Spike-specific IgG seroconversion rate was 55.2% (95% CI: 48.1–62.2) after the third dose and 81.7% (95% CI: 29.0–100.0) after the fourth dose (Supplementary Figure 4). Meta-regression analysis showed that a higher vaccine dosage was significantly correlated with a high seroconversion rate (regression coefficient, 0.231; 95% CI: 0.181–0.282; P <10−5; Supplementary Figure 5). No significant associations were detected for sample size (P = 0.93) and study design (P = 0.25).
Fig. 1.
Serological response in SOTRs. Boxplots of positive serological response rates (%) in SOTRs and healthy controls after different dose of COVID-19 mRNA vaccination (first dose in SORTRs as reference group) (A), positive serological response rates (%) by organ type after partial COVID-19 vaccination and after complete COVID-19 vaccination (liver transplantation receipts as reference group) (B). Summary ORs for anti-spike IgG seronegativity of immunosuppressive treatment (C). Each point indicates a study cohort where data were available. Pairwise comparisons are based on the non-parametric Mann-Whitney U independent-samples test (**<0.01, ***<0.0001).
Seropositivity was significantly higher in LTRs when compared with KTRs and TTRs after partial and complete immunization (Fig. 1B). Single dose-response rate of LTRs (22.4%; 95% CI: 14.2–31.6; Supplementary Figure 6A) was higher than KTRs (6.7%; 95% CI: 3.6–10.5; Supplementary Figure 6B) and TTRs (8.9%; 95% CI: 5.1–13.5; Supplementary Figure 6C). After complete vaccination, seroconversion rates was 60.8% (95% CI: 50.4–70.7; Supplementary Figure 7A) for LTRs, 38.4% (95% CI: 33.9–42.9; Supplementary Figure 7B) for KTRs and 30.0% (95% CI: 21.1–39.7; Supplementary Figure 7C) for TTRs. In addition, a higher seroconversion rate among young SOTRs with completed vaccination regimen (66.6%; 95% CI: 56.4–76.1; Supplementary Figure 8) was documented when compared with older SOTRs (40.1%).
As for immunosuppressive treatment, antimetabolite (OR = 4.29, 95% CI: 3.43–5.38, P < 10−5; Supplementary Figure 9) and Belatacept therapy (OR = 5.43, 95% CI: 3.4–8.66, P < 10−5; Supplementary Figure 10) were significantly associated anti-spike IgG seronegativity, while mTOR inhibitor was siginificantly assocaited with seropositivity (Fig. 1C). In addition, the summary OR for positive antibody response was 1.031 (95% CI: 1.025–1.036, P < 10−5; Supplementary Figure 11) per 1 mL/min increment in estimated glomerular filtration rate.
Owning to the heterogenicity in serological immunoassays and the difference in results in the 30 datasets reporting data on IgG titres, meta-analysis could not be performed. Overall, significantly lower anti-S IgG titres were documented in SOTRs patients after partial and complete immunization compared with healthy controls (Supplementary Table 2).
A pooled analysis of 21 studies including 315 SOTRs with incomplete and 1310 SOTRs with complete vaccination regimens showed that 14.0% (95% CI: 7.7–21.6) and 38.8% (95% CI: 27.7–50.5) developed T-cell response, respectively (Supplemental Figure 12).
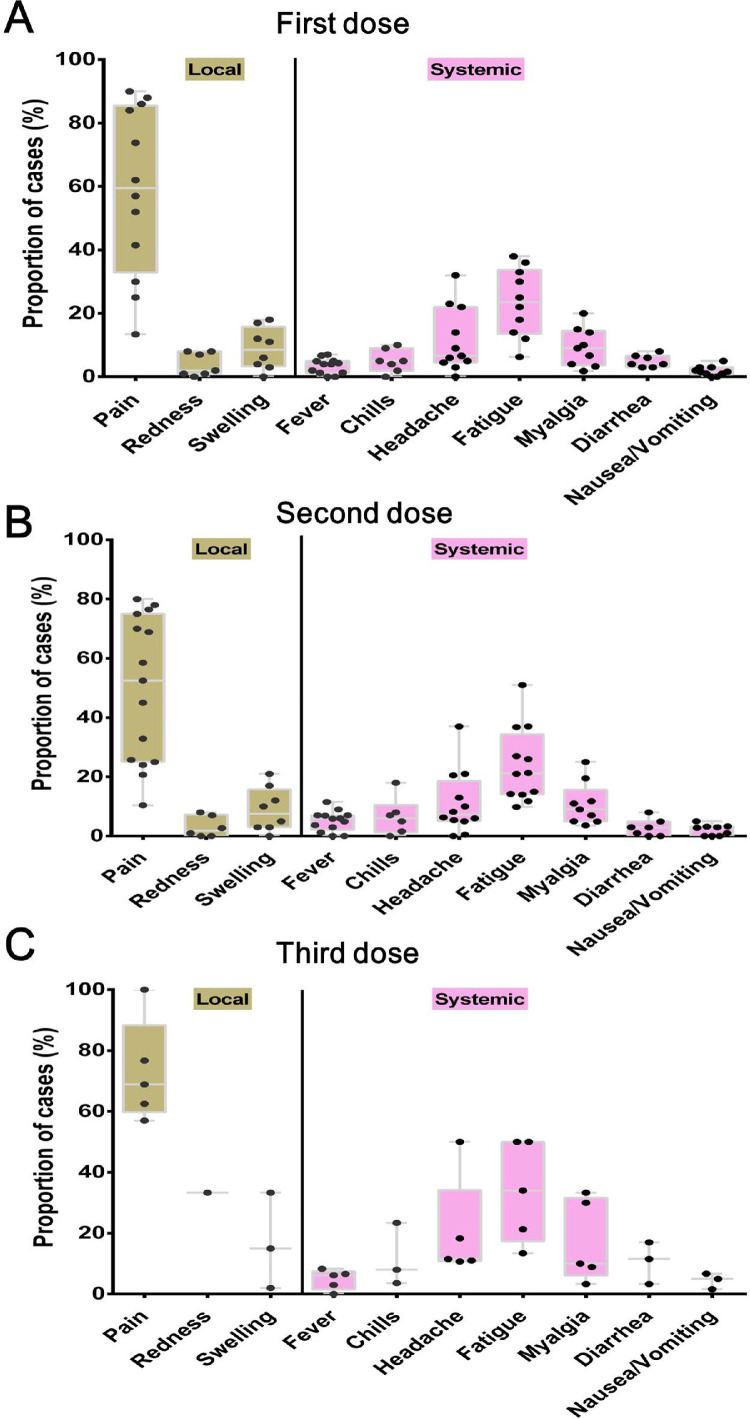
No cases of acute rejection, allograft dysfunction or allograft failure was observed. The overall local and systemic reactions after COVID-19 vaccination in SOTRs patients are shown in Fig. 2 . A similar pattern was observed after the first, second and third dose, with pain (∼47–63%) and swelling (∼9%) being the main local reactions and fatigue (∼23%) and headache (∼7%) the systemic reactions.
Fig. 2.
Local and systemic toxicities reported in SOTRs after first dose (A), second (B) and third (C) dose vaccination. Each point indicates a study cohort where data were available.
The shape of the funnel plots were symmetrical (Supplementary Figure 13), and Egger's test did not reveal publication bias (P = 0.84).
In summary, we provide the first and the largest meta-analysis to address the serological and safety data after COVID-19 mRNA vaccination of SOTRs. Results of this study demonstrate that a significantly lower seroconversion rate and anti-S IgG titres were achieved among SOTRs after 2 doses of COVID-19 mRNA vaccine compared with healthy controls. Therefore, the clinical effectiveness might remain sub-optimal compared to healthy population. The seroconversion after 2 doses was 40.1%, much lower than the rates reported in other vulnerable population, such as patients with immune-mediated inflammatory diseases (73.2%)8, cancer patients (51%)9, and patients with end-stage kidney disease (86%)10. Thus, SOTRs should receive the complete vaccination regimen without delay.
A higher serologic response to a 3-dose (55.2%) and 4-dose (81.7%) vaccine strategy suggests SOTRs should be considered to receive booster dose regimens. Since antimetabolite and Belatacept therapy was associated with impaired immune response, alternative vaccine platforms (ie. inactive SARS-CoV-2 vaccine, recombinant adenovirus vaccine), temporary adjustment of immunosuppressive regimens may be required. The effect of augmented vaccination schedules, monoclonal antibodies and new oral antiviral agents should be assessed for SOTRs of future study.
Robust results confirmed the safety of COVID-19 mRNA vaccines and support the prioritization of SOTRs to receive their first and second doses to enhance their immune response, and highlight benefits of a third and fourth booster, especially KTRs and TTRs. More importantly, increasing opportunity of information and concerns sharing among health-care workers and SOTRs patients remain the key to decrease patients’ hesitancy and thus increase adherence to vaccination schemes.
Funding
This work was supported by grant from the Research Start-up Fund in Changning Maternity and Infant Health Hospital (2021Y-15).
Conflict of Interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.02.016.
Appendix. Supplementary materials
References
- 1.Belsky J.A., Tullius B.P., Lamb M.G., Sayegh R., Stanek J.R., Auletta J.J. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. Mar 2021;82(3):329–338. doi: 10.1016/j.jinf.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducloux D., Bamoulid J., Chabannes M., Colladant M., Munshi A., Roubiou C., Seibel J., Tachikart A., Yannaraki M., Crepin T., Courivaud C. Current vaccine strategies against SARS_CoV-2 only poorly protect kidney transplant recipients. J Infect. 2022 Jan 21 doi: 10.1016/j.jinf.2022.01.020. S0163-4453(22)00020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsapepas D., Husain S.A., King K.L., Burgos Y., Cohen D.J., Mohan S. Perspectives on COVID-19 vaccination among kidney and pancreas transplant recipients living in New York City. Am J Health Syst Pharm. 2021;78(22):2040–2045. doi: 10.1093/ajhp/zxab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tharmaraj D., Dendle C., Polkinghorne K.R., Mulley W.R. Kidney transplant recipients' attitudes toward COVID-19 vaccination and barriers and enablers to vaccine acceptance. Transpl Infect Dis. 2021 Oct 25:e13749. doi: 10.1111/tid.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prendecki M., Thomson T., Clarke C.L., Martin P., Gleeson S., De Aguiar R.C., et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398(10310):1482–1484. doi: 10.1016/S0140-6736(21)02096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakuraba A., Luna A., Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):88–108.e9. doi: 10.1053/j.gastro.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., Mesa-Chavez F., Barrientos-Gutiérrez T., Tagliamento M., et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J.J., Lee T.H., Tian Y.C., Lee C.C., Fan P.C., Chang C.H. Immunogenicity rates after SARS-CoV-2 vaccination in people with end-stage kidney disease: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.