Abstract
We examined the genetic diversity of serotype V group B streptococcus (GBS) isolates in the Paris area and compared them with the predominant American serotype V clone. Pulsed-field gel electrophoresis yielded 11 patterns for 64 French GBS. One pattern was obtained with 60% of the isolates tested and was indistinguishable from that of the predominant American clone.
Group B streptococci (GBS) are the main cause of severe infection in both infants and adults (2, 20, 21). GBS are serotyped on the basis of their capsular polysaccharide, of which nine different serotypes have been described (10, 15). The classical serotypes Ia, Ib, II, and III are the predominant cause of disease in neonates. Recently, serotype V GBS have emerged as a new cause of GBS infection or colonization in children and adults (13, 18, 19). Indeed, population-based surveillance of GBS in the 1990s indicated that serotype V was responsible for 10 to 15% of neonatal GBS infections in the United States (7, 13, 17) and was the most common serotype isolated from nonpregnant adults with invasive disease (13). Moreover, data from the Centers for Disease Control and Prevention (Atlanta, Ga.) have shown the emergence of a serotype V clonal type (8).
In France, too, recent studies point to a significant shift in the distribution of GBS serotypes, with serotype V emerging as one of the major serotypes recovered from neonates (1, 11, 16).
We examined the genetic diversity of French serotype V GBS clinical isolates in the Paris area and compared them with the predominant American clone.
We studied a collection of 64 serotype V GBS isolates recovered between January 1998 and January 2000 in the Paris area. The isolates were obtained from genital specimens from pregnant women (n = 30) or from gastric fluid or ear specimens from colonized or infected newborns (n = 34). GBS isolates were confirmed at the species level by standard laboratory methods and were serotyped with a commercial latex agglutination kit (Streptex; Murex Diagnostics UK). The isolates were stored in Todd-Hewitt broth with 20% glycerol at −80°C until further analysis was done.
Pulsed-field gel electrophoresis (PFGE) of serotype V GBS strains was performed as previously described (12). SmaI restriction enzyme chromosomal digests were separated with a Bio-Rad contour-clamped homogenous electric field mapper with a switch time of 0.85 to 35.38 s for 22 h and 35 min at a 120° angle with a voltage gradient of 6 V/cm at 14°C. The DNA size standard was a lambda DNA ladder (Bio-Rad). The gels were stained with ethidium bromide and photographed under UV light. PFGE banding patterns were compared visually. Strains were considered genetically distinguishable if their restriction patterns differed by three or more bands (24). Banding patterns were also compared using a computer system (Biocapt; Vilber-Lourmat) and whole-band analyzer software (Biogène; Vilber-Lourmat). Cluster analysis (unweighted pair group average) was used to calculate similarity and dissimilarity among GBS isolates. A difference was considered significant if the similarity coefficient was <80%. The results were compared with those obtained with the predominant American clonal serotype V GBS strain (8).
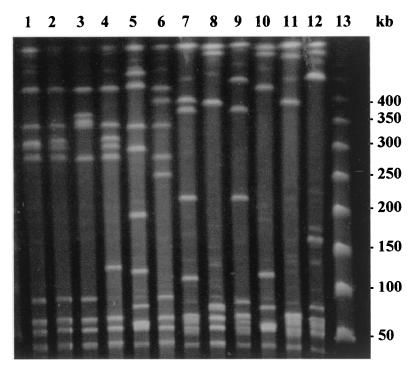
PFGE typing of the 64 French serotype V GBS isolates yielded 11 distinct patterns (B to L) and a total of 28 subtypes. Representative SmaI digest patterns for the serotype V strains are shown in Fig. 1. The most common PFGE pattern was B (five subtypes), which was obtained with 60% of the isolates tested. Indeed, 39 of the 64 isolates were highly related, having similarity coefficients of >80% (data not shown). This pattern was genetically related to the predominant U.S. serotype V GBS clone (pattern A) (Fig. 1). PFGE patterns C, D, F, G, H, K, and L were shared by four, three, four, two, two, four, and three isolates, respectively. PFGE patterns E, I, and J were each represented by a single isolate.
FIG. 1.
Representative major PFGE patterns of serotype V GBS isolates. Lane 1, pattern A (predominant American clonal serotype V GBS); lane 2, pattern B (39 isolates); lane 3, pattern C (4 isolates); lane 4, pattern D (3 isolates); lane 5, pattern E (1 isolate); lane 6, pattern F (4 isolates); lane 7, pattern G (2 isolates); lane 8, pattern H (2 isolates); lane 9, pattern I (1 isolate); lane 10, pattern J (1 isolate); lane 11, pattern K (4 isolates); lane 12, pattern L (3 isolates); lane 13, DNA size marker (Roche).
GBS have emerged as an important cause of morbidity and mortality among neonates, pregnant women, and other adults (9). In previous studies, serotypes Ia, Ib, II, and III were isolated from neonates with early-onset disease and from pregnant women with vaginal GBS colonization (4). Late-onset neonatal disease was due primarily to serotype III (25).
Serotype V GBS was first isolated in 1976 in the United States and was initially identified as NT1 (nontypeable type 1) (27); it was assigned type V status in 1985 (14). Serotype V appears to have emerged recently, because studies done before serotype V typing serum was available showed small percentages of nontypeable isolates (3). Serotype V seems to have been common since at least the mid-1980s in the United States (6) and also in Japan (23), Indonesia (26), The Gambia (22), Sweden (4), and France (1, 11, 16). Serotype V was the most common serotype recovered from nonpregnant adults with invasive GBS disease and the second and third most common serotype recovered from pregnant women and neonates with early-onset disease (6). French reports indicate that serotype V accounted for about 15% of isolates recovered from neonates (1, 16). Similar proportions (14 and 12%) of serotype V strains causing invasive infections were observed in neonates from Atlanta (6) and Maryland (13).
Molecular methods have been useful for discriminating among isolates of the same serotype (5, 7, 12). PFGE is a powerful technique for studying chromosomal relatedness among bacterial isolates. Blumberg et al. found that serotype V isolates recovered from patients in the Atlanta area were highly related (6). In our PFGE study, we obtained 11 patterns for the 64 serotype V GBS isolates, with one predominant. Genetic diversity among serotype V isolates had already been reported by Elliott et al. (8). However, these investigators have shown that 56% of 45 serotype V GBS isolates collected from 1986 to 1996 in the United States were genetically related (8). By PFGE, we found that this American clonal type, also found in Argentina (8), was indistinguishable from our predominant strain. Our study provides compelling evidence that the predominant isolates present in the United States and France are clonally related. Thus, the isolates in these two geographic locations are genetically diverse and have similar clonal structures.
Acknowledgments
We thank J. A. Elliott for providing the predominant American clonal serotype V GBS strain and D. Facklam for critical review of the manuscript.
REFERENCES
- 1.Adam M N, Le Pennec M P, Vandemeulebroucke E, Giacomini T. Serotyping of group B Streptococcus in microbiological samples at the Robert-Ballanger hospital. Pathol Biol. 1994;42:544–546. [PubMed] [Google Scholar]
- 2.Baker C J. Summary of the workshop on perinatal infections due to group B Streptococcus. J Infect Dis. 1977;136:137–152. doi: 10.1093/infdis/136.1.137. [DOI] [PubMed] [Google Scholar]
- 3.Baker C J, Barrett F F. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230:1158–1160. [PubMed] [Google Scholar]
- 4.Berg S, Trollfors B, Lagergard T, Zackrisson G, Claesson B A. Serotypes and clinical manifestations of group B streptococcal infections in western Sweden. Clin Microbiol Infect. 2000;6:9–13. doi: 10.1046/j.1469-0691.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 5.Bingen E, Denamur E, Lambert-Zechovsky N, Aujard Y, Brahimi N, Geslin P, Elion J. Analysis of DNA restriction fragment length polymorphism extends the evidence for breast milk transmission in Streptococcus agalactiae late-onset neonatal infection. J Infect Dis. 1992;165:569–573. doi: 10.1093/infdis/165.3.569. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliot J, Facklam R R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg H M, Stephens D S, Licitra C, Pigott N, Facklam R, Swaminathan B, Wachsmuth I K. Molecular epidemiology of group B streptococcal infections: use of restriction endonuclease analysis of chromosomal DNA and DNA restriction fragment length polymorphisms of ribosomal RNA genes (ribotyping) J Infect Dis. 1992;166:574–579. doi: 10.1093/infdis/166.3.574. [DOI] [PubMed] [Google Scholar]
- 8.Elliott J A, Farmer K D, Facklam R R. Sudden increase in isolation of group B streptococci, serotype V, is not due to emergence of a new pulsed-field gel electrophoresis type. J Clin Microbiol. 1998;36:2115–2116. doi: 10.1128/jcm.36.7.2115-2116.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farley M M, Harvey R C, Stull T, Smith J D, Schuchat A, Wenger J D, Stephens D S. A population-based assessment of invasive disease due to group B streptococcus in nonpregnant adults. N Engl J Med. 1993;328:1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri P, Flore A E. Surface protein expression in group B streptococcal invasive isolates. In: Horaud T, Sicard M, Bouvet A, LeClerq R, DeMontclos H, editors. Streptococci and the host: proceedings of the XIII Lancefield International Symposium. New York, N.Y: Plenum Publishing; 1997. pp. 635–637. [Google Scholar]
- 11.Geslin P, Sissia G, Jelinkova J, Fremaux A, Motlova J. Serotype distribution of group B streptococci isolated from human source in France over a 10-year period (1980–1989). New perspectives on streptococci and streptococcal infections. Zentbl Bakteriol. 1992;22(Suppl.):484–485. [Google Scholar]
- 12.Gordillo M E, Singh K V, Baker C J, Murray B E. Typing of group B streptococci: comparison of pulsed-field gel electrophoresis and conventional electrophoresis. J Clin Microbiol. 1993;31:1430–1434. doi: 10.1128/jcm.31.6.1430-1434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison L H, Ellilot J A, Dwyer D M, Libonati J P, Ferrieri P, Billmann L, Schuchat A. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J Infect Dis. 1998;177:998–1002. doi: 10.1086/515260. [DOI] [PubMed] [Google Scholar]
- 14.Jelinkova J, Motlova J. Worldwide distribution of two new serotypes of group B streptococci: type IV and provisional type V. J Clin Microbiol. 1985;21:361–362. doi: 10.1128/jcm.21.3.361-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogan G, Uhrin D, Brisson J R, Paoletti L C, Blodgett A E, Kasper D L, Jennings H J. Structural and immunochemical characterization of the type VIII group B streptococcus capsular polysaccharide. J Biol Chem. 1996;271:8786–8790. doi: 10.1074/jbc.271.15.8786. [DOI] [PubMed] [Google Scholar]
- 16.Le Thomas I, Lepercq J, Bergeret M, Francoual C, Raymond J. Role of group B streptococcus serotype V in materno-fetal infections. Arch Pediatr. 1997;4:1074–1078. doi: 10.1016/s0929-693x(97)88971-3. [DOI] [PubMed] [Google Scholar]
- 17.Lin F Y, Clemens J D, Azimi P H, Regan J A, Weisman L E, Philips III J B, Rhoads G G, Clark P, Brenner R A, Ferrieri P. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J Infect Dis. 1998;177:790–792. doi: 10.1086/517810. [DOI] [PubMed] [Google Scholar]
- 18.Moylett E H, Fernandez M, Rench M A, Hickman M E, Baker C J. A 5-year review of recurrent group B streptococcal diseases: lessons from twin infants. Clin Infect Dis. 2000;30:282–287. doi: 10.1086/313655. [DOI] [PubMed] [Google Scholar]
- 19.Rench M A, Baker C J. Neonatal sepsis caused by a new group B streptococcal serotype. J Pediatr. 1993;122:638–640. doi: 10.1016/s0022-3476(05)83554-1. [DOI] [PubMed] [Google Scholar]
- 20.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz B, Schuchat A, Oxtoby M J, Cochi S L, Hightower A, Broome C V. Invasive group B streptococcal disease in adults—a population-based study in metropolitan Atlanta. JAMA. 1991;266:1112–1114. [PubMed] [Google Scholar]
- 22.Suara R O, Adegbola R A, Baker C J, Secka O, Mulholland E K, Greenwood B M. Carriage of group B streptococci in pregnant Gambian mothers and their infants. J Infect Dis. 1994;170:1316–1319. doi: 10.1093/infdis/170.5.1316. [DOI] [PubMed] [Google Scholar]
- 23.Takazawa Y, Tomizawa I. Serotypes and antibiotic susceptibility of group A and B streptococci clinically isolated in Sapporo in the last five years. J Jpn Assoc Infect Dis. 1991;65:938–944. doi: 10.11150/kansenshogakuzasshi1970.65.938. [DOI] [PubMed] [Google Scholar]
- 24.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenger J D, Hightower A W, Facklam R R, Gaventa S, Broome C V. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. J Infect Dis. 1990;162:1316–1323. doi: 10.1093/infdis/162.6.1316. [DOI] [PubMed] [Google Scholar]
- 26.Wibawan I W T, Lammler C, Lautrou Y, Warsa U C. Serotyping and further characterization of group B streptococcal isolates from Indonesia. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;277:260–266. doi: 10.1016/s0934-8840(11)80621-3. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson H W. Nontypeable group B streptococci isolated from human sources. J Clin Microbiol. 1977;6:183–184. doi: 10.1128/jcm.6.2.183-184.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]