Abstract
Background
Inhaled corticosteroids (ICS) afford therapeutic benefits in some COPD patients, but their widespread use is cautioned due to an increased risk of developing pneumonia. Subclass variations exist, and the risk profile differs for individual ICS. Formulation particle size has been identified as a potential effect modifier. The present study compared the risk of pneumonia among new COPD users of fixed-dose combination inhalers containing fine-particle fluticasone (fp-FDC-F) versus extrafine particle beclometasone (ef-FDC-BDP).
Methods
A propensity matched historical cohort study was conducted using data from the Optimum Patient Care Research Database. COPD patients aged ≥40 years with ≥1 year of continuous medical data who initiated fp-FDC-F or ef-FDC-BDP were compared. The primary outcome was time to pneumonia event, as treated, using either sensitive (physician diagnosed) or specific (physician diagnosed and x-ray or hospital admission confirmed) definitions.
Results
A total of 13,316 patients were matched. Initiation of fp-FDC-F (mean dosage furoate 99 µg; propionate 710 µg) was associated with an increased risk of pneumonia versus ef-FDC-BDP (mean beclometasone dose 395 µg), irrespective of definition (sensitive HR 1.38 95% CI 1.14–1.68; specific HR 1.31 95% CI 1.05–1.62).
Conclusion
In the current investigation, we found that in comparison to extrafine beclomethasone, commencing a formulation containing fluticasone is associated with an increased risk of developing pneumonia. These observations support the idea that not all ICS are equal in their adverse effects and subclass variations exist and should be carefully considered in the treatment choice.
Keywords: inhaled corticosteroids, pneumonia, COPD, extrafine beclomethasone, fluticasone
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by a chronic inflammatory response that compromises parenchymal tissue integrity and normal repair mechanisms. This ultimately results in emphysema, remodelling and fibrosis of the small airways, manifesting as gas trapping and poorly reversible airflow limitation.1 Long-acting bronchodilators (LABD), including long-acting beta-2 agonists (LABA) or long-acting muscarinic antagonists (LAMA), are the primary drug classes used to manage this condition, either in isolation or as a combined formulation.2,3 In patients at higher risk of exacerbations, inhaled corticosteroids (ICS) can be combined with LABD to form either a dual or triple therapy.3 The use of ICS/LABD combinations in managing severe COPD confers significant benefits. Namely, affording greater symptom control, improved pulmonary function and health status, and reduced exacerbations more effectively than either drug class administered in isolation.4,5
Despite the benefits afforded by ICS, their widespread use is cautioned due to an increased risk of pneumonia. The first major investigation to highlight this relationship was the TORCH study6 and numerous others have since supported this observation.7–12 In relation to effect modifiers, current smoking status, age, body mass index, exacerbation history and disease severity have been shown to impact the risk profile of developing pneumonia in COPD.1
There is also evidence to suggest subclass variations exist and the risk profile of developing pneumonia following ICS use could be impacted by the subtype of corticosteroid used,11,13–17 as reported by both the UPLIFT11 and PATHOS13 studies, as well as numerous others. In highlighting these observations, it should be noted that irrespective of the ICS subtype used, they all carry an increased risk of pneumonia when compared to LABD therapy in insolation, as evidence in the ETHOS, TRINITY and FORWARD studies.12,18,19
In addition to subclass variations, ICS particle size has been suggested to impact on pneumonia risk. In a recent publication, the terms extrafine and fine, as they relate to ICS particle size, have been defined as a mass median aerodynamic diameter (MMAD) of <2.1µm and 2.1–5µm respectively.20 Notably, extrafine formulations have been observed to have a significant reduction in odds ratio for pneumonia diagnosis when compared to fine particle preparations.15 With the reduced risk associated with extrafine formulations being attributed to greater deposition within small airways, and smaller doses needed to achieve a therapeutic effect.15 This is an important consideration as a clear relationship between ICS dose and pneumonia risk has been established.12 Collectively, there is a requirement for a real-world study comparing the use of extrafine particle fixed dose combination beclometasone dipropionate (ef-FDC-BDP; with a MMAD of 1.1µm in FOSTER® and TRIMBOW® NEXTHALER® DPIs and 1.3µm in FOSTER® pMDI) with other ICSs, such as fine particle fluticasone (fp-FDC-F; with a MMAD of 3.9µm and 3.2µm for propionate (SERETIDE® DISKUS®) and furoate (RELVAR® ELLIPTA®) esters respectively).21–23 The aim of the present study was to compare the risk of pneumonia in COPD among new users of fixed-dose dual or triple combination inhalers containing fine-particle fluticasone (fp-FDC-F) versus ef-FDC-BDP, and to assess if differences between fluticasone esters can be identified.
Materials and Methods
Study Design and Ethical Approval
The present investigation was a historical propensity matched cohort study, including a broad real-life population of patients with active COPD in the UK. The baseline period was one year prior to the index date, which was defined as the initiation date of either an extrafine particle fixed dose combination containing beclometasone (Foster and Trimbow) or a fine particle fixed dose combination containing fluticasone (Relvar Ellipta, Trelegy Ellipta, Seretide, Sirdupla and Airflusal Forspiro). Data were obtained from the Optimum Patient Care Research Database (OPCRD; https://opcrd.co.uk/). The OPCRD dataset comprises medical records of more than 12 million patients from over 800 general practices across the UK (approximately 10% of the total UK population), drawn from all UK clinical systems (EMIS, TPP SystmOne, InPS Vision, Microtest Evolution). It benefits from a long retrospective period (median time in the database is 13 years, going back to birth for summary diagnostic data in many cases), and contains linked patient-completed respiratory questionnaires. Respiratory-related outcome measures within the OPCRD have been validated using patient reported outcomes.24 The study protocol was established prior to data extraction, in accordance with the criteria for the European Network Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) and follows the ENCePP code of conduct (2014). Registration of the study with the European Union electronic Register of Post-Authorization studies was also undertaken (EUPAS35439). As noted, the dataset was derived from the OPCRD, which has ethical approval from the National Health Service Research Authority to hold and process anonymised research data (Research Ethics Committee reference: 15/EM/0150). Approval for this study was granted by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee – the independent scientific advisory committee for the OPCRD (ADEPT0820). The authors do not have permission to give public access to the study dataset; requests to access OPCRD can be made via the OCPRD website (https://opcrd.co.uk/our-database/data-requests/) or via the enquiries email info@opcrd.co.uk.
Inclusion and Exclusion Criteria
Requirements for inclusion in the current study were age >40 years at the date of COPD diagnosis, which was not followed by a COPD resolved diagnostic disease code, >1 year of continuous data in the electronic health record prior to index date, commencement of either ef-FDC-BDP or fp-FDC-F during the study period (2015–2019), without prior use of a FDC ICS/LABD combination and a second prescription within 90 days of the first. Exclusion criteria were only having recorded a “never smoked status”, a diagnostic read code for other chronic lower respiratory tract conditions and having a pneumonia event or respiratory-related bacterial infection in the 28 days prior to the index date.
Outcomes
The primary outcome was time to pneumonia event, using sensitive and specific definitions. Sensitive definition pneumonia was any physician diagnosed pneumonia, and specific definition pneumonia was physician diagnosed pneumonia confirmed with chest radiograph, or hospital admission for pneumonia within one month of pneumonia diagnosis. The secondary outcome was time to a respiratory infection defined as either upper-, lower-respiratory tract infection or antibiotic prescription with evidence of either upper- or lower-respiratory tract infection on the same day. Read codes used to define upper- and lower-respiratory tract infections are included in Appendices 1 and 2. Exploratory analysis examining time to event for acute OCS use, antibiotics prescription, exacerbation, primary care recorded hospitalisation and pneumonia-related hospitalisation were also undertaken.
Confounders and Propensity Matching
To account for confounders, propensity score matching25 was undertaken prior to statistical analysis. Covariates were required to have no more than 30% missing data. Where data were missing, a variable was encoded into a categorical variable with a category added for the observations with missing values. The propensity score was generated from logistic regression modelling using all available patient-level baseline characteristics. In line with Austin (2011),25 the logit of the propensity score was used as the matching scale with a calliper width equal to 0.2 of the standard deviation of the logit of the propensity score.
Minimum criteria for accepting a matching set were based on matching rate (>60% of smaller treatment group matched) and multivariable balance (<90% of baseline variables showing <10% standardised mean difference). Residual bias potential after propensity matching was assessed using the relative change in coefficient of the treatment when each baseline characteristic was added into the outcome model fitted to the propensity score matched samples. Where bias statistics were at least 2%, baseline variables were added to the outcome model in a forward selection approach, in descending order of highest bias potential.
Statistical Analysis
Superiority of fp-FDC-F was assessed against ef-FDC-BDP using per protocol analysis. Patients were censored at the end of data availability, 4 weeks after the last prescription containing ICS or 4 weeks after switching to the comparator. The 4-week period ensures a pneumonia event was captured even if early symptoms caused discontinuation of ICS or switching to other medications.
Cox regression was used for time to event analysis. In the primary analysis, this assessed the association between ICS treatment and time to the first pneumonia event following initiation of fp-FDC-F or ef-FDC-BDP. Analyses were repeated in unmatched, and propensity score matched samples to quantify the impact of measured confounders. A similar approach was adopted for secondary and exploratory outcomes. Exacerbations occurring within 28 days of a previous event were considered part of a single episode. From this modelling, hazard ratios and 95% confidence intervals for each effect were generated. All statistical analysis was performed using R,26 with propensity score matching performed using the Matching package.27
Results
Comparison of ef-FDC-BDP and fp-FDC-F
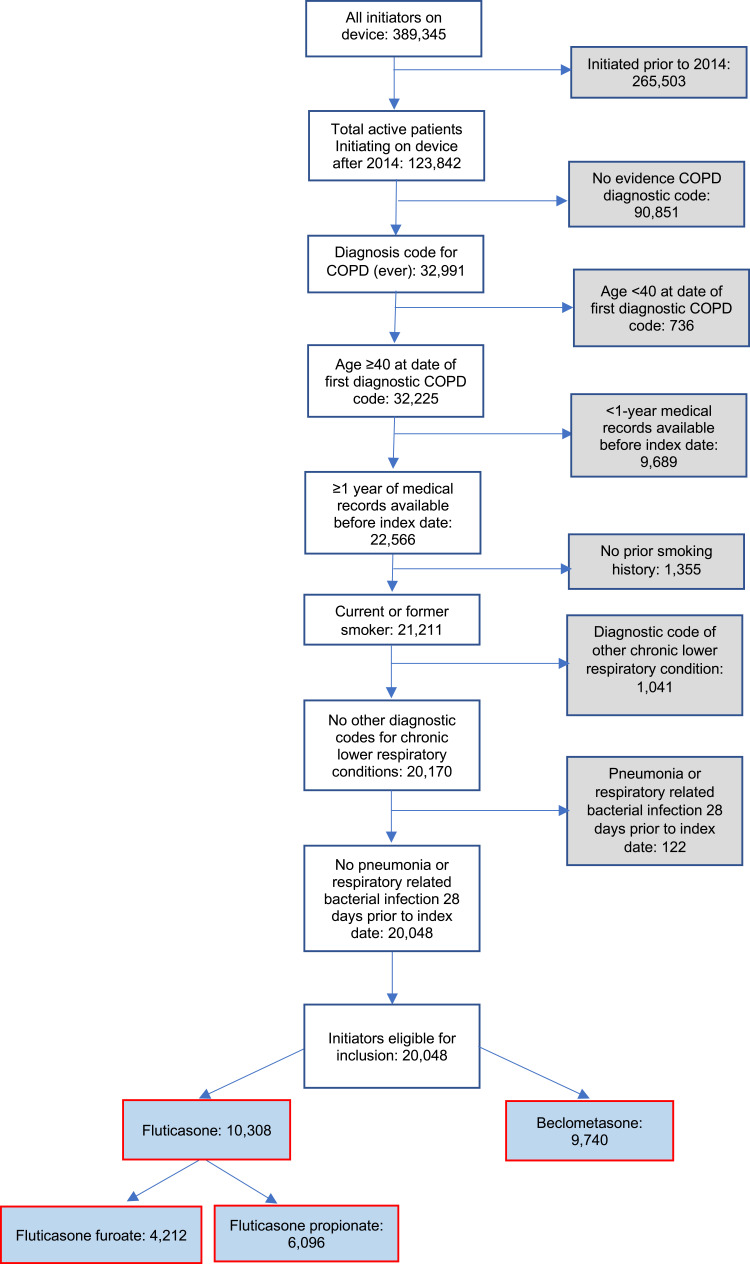
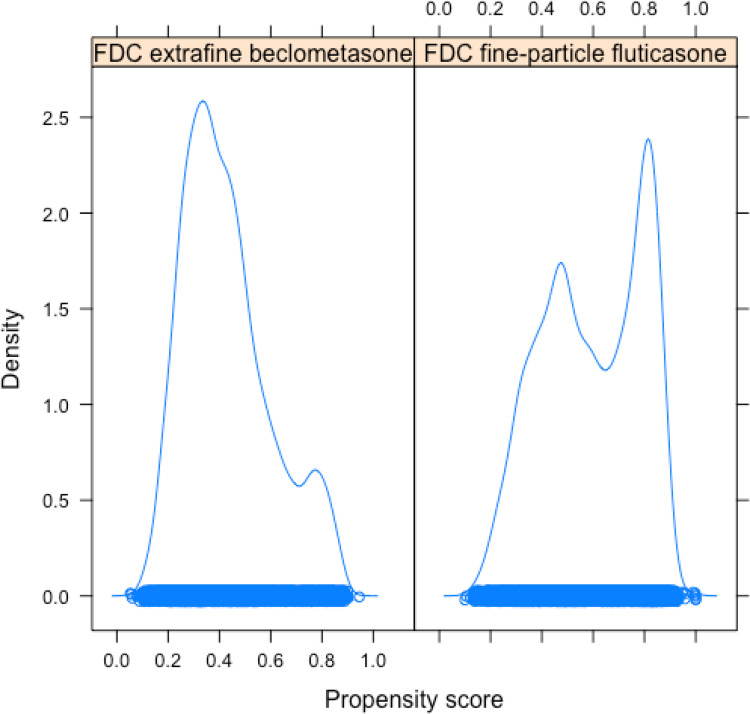
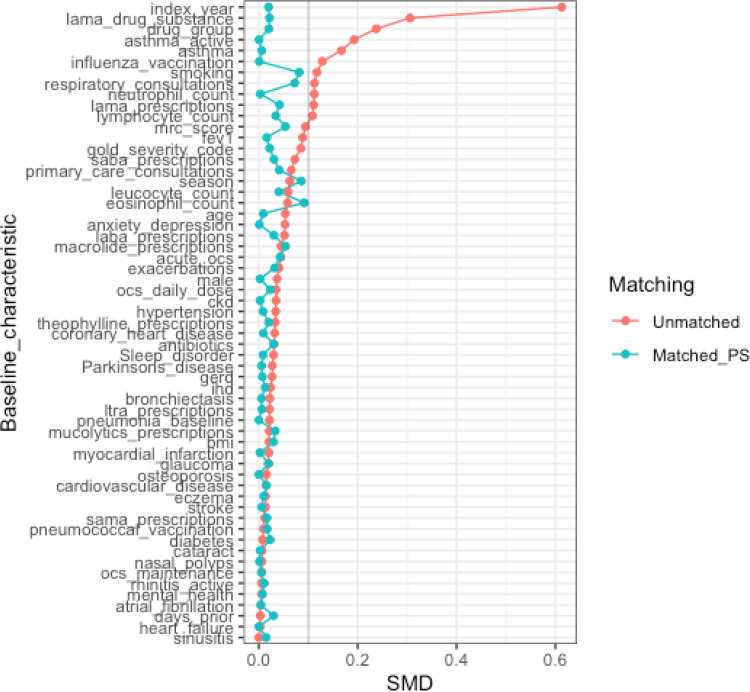
A total of 20,048 patients were eligible for inclusion in the present study, 9740 commencing ef-FDC-BDP and 10,308 commencing fp-FDC-F (Figure 1). Of these, 13,316 patients were matched, 6658 commencing beclometasone and 6658 commencing fluticasone. The baseline characteristics of the unmatched and propensity matched cohorts for new uses of ef-FDC-BDP and fp-FDC-F are shown in Table 1 and Appendix 3. The distribution of propensity scores had a broad region of common support (0.10 < propensity score <0.95) (Figure 2) and the standardized mean difference was below the 10% threshold for all baseline characteristics (Figure 3).
Figure 1.
Flow diagram of patients eligible for propensity matching.
Abbreviations: COPD, chronic obstructive pulmonary disease; ef-FDC-BDP, extrafine particle fixed dose beclometasone; fp-FDC-F, fine-particle fixed dose fluticasone; fp-FDC-FF, fine-particle fixed dose fluticasone furoate; fp-FDC-FP, fine-particle fixed dose fluticasone propionate.
Table 1.
Demographic features of unmatched and matched populations commencing either extrafine fixed dose beclometasone or fine fixed dose fluticasone
| Variable | Unmatched | Matched | |||
|---|---|---|---|---|---|
| Initiating ef-FDC- BDP n=9740 | Initiating fp-FDC-F n=10,308 | Initiating ef-FDC-BDP n=6658 | Initiating fp-FDC-F n=6658 | ||
| Age (years) | Mean (SD) | 67.60 (11.43) | 68.20 (11.06) | 67.89 (11.25) | 67.79 (11.20) |
| Male gender | Male n (%) | 5077 (52.1) | 5563 (54.0) | 3529 (53.0) | 3521 (53.0) |
| Smoking status | N (% non-missing) | 9421 (96.7) | 9777 (94.8) | 6511 (97.8) | 6529 (98.1) |
| Ex-smoker n (%) | 4374 (46.4) | 4436 (45.4) | 2871 (44.1) | 2953 (45.2) | |
| Current smoker n (%) | 5047 (53.6) | 5341 (54.6) | 3508 (53.9) | 3368 (51.6) | |
| Index year | <2014, n (%) | 843 (8.7) | 3081 (29.9) | 843 (12.7) | 856 (12.9) |
| 2015, n (%) | 1458 (15.0) | 1891 (18.3) | 1344 (20.2) | 1346 (20.2) | |
| 2016, n (%) | 1502 (15.4) | 1252 (12.1) | 1092 (16.4) | 1100 (16.5) | |
| 2017, n (%) | 1562 (16.0) | 1129 (11.0) | 955 (14.3) | 947 (14.2) | |
| 2018, n (%) | 1715 (17.6) | 1005 (9.7) | 866 (13.0) | 838 (12.6) | |
| ≥ 2019, n (%) | 2660 (27.3) | 1950 (18.9) | 1558 (23.4) | 1571 (23.6) | |
| BMI (kg/m2) | N (% non-missing) | 9296 (95.4) | 9850 (95.6) | 6296 (94.6) | 6277 (94.3) |
| Underweight <18.5, n (%) | 425 (4.6) | 486 (4.9) | 300 (4.8) | 326 (5.2) | |
| Normal ≥18.5 <25, n (%) | 3178 (34.2) | 3382 (34.3) | 2165 (34.4) | 2122 (33.8) | |
| Overweight ≥25 <30, n (%) | 2818 (30.3) | 2931 (29.8) | 1923 (30.5) | 1872 (29.8) | |
| Obese ≥30, n (%) | 2875 (30.9) | 3051 (31.0) | 1907 (30.3) | 1956 (31.2) | |
| Asthma diagnosis ever | Yes, n (%) | 2511 (25.8) | 1943 (18.8) | 1438 (21.6) | 1422 (21.4) |
| Asthma diagnosis ever - Smoking status | N (% non-missing) | 2461 (25.3) | 1874 (18.2) | 1435 (99.8) | 1419 (99.8) |
| Ex-smoker n (%) | 1301 (52.9) | 966 (51.5) | 728 (50.6) | 702 (49.4) | |
| Current smoker n (%) | 1160 (47.1) | 908 (48.5) | 674 (46.9) | 676 (47.5) | |
| Active Asthma | Yes, n (%) | 2204 (22.6) | 1588 (15.1) | 1224 (18.4) | 1225 (18.4) |
| Comorbidities | |||||
| Anxiety or depression | Yes, n (%) | 3893 (40.0) | 3854 (37.4) | 2498 (37.5) | 2501 (37.6) |
| Allergic/non-allergic rhinitis | Yes, n (%) | 449 (4.6) | 431 (4.2) | 794 (11.9) | 795 (11.9) |
| Eczema | Yes, n (%) | 232 (2.4) | 266 (2.6) | 162 (2.4) | 170 (2.6) |
| Gastro-oesophageal reflux disease | Yes, n (%) | 205 (2.1) | 179 (1.7) | 128 (1.9) | 127 (1.9) |
| Chronic rhinosinusitis | Yes, n (%) | 259 (2.7) | 274 (2.7) | 171 (2.6) | 187 (2.8) |
| Nasal polyps, ever before | Yes, n (%) | 204 (2.1) | 207 (2.0) | 126 (1.9) | 127 (1.9) |
| Bronchiectasis | Yes, n (%) | 221 (2.3) | 201 (1.9) | 134 (2.0) | 139 (2.1) |
| Hypertension | Yes, n (%) | 3585 (36.8) | 3964 (38.5) | 2411 (36.2) | 2384 (35.8) |
| Cardiovascular disease | Yes, n (%) | 2766 (28.4) | 2995 (29.1) | 1850 (27.8) | 1804 (27.1) |
| Coronary heart disease | Yes, n (%) | 1230 (13.4) | 1392 (14.3) | 894 (13.4) | 873 (13.1) |
| Myocardial infarction | Yes, n (%) | 700 (7.2) | 796 (7.7) | 480 (7.2) | 476 (7.1) |
| Cerebrovascular accident | Yes, n (%) | 486 (5.0) | 545 (5.3) | 327 (4.9) | 319 (4.8) |
| Heart failure | Yes, n (%) | 428 (4.4) | 447 (4.3) | 279 (4.2) | 279 (4.2) |
| Ischaemic heart disease | Yes, n (%) | 1394 (14.3) | 1562 (15.2) | 974 (14.6) | 944 (14.2) |
| Diabetes diagnosis or medication | Yes, n (%) | 1438 (14.8) | 1549 (15.0) | 1007 (15.1) | 953 (14.3) |
| Osteoporosis | Yes, n (%) | 525 (5.4) | 592 (5.7) | 350 (5.3) | 350 (5.3) |
| Parkinson disease | Yes, n (%) | 23 (0.2) | 40 (0.4) | 21 (0.3) | 19 (0.3) |
| Sleep disorder | Yes, n (%) | 1330 (13.7) | 1303 (12.6) | 858 (12.9) | 839 (12.6) |
| Drug treatment category in the year prior to the index date | No therapy | 1574 (16.2) | 1922 (18.6) | 1350 (20.3) | 1355 (20.4) |
| SABA/SAMA | 1890 (19.4) | 2057 (20.0) | 1328 (19.9) | 1346 (20.2) | |
| ICS | 2144 (22.0) | 1533 (14.9) | 1195 (17.9) | 1171 (17.6) | |
| ICS + LABA | 353 (3.6) | 257 (2.5) | 198 (3.0) | 198 (3.0) | |
| ICS + LAMA | 640 (6.6) | 508 (4.9) | 360 (5.4) | 353 (5.3) | |
| ICS + LABA + LAMA | 267 (2.7) | 286 (2.8) | 174 (2.6) | 180 (2.7) | |
| LABA | 260 (2.7) | 280 (2.7) | 163 (2.4) | 179 (2.7) | |
| LABA + LAMA | 921 (9.5) | 1268 (12.3) | 662 (9.9) | 653 (9.8) | |
| LAMA | 1691 (17.4) | 2197 (21.3) | 1228 (18.4) | 1223 (18.4) | |
| Eosinophil count (10^9/L) | N (% non-missing) | 7968 (81.8) | 8363 (81.1) | 5308 (79.8) | 5259 (79.0) |
| <0.15, n (%) | 2487 (31.2) | 2599 (31.1) | 1663 (31.3) | 1670 (31.8) | |
| 0.15 <0.35, n (%) | 3893 (48.9) | 4021 (48.1) | 2584 (48.7) | 2517 (47.8) | |
| ≧0.35, n (%) | 1588 (19.9) | 1743 (20.9) | 1061 (20.0) | 1072 (20.4) | |
| GOLD group | N (% non-missing) | 6876 (70.6) | 7571 (73.4) | 4618 (69.4) | 4610 (69.2) |
| A, n (%) | 2385 (34.7) | 2586 (34.2) | 1613 (24.2) | 1618 (24.3) | |
| B, n (%) | 1880 (27.3) | 2235 (29.5) | 1337 (20.1) | 1306 (19.6) | |
| C, n (%) | 1452 (21.1) | 1431 (18.9) | 884 (13.3) | 917 (13.8) | |
| D, n (%) | 1159 (16.9) | 1319 (17.4) | 784 (11.8) | 769 (11.6) | |
Abbreviations: BMI, body mass index; ef-FDC-B, extrafine fixed dose combination beclometasone; fp-FDC-F, fine-particle fixed dose fluticasone; GOLD, global initiative for chronic obstructive lung disease; ICS, inhaled corticosteroid; ICS+LABA, inhaled corticosteroid + long acting bronchodilator; ICS+LAMA, inhaled corticosteroid + long acting muscarinic antagonist; ICS + LABA+LAMA, inhaled corticosteroid + long acting beta agonist + long acting muscarinic antagonist; LABA, long acting beta agonist; LABA+LAMA, long acting beta agonist + long acting muscarinic antagonist; LAMA, long acting muscarinic antagonist; SABA/SAMA, short acting beta agonist/short acting muscarinic antagonists; SD, standard deviation.
Figure 2.
Density plots showing the distribution of propensity scores for patients treated with extrafine particle fixed dose beclometasone (ef-FDC-BDP) and fine-particle fixed dose fluticasone (fp-FDC-F). The propensity score represents the estimated probability that each patient is assigned to fp-FDC-F treatment, based on their baseline characteristics (with possible values ranging from 0 to 1). A rug plot is shown along the x-axis, with a circle representing the propensity score for each patient, providing a compact visualisation of the range of propensity score values for each treatment (range of propensity scores for ef-FDC-BDP: 0.09–0.94; range of propensity scores for fp-FDC-F: 0.12–0.96).
Abbreviation: FDC, fixed dose combination.
Figure 3.
Covariate plot showing standardised mean differences (SMD) for comparison of baseline characteristics for new users of inhaled fixed dose combinations with fine particle fluticasone or extrafine particle beclometasone before and after propensity score matching.
Notes: Acute_ocs, number of acute oral steroid prescriptions; days_prior, number of days available in-patient record prior to index date; drug_group, COPD drug group classification in baseline period (No therapy, SABA/SAMA, LABA, LABA + LAMA, LAMA); ocs_maintenance, number of maintenance oral steroid prescriptions in baseline period.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; FEV1, forced expiratory volume in one second; GERD, Gastro-eosophageal reflux disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IHD, ischemic heart disease; LAMA, long acting muscarinic antagonist; LTRA, Leukotriene receptor antagonists; MRC, Medical Research Council; OCS, oral corticosteroids; PS, propensity score; SABA, short-acting beta-agonist; SAMA, short acting muscarinic antagonist; SMD, standardised mean difference.
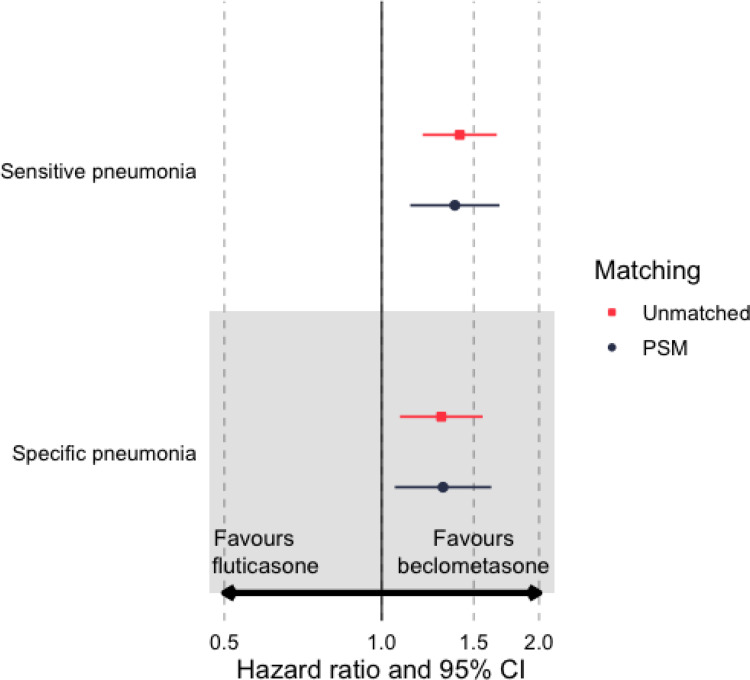
In comparison to ef-FDC-BDP, initiation of fp-FDC-F was associated with an increased risk of developing sensitive definition pneumonia, for both the propensity matched (HR 1.38; 95% CI 1.14–1.68) and unmatched (HR 1.41; (95% CI 1.20–1.66)) cohorts (Table 2 and Figure 4). Similarly, commencing fp-FDP-F was associated with an increase in the risk of developing specific definition pneumonia for the propensity matched (HR 1.31; 95% CI 1.05–1.62) and unmatched cohorts (HR 1.30; 95% CI 1.09–1.56).
Table 2.
Hazard ratios for time-to-event uutcomes for new users of fine-particle fixed dose fluticasone and extra fine fixed dose beclometasone in propensity score matched samples
| Outcome | Number of Patients | Number of Patients with ≥ 1 Event (%) | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Sensitive pneumonia definition | |||||
| Unmatched | 20,048 | 584 (2.9%) | 1.41 | 1.20–1.66 | P < 0.001 |
| Propensity score matched | 13,316 | 399 (3.0%) | 1.38 | 1.14–1.68 | P = 0.001 |
| Specific pneumonia definition | |||||
| Unmatched | 20,048 | 471 (2.3%) | 1.30 | 1.09–1.56 | P = 0.004 |
| Propensity score matched | 13,316 | 322 (2.4%) | 1.31 | 1.05–1.62 | P = 0.015 |
| Secondary outcomes | |||||
| Respiratory outcome | |||||
| URTI & LRTI | |||||
| Unmatched | 20,048 | 5327 (26.6%) | 1.09 | 1.03–1.15 | P = 0.002 |
| Propensity score matched | 13,316 | 3535 (26.8%) | 1.08 | 1.01–1.16 | P = 0.019 |
| LRTI only | |||||
| Unmatched | 20,048 | 4817 (24.1%) | 1.09 | 1.03–1.15 | P = 0.004 |
| Propensity score matched | 13,316 | 3200 (24.3%) | 1.08 | 1.01–1.15 | P = 0.036 |
| Exploratory outcomes | |||||
| Acute OCS use | |||||
| Unmatched | 20,048 | 7099 (35.5%) | 1.06 | 1.01–1.11 | P = 0.023 |
| Propensity score matched | 13,316 | 4795 (36.1%) | 1.04 | 0.98–1.10 | P = 0.178 |
| Antibiotic prescription | |||||
| Unmatched | 20,048 | 6502 (32.5%) | 1.08 | 1.03–1.13 | P = 0.003 |
| Propensity score matched | 13,316 | 4351 (32.8%) | 1.03 | 0.97–1.10 | P = 0.294 |
| Exacerbation | |||||
| Unmatched | 20,048 | 8998 (45.1%) | 1.09 | 1.05–1.14 | P<0.001 |
| Propensity score matched | 13,316 | 6042 (45.5%) | 1.06 | 1.01–1.11 | P=0.028 |
| Primary care recorded hospitalization | |||||
| Unmatched | 20,048 | 6567 (32.5%) | 0.98 | 0.94–1.03 | P = 0.445 |
| Propensity score matched | 13,316 | 4459 (33.9%) | 1.02 | 0.96–1.08 | P = 0.493 |
| Pneumonia related hospitalization | |||||
| Unmatched | 20,048 | 302 (2.5%) | 1.41 | 1.12–1.77 | P = 0.003 |
| Propensity score matched | 13,316 | 223 (1.7%) | 1.44 | 1.11–1.88 | P = 0.007 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LTRI, Lower Tract Respiratory Infection; OCS, oral corticosteroids; UTRI, Upper Tract Respiratory Infection.
Figure 4.
Hazard ratios for comparing time-to-event outcomes, sensitive and specific definition pneumonia, for new users of fine particle fixed dose fluticasone and extrafine fixed dose beclometasone in propensity score matched samples.
Abbreviations: CI, confidence interval; PSM, propensity score matched.
Initiation of fp-FDC-F was also associated with an increased risk of developing upper- and lower-respiratory tract infections (HR 1.08; 95% CI 1.01–1.16) as well as lower respiratory tract infections in isolation (HR 1.08; 95% CI 1.01–1.15) when compared to ef-FDC-BDP, for the propensity matched cohort (Table 2). Similar observations were also observed for the unmatched cohort.
Assessment of exploratory outcomes indicates the commencement of fp-FDC-F was associated with an increased risk of a COPD exacerbation (HR 1.06; 95% CI 1.01–1.11) and pneumonia-related hospitalisation (HR 1.44; 95% CI 1.11–1.88) for the propensity matched cohort when compared to ef-FDC-BDP (Table 2). No significant differences were observed for acute OCS use, antibiotic prescription or primary care recorded hospitalisation.
Subgroup Analysis of Fluticasone Propionate
Fifteen thousand eight hundred and thirty-six patients were eligible for inclusion in the subgroup analysis of fixed-dose combination inhaler treatment containing fine-particle fluticasone propionate (fp-FDC-FP) (Figure 1). Of these, 9740 patients were commencing beclometasone and 6096 were commencing fluticasone propionate. A total of 7616 patients were matched, 3808 commencing ef-FDC-BDP (mean extrafine beclometasone dose 395 µg) and 3808 commencing fp-FDC-FP (mean fluticasone propionate dose 710 µg; mean extrafine beclometasone dose equivalent 568 µg).
In comparison to ef-FDC-BDP, the initiation of fp-FDC-FP was associated with an increased risk of developing both sensitive (HR 1.64; 95% CI 1.26–2.12) and specific (HR 1.45; 95% CI 1.09–1.94) definition pneumonia for the propensity matched cohort (Table 3). Similarly, commencement of fp-FDC-FP was also associated with an increased risk of developing upper and lower-respiratory tract infections in the propensity matched (HR 1.12; 95% CI 1.04–1.22) and unmatched (HR 1.18; 95% CI 1.12–1.26) cohorts. The risk of developing only a lower-respiratory tract infection after the commencement of ef-FDC-FP was also increased in both the propensity matched (HR 1.14; 95% CI 1.05–1.25) and unmatched (HR 1.18; 95% CI 1.11–1.26) cohorts.
Table 3.
Hazard ratios for comparing time-to-event uutcomes for new users of fine-particle fixed dose fluticasone-propionate (fp-FDC-FP) or -furoate (fp-FDC-FF) and extra fine fixed dose beclometasone (ef-FDC-BDP) in propensity score matched samples
| Outcome | Fine Fixed Dose Fluticasone Propionate | Fine Fixed Dose Fluticasone Furoate | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Patients | HR | 95% CI | p-value | Number of Patients | HR | 95% CI | p-value | |
| Primary outcomes | ||||||||
| Sensitive pneumonia definition | ||||||||
| Unmatched | 9740 ef-FDC-BDP, 6096 fp-FDC-FP | 1.43 | 1.19–1.72 | P < 0.001 | 9740 ef-FDC-BDP, 4212 fp-FDC-FF | 1.37 | 1.11–1.70 | P = 0.004 |
| Propensity score matched | 3808 ef-FDC-BDP, 3808 fp-FDC-FP | 1.64 | 1.26–2.12 | P < 0.001 | 3449 ef-FDC-BDP, 3449 fp-FDC-FF | 1.34 | 1.01–1.78 | P = 0.040 |
| Specific pneumonia definition | ||||||||
| Unmatched | 9740 ef-FDC-BDP, 6096 fp-FDC-FP | 1.22 | 0.99–1.51 | P = 0.059 | 9740 ef-FDC-BDP, 4212 fp-FDC-FF | 1.43 | 1.14–1.80 | P = 0.002 |
| Propensity score matched | 3808 ef-FDC-B, 3808 fp-FDC-FP | 1.45 | 1.09–1.94 | P = 0.012 | 3449 ef-FDC-BDP, 3449 fp-FDC-FF | 1.30 | 0.96–1.77 | P = 0.085 |
| Secondary outcomes | ||||||||
| Respiratory outcome | ||||||||
| URTI & LRTI | ||||||||
| Unmatched | 9740 ef-FDC-BDP, 6096 fp-FDC-FP | 1.18 | 1.12–1.26 | P < 0.001 | 9740 ef-FDC-BDP, 4212 fp-FDC-FF | 0.95 | 0.88–1.02 | P = 0.135 |
| Propensity score matched | 3808 ef-FDC-BDP, 3808 fp-FDC-FP | 1.12 | 1.04–1.22 | P = 0.006 | 3449 ef-FDC-BDP, 3449 fp-FDC-FF | 1.08 | 0.99–1.19 | P = 0.092 |
| LRTI only | ||||||||
| Unmatched | 9740 ef-FDC-BDP, 6096 fp-FDC-FP | 1.18 | 1.11–1.26 | P < 0.001 | 9740 ef-FDC-BDP, 4212 fp-FDC-FF | 0.95 | 0.88–1.03 | P = 0.195 |
| Propensity score matched | 3808 ef-FDC-BDP, 3808 fp-FDC-FP | 1.14 | 1.05–1.25 | P = 0.002 | 3449 ef-FDC-BDP, 3449 fp-FDC-FF | 1.06 | 0.96–1.17 | P = 0.280 |
Abbreviations: CI, confidence interval; ef-FDC-B, extrafine fixed dose combination beclometasone; fp-FDC-FP, fine-particle fixed dose fluticasone furoate; fp-FDC-FP, fine-particle fixed dose fluticasone propionate; HR, hazard ratio; LRTI, Lower Tract Respiratory Infection; URTI, Upper Tract Respiratory Infection.
Subgroup Analysis of Fluticasone Furoate
In the subgroup analysis of fluticasone furoate, 13,952 patients were eligible for inclusion, 9740 commencing ef-FDC-BDP and 4212 commencing fixed-dose combination inhaler treatment containing fine-particle fluticasone furoate (fp-FDC-FF) (Figure 1). A total of 6898 patients were matched, 3449 commencing ef-FDC-BDP (mean beclometasone dose 395 µg) and 3449 commencing fp-FDC-FF (mean fluticasone furoate dose 99 µg; mean extrafine beclometasone dose equivalent 432 µg).
Commencing fp-FDC-FF was associated with an increased risk of developing sensitive definition pneumonia in both propensities matched (HR 1.34; 95% CI 1.01–1.78) and unmatched (HR 1.37; 95% CI 1.11–1.70) cohorts when compared to ef-FDC-BDP (Table 3). Initiating ef-FDC-FF therapy was also associated with an increased risk of developing specific definition of pneumonia in the unmatched cohort (HR 1.43; 95% CI 1.14–1.80).
Discussion
The current investigation demonstrates that in comparison to extrafine beclometasone, the initiation of fine-particle fluticasone was associated with an increased risk of developing pneumonia, either sensitive or specific definition. New users of fluticasone-containing formulations also had a higher risk of developing upper-respiratory tract infections and non-pneumonia lower-respiratory tract infections as well as exacerbations and pneumonia-related hospitalisations. There was no observable difference between either drug in relation to OCS-treated exacerbations, suggesting extrafine beclometasone’s advantages are driven by its lower risk of developing respiratory tract infections rather an impact on the underlying pathophysiology associated with COPD. Sub-group analysis additionally indicates that both fine-particle fluticasone esters, propionate and furoate, were associated with an increased risk of sensitive definition pneumonia, when compared to extrafine beclometasone. Similar findings were observed for specific definition pneumonia, although the effect failed to reach statistical significance for fluticasone furoate. Commencement of fine-particle fluticasone propionate was also associated with a higher risk of developing upper- and lower-respiratory tract infections.
Both fluticasone esters, propionate and furoate, have previously been linked to an increased risk of developing pneumonia, however these prior investigations are not without limitations ranging from a lack of objectivity when defining what constitutes a COPD diagnosis, analysis conducted on an intention to treat basis, to a lack of matching prior to randomisation into treatment arms.10,11,14,17 Namely, the current study did not have these limitations, allowing for a reliable, direct comparison between new users of fluticasone esters and beclometasone. Similarly, the observation that extrafine formulations of ICS for the treatment of COPD are associated with a reduced risk of developing pneumonia and adverse respiratory events when compared to fine particle therapies has also been documented.15 However, a limitation of this former study was that there was no direct comparison between different pharmacological compounds, meaning the current investigation is the first to directly compare the risk of developing pneumonia when initiating ef-FDC-BDP and fp-FDC-F. One notable publication has reported that the use of ef-FDC-BDP at a significantly lower dose was comparable in managing exacerbation rates as higher doses of fp-FDC-F.28 Additionally, ef-FDC-BDP had better odds of 2-year treatment stability when compared to fp-FDC-F.28 In this regard, the use of ef-FDC-BDP has been shown to confer therapeutic advantages over fp-FDC-F, however absent from former investigations was the ability to reliably assess the risk of adverse respiratory events occurring, thus highlighting the value of the present investigation.
It is possible the proposed mechanisms underlying treatment advantages and reduced risk profiles associated with ef-FDC-BDP use in the treatment of COPD, when compared to fp-FDC-F are attributed to the pharmacokinetics, pharmacodynamics, and chemical attributes of these compounds.15,29–31 Prior investigations have established fluticasone has a larger particle size and greater binding affinity for the glucocorticoid receptor, when compared to beclometasone.29,30 The smaller particle size of beclometasone has been suggested to increase deposition in the bronchi and bronchioles, and potentially require lower doses to mediate therapeutic effects with extrafine particle formulations.29,30 These suggestions are consistent with present findings, whereby the mean extrafine beclometasone equivalent dose was lower for patients commencing ef-FDC-BDP when compared to fp-FDC-FF and fp-FDC-FP. The ability to administer lower ICS doses and still achieve therapeutic effects also helps to minimise the risk of adverse events. This is of particular importance because increased ICS dosing, irrespective of subclass, has a positive relationship with the development of pneumonia.12,15 In addition to dosage, binding affinity with glucocorticoid receptors can impact upon the therapeutic and safety profile of an ICS as both the positive respiratory effects and local and systemic side effects are mediated through the same receptor.29 Thus, it is possible that fluticasone’s increased relative receptor affinity, in combination with greater doses that were administered, by comparison to beclometasone, contributed to the risk profile in COPD patients.
In relation to differences in pharmacokinetics, the active moieties of beclometasone have a lower lipophilicity, in comparison to fluticasone.29,31 Lower lipophilicity in an ICS is associated with a reduced risk of pneumonia because it leads to a shorter retention time and consequently, less localised immunosuppression.31,32 Importantly, these interclass variations in ICS exemplify an important clinical concept. That is, selective prescription of these drugs in COPD patients can achieve therapeutic goals whilst minimising the risk of adverse events. This concept of ef-FDC-BDP having a lower risk of developing severe pneumonia when compared to fluticasone has also been reported in randomised controlled trials.31 However, it should be noted that the use of extrafine ICS formulations does not completely mitigate the risk of pneumonia when compared to LABD in isolation, as demonstrated in the TRINITY and FORWARD studies.18,19 In acknowledging the risk of ICS/LABD combination when compared to LABD, their use in managing COPD should not be discouraged, as they still afford benefits above either compound in isolation, as exemplified by reduced exacerbation rates and improved pre-dose FEV1.18,19 Moving forward, it would be beneficial to compliment the current findings with direct comparisons between extrafine particle ICS/LABD formulations versus LABD in isolation to further determine the safety and potential usefulness in wider spread management of COPD patients.
Key strengths of the current investigation include a robust study population followed over an extended period and the presentation of as-treated data which assesses the actual period during which patients were at risk from adverse effects of their treatment. In terms of limitations, the present investigation was not designed to allow for a direct comparison between fluticasone furoate and fluticasone propionate. Whilst both compounds had an increased risk profile in comparison to beclometasone, individual differences in the risk profile between them cannot be excluded. Specifically, fp-FDC-FP is prescribed in much higher doses for COPD in Europe when compared to the other countries33 and is also prescribed at a higher dose in comparison to fp-FDC-FF. This could potentially account for the results noted in the subgroup analysis whereby fp-FDC-FP had a higher risk of pneumonia in comparison to fp-FDC-FF. Prior publications have shown that when administered at smaller doses the effects of fp-FDC-FP are comparable33 which exemplifies why direct comparison of subgroups in this study should be done with this limitation in mind. Use of propensity score matching allowed control for a wide range of possible confounding factors but the potential for residual confounding due to unmeasured baseline differences cannot be excluded. Additionally, time to event analysis was only conducted for first event and the exploratory analysis of time to event for acute OCS use and antibiotics prescriptions were not qualified specifically for COPD exacerbations and pneumonia, respectively. COPD patients who had no smoking history were also excluded from this study as they have been shown to have milder symptoms, less inflammation and fewer comorbidities than current or former smokers.34 Finally, when examining comorbidities, only patients with active rhinitis were captured. In the current investigation, active rhinitis was defined as having a diagnosis or pharmacological intervention during the baseline period, resulting in lower prevalence rates observed in the study population.
Conclusions
In comparison to ef-FDC beclometasone, commencement of fp-FDC fluticasone is associated with an increased risk of developing pneumonia, and upper- and lower-respiratory tract infections in COPD patients. The reduced risk profile associated with beclometasone use in COPD patients relative to fluticasone esters, may be attributed to the smaller particle size, pharmacokinetic and pharmacodynamic properties. Ultimately allowing smaller doses to be administered whilst still mainlining therapeutic benefits. The observations from the present investigation further support the idea that not all ICS are equal in their therapeutic and adverse effects and subclass variations exist. In this regard, with careful consideration, the use of ICS as part of a dual or triple therapy for the management of COPD can potentially achieve desirable therapeutic effects whilst limiting adverse respiratory outcomes.
Acknowledgments
Professor Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC). Jaco Vooriham is acknowledged for his contribution to protocol development. We would also like to acknowledge Ms. Shilpa Suresh (MSc) of the Observational and Pragmatic Research Institute (OPRI), Singapore, for editorial and formatting assistance which supported the development of this publication.
Funding Statement
This study was conducted by the Observational and Pragmatic Research Institute (OPRI) Pte Ltd and was funded by Chiesi Farmaceutici S.p.A.
Abbreviations
ADEPT, Anonymised Data Ethics Protocols and Transparency; BMI, body mass index; COPD, Chronic Obstructive Pulmonary Disease; ef-FDC-BDP, extrafine particle fixed dose beclomethasone; ENCePP, European Network Centers for Pharmacoepidemiology and Pharmacovigilance; fp-FDC-F, fine-particle fixed dose fluticasone; fp-FDC-FF, fine-particle fixed dose fluticasone furoate; fp-FDC-FP, fine-particle fixed dose fluticasone propionate; HR, Hazard Ratio; ICS, inhaled corticosteroids; LABA, long-acting beta-2 agonists; LABD, long-acting bronchodilators; LAMA, long-acting muscarinic antagonists; OPCRD, Optimum Patient Care Research Database.
Ethics Approval
The study protocol was established prior to data extraction, in accordance with the criteria for the European Network Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) and follows the ENCePP code of conduct (2014). Registration of the study with the European Union electronic Register of Post-Authorization studies was also undertaken (EUPAS35439). As noted, the dataset was derived from the OPCRD, which has ethical approval from the National Health Service Research Authority to hold and process anonymised research data (Research Ethics Committee reference: 15/EM/0150). Approval for this study was granted by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee – the independent scientific advisory committee for the OPCRD (ADEPT0820). The authors do not have permission to give public access to the study dataset; requests to access OPCRD can be made via the OCPRD website (https://opcrd.co.uk/our-database/data-requests/) or via the enquiries email info@opcrd.co.uk.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All authors took part in drafting the article or revising it critically for important intellectual content. All authors agreed to submit to the current journal. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
David B Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Thermofisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
Derek Skinner, Rebecca Vella and Victoria Carter are affiliated with the Observational and Pragmatic Research Institute.
William Henley is affiliated with the Observational and Pragmatic Research Institute and reports travel support from Eisai Limited.
Leonardo M. Fabbri reports lecture fees and/or consultancies from Alfasigma, AstraZeneca, Chiesi, Boehringer Ingelheim, GlaxoSmithKline, Lusofarmaco, Merck, Novartis, Zambon, and Verona Pharma.
Huib AM Kerstjens reports grants and consultancy/advisory board participation from/for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis, all outside the submitted work. All were paid to his institution.
Alberto Papi is on the boards for and has received research and travel support and consultancy and lecture fees from Chiesi Farmaceutici, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Merck Sharp & Dohme, Takeda, Mundipharma Research Limited, and Teva; has received lecture fees and travel support from Menarini, Novartis, and Zambon; is on the boards for and has received lecture fees and travel support from Pfizer and has received research support from Sanofi.
Nicolas Roche reports grants and personal fees from Boehringer Ingelheim, Novartis, GSK, and Pfizer, and personal fees from Teva, GSK, AstraZeneca, Chiesi, Sanofi, and Zambon.
Dave Singh has received personal fees from GSK, Cipla, Genentech and Peptinnovate, and personal fees and grant support from AstraZeneca, Boehringer Ingelheim, Chiesi, Glenmark, Gossamerbio, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Theravance and Verona..
Claus F Vogelmeier gave presentations at symposia and/or served on scientific advisory boards sponsored by Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Menarini, Novartis, Nuvaira, and MedUpdate.
Elif Şen report no conflict of interest.
José Eduardo Delfini Cançado reports grants and personal fees from Aché, AstraZeneca, Boehringer Ingelheim, Chiesi, Eurofarma, GSK, Glenmarkpharma, Novartis, Sanofi and Zambon.
Elena Nudo, Sara Barile and George Georges are employees of Chiesi Farmaceutici, S.p.A. George Georges reports stock ownerships from GSK, Sanofi, Insmed, Regeneron, Gilead, Vertex, Ultragenyx, and Fibrogen. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed January 24, 2022.
- 2.Volgelmeir CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 3.Cazzola M, Rogliani P, Stolz D, Matera MG. Pharmacological treatment and current controversies in COPD. F1000Res. 2019;8:1533. doi: 10.12688/f1000research.19811.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:469–479. doi: 10.2147/COPD.S48492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calzetta L, Cazzola M, Matera MG, Rogliani P. Adding a LAMA to ICS/LABA therapy, a meta-analysis of triple combination therapy in COPD. Chest. 2019;155:758–770. doi: 10.1016/j.chest.2018.12.016 [DOI] [PubMed] [Google Scholar]
- 6.Calverley PMA, Anderson JA, Celli B, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 7.Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–223. doi: 10.1016/S2213-2600(13)70040-7 [DOI] [PubMed] [Google Scholar]
- 8.Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12:27–34. doi: 10.1513/AnnalsATS.201409-413OC [DOI] [PubMed] [Google Scholar]
- 9.Janson C, Johansson G, Ställberg B, et al. Identifying the associated risks of pneumonia in COPD patients: arctic an observational study. Resp Research. 2018;19:172. doi: 10.1186/s12931-018-0868-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suissa S, Patenaude V, Lapi F, Ernest P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. doi: 10.1136/thoraxjnl-2012-202872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morjaria JB, Rigby A, Morice AH. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT Study. Lung. 2017;195:281–288. doi: 10.1007/s00408-017-9990-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 13.Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS). BMJ. 2013;346:f3306. doi: 10.1136/bmj.f3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Li S, Zhou W, Yang X, Li J, Cao J. Risk of pneumonia with different inhaled corticosteroids in COPD patients: a meta-analysis. COPD. 2020;17:462–469. doi: 10.1080/15412555.2020.1787369 [DOI] [PubMed] [Google Scholar]
- 15.Sonnappa S, Martin R, Israel E, et al. Risk of pneumonia in obstructive lung disease: a real-life study comparing extra-fine and fine-particle inhaled corticosteroids. PLoS One. 2017;12:e0178112. doi: 10.1371/journal.pone.0178112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–1084. doi: 10.1016/S0140-6736(18)30206-X [DOI] [PubMed] [Google Scholar]
- 17.Solidoro P, Patrucco F, Bagnasco D. Comparing a fixed combination of budesonide/formoterol with other inhaled corticosteroid plus long-acting beta agonist combinations in patients with chronic obstructive pulmonary disease: a review. Expert Rev Respir Med. 2019;13:1087–1094. doi: 10.1080/17476348.2019.1665514 [DOI] [PubMed] [Google Scholar]
- 18.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–1929. doi: 10.1016/S0140-6736(17)30188-5 [DOI] [PubMed] [Google Scholar]
- 19.Wedzicha JA, Singh D, Vestbo J, et al.; for the FORWARD Investigators. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Resp Med. 2014;108:1153–1162. doi: 10.1016/j.rmed.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 20.Hillyer EV, Price DB, Chrystyn H, et al.; Roche N on behalf of the Respiratory Effectiveness Group, Small Airways Study Group. Harmonizing the nomenclature for therapeutic aerosol particle size: a proposal. J Aerosol Med Pulm Drug Deliv. 2018;31:1–3. doi: 10.1089/jamp.2017.1396 [DOI] [PubMed] [Google Scholar]
- 21.Lavorini F, Janson C, Braido F, Stratelis G, Løkke A. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther Adv Respir Dis. 2019;13:1–28. doi: 10.1177/1753466619884532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Backer W, Devolder A, Poli G, et al. Lung deposition of BDP/Formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010;23:137–148. doi: 10.1089/jamp.2009.0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usmani OS, Mignot B, Kendall I, et al. Predicting lung deposition of extrafine inhaled corticosteroid-containing fixed combination in patients with chronic obstructive pulmonary disease using functional respiratory imaging: an in silico study. J Aerosol Med Pulm Drug Deliv. 2021;34:204–211. doi: 10.1089/jamp.2020.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collice G, Chisholm A, Dima AL, et al. Performance of database-derived severe exacerbations and asthma control measures in asthma: responsiveness and predictive utility in a UK primary care database with linked questionnaire data. Pragmat Obs Res. 2018;9:29–42. doi: 10.2147/POR.S151615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2020. Available from: https://www.R-project.org/. Accessed January 24, 2022.
- 27.Sekhon JC. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J Stat Softw. 2011;42:1–52. doi: 10.18637/jss.v042.i07 [DOI] [Google Scholar]
- 28.Postma DS, Roche N, Colice G, et al. Comparing the effectiveness of small-particle versus large-particle inhaled corticosteroid in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:1163–1186. doi: 10.2147/COPD.S68289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28:1042–1050. doi: 10.1183/09031936.00074905 [DOI] [PubMed] [Google Scholar]
- 30.Hübner M, Hochhaus G, Hartmut D. Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin North Am. 2005;25:469–488. doi: 10.1016/j.iac.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 31.Chang TY, Chien JY, Wu CH, Dong YH, Lin FJ. Comparative safety and effectiveness of inhaled corticosteroid and long-acting β2-agonist combinations in patients with COPD. Chest. 2020;157:1117–1129. doi: 10.1016/j.chest.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 32.Lipworth B, Kuo CR, Jabbal S. Current appraisal of single inhaler triple therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3003–3009. PMID: 30319248; PMCID: PMC6167973. doi: 10.2147/COPD.S177333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones R, Martin J, Thomas V, et al. The comparative effectiveness of initiating fluticasone/salmeterol combination therapy via pMDI versus DPI in reducing exacerbations and treatment escalation in COPD: a UK database study. Int J Chron Obstruct Pulmon Dis. 2017;12:2445–2454. doi: 10.2147/COPD.S141409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med. 2013;1:543–550. doi: 10.1016/S2213-2600(13)70137-1 [DOI] [PubMed] [Google Scholar]