Abstract
We compared the clinical and laboratory features of human immunodeficiency virus (HIV)- and non-HIV-infected patients with penicilliosis marneffei. HIV-infected patients had a higher incidence of fungemia. A total of 85.7% of the HIV-negative patients had underlying diseases including hematologic malignancies or had received therapy with corticosteroids or cytotoxic agents. By a Penicillium marneffei-specific mannoprotein Mp1p enzyme-linked immunosorbent assay, serum antigen titers were found to be higher in HIV-positive patients, whereas serum antibody levels were found to be higher in HIV-negative patients.
Penicilliosis marneffei is a unique dimorphic fungal infection endemic in Southeast Asia. It is one of the commonest opportunistic infections among AIDS patients in areas of endemicity and is considered an indicator disease for AIDS (8, 14). The clinical manifestations of penicilliosis in AIDS patients have been well described, whereas reports that have described penicilliosis in non-AIDS patients are limited. A comparison of the clinical features of the disease between AIDS and non-AIDS patients has not been reported so far.
The diagnosis of penicilliosis is traditionally confirmed by isolation of the fungus from clinical specimens. The problem of the prolonged incubation which may be required for culture has been addressed previously (18). A number of antigen and antibody detection assays have therefore been described in recent years. We previously reported on an indirect immunofluorescent assay for antibody detection in patients with penicilliosis (20). Subsequently, a novel gene, MP1, was cloned. MP1 was found to encode an immunogenic mannoprotein, Mp1p (2). This allowed us to use this gene product for antigen and antibody detection in patients with suspected penicilliosis. In this article, we report on the microbiological diagnosis of penicilliosis by conventional culture and the serodiagnosis of penicilliosis in human immunodeficiency virus (HIV)- and non-HIV-infected patients and on the clinical features of HIV- and non-HIV-infected patients with penicilliosis.
Patients with culture-documented penicilliosis in Queen Mary Hospital, Hong Kong, from 1994 to 1999 were reviewed. Only patients for whom adequate clinical information and specimens for analysis were available were included in the study. Mortality was attributable to penicilliosis if death occurred within 14 days of diagnosis or if there were persistent positive fungal cultures at the time of death. There must have been no other concurrent diseases that might have contributed to the mortality. Serial serum samples were collected whenever possible and were stored at −70°C until use. Blood cultures were performed with the BACTEC 9240 system (Becton Dickinson, Sparks, Md.). The specimens were incubated for 14 days before being reported as negative. Positive fungal cultures were confirmed by Gram staining of a smear of the blood culture broth, followed by subculture onto Sabouraud dextrose agar (SDA) without cycloheximide with incubation at 25 and 37°C in room air. Penicillium marneffei was identified by the following criteria: (i) demonstration of thermal dimorphism by showing a conversion from the yeast form at 37°C to the mold form at 25°C, (ii) production of a diffusible red pigment from the mold form when it was cultured at 25°C on SDA, and (iii) the microscopic morphology of the mycelia including the presence of conidiophore-bearing biverticillate penicilli, with each penicillus being composed of four to five metulae with smooth-walled conidia (16). Clinical specimens other than blood were examined microscopically both by Gram staining and, after digestion with 20% KOH, for the presence of fungal elements. The specimens were then cultured on SDA at 25 and 37°C.
Detection of P. marneffei antigen (Mp1p) and antibody in serum was performed by an enzyme-linked immunosorbent assay (ELISA) by previously published protocols (3, 4). Briefly, for Mp1p antigen detection, standard 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with guinea pig anti-Mp1p serum at a dilution of 1:5,000 in bicarbonate coating buffer (pH 9.6) after overnight incubation at 4°C, followed by further blocking in phosphate-buffered saline (pH 7.4) with 2% bovine serum albumin. Purified Mp1p protein or human serum samples diluted to 1:20 were added to the wells, and the plates were incubated at 37°C for 2 h. The wells were then washed with washing buffer (phosphate-buffered saline at pH 7.4 with 0.05% Tween 20). Rabbit anti-Mp1p serum was added at a dilution of 1:500, and the plates were incubated at 37°C for 1 h. The wells were then washed again, and 1:2,000-diluted, alkaline phosphatase-conjugated goat anti-rabbit antibody was added. Detection was carried out with p-nitrophenyl phosphate substrate (Sigma Chemical Co., St. Louis, Mo.), and the optical density was read at 450 nm with an ELISA plate reader (Labinstruments, Grödig, Austria). For anti-Mp1p antibody detection, microtiter plates were coated with 0.5 ng of purified glutathione S-transferase–Mp1p protein in bicarbonate coating buffer (pH 9.6) by overnight incubation at 4°C, followed by blocking in phosphate-buffered saline (pH 7.4) with 2% bovine serum albumin. One hundred microliters of diluted human serum specimens (1:100 dilution) were added to the wells, and the plates were incubated at 37°C for 2 h. After the plates were washed with washing buffer (phosphate-buffered saline [pH 7.4] with 0.05% Tween 20), 1:4,000-diluted, alkaline phosphatase-conjugated goat anti-human antibody (Cappel ICN Pharmaceuticals, Aurora, Ill.) was added. Detection was carried out with p-nitrophenyl phosphate substrate, and the optical density was read at 450 nm with an ELISA plate reader.
A full dilution range of the standard positive serum sample was made and titrated under conditions identical to those used for the single dilutions of the test sera. At the end of the test, a standard curve relating the optical density to the dilution of the standard positive serum sample was constructed. The titers of the test samples were then read from this curve so that a semiquantitative result could be obtained. The same standard positive serum sample was used when standardization between laboratories or different batches of a run were required. The cutoff value for the ELISA was calculated by studying 50 serum samples from healthy blood donors.
Fifteen culture-documented patients with penicilliosis were analyzed. Their clinical features are summarized in Table 1 according to their HIV antibody status. There were more males than females among the HIV-positive patients, but no significant difference in the age distribution of the patients was observed. HIV-positive patients were more likely to have fungemia than HIV-negative patients, while the latter group frequently required tissue biopsies for confirmation of the infection. There was a significant difference in the delay in the time to diagnosis: a median delay of 1 week for HIV-positive patients compared with a median delay of 5.5 weeks for HIV-negative patients (P < 0.01 by the Mann-Whitney U test). In both categories of patients, pulmonary involvement was the most common manifestation at presentation, followed by pyrexia of unknown origin and cutaneous manifestations. Tuberculosis was the most common presumptive diagnosis made prior to definitive mycological diagnosis. The rates of mortality directly attributable to penicilliosis were similar in both groups of patients.
TABLE 1.
Clinical features of 15 patients with culture-documented penicilliosis marneffei in the Queen Mary Hospital from 1994 to 1999
| Clinical feature | HIV-positive patients (n = 8) | HIV-negative patients (n = 7) |
|---|---|---|
| No. of males:no. of females | 8:0 | 2:5 |
| Median (range) age (yr) | 37.5 (10–61) | 45 (23–73) |
| Underlying disease(s) (no. of patients) | HIV infection (8) | Hemic malignancya (n = 2), autoimmune diseasesb (n = 3), diabetes mellitus (n = 1), none (n = 1) |
| Sites of isolation (no. of patients)c | ||
| Blood | 4 | 2 |
| Lymph node | 2 | 0 |
| Bronchoalveolar lavage fluid | 3 | 3 |
| Skin biopsy specimen | 1 | 2 |
| Others | 1 (large bowel biopsy) | 3 (pleural fluid, bone, subcutaneous abscess [n = 1 each]) |
| Presenting syndrome (no. of patients) | ||
| Pyrexia of unknown origin | 1 | 1 |
| Pulmonary involvement | 3 | 5 |
| Cutaneous lesions | 2 | 1d |
| Lymphadenopathy | 2 | 0 |
| Peritonitis | 0 | 1 |
| Initial diagnosis prior to confirmation of penicilliosis marneffei (no. of patients) | ||
| Pyrexia of unknown origin | 2 | 1 |
| Tuberculosis | 5 | 3 |
| Autoimmune disease | 1 | |
| Typhoid fever | 2 | |
| Melioidosis | 2 | |
| Others | 2 (carcinoma, chicken pox [n = 1 each]) | 3 (cryptococcosis, pneumonia, peritonitis [n = 1 each]) |
| Median (range) delay in diagnosis (wk) | 1 (1–4) | 5.5 (2–15) |
| Outcome (no. of patients) | ||
| Recovered | 5 | 3 |
| Died | 3 | 4 |
| Cause of death (no. of patients) | Penicilliosis (n = 1), Mycobacterium avium complex infection (n = 1), and other intercurrent infections 1 yr later (n = 1) | Penicilliosis (n = 2), nosocomial pneumonia (n = 1), and nosocomial staphylococcal bacteremia (n = 1) |
T-cell lymphoma (n = 1), Waldenström's macroglobulinemia (n = 1).
A patient with Sjögren's syndrome receiving corticosteroids and cyclophosphamide, a patient with systemic lupus erythematosus receiving azathioprine, and a patient with autoimmune hemolytic anemia receiving corticosteroid.
The fungus may be isolated from multiple sites of the same patient.
One patient had simultaneous pulmonary and cutaneous involvement on presentation.
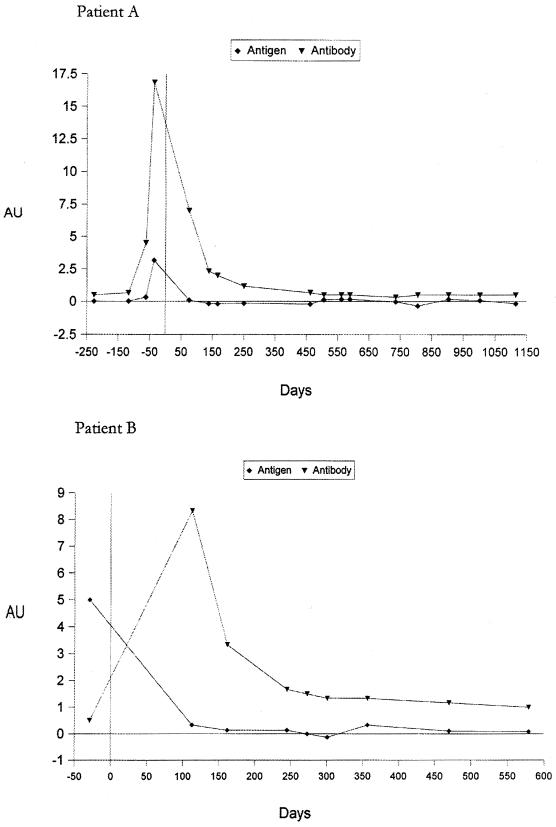
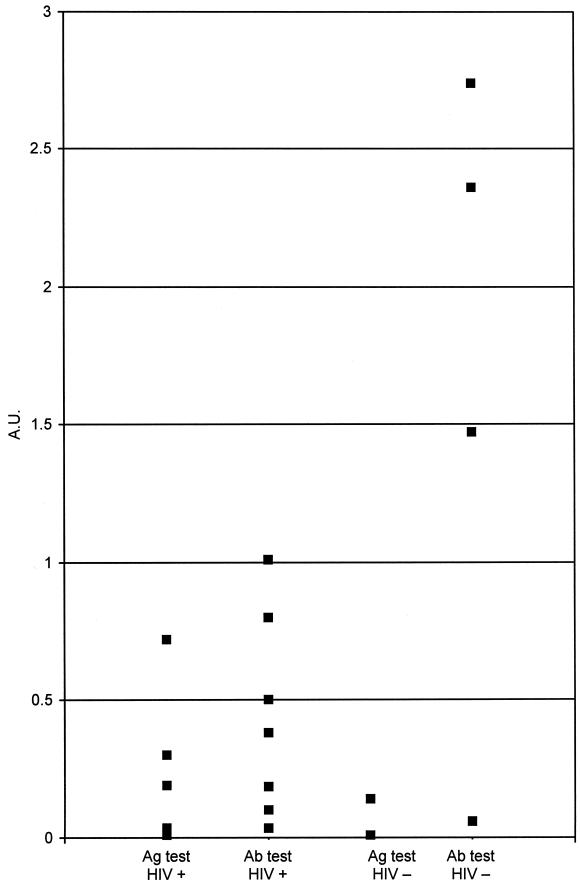
The serological results for two representative patients with penicilliosis are shown in Fig. 1. Both patients were HIV positive. The day when the first blood sample positive by culture was collected is designated day 0. The blood culture became positive after 7 days of incubation, at which time the patients were started on amphotericin B (0.6 mg/kg of body weight once daily) for 2 weeks, followed by oral itraconazole maintenance therapy (200 mg twice a day for 10 weeks). The titers of antigen and antibody against P. marneffei in serum were elevated as early as 30 days before blood cultures became positive. Both serum antigen and antibody titers dropped after antifungal therapy. Upon subsequent follow-up, there was no clinical or mycological evidence of relapse. ELISAs for both antigen and antibody in serum remained negative 1,115 and 579 days after the initial diagnosis in patients A and B, respectively. Figure 2 shows the serum antigen and antibody levels in the two groups of patients. The HIV-positive patients tended to have higher antigen titers and lower antibody titers, while the converse was true for the HIV-negative patients.
FIG. 1.
Serum P. marneffei antigen and antibody levels in two HIV-positive patients with culture-documented penicilliosis marneffei over time. Day 0 was the day of the first positive fungal culture. AU, arbitrary units.
FIG. 2.
Serum P. marneffei antigen (Ag) and antibody (Ab) titers in patients with (HIV+) and without (HIV−) HIV infection. A.U., arbitrary units.
Penicilliosis marneffei in AIDS patients consists of characteristic molluscum contagiosum-like lesions, diffuse pulmonary involvement, and frequently, concomitant fungemia (8). Infections are less commonly described in HIV-negative patients; cases have been reported among patients with alcoholism (11), tuberculosis (11), and systemic lupus erythematosus (9, 10); patients receiving corticosteroid or other forms of immunosuppressive therapy (5, 9); and even patients with no apparent underlying disease (12). Manifestations in HIV-negative patients include lymphadenopathy (21), osteomyelitis and septic arthritis (11), pulmonary infection (12), and disseminated infection with multiorgan involvement (11). We observed a predominance of male patients in the HIV-positive group, whereas the majority of HIV-negative patients were females. The biological and clinical significance of this finding is unknown; whether this is related to occupational or environmental exposure also remains to be determined. In the present series, 46.7% of our patients were HIV negative. All except one of the patients in the HIV-negative group had underlying diseases, including hematologic malignancies, or were receiving treatments that might impair their cell-mediated immunity, such as immunosuppressive therapy with antineoplastic agents or corticosteroids. It is therefore important for clinicians working in areas of endemicity to consider penicilliosis when managing febrile patients with possible defects in cellular immunity.
In the absence of underlying HIV infection and characteristic clinical findings, a diagnosis of penicilliosis may be much delayed. This was seen in our study, in which the time to diagnosis was significantly longer for HIV-negative patients than for HIV-positive patients. The initial differential diagnoses reflected the epidemiologies of locally important infectious and noninfectious diseases such as typhoid fever, melioidosis, autoimmune diseases, and malignant fever. Patients with HIV infection tended to have a higher incidence of fungemia, whereas a tissue specimen was often necessary for diagnosis in HIV-negative hosts. This is unlikely to be due to more intensive investigations in HIV-positive patients, because as part of the initial workup, at least two sets of blood samples for culture were collected from any patient, regardless of the HIV infection status, presenting with pyrexia of unknown origin. Blood culture is repeated if the fever does not respond to therapy. Furthermore, all HIV-positive patients in the present study had a positive blood culture on admission. Nonetheless, the rates of mortality attributable to penicilliosis were similar in the two groups of patients. This is likely due to the fact that the fungus is very susceptible to presently available antifungal agents. As long as the correct diagnosis can be made promptly, amphotericin B followed by itraconazole maintenance therapy is generally curative or effective in preventing a relapse of disease (13).
An early etiological diagnosis is thus of paramount importance. Fungemia could be detected in at least 55% of the HIV-positive patients (8). The HIV-negative patients pose a more difficult situation. In our series, 28.6% of the HIV-negative patients did not have fungemia at the time of presentation, thus necessitating more invasive procedures to obtain samples of deep tissue. A number of serological tests for the detection of P. marneffei antigen or antibodies in body fluids have been described in recent years. In 1998 we described the MP1 gene, which encodes a cell wall mannoprotein in P. marneffei (2). Antigen and antibody ELISAs that use the Mp1p protein and antibody against Mp1p were subsequently constructed (3, 4). Figure 1 shows representative serological results of a study based on this assay for two HIV-positive patients with culture-documented penicilliosis. The serum antigen and antibody titers rose at the time of active infection. It is of interest that the antibody and antigen assays were already positive at least 30 days prior to the day of positive culture of clinical specimens. With appropriate antifungal therapy, both the antibody and antigen titers remained negative. HIV-positive patients tended to have a lower serum antibody level, probably as a result of the underlying immune defects associated with HIV infection (Fig. 2). On the other hand, their serum antigen levels were usually markedly higher, presumably as a result of a higher fungal load secondary to the immune defects. Therefore, the detection of antigen and the detection of antibody in patients with suspected penicilliosis complement each other. For the two representative patients whose results are shown in Fig. 1, although detectable antibody levels may have appeared before or after the time that a blood culture became positive for fungi, antigen was consistently detectable in serum by ELISA before fungemia was documented. The serum antigen level could therefore be of value for immunocompromised patients. The test is potentially useful for the detection of relapses of penicilliosis although none of the HIV-positive patients with documented penicilliosis in our series had a relapse owing to the use of itraconazole prophylaxis. At present, on presentation all our HIV-positive patients receive routine screening for cryptococcal antigen, P. marneffei antigen and antibody, and galactomannan antigen. Hence, they can be started on antifungal prophylaxis if it is so indicated by a positive test result. A prospective double-blind evaluation of the test is therefore not feasible in this clinical setting.
A number of antigen and antibody assays have been described in recent years for the diagnosis of penicilliosis and other endemic mycoses. However, the specificities of most assays that use polyclonal antibodies and antigens are limited by cross-reactions with other human pathogens. Desakorn et al. (7) used polyclonal hyperimmune sera raised from rabbits immunized with whole arthroconidia of P. marneffei in an ELISA for the detection of P. marneffei antigen in urine. A significant proportion of patients with infections other than penicilliosis (e.g., other systemic mycoses and melioidosis) also had positive results, albeit at a lower titer. Wheat et al. (17) also showed cross-reactivity between penicilliosis and histoplasmosis in urinary antigen detection by a rabbit immunoglobulin G-based assay for Histoplasma capsulatum var. capsulatum. Similarly, the galactomannan of Aspergillus fumigatus cross-reacts with P. marneffei in a latex agglutination test and immunohistochemical staining (15). These antigen- or antibody-based tests might be of utility for the diagnosis of mycoses in areas of low endemicity (e.g., for the diagnosis of suspected infections in returning travelers), whereas for residents of areas of endemicity, background titers could be an important consideration in interpreting the test results. ELISA with Mp1p represents another approach to the serodiagnosis of systemic mycoses: the use of monospecific antigens and antibodies. Other examples include the use of the ASPND1r recombinant antigen of Aspergillus nidulans (1), a recombinant complement-fixing antigen of Coccidioides immitis (19), and a recombinant H antigen from H. capsulatum (6). It is hoped that these types of tests will help laboratorians provide a rapid diagnosis of emerging opportunistic infections, such as penicilliosis marneffei, in time to have a proactive effect on the therapeutic outcome of the patient.
Acknowledgments
We are most grateful to Michael McNeil, Division of Bacterial and Mycotic Diseases, Centers for Disease Control and Prevention, Atlanta, Ga., for critical comments on the manuscript.
The project was partly supported by funds from the Research Grants Council (grant HKU 498/96 M), the Committee of Research and Conference Grants, and the AIDS Trust Fund, Hong Kong.
REFERENCES
- 1.Calera J A, Ovejero M C, Lopez-Medrano R, Lopez-Aragon R, Puente P, Leal F. The recombinant antigen ASPND1r from Aspergillus nidulans is specifically recognized by sera from patients with aspergilloma. Microbiology. 1998;144(Pt 2):561–567. doi: 10.1099/00221287-144-2-561. [DOI] [PubMed] [Google Scholar]
- 2.Cao L, Chan C M, Lee C, Wong S S Y, Yuen K Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun. 1998;66:966–973. doi: 10.1128/iai.66.3.966-973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L, Chan K M, Chen D L, Vanittanakom N, Lee C, Chan C M, Sirisanthana T, Tsang D N C, Yuen K Y. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J Clin Microbiol. 1999;37:981–986. doi: 10.1128/jcm.37.4.981-986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Chen D L, Lee C, Chan C M, Chan K M, Vanittanakom N, Tsang D N C, Yuen K Y. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J Clin Microbiol. 1998;36:3028–3031. doi: 10.1128/jcm.36.10.3028-3031.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chim C S, Fong C Y, Ma S K, Wong S S, Yuen K Y. Reactive hemophagocytic syndrome associated with Penicillium marneffei infection. Am J Med. 1998;104:196–197. doi: 10.1016/s0002-9343(97)00253-2. [DOI] [PubMed] [Google Scholar]
- 6.Deepe G S, Jr, Durose G G. Immunobiological activity of recombinant H antigen from Histoplasma capsulatum. Infect Immun. 1995;63:3151–3157. doi: 10.1128/iai.63.8.3151-3157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desakorn V, Smith M D, Walsh A L, Simpson A J H, Sahassananda D, Rajanuwong A, Wuthiekanun V, Howe P, Angus B J, Suntharasamai P, White N J. Diagnosis of Penicillium marneffei infection by quantitation of urinary antigen by using an enzyme immunoassay. J Clin Microbiol. 1999;37:117–121. doi: 10.1128/jcm.37.1.117-121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong T A. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Lam K Y, Cheung F, Yam L Y C, Lee C H, Fung K H. Atypical presentations in a patient with systemic lupus erythematosus. J Clin Pathol. 1997;50:174–176. doi: 10.1136/jcp.50.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo C Y, Chan D T M, Yuen K Y, Li F K, Cheng K P. Penicillium marneffei infection in a patient with SLE. Lupus. 1995;4:229–231. doi: 10.1177/096120339500400313. [DOI] [PubMed] [Google Scholar]
- 11.Louthrenoo W, Thamprasert K, Sirisanthana T. Osteoarticular penicilliosis marneffei, a report of eight cases and review of the literature. Br J Rheumatol. 1994;33:1445–1450. doi: 10.1093/rheumatology/33.12.1145. [DOI] [PubMed] [Google Scholar]
- 12.So S Y, Chau P Y, Jones B M, Wu P C, Pun K K, Lam W K, Lawton J W M. A case of invasive penicilliosis in Hong Kong with immunologic evaluation. Am Rev Respir Dis. 1985;131:662–665. doi: 10.1164/arrd.1985.131.4.662. [DOI] [PubMed] [Google Scholar]
- 13.Supparatpinyo K, Perriens J, Nelson K E, Sirisanthana T. A controlled trial of itraconazole to prevent relapse of Penicillium marneffei infection in patients infected with the human immunodeficiency virus. N Eng J Med. 1998;339:1739–1743. doi: 10.1056/NEJM199812103392403. [DOI] [PubMed] [Google Scholar]
- 14.Tsang D N C, Li P C K, Tsui M S, Lau Y T, Mak K F, Yeoh E K. Penicilliosis marneffei: another pathogen to consider in patients infected with human immunodeficiency virus. Rev Infect Dis. 1991;13:766–767. doi: 10.1093/clinids/13.4.766-a. [DOI] [PubMed] [Google Scholar]
- 15.van Cutsem J, Meulemans L, van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33:61–69. doi: 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]
- 16.Viviani M A, Tortorano A M. Penicillium marneffei. In: Ajello L, Hay R J, editors. Medical mycology, vol. 4. Topley and Wilson's microbiology and microbial infections. 9th ed. London, United Kingdom: Arnold; 1998. pp. 409–419. [Google Scholar]
- 17.Wheat J, Wheat H, Connolly P, Kleiman M, Supparatpinyo K, Nelson K, Bradsher R, Restrepo A. Cross reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urinary samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 18.Wong S S Y, Cao L, Yuen K Y. Management of penicilliosis marneffei. JAMA Southeast Asia. 1998;14:7–9. [Google Scholar]
- 19.Yang M C, Magee D M, Kaufman L, Zhu Y, Cox R A. Recombinant Coccidioides immitis complement-fixing antigen: detection of an epitope shared by C. immitis, Histoplasma capsulatum, and Blastomyces dermatitidis. Clin Diagn Lab Immunol. 1997;4:19–22. doi: 10.1128/cdli.4.1.19-22.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen K Y, Wong S S Y, Tsang D N C, Chau P Y. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]
- 21.Yuen W C, Chan Y F, Loke S L, Seto W H, Poon G P. Chronic lymphadenopathy caused by Penicillium marneffei: a condition mimicking tuberculous lymphadenopathy. Br J Surg. 1986;73:1007–1008. doi: 10.1002/bjs.1800731224. [DOI] [PubMed] [Google Scholar]