Abstract
Delayed cerebral ischemia (DCI) is identified as one of the significant contributors to poor patient outcome after aneurysmal subarachnoid hemorrhage (SAH). We previously reported that a supratherapeutic dose of isoflurane conditioning (2%) provided robust protection against SAH-induced DCI. The aim of our current study is to compare the efficacy of the supratherapeutic dose of isoflurane to that typically used to establish general anesthesia or sedation. After IRB approval for animal studies, ten to fourteen-week-old wild-type male mice (C57BL/6) were divided into five groups – sham, SAH alone, or SAH with isoflurane conditioning (0.5%, 1%, and 2%). Conditioning was performed with one-hour of isoflurane initiated one-hour after induction of SAH via endovascular perforation technique. Vasospasm measurement in the middle cerebral artery was assessed 72 h after SAH. Neurological assessment was performed at baseline and for next three days after SAH. It was identified that all tested doses of isoflurane conditioning (0.5%, 1%, and 2%) significantly attenuated large artery vasospasm and markedly improved neurological deficits following SAH. No significant differences in neurovascular outcome were noted between the three doses of isoflurane conditioning. Our data show that isoflurane dosing typically used for general anesthesia (1%) or sedation (0.5%) provide similar levels of DCI protection in SAH as that provided by a supratherapeutic dose (2%). This result has important implications for future translational studies. Additional studies examining the therapeutic potential of anesthetic conditioning for SAH are therefore warranted.
Keywords: Isoflurane concentration, DCI, Neurologic outcome, Aneurysmal subarachnoid hemorrhage
1. Introduction
Aneurysmal Subarachnoid hemorrhage (SAH) affects approximately 30,000 people in the US per year (Mayberg et al., 1994). Morbidity and mortality remain unacceptably high (Sudlow and Warlow, 1997). Apart from the initial hemorrhage severity after SAH, the secondary brain injury due to delayed cerebral ischemia (DCI) plays an important role in the outcomes of SAH patients (Broderick et al., 1994). Many new strategies to prevent DCI have been explored in recent years and none have proven efficacious, likely due to targeting individual elements of what has proven to be a multifactorial pathophysiological process.
Conditioning is a therapeutic strategy that is not only powerful, but also remarkably pleiotropic with protective effects on all major CNS cell types including neurons, glia, and vascular cells (Gidday, 2006; Dirnagl et al., 2009; Trendelenburg and Dirnagl, 2005). Previous preclinical and clinical studies have shown that inhalational anesthetics have a potential to attenuate SAH-induced DCI (Milner et al., 2015; Athiraman et al., 2019, 2020). Athiraman et al and Milner et al showed in an endovascular perforation mouse model of SAH that a single one-hour exposure of 2% isoflurane beginning one hour after SAH provided multifaceted protection against DCI including improved short-term neurological deficits (Athiraman et al., 2020; Milner et al., 2015). This neurovascular protection was causally implicated to hypoxia inducible factor-1α (HIF-1α) and endothelial nitric oxide synthase (eNOS) when they showed that pharmacologic and genetic inhbition of HIF-1α and NOS prevented isoflurane-induced DCI protection. The dose of isoflurane used in both studies are equivalent to 1.8 MAC (MAC = minimum alveolar concentration of anesthetic required for 50% of patients to not respond to a surgical stimulus), which is a supratherapeutic dose for achieving general anesthesia in both mice (Sonner et al., 1999) and patients (Nickalls and Mapleson, 2003). Exposure of a supratherapeutic dose of isoflurane to SAH patients could lead to serious hemodynamic challenges that would make translation of an isoflurane conditioning-based therapy difficult. Therefore, additional studies to define the lowest effective isoflurane dose to achieve maximal neurovascular protection in SAH is required, as this is a critical and necessary next step towards translating an isoflurane conditioning-based therapy for SAH to the clinic. The aim of our current study is to compare the effects of standard anesthetic and subanesthetic isoflurane dosing on SAH-induced neurovascular deficits to that associated with a supratherapeutic isoflurane dose.
2. Results:
2.1. Isoflurane conditioning and physiological variables: (Table 1)
Table 1.
Physiological variables from arterial blood: Mice underwent sham or SAH surgery and exposed to isoflurane 0.5%, 1% or 2% for 1-hour, one-hour post SAH. Measurements were performed at the end of two-hour period after sham or SAH surgery. n = 3–4 in each group. Values are represented as mean ± SEM. Differences were analyzed by ANOVA followed by Newman-Keuls post hoc test. P < 0.05 is considered statistically significant. SBP - #p < 0.05 SAH vs SAH + Isoflurane (0.5%, 1% and 2%), DBP - #p < 0.05 SAH vs SAH + Isoflurane (0.5%, 1% and 2%), &p < 0.05 Sham vs SAH + Isoflurane 2%, MBP - #p < 0.05 SAH vs SAH + Isoflurane (0.5%, 1% and 2%), *p < 0.05 Sham vs SAH + Isoflurane 2%. pCO2- partial pressure of carbondioxide, pO2- partial pressure of oxygen, sO2- oxygen saturation, Hb- hemoglobin, SBP- Systolic blood pressure, DBP- diastolic blood pressure, MBP- Mean blood pressure, HR- Heart rate.
| Parameters | Sham | SAH | SAH + Isoflurane 0.5% | SAH + Isoflurane 1% | SAH + Isoflurane 2% |
|---|---|---|---|---|---|
| pH | 7.35 ± 0.03 | 7.32 ± 0.03 | 7.41 ± 0.01 | 7.37 ± 0.03 | 7.44 ± 0.02 |
| pCO2 (mmHg) | 37.27 ± 1.71 | 41.23 ± 1.31 | 31.67 ± 3.03 | 35.35 ± 3.40 | 30.57 ± 2.58 |
| pO2 (mmHg) | 109 ± 2.89 | 97.27 ± 4.45 | 101.27 ± 4.68 | 97.90 ± 4.10 | 105.30 ± 6.03 |
| sO2 (%) | 95.63 ± 0.5 | 93.60 ± 1.29 | 97.70 ± 1.29 | 94.87 ± 0.58 | 96.47 ± 1.80 |
| Hb (g/dl) | 13.6 ± 0.4 | 14.40 ± 0.06 | 13.30 ± 0.72 | 13.63 ± 0.81 | 13.77 ± 0.28 |
| SBP (mmHg) | 120 ± 3 | 143 ± 7 | 103 ± 11# | 103 ± 9# | 92 ± 2# |
| DBP (mmHg) | 116 ± 1 | 132 ± 5 | 98 ± 11# | 97 ± 9# | 86 ± 3#& |
| MBP (mmHg) | 119 ± 3 | 136 ± 6 | 104 ± 10# | 100 ± 9# | 87 ± 2#* |
| HR | 530 ± 15 | 566 ± 32 | 476 ± 11 | 492 ± 17 | 458 ± 34 |
No significant differences were seen between groups in the following physiological parameters: pH, pC02, pO2, sO2, Hb, and HR. In contradistinction, significant differences were seen with blood pressure between the SAH group and the SAH: Isoflurane groups (0.5%, 1%, and 2%), which is consistent with the known impact of isoflurane anesthesia on blood pressure.
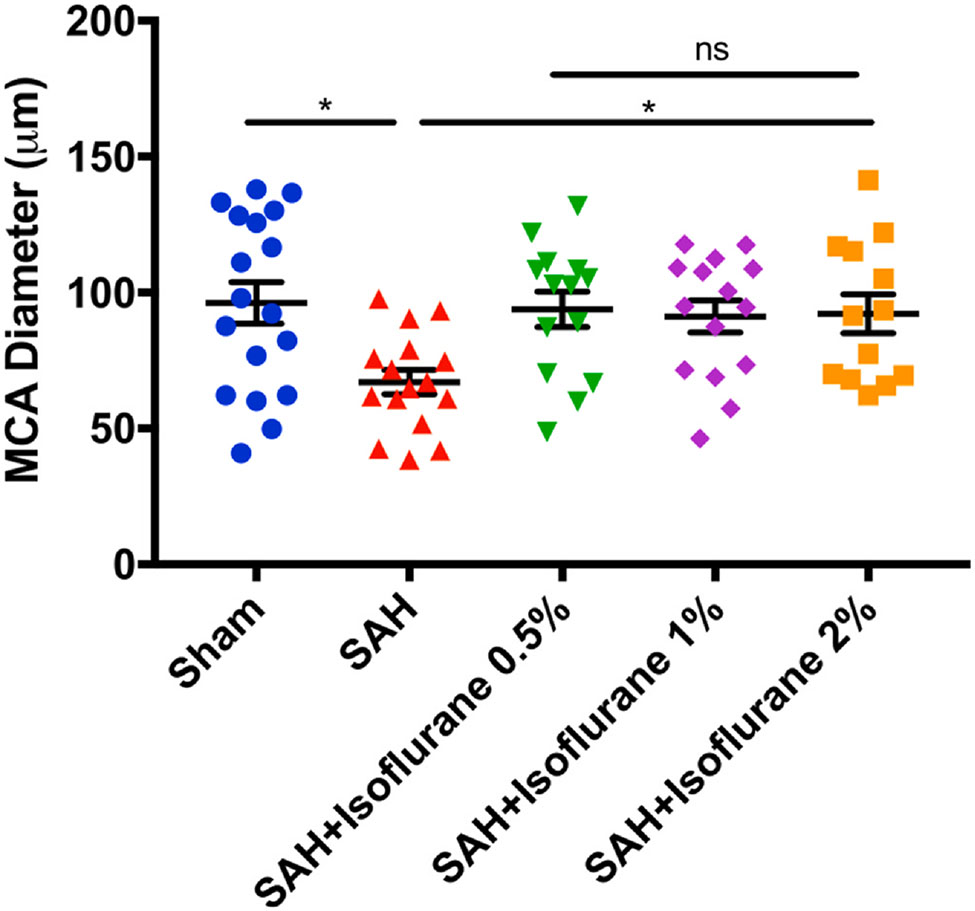
2.2. Isoflurane conditioning attenuated SAH-induced large artery vasospasm in wild type mice: (Fig. 1)
Fig. 1.
Different doses of isoflurane conditioning attenuates SAH-induced vasospasm in wild type mice: Mice underwent sham or SAH surgery and exposed to isoflurane 0.5%, 1% or 2% for 1-hour, one-hour post SAH. On post-surgery day 3, mice were perfused with ROX-SE staining and vessel diameter in the proximal MCA ipsilateral to suture perforation was measured. Data indicate mean ± SEM. *p < 0.05 sham vs. SAH, SAH vs. SAH + Isoflurane conditioning, by ANOVA and Newman-Keuls multiple comparison test. SAH- subarachnoid hemorrhage, ROX-SE, 5-(and-6)-Carboxy-X-rhodamine, succinimidyl ester, MCA- middle cerebral artery, ANOVA- analysis of variance.
Out of a total 82 wild-type mice used in the experiment, 2 animals died in the SAH group, 3 animals died in the isoflurane conditioning group, and none died in the sham group. Out of the 3 animals that died in the isoflurane conditioning group, 1 died in the 1% isoflurane group and 2 died in the 2% isoflurane group. No animals died in the 0.5% isoflurane group, and mice in this group were sedated and not fully anesthetized as noted by spontaneous movements inside the anesthetic induction chamber during treatment and ability to regain normal activity immediately after cessation of anesthetic exposure. In contradistinction, animals in the other two isoflurane groups (1% and 2%) were completely anesthetized during treatment and took several minutes to regain normal activity after cessation of anesthetic exposure. Animals subjected to SAH surgery were found to have SAH at the time of animal sacrifice and none in sham group were noted to have SAH. Significant large artery vasospasm was noted in mice subjected to SAH as compared to sham surgery (p < 0.05, Fig. 1). All three doses of isoflurane conditioning (0.5%, 1%, and 2%) were found to provide robust protection against SAH-induced vasospasm (p < 0.05, Fig. 1). No significant difference in vasospasm protection was noted within the isoflurane conditioning groups and between sham and isoflurane conditioning groups (p > 0.05, Fig. 1).
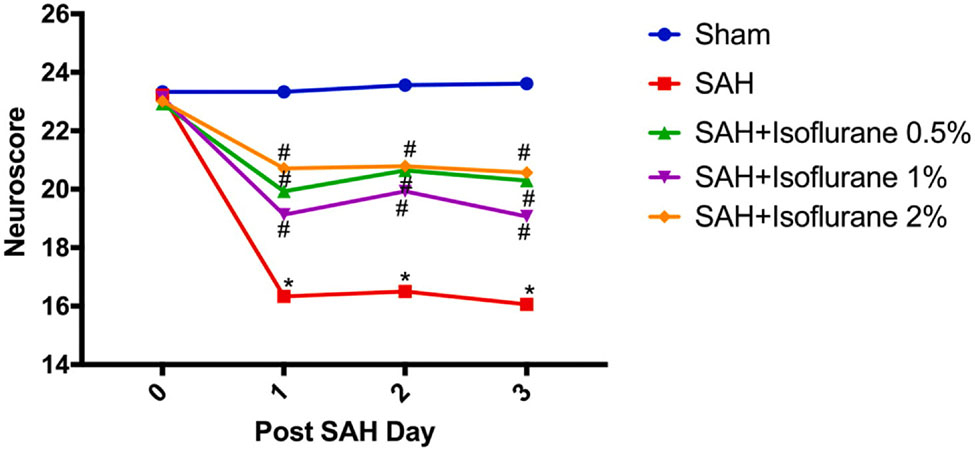
2.3. Isoflurane conditioning improved neurological outcomes after SAH in wild type mice: (Fig. 2)
Fig. 2.
Different doses of isoflurane conditioning improves SAH-induced neurological deficits in wild type mice: Wild type mice underwent SAH or sham surgery followed 1 h later by exposure to 0.5%, 1% or 2% isoflurane or room air for 1 h. Neuroscore was assessed daily and until three days after SAH. Data indicate mean ± SEM. *p < 0.05 Sham vs SAH; #p < 0.05 SAH vs. SAH + Isoflurane conditioning by two-way repeated measures ANOVA followed by Newman-Keuls multiple comparison test. SAH- subarachnoid hemorrhage, ANOVA- analysis of variance
Next, we wanted to determine the extent of neurological protection afforded by different doses of isoflurane conditioning. Mice subjected to SAH surgery were noted to have significant neurological deficits as compared to sham surgery (p < 0.05, Fig. 2). All three doses of isoflurane conditioning (0.5%, 1%, and 2%) were found to markedly attenuate SAH-induced neurological deficits (p < 0.05, Fig. 2). No significant difference in neurological protection was noted within the isoflurane conditioning groups (p > 0.05, Fig. 2).
3. Discussion
The key findings of our study are as follows: 1) Conditioning with a supratherapeutic dose of isoflurane (2%) provides strong protection against SAH-induced vasospasm and neurological deficits in wild-type mice, consistent with previous reports from independent laboratories; (Milner et al., 2015; Mutoh et al., 2016; Athiraman et al., 2020) and 2) Conditioning with an anesthetic dose of isoflurane (1%) as well as a subanesthetic dose of isoflurane (0.5%) provides similarly strong protection against SAH-induced vasospasm and neurological deficits in wild-type mice. The latter findings are novel and important as they demonstrate that anesthetic conditioning has great promise as a novel therapeutic strategy for improving patient outcome after SAH.
We previously reported in two preclinical studies that a brief exposure to a supratherapeutic dose of isoflurane (2% for one-hour) when started one-hour after SAH markedly reduced several characteristic features of DCI, including large artery vasospasm and neurological deficits (Milner et ah, 2015; Athiraman et ah, 2020). More recently, we and others have shown in clinical studies that a conditioning effect of inhalational anesthetics on SAH-induced vasospasm and DCI likely exists. First, Lee et al. noted that SAH patients who underwent desflurane anesthetic for aneurysm clipping developed less transcranial doppler-evident vasospasm than SAH patients who received propofol anesthetic (Lee et al., 2018). Second, we showed in a relatively small patient cohort that SAH patients who received inhalational anesthetics only during aneurysm clipping/coiling developed less angiographic vasospasm than SAH patients who received combined inhalational and intravenous anesthetic (Athiraman et al., 2019). Third, we showed in a larger patient cohort that SAH patients who received inhalational anesthetics only during aneurysm clipping/coiling developed not only less angiographic vasospasm but also less DCI than SAH patients who received combined inhalational and intravenous anesthetic (Athiraman et al., 2020).
Results from our current study confirm and extend upon past preclinical and clinical studies in several important ways. First, it validates our initial observation that a single brief exposure to a supratherapeutic concentration of isoflurane (2%) protects against SAH-induced vasospasm and neurological deficits. Second, it shows that an anesthetic dose of isoflurane (1%) provides similar levels of protection against SAH-induced vasospasm and neurological deficits – a result consistent with the aforementioned clinical studies in which anesthetic doses of inhalational anesthetics were associated with less vasospasm or DCI in SAH patients. Third, it shows that a subanesthetic dose of isoflurane (0.5%) also provides significant protection against SAH-induced vasospasm and neurological deficits – a result that opens up the possibility that an anesthetic conditioning-based therapy could be applied to SAH patients outside of the operating room. Taken together, these results provide essential support for two applications of anesthetic conditioning in SAH patients.
3.1. Isoflurane (1%) and DCI protection:
First, one-time exposure to an anesthetic dose of isoflurane (1%) could be easily applied to this patient population, as the vast majority of SAH patients undergo general anesthesia for repair of the ruptured aneurysm (either via clipping or coiling). Choosing an inhalational anesthetic with proven protective effects against DCI for these procedures would be imminently feasible. Rigorous clinical trials to examine the potential benefit of selecting isoflurane or other inhalational anesthetic over intravenous anesthetic agents such as Propofol during aneurysm repair would be required to examine this intriguing possibility. Issues of dose and timing of inhalational anesthetic conditioning to achieve maximal early brain injury (EBI) protection and long-term neurological protection in SAH patients would also need to be examined.
3.2. Isoflurane (0.5%) and DCI protection
Second, one-time exposure to a subanesthetic dose of isoflurane (0.5%) could also be considered as an application of anesthetic conditioning to SAH patients. Our finding that conditioning with 0.5% isoflurane produced significant reduction in vasospasm and improved neurological outcome in a mouse model of SAH lends support to this idea. Given that subanesthetic doses of isoflurane are intended to produce sedation and not general anesthesia, the efficacy of this dose of isoflurane conditioning would open a pathway to the use of anesthetic conditioning in settings outside the operating room by removing the need for invasive hemodynamic and ventilatory monitoring. Notably, this could include locations typically encountered by SAH patients such as the Emergency Department and the Intensive Care Unit (ICU). In fact, two past studies have started to explore the effects of isoflurane sedation on SAH patients in the ICU, albeit not in the context of isoflurane serving as a conditioning agent to prevent or reduce EBI. Villa et al. noted that administration of 0.8% isoflurane sedation to SAH patients for one-hour in the ICU increased regional cerebral blood flow, increased jugular vein oxygen saturation, and decreased cerebral metabolic rate without increasing intracranial pressure (ICP) (Villa et al., 2012). Bosel et al. showed that isoflurane sedation (0.5 to 0.8 MAC) is safe in hemorrhagic and ischemic stroke patients in the ICU (Bösel et al., 2012). Specifically, they noted that one hour of isoflurane sedation decreased cerebral oxygen extraction without increasing ICP. However, they did find that longer exposures of isoflurane sedation (6 and 12 h) impacted hemodynamics including reducing mean arterial pressure and cerebral perfusion pressure. Taken together, these two studies suggest that short exposure (1hr) of subanesthetic doses of isoflurane (< 1%) is likely well-tolerated in SAH patients, raising the prospect that a conditioning-based therapy with a sedative dose of isoflurane could be safely applied and tested in SAH patients.
3.3. Purported mechanisms of inhalational anesthetic conditioning-induced DCI protection:
Milner et al., not only showed that isoflurane conditioning protects against DCI in an animal model of SAH, but they also provided pharmacologic and genetic evidence that this protection is mediated via HIF-1α (Milner et al., 2015), a well-described transcription factor with multiple downstream molecular targets. Though the mechanism by which HIF-1α exerts its protective effect on vasospasm and DCI remains to be determined, several HIF-1α-dependent molecules have been causally linked to DCI pathophysiology including eNOS (Vellimana et al., 2011; Athiraman et al., 2020). Athiraman et al., recently showed that isoflurane conditioning afforded significant protection against SAH-induced DCI in a mice endovascular perforation model and also provided genetic evidence that this DCI protection was at least in part mediated via eNOS (Athiraman et al., 2020). Importantly, these results with isoflurane have been validated by an independent laboratory (Mutoh et al., 2016), and other inhalational anesthetics have also been shown to upregulate HIF-1α and eNOS (Zhi et al., 2012; Li et al., 2015; Redel et al., 2013).
Another potential mechanism by which inhalational anesthetic conditioning exerts its protective effect against DCI includes down-regulation of endothelin-1 (ET-1), a potent cerebral vasoconstrictor with strong pathophysiologic links to vasospasm and DCI (Zimmermann and Seifert, 1998). Experimentally, Park et al., has shown that administration of isoflurane significantly attenuates ET-1-induced vasoconstriction of cortical microvessels (Park et al., 2002). Clinically, Wang et al. showed that SAH patients undergoing desflurane anesthesia for aneurysm clipping experienced a significant reduction in ET-1 levels in the perioperative period (Wang et al., 2004).
In total, our findings show that anesthetic (1%) and subanesthetic (0.5%) doses of isoflurane conditioning robustly reduce large artery vasospasm and improve neurological outcome after experimental SAH, and do so to a similar degree as that seen with isoflurane conditioning using a supratherapeutic dose (2%). These results are important and impactful in that they provide an important foundation upon which future translational studies examining the safety and efficacy of inhalational anesthetic conditioning in SAH patients can be based.
3.4. Limitations
Our study has several limitations. First, though the endovascular perforation model closely mimics the features of SAH in humans, our findings will need validation in alternate models of experimental SAH. Second, our study focused on vasospasm-induced DCI and neurological deficits; the impact of conditioning with anesthetic and subanesthetic doses of isoflurane on non-vasospasm elements of DCI (e.g. micro-circulatory dysfunction) will require additional study. Third, our current study did not explore underlying mechanism, as our focus was primarily on assessing the translational potential of inhalational anesthetic conditioning for SAH. Fourth, the impact of isoflurane conditioning on long-term endpoints after SAH (e.g. neurobehavioral deficits) will be required before translational studies in humans can be pursued.
4. Conclusion
We conclude that conditioning with anesthetic and subanesthetic doses of isoflurane provides robust protection against vasospasm-induced DCI and neurological deficits in an experimental model of SAH. These results, when combined with the known safety profile of these doses of isoflurane in humans, strongly suggest inhalational anesthetic conditioning can be safely applied to SAH patients inside and potentially outside of the operating room. Additional studies rigorously examining the therapeutic potential of inhalational anesthetic conditioning in SAH patients is therefore warranted.
5. Methods and materials
5.1. Ethical statement, study design and animals
Our experimental protocols were approved by the institutional animal care and use committee (IACUC) at our institution. All experiments were randomized and blinded such that the investigator assessing vasospasm and neurological outcome was blinded to treatment group. Animals used in the experiment were housed in an AAALAC-accredited facility in temperature- and humidity-controlled rooms with a 12-h light-dark cycle. Mice were housed five to a cage and had ad libitum access to laboratory chow and tap water. Ten to fourteen-week-old male wild type mice (C57BL/6J) were obtained from Jackson laboratories (Bar Harbor, ME).
5.2. Experimental groups
Animals underwent either sham surgery (n = 18), SAH surgery alone (n = 19) or SAH surgery with isoflurane conditioning (n = 45). As detailed below, isoflurane conditioning was divided into three separate groups 0.5% (n = 14), 1% (n = 16) and 2% (n = 15).
5.3. SAH procedure
SAH was induced by endovascular perforation model as previously described (Athiraman et al., 2020; Milner et al., 2015, 2014; Vellimana et al., 2011; Han et al., 2012). Briefly, a midline incision was made in the neck and the external carotid artery (ECA) was exposed and a 5–0 nylon suture was introduced through it and advanced distally through the internal carotid artery (ICA) until reaching the ICA bifurcation. The suture was advanced further to induce SAH, and then removed and the ECA was ligated. Mice undergoing sham operation underwent the same steps except for perforation. Mice was recovered in a heated incubator and was returned to their cages when fully ambulatory, typically within 2 h of induction.
5.4. Isoflurane conditioning
Anesthetic conditioning was performed using 1-hour exposure of different doses of isoflurane (0.5%, 1% and 2%) beginning 1 h after induction of SAH in separate cohorts. Briefly, mice were placed in an anesthetic induction chamber and perfused with isoflurane and room air for one hour. The sham group was placed in the same induction chamber but exposed only to room air for one hour. Isoflurane concentration in the anesthetic induction chamber was measured by an anesthetic gas analyzer (Datex Ohmeda, Capnomac Ultima, USA). Normothermia was maintained with a homeothermic blanket.
5.5. Vasospasm assessment
SAH -induced large artery vasospasm was measured at 72 h after SAH by a pressure controlled cerebrovascular casting with ROX-SE (5-(and-6)-Carboxy-X-rhodamine, succinimidyl ester) technique as previously described (Aum et al., 2017). Briefly, mice were anesthetized and transcardially perfused with 1X phosphate buffered saline, 10% formalin and ROX-SE. After removing the brains, blood vessels in the circle of Willis were imaged under a fluorescent microscope using a charge coupled-device camera (CCD camera, CoolSNAP EZ, Photometrics, Tucson, AZ) and MetaMorph® software (Universal Imaging, West Chester, PA). Vasospasm measurement for each brain sample was obtained by recording the narrowest diameter within the first 1000 μm segment of the left (ipsilateral) middle cerebral artery (MCA).
5.6. Neurobehavioral outcome assessment
Neurological outcome was measured at baseline and for next three days after sham/SAH surgery until animals were sacrificed as previously described (Athiraman et al., 2020; Milner et al., 2015, 2014; Vellimana et al., 2011; Han et al., 2012; Aum et al., 2017). Briefly, neurological function was graded based on a motor score (0 to 12) that evaluates spontaneous activity (0–3, 0- no movement, 1-animal barely moves, 2-animal moves around cage, 3-normal exploration) symmetry of limb movements (0–3, 0- no movement of either forelimb, 1-minimal movement of either forelimb, 2-one forelimb outstretches less than the other forelimb, 3-all extremities extend symmetrically), climbing (0–3, 0-unable to hang from mesh, 1- able to hang on mesh for few seconds but falls, 2-able to hang but unable to displace on mesh, 3- able to hang and displace on mesh), balance and coordination (0–3, 0- unable to hold the pole, 1-able to hold for a few seconds but falls, 2-able to hold on pole and get to the top surface but unable to walk, 3-able to hold on pole and get to the top surface and walk), and a sensory score (4 to 12) that evaluates body proprioception (1–3, 1- no reaction to stimulus on one side or both sides, 2- decreased & slower head turning to stimulus on one side compared to the other side, 3- symmetric head turning to stimulus on both sides), vibrissae touch (1–3, 1- no reaction to stimulus on one side or both sides, 2 - slow reaction to stimulus on one side or both sides, 3- normal speed bilaterally), visual response (1–3, 1- no reaction to visual stimulus, 2- blinking by stimulation in one visual field only, 3- normal blinking by stimulation of both visual fields), and tactile responses (1–3, 1- no withdrawal. No reaction to stimulus on one or both sides, 2- slow withdrawal. Slow reaction to stimulus on one side compared to the other side, 3- Quick withdrawal. Normal speed bilaterally).
5.7. Assessment of physiological variables
Femoral artery cannulation was performed in a subset of mice to assess the physiological variables in all the treatment groups. Following parameters were assessed from the arterial line – pH, partial pressure of carbondioxide (pCO2), partial pressure of oxygen (pO2), oxygen saturation (sO2), hemoglobin (Hb), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP) and heart rate (HR). These parameters were assessed at the end of a two-hour period after sham or SAH surgery in all groups. Blood pressure values were recorded over a period of 10 min and the average values are presented.
5.8. Statistical analysis
Data are represented as the mean ± SEM. Large artery vasospasm measured from the MCA was analyzed by ANOVA followed by Newman-Keuls multiple comparison test. Neurological outcome was measured by two-way repeated measures ANOVA followed by a Newman-Keuls multiple comparison test. Statistical significance was set at P < 0.05.
HIGHLIGHTS.
Anesthetic dose of isoflurane conditioning provides protection against DCI.
Subanesthetic dose of isoflurane conditioning provides protection against DCI.
This finding may have wide therapeutic implications in SAH patients.
Acknowledgements
We thank Ernesto Gonzales for performing subarachnoid hemorrhage surgery.
Funding
This work was supported by the Brain Aneurysm Foundation grant awarded to Dr. Athiraman; McDonnell Center for Cellular and Molecular Neurobiology grant awarded to Dr. Athiraman; William L. Young Neuroscience Research Award grant awarded to Dr. Athiraman, NIH T32 training grant in Anesthesiology research awarded to Dr. Athiraman and National Institutes of Health grants R01 NS091603 awarded to Dr. Zipfel and R25 NS090978 awarded to Dr. Zipfel.
Footnotes
CRediT authorship contribution statement
Umeshkumar Athiraman: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Meizi Liu: Validation, Formal analysis, Investigation, Data curation, Writing - review & editing. Keshav Jayaraman: Validation, Investigation, Data curation, Writing - review & editing. Jane Yuan: Validation, Writing - review & editing. Jogender Mehla: Validation, Writing - review & editing. Gregory J. Zipfel: Validation, Resources, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, Sternau LL, Torner J, Adams HP Jr., Feinberg W, 1994. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Stroke 25, 2315–2328. [DOI] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP, 1997. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International stroke incidence collaboration. Stroke 28, 491–499. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A, 1994. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25, 1342–1347. [DOI] [PubMed] [Google Scholar]
- Gidday JM, 2006. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci 7, 437–448. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A, 2009. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 8, 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg G, Dirnagl U, 2005. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia 50, 307–320. [DOI] [PubMed] [Google Scholar]
- Milner E, Johnson AW, Nelson JW, Harries MD, Gidday JM, Han BH, Zipfel GJ, 2015. HIF-1α mediates isoflurane-induced vascular protection in subarachnoid hemorrhage. Ann. Clin. Transl. Neurol 2, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athiraman U, Aum D, Vellimana AK, Osbun JW, Dhar R, Tempelhoff R, Zipfel GJ, 2019. Evidence for a conditioning effect of inhalational anesthetics on angiographic vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg 14, 1–7. [DOI] [PubMed] [Google Scholar]
- Athiraman U, Dhar R, Jayaraman K, Karanikolas M, Helsten D, Yuan J, Lele A, Rath GP, Tempelhoff R, Roth S, Zipfel GJ., 2020. Conditioning Effect of Inhalational Anesthetics on Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Neurosurgery. 2020 Aug 28; nyaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athiraman U, Jayaraman K, Liu M, Giri T, Yuan J, Zipfel GJ, 2020. Role of endothelial nitric oxide synthase in isoflurane conditioning-induced neurovascular protection in subarachnoid hemorrhage. J. Am. Heart Assoc 8, e017477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner JM, Gong D, Li J, Eger EI 2nd, Laster MJ, 1999. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg. 89, 1030–1034. [DOI] [PubMed] [Google Scholar]
- Nickalls RW, Mapleson WW, 2003. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 91, 170–174. [DOI] [PubMed] [Google Scholar]
- Vellimana AK, Milner E, Azad TD, Harries MD, Zhou M, Gidday JM, Han BH, Zipfel GJ, 2011. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke 42, 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner E, Holtzman JC, Friess S, Hartman RE, Brody DL, Han HB, Zipfel GJ, 2014. Endovascular perforation subarachnoid hemorrhage fails to cause Morris water maze deficits in the mouse. J. Cereb. Blood Flow Metab 9, 1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Vellimana AK, Zhou ML, Milner E, Zipfel GJ, 2012. Phosphodiesterase 5 inhibition attenuates cerebral vasospasm and improves functional recovery after experimental subarachnoid hemorrhage. Neurosurgery 1, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aum DJ, Vellimana AK, Singh I, Milner E, Nelson JW, Han BH, Zipfel GJ, 2017. A novel fluorescent imaging technique for assessment of cerebral vasospasm after experimental subarachnoid hemorrhage. Sci Rep. 7, 9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Mutoh T, Sasaki K, Yamamoto Y, Tsuru Y, Tsubone H, Ishikawa T, Taki Y, 2016. Isoflurane postconditioning with cardiac support promotes recovery from early brain injury in mice after severe subarachnoid hemorrhage. Life Sci. 153, 35–40. [DOI] [PubMed] [Google Scholar]
- Lee JW, Woo JH, Baik HJ, Kim DY, Chae JS, Yang NR, Seo EK, 2018. The effect of anesthetic agents on cerebral vasospasms after subarachnoid hemorrhage: a retrospective study. Medicine (Baltimore) 97, e11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa F, Iacca C, Molinari AF, Giussani C, Aletti G, Pesenti A, Citerio G, 2012. Inhalation versus endovenous sedation in subarachnoid hemorrhage patients: effects on regional cerebral blood flow. Crit Care Med. 40, 2797–2804. [DOI] [PubMed] [Google Scholar]
- Bösel J, Purrucker JC, Nowak F, Renzland J, Schiller P, Pérez EB, Poll S, Brunn B, Hacke W, Steiner T, 2012. Volatile isoflurane sedation in cerebrovascular intensive care patients using AnaConDa(®): effects on cerebral oxygenation, circulation, and pressure. Intensive Care Med. 38, 1955–1964. [DOI] [PubMed] [Google Scholar]
- Zhi Y, Qulian G, Pingping X, Na W, Wang E, Yajing Y, 2012. Sevoflurane postconditioning involves an up-regulation of HIF-1α and HO-1 expression via PI3K/Akt pathway in a rat model of focal cerebral ischemia. Brain Res. 1463, 63–74. [DOI] [PubMed] [Google Scholar]
- Li S, Xu J, Yao W, Li H, Liu Q, Xiao F, Irwin MG, Xia Z, Ruan W, 2015. Sevoflurane pretreatment attenuates TNF-α-induced human endothelial cell dysfunction through activating eNOS/NO pathway. Biochem. Biophys. Res. Commun 460 (3), 879–886. [DOI] [PubMed] [Google Scholar]
- Redel A, Stumpner J, Smul TM, Lange M, Jazbutyte V, Ridyard DG, Roewer N, Kehl F, 2013. Endothelial nitric oxide synthase mediates the first and inducible nitric oxide synthase mediates the second window of desflurane-induced preconditioning. J .Cardiothorac. Vase. Anesth 27 (3), 494–501. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Seifert V, 1998. Endothelin and subarachnoid hemorrhage: an overview. Neurosurgery 43, 863–875. [DOI] [PubMed] [Google Scholar]
- Park KW, Dai HB, Metais C, Comunale ME, Sellke FW, 2002. Isoflurane does not further impair microvascular vasomotion in a rat model of subarachnoid hemorrhage. Can. J. Anaesth 49, 427–433. [DOI] [PubMed] [Google Scholar]
- Wang T, Luo F, Shan R, Zhen Y, Zhao J, Zhang S, 2004. Changes of endothelin and calcitonin gene-related peptide during desflurane anesthesia in patients undergoing intracranial aneurysm clipping. J. Neurosurg. Anesthesiol 16, 236–239. [DOI] [PubMed] [Google Scholar]