Abstract
The epidemiologic correlation between the poor prognosis of SARS-CoV-2 infection and vitamin D deficiency has been observed worldwide, however, their molecular mechanisms are not fully understood. In this study, we used combined molecular docking, molecular dynamics simulations and binding free energy analyses to investigate the potentials of vitamin D3 and its hydroxyderivatives as TMPRSS2 inhibitor and to inhibit the SARS-CoV-2 receptor binding domain (RBD) binding to angiotensin-converting enzyme 2 (ACE2), as well as to unveil molecular and structural basis of 1,25(OH)2D3 capability to inhibit ACE2 and SARS-CoV-2 RBD interactions. The results show that vitamin D3 and its hydroxyderivatives are favorable to bind active site of TMPRSS2 and the binding site(s) between ACE2 and SARS-CoV2-RBD, which indicate that vitamin D3 and its biologically active hydroxyderivatives can serve as TMPRSS2 inhibitor and can inhibit ACE2 binding of SARS-CoV-2 RBD to prevent SARS-CoV-2 entry. Interaction of 1,25(OH)2D3 with SARS-CoV-2 RBD and ACE2 resulted in the conformation and dynamical motion changes of the binding surfaces between SARS-CoV-2 RBD and ACE2 to interrupt the binding of SARS-CoV-2 RBD with ACE2. The interaction of 1,25(OH)2D3 with TMPRSS2 also caused the conformational and dynamical motion changes of TMPRSS2, which could affect TMPRSS2 to prime SARS-CoV-2 spike proteins. Our results propose that vitamin D3 and its biologically active hydroxyderivatives are promising drugs or adjuvants in the treatment of COVID-19.
Keywords: COVID-19, vitamin D3 and its hydroxyderivatives, ACE2, SARS-CoV-2 RBD, TMPRSS2, molecular docking, molecular dynamics simulation, binding free energy analysis
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 presents a great threat to public health (Dong et al., 2020). One important epidemiologic correlation with SARS-CoV-2 infection (Alipio, 2020; Rhodes et al., 2021) is the prevalence of vitamin D deficiency in patients with severe COVID-19 symptoms (D’Avolio et al., 2020; Ebadi & Montano-Loza, 2020; Grant et al., 2020; Jakovac, 2020; Meltzer et al., 2020; Merzon et al., 2020; Rhodes et al., 2021; Slominski et al., 2020b, 2020c). This observation is not limited to a region or a particular type of population, instead is noticeable worldwide (Lau et al., 2020; Rhodes et al., 2021). Vitamin D belongs to a group of fat-soluble secosteroids of which enzymatic activation leads to the production of hormonally active hydroxy-metabolites (Jenkinson, 2019; Tuckey et al., 2019) that play a central role in body regulation of calcium, magnesium, and phosphate homeostasis and which control the expression of roughly 5% of human genes (Bikle & Christakos, 2020; Holick, 2003; 2007), and regulate cell proliferation and differentiation, immune-regulation, interaction with viral factors, autophagy and apoptosis (Aygun, 2020; Chen et al., 2020; Samuel & Sitrin, 2008; Slominski et al., 2014b, 2018; Zhang et al., 2020b; Zmijewski & Carlberg, 2020). In the canonical pathway, vitamin D3 is activated through two sequential hydroxylations at position C25 by CYP2R1 and CP27A1 with following hydroxylation at C1α by CYP27B1 to produce biologically active 1,25(OH)2D3 (Bikle & Christakos, 2020; Holick, 2003). 1,25(OH)2D3 regulates calcium body homeostasis and has important pleiotropic effects on multiple body functions including immune system (Bikle, 2008, 2010a, 2010b, 2011; Holick, 2003, 2007; Plum & DeLuca, 2010). An alternative, non-canonical pathway of vitamin D activation is initiated by series of hydroxylation of its side chain by CYP11A1 (Slominski et al., 2015, 2020a) with additional hydroxylations by other CYP enzymes (Slominski et al., 2020a; Tuckey et al., 2019). Metabolites of this alternative pathway are produced in vivo and are detectable in the human body (Slominski et al., 2012, 2015). They are biologically active with the mechanism of action being both different and overlapping with classical 1,25(OH)2D3 (Slominski et al., 2014a, 2014b, 2017, 2018, 2020a). Of interest, CYP11A1 is also expressed in immune cells in a context dependent fashion (Slominski et al., 2020d). Using high doses of vitamin D3 and its hydroxyderivatives for SARS-CoV-2 prevention and therapy has been proposed (Entrenas Castillo et al., 2020; Grant et al., 2020; Kaufman et al., 2020; Muralidharan Jothimani et al., 2021; Quesada-Gomez et al., 2020; Slominski et al., 2020b, 2020c) but the exact mechanism of this action remains unknown.
Acute respiratory distress syndrome (ARDS) develops because of SARS-CoV-2 infection causing multiple organ damage in patients (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020; Huang et al., 2020; Mehta et al., 2020). SARS-CoV-2 patients have increased immune responses with high concentrations of proinflammatory interleukins (ILs), tumor necrosis factor-α (TNF-α), gamma interferon (IFN-γ) and nuclear factor-κB (NFκB) (Conti et al., 2020; Huang et al., 2020; Zhang et al., 2020a) – leading to a phenomenon called the cytokine storm caused by triggered Th1 cell response (Cantorna & Mahon, 2005; Conti et al., 2020; Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020; Huang et al., 2020; Mehta et al., 2020; Schleithoff et al., 2006; Zhang et al., 2020a). 1,25(OH)2D3 and CYP11α1-derived vitamin D metabolites decrease Th1 cell responses and IL-17 production (Ardizzone et al., 2009; Bikle & Christakos, 2020; Cantorna & Mahon, 2005; Chen et al., 2013; Janjetovic et al., 2009; 2010; Provvedini et al., 1983; Schleithoff et al., 2006; Slominski et al., 2014a, 2014b; Sun et al., 2018; Tsoukas et al., 1984). NFκB activity and TNF-α, IFN-γ and IL-2 production are also reduced by active forms of vitamin D.
The cellular entry of SARS-CoV-2 involves binding of receptor binding domain (RBD) of spike protein in SARS-CoV2 with angiotensin-converting enzyme 2 (ACE2) receptor, and cellular serine protease TMPRSS2 primes viral spike proteins in SARS-CoV-2 (Hoffmann et al., 2020). SARS-CoV-2 spike protein has lower binding affinity to ACE2 compared to SARS-CoV spike protein, but SARS-CoV-2 RBD has higher ACE2 binding affinity than SARS-CoV RBD (Perrotta et al., 2020). SARS-CoV and SARS-CoV-2 RBD are typically in standing up state and resting state, respectively. Although SARS-CoV-2 RBD has higher binding affinity, its lying-down state makes it less accessible to ACE2 and other inhibitory or neutralizing agents. To overcome this and maintain its high binding affinity, SARS-CoV-2 RBD has developed a strategy which involves the use of host protease for its activation. Furin, along with TMPRSS2 and cathepsins, have roles in viral entry of human cells (Perrotta et al., 2020; Shang et al., 2020). The hidden RBD of SARS-CoV-2 is critical to both vaccination and antibody neutralization due to its limited accessibility (Perrotta et al., 2020; Shang et al., 2020). TMPRSS2 inhibitors approved for clinical use blocked viral entry (Hoffmann et al., 2020). Molecules that would inhibit the interactions of SARS-CoV-2 RBD and ACE2 and would serve as TMPRSS2 inhibitors could be an effective therapy to constrain cellular entry of SARS-CoV-2.
In this study, we aim to determine the potential of vitamin D3 and its hydroxyderivatives to act as TMPRSS2 inhibitors and to inhibit ACE2 and SARS-CoV-2 RBD interaction using molecular docking, molecular dynamics (MD) simulations and binding free energy analyses. The results suggest that vitamin D3 and its hydroxyderivatives can serve as promising therapeutics or adjuvants against COVID-19.
Materials and methods
Protein receptors and ligands
ACE2 and SARS-CoV-2-RBD structures and the binding sites between ACE2 and SARS-CoV-2-RBD
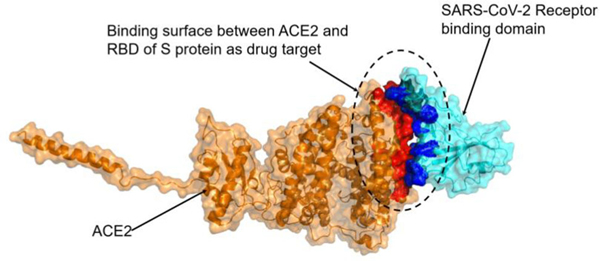
Electron microscopy structure of ACE2 and SARS-CoV-2-RBD complex (PDB ID: 6M17, resolution: 2.90 Å) (Yan et al., 2020) was obtained from protein data bank (https://www.rcsb.org/). Monomer structures of ACE2 and SARS-CoV-2-RBD were used for determining if and how vitamin D3 and its derivatives interact with ACE2 or SARS-CoV-2-RBD. The binding sites between ACE2 and SARS-CoV-2-RBD were determined using contact amino acid function in PyMOL based on the complex structure of ACE2 and SARS-CoV-2-RBD. The binding sites between ACE2 and RBD of spike protein primarily occur on the N-terminal alpha helix of ACE2 and the arch at the top of the RBD of spike (Yan et al., 2020). Specifically, the residues Tyr41, Gln42, Lys353, Arg357, Asp30, His34, Gln24 and Met82 from ACE2, and the residues Gln498, Thr500, Asn501, Lys417, Tyr453, Gln474 and Phe486 from SARS-CoV-2-RBD have been shown to interact (Yan et al., 2020). This binding site was used for molecular docking of vitamin D3 and its hydroxyderivatives with ACE2 and SARS-CoV2-RBD (red and blue region in Figure 1, respectively). The contact surface between ACE2 and SARS-CoV-2-RBD is shown in Figure 1.
Figure 1.
Binding surfaces between ACE2 and SARS-CoV-2-RBD. Contact surface of ACE2 shown as red and contact surface of SARS-coV-2 RBD shown as blue.
TMPRSS2 structure
TMPRSS2 structure is not available in protein data bank. Homologous modeling was used with MODELLER (Eswar et al., 2006) to predict the structure of TMPRSS2 based on the crystal structure of TMPRSS1 (PDBID: 1Z8G (Herter et al., 2005), resolution: 1.55 Å), a homologous protein of TMPRSS2 that has a 51.3% sequence similarity. TMPRSS2 model construction and evaluation were detailed in Supplemental Materials. The active site of TMPRSS2 is composed of a catalytic triad, consisting of His296, Asp345 and Ser441, and a substrate binding residue Asp435, at the bottom of the pocket (Meng et al., 2020; Wilson et al., 2005). This active site was used for molecular docking of vitamin D3 and its hydroxyderivatives with TMPRSS2.
Vitamin D3 and its hydroxyderivatives
Vitamin D3 and its hydroxyderivatives including 1α,25(OH)2D3, 25(OH)D3, 20S(OH)D3, 1α,20S(OH)2D3, 20S,23S(OH)2D3, 1α,20S,23S(OH)2D3, 20S,23R(OH)2D3 are the ligands with their structures shown in Table 1. They are used in this study to evaluate their potential as TMPRSS2 inhibitor and to inhibit ACE2 and SARS-CoV-2 RBD interactions.
Table 1.
Molecular docking and MM-PBSA binding free energy results for the complexes of vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2 (kcal/mol).
| Compounds╲Protein receptor | Binding affinity from molecular docking (kcal/mol) | MM-PBSA binding free energy (kcal/mol)a | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| TMPRSS2 | ACE2 | SARS-CoV-2 RBD | TMPRSS2 | ACE2 | SARS-CoV-2 RBD | ||
|
| |||||||
| Vitamin D3 |
|
−6.3 | −5.7 | −6.0 | −16.58 | −17.60 | −20.33 |
| 1α,25(OH)2D3 |
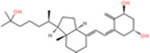
|
−6.6 | −5.9 | −4.6 | −12.97 | −30.45 | −22.39 |
| 25(OH)D3 |
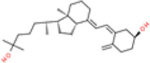
|
−6.4 | −6.1 | −6.3 | −14.38 | −25.35 | −24.18 |
| 20S(OH)D3 |
|
−6.3 | −5.9 | −3.1 | −17.16 | −19.25 | −4.44 |
| 1α,20S(OH)2D3 |
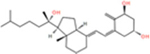
|
−6.0 | −5.7 | −6.6 | −16.14 | −14.92 | −16.02 |
| 20S,23R(OH)2D3 |
|
−6.0 | −5.8 | −6.0 | −12.75 | −3.34 | −11.36 |
| 20S,23S(OH)2D3 |
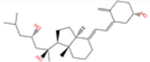
|
−5.4 | −7.7 | −6.0 | −10.37 | −25.11 | −15.95 |
| 1α,20S,23S(OH)3D3 |
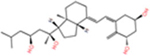
|
−6.0 | −7.6 | −5.5 | −27.74 | −12.75 | −14.60 |
MM-PBSA binding free energy was calculated based on the top-ranked docking complex, in which entropy was not included. Molecular docking binding affinity < −5.0 kcal/mol and MM-PBSA < −10 kcal/mol are considered favorable binding.
Molecular docking of vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2
We performed the molecular docking using AutoDock Vina software (Trott & Olson, 2010). Vitamin D3 and its hydroxyderivatives (1α,25(OH)2D3, 25(OH)D3, 20S(OH)D3, 1α,20S(OH)2D3, 20S,23S(OH)2D3, 1α,20S,23S(OH)2D3, 20S,23R(OH)2D3) are the ligands, and ACE2, SARS-CoV2-RBD and TMPRSS2 were receptors for molecular docking. Autodock vina is supported by Autodock tools program (Morris et al., 2009) that was used for protein optimization by removing water and other molecules and adding polar hydrogen atoms and Kollman charges. The 3D structures of the ligands were also loaded into AutoDock Tools (Morris et al., 2009), where nonpolar hydrogen atoms were removed and torsion tree root was detected. With the ligands treated as flexible with rotatable bonds while the receptor protein treated as rigid, ligand docking of the protein at the binding site was performed using AutoDock Vina. Docking results of ligand–receptor complexes with each receptor were ranked according to the calculated binding affinities (kcal/mol).
To further evaluate binding free energy of the top-ranked ligand–receptor complex, we calculated its binding free energy using Molecular Mechanics Poisson–Boltzmann Surface Area (MM-PBSA) method (Ganoth et al., 2006; Huang et al., 2020; Kollman et al., 2000; Suever et al., 2008; Wang et al., 2001), which was implemented using the AMBER 18 MD Software Package (Case et al., 2018). MM-PBSA binding free energy was calculated by finding the energy difference between the complex and the receptor and ligand. The binding free energy (ΔGbinding) was calculated by summing the molecular mechanics energy change including the electrostatic energy (ΔEelec) and the van der Waals energy (ΔEvdW), and the solvation free energy including a polar part (ΔGpol) and a nonpolar part (ΔGnonpol).
MD simulation and binding free energy analyses of 1,25(OH)2D3 in complex with ACE2, SARS-CoV-2 RBD and TMPRSS2
The classical active form of vitamin D3, 1,25(OH)2D3, regulates calcium body homeostasis and has important pleiotropic effects on multiple body functions including immune system (Bikle, 2008, 2010a, 2010b, 2011; Holick, 2003; 2007; Plum & DeLuca, 2010). To determine the molecular and structural basis of 1,25(OH)2D3 to inhibit ACE2 interaction with SARS-CoV-2 RBD and, to attenuate the activity of TMPRSS2, we performed 100 ns MD simulation for six systems: complexes of 1,25(OH)2D3 with SARS-CoV2-RBD, ACE2 and TMPRSS2, and for SARS-CoV2-RBD, ACE2 and TMPRSS2 by themselves. All six 100 ns MD simulation systems were performed in duplicate for reproducibility. AMBER force field was used for the simulated systems. The AMBER ff14SB protein force field was used as parameters for the receptors. We have obtained the force field and charge parameter for 1,25(OH)2D3 in our earlier study (Slominski et al., 2021).
MD simulations
We performed MD simulation for the studied systems using a standard protocol as described previously (Lee et al., 2008; Liu et al., 2008; Pan et al., 2011; Song et al., 2005; Suever et al., 2008; Sung et al., 2010; Wang et al., 2017; 2019; Yan et al., 2010, 2011; Yang & Song, 2016). Each complex was first minimized and solvated with TIP3P water molecules and 150 mM NaCl (physiological salt concentration) in a periodic box. At least 20 Å distance between the protein complex and the periodic box boundaries was ensured to reduce potential artifacts arising from periodicity. Additional ions of Na+ or Cl− were added into the periodic box to neutralize the charge of protein–ligand complex. The solvated system was first energy-minimized with the protein complex and ions restrained but water unrestrained. The solvent was further equilibrated with the protein complex and ions restrained at a constant number–pressure–temperature (NPT) at 50 K and 1 atm for 10 ps. The simulated system was warmed up via constant number–volume–temperature (NVT) to 300 K by steps of 50 K lasting 10 ps each with SHAKE constraints and a time step of 2 fs. Production MD simulation of 100 ns at NPT of 300 K and 1 atm was performed. In the production simulations, electrostatics interaction was calculated using the particle-mesh Ewald method with interpolation of order 4. Lennard–Jones cutoffs set at 1.0 nm. SHAKE constraint was used on all hydrogen-heavy atom bonds to permit a dynamics time step of 2 fs. To determine the simulated systems’ equilibration tendencies, root mean square deviation (RMSD) of protein backbone atoms over time were analyzed.
MM/PBSA binding free energy analysis based on MD simulation trajectories
To better understand the binding characteristics of ACE2, SARS-CoV-2 RBD and TMPRSS2 with 1α,25(OH)2D3, binding free energies were calculated for the complexes of 1,25(OH)2D3 with SARS-CoV2-RBD, ACE2 and TMPRSS2. The binding free energy was determined with the MM-PBSA method as described in previous studies (Ganoth et al., 2006; Kollman et al., 2000; Suever et al., 2008; Wang et al., 2001), which was implemented using the AMBER 18 MD Software Package (https://ambermd.org/). The MM-PBSA method combines molecular mechanics, continuum electrostatics, solvent accessible surface area calculations to calculate the binding free energy from a series of snapshots obtained from the trajectories of the MD simulations. The binding free energy was calculated by finding the energy difference between the complex and the receptor and ligand (Equation (1)). The complex for each of the frames in the production trajectory was decomposed into receptor and ligand components. The resulting three components (complex, receptor and ligand) were then used for the binding free energy analysis. Binding free energy of the complex structure over the cumulative time was also used to verify the equilibration of the complex structure during the MD simulations
| (1) |
Snapshots at a time interval of 200 ps from the last 70 ns MD simulation trajectories after the system equilibration were used to calculate all components of binding free energy. The means and standard deviations of the binding free energy over the MD simulation trajectories were calculated after the system reached initial equilibration using the bootstrap statistical method (Efron & Tibshirani, 1998).
Conformation, dynamical motion and electrostatic potential analyses based on MD simulation trajectories
To determine the simulated systems’ equilibration tendencies and their convergences, RMSD of protein backbone atoms over time and binding free energy of the complex structure over the cumulative time were analyzed. Based on equilibrated MD simulation trajectories, we performed a series of analyses to better understand conformational stability, secondary structure and dynamical motion and electrostatic potential characteristic of ACE2, SARS-CoV2-RBD or TMPRSS2 in complex with 1,25(OH)2D3.
Representative ligand–receptor complex structure
The dbscan (density-based spatial clustering of applications with noise) program (Ester et al., 1996) in Amber 18 was used for the clustering analyses of the equilibrated MD simulation trajectories. In dbscan, points are considered as part of a single cluster if there are at least some other points within a neighborhood radius ε. The dbscan algorithm also generates an average structure for the population of each cluster called a medoid. The medoid structure in the largest cluster was chosen as a representative structure for electrostatic potential analysis and to evaluate the conformation changes of TPRSS2, ACE2 and SARS-CoV-2-RBD by binding with 1,25(OH)2D3.
System equilibration
To determine the simulated systems’ equilibration tendencies and its convergence, RMSD of protein backbone atoms over time of all systems were analyzed.
Conformational stability and secondary structure analyses
To characterize the conformational fluctuation of ACE2, SARS-CoV2-RBD and TMPRSS2 by binding with 1,25(OH)2D3, root mean square fluctuations (RMSF) were calculated on a residue-by-residue basis, and averaged over the production simulation trajectories after the systems reached equilibrium. To characterize the change of the secondary structure of ACE2, SARS-CoV2-RBD and TMPRSS2 by binding with 1,25(OH)2D3, we evaluated the secondary structure using the DSSP method in the cpptraj program of AMBER18 (Kabsch & Sander, 1983). For ACE2, SARS-CoV2-RBD or TMPRSS2 in complex with 1,25(OH)2D3, the occupancy of each residue in the secondary structure was determined based on the percentage of time that the residue existed in the secondary structure over the equilibrated MD simulations.
Solvent accessible surface area analyses (SASA)
To determine the change of SASA for the binding areas for ACE2 and SARS-CoV-2-RBD by binding with 1,25(OH)2D3, average SASA was calculated through the equilibrated MD simulation trajectories using the molsurf function in Amber 18. ACE2 binding surface for SARS-CoV-2 RBD includes residues 21–101 and 323–356 and SARS-CoV-2 RBD binding surface for ACE2 includes residues 470–504.
Dynamic motion analyses
To determine the dynamic motion changes of the binding surfaces between ACE2 and SARS-CoV-2-RBD by binding with 1,25(OH)2D3, we performed principal component analysis (PCA) (Hayward et al., 1995) based on the equilibrated MD simulation trajectory. PCA was performed to decompose the motions of binding surfaces of ACE2 and SARS-CoV-2 RBD by binding with 1,25(OH)2D3 into a few principal motions that are described by eigenvectors and eigenvalues. Eigenvalue characterizes a principal mode of the total motion of the system. The first PCA mode is usually interpreted as the direction of the largest conformational fluctuation of the system during MD simulations (Berendsen & Hayward, 2000). Porcupine plots were used to visualize the collective dynamic modes calculated from PCA analysis. To analyze the degree of correlated motions between residues for the TMPRSS2 by binding with 1,25(OH)2D3 over the equilibrated MD simulation trajectories, we generated a dynamic cross-correlation maps (ΔCCM) to compare dynamic motion of TMPRSS2 with and without binding with 1,25(OH)2D3.
Electrostatic potential analyses
We performed electrostatic potential analyses using APBS software (Baker et al., 2001; Jurrus et al., 2018) to evaluate the electrostatic interactions of 1,25(OH)2D3 with ACE2, SARS-CoV-2 RBD and TMPRSS2. PDB2PQR software (Dolinsky et al., 2004, 2007) was used to convert representative complex structures of 1,25(OH)2D3 with ACE2, SARS-CoV-2 RBD and TMPRSS2 in PDB format into PQR format that included AMBER radii and charges for each atom, which were then used to generate surface electrostatic potentials for the representative complex structure of ACE2, SARS-coV-2 RBD and TMPRSS2 with 1,25(OH)2D3 using APBS software.
Statistical methods
To compare the differences of conformations and dynamical motions changes of ACE2, SARS-coV-2 RBD and TMPRSS2 with and without binding 1,25(OH)2D3, both the average values and the standard deviations of the analyzed variables were calculated. Because the adjacent snapshots from the MD trajectory have the tendency to be correlated with each other, the autocorrelation times (Lee et al., 2008) for the studied variables were obtained to resample the trajectories into statistically independent periods to calculate the standard deviations for the studied variables as our previous studies (Lee et al., 2008; Liu et al., 2008; Pan and Song, 2010; Song et al., 2005; Suever et al., 2008; Wang et al., 2014; Yan et al., 2010, 2011). With the obtained decorrelation time for the studied variable, bootstrap analyses (Efron & Tibshirani, 1998) were performed to resample the trajectories to obtain statistically independent samples. The newly generated sample datasets were used to calculate the averages and the standard deviation for the interested variables. The Student’s t test (Bailey, 1995) with 95% confidence was used to determine whether the mean and standard deviations for the interested variables were significantly different. The protocol of the statistical methods used in this study has also been used in our published studies (Lee et al., 2008; Liu et al., 2008; Pan and Song, 2010; Song et al., 2005; Suever et al., 2008; Wang et al., 2014; Yan et al., 2010, 2011).
Results and discussion
Constructed structure of TMPRSS2
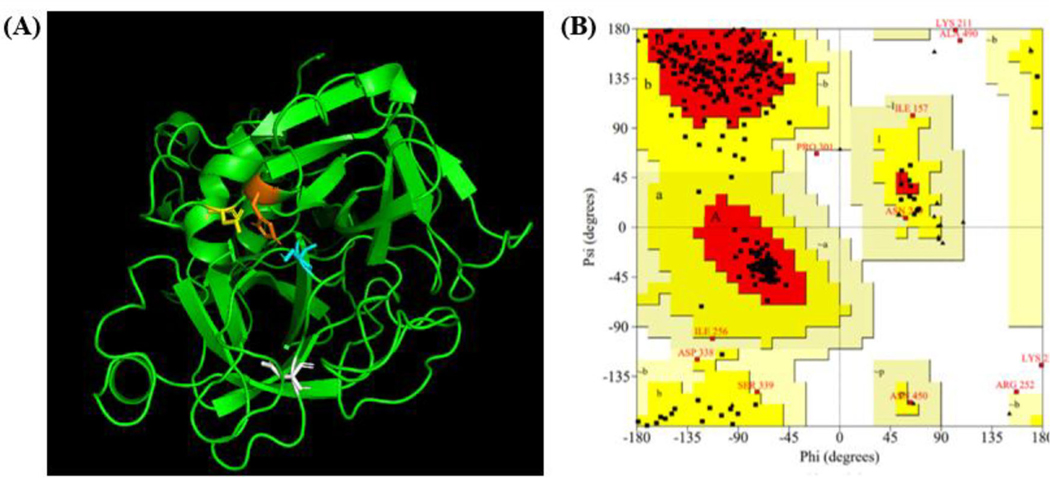
To construct TMPRSS2 structure, a template structure needs to be identified. Sequence alignment of TMPRSS2 with its homologous protein TMPRSS1 using T-Coffee (Chang et al., 2014; 2015; Di Tommaso et al., 2011; Notredame et al., 2000) showed that the two proteins had 31.7% sequence identity and 51.3% sequence similarity (Figure S1), making TMPRSS1 structure a good template for constructing the structure of TMPRSS2. With the crystal structure of TMPRSS1 (PDBID: 1Z8G (Herter et al., 2005), resolution: 1.55 Å) as template, the TMPRSS2 structure was constructed with MODELLER (Eswar et al., 2006). To generate the TMPRSS2 structures, an alignment was first created between TMPRSS1 and TMPRSS2. The alignment along with the TMPRSS1 PDB file were used to generate predictive structures of TMPRSS2. The quality of the constructed TMPRSS2 structures were evaluated with ERRAT (Colovos & Yeates, 1993), PROCHECK (Laskowski et al., 1993, 1996), and WHATCHECK (Hooft et al., 1996) (Table S1). The constructed TMPRSS2 structure with the best structure quality evaluation is shown as Figure 2(A) along with this structure’s Ramachandran plot (Figure 2(B)). The catalytic triad from the constructed TMPRSS2 structure, consisting of His296, Asp345 and Ser441, were grouped close together with the R groups of these three residues all pointing to a central position, and the substrate binding residue Asp435 remains near the catalytic triad on a random loop (Figure 2(A)). Position of the catalytic triad and Asp435 further validates the constructed TMPRSS2 structure. The Ramachandran plot, generated by PROCHECK (Laskowski et al., 1993, 1996), shows that the majority of residues in the TMPRSS2 structure lie within the most favored region (red), with most of the other residues lying in the allowed region (Δark yellow) (Figure 2(B)). A combined 96.6% of TMPRSS2’s residues lie within the most favored and allowed regions, showing the constructed TMPRSS2 structure with good quality. Inhibiting the catalytic triad could help prevent SARS-CoV-2 from infecting a cell. This constructed structure of TMPRSS2 was used for molecular docking targeting the catalytic triad with vitamin D3 and its classical (25(OH)D3, 1,25(OH)2D3) and CYP11α1-derived (20S(OH)D3, 1α,20S(OH)2D3, 20S,23S(OH)2D3, 1α,20S,23S(OH)2D3) hydroxyderivatives and chemically synthesized 20S,23R(OH)2D3, and for MD simulations of TMPRSS2 with 1,25(OH)2D3.
Figure 2.
Constructed structure of TMPRSS2. (A) The active site of the catalytic triad consists of His296 (orange), Asp345 (yellow), and Ser441 (blue). Substrate binding residue Asp435 in white. The figure was generated with PyMOL. (B) The Ramachandran plot, generated by PROCHECK (Laskowski et al., 1996; Laskowski et al., 1993), shows a vast majority of residues lying within the most favored (red) and allowed (Δark yellow) regions. Six residues lie within the generously allowed region (pale yellow) and four lie within the disallowed region (white).
Favorable binding of vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2
After molecular docking with AutoDock Vina (Trott & Olson, 2010), the top-scored docking complexes of vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2 were manually inspected and the binding free energy of each top-scored complex was calculated with MM-PBSA method (Ganoth et al., 2006; Huang et al., 2020; Kollman et al., 2000; Suever et al., 2008; Wang et al., 2001). In Table 1, we show the molecular docking results of vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2 and MM-PBSA binding free energy result for top-scored docking complexes of vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2. Results showed that vitamin D3 and its hydroxyderivatives of 1α,25(OH)2D3, 25(OH)D3, 20S(OH)D3, 1α,20S(OH)2D3, 20S,23R(OH)2D3, 20S,23S(OH)2D3, 1α,20S,23S(OH)3D3 are all favorable to bind to the active site of TMPRSS2, further serving as TMPRSS2 inhibitor to block SARS-CoV-2 entry.
For ACE2 binding site for SARS-CoV2-RBD, vitamin D3 and its hydroxyderivatives, 1α,25(OH)2D3, 25(OH)D3, 20S(OH)D3, 1α,20S(OH)2D3, 20S,23S(OH)2D3 and 1α,20S,23S(OH)3D3 are all favorable to interact with ACE2, the exception was 20S,23R(OH)2D3. For SARS-CoV2-RBD binding site for ACE2, except 20S(OH)D3, vitamin D3 and its hydroxyderivatives of 1α,25(OH)2D3, 25(OH)D3, 1α,20S(OH)2D3, 20S,23R(OH)2D3, 20S,23S(OH)2D3 and 1α,20S,23S(OH)3D3 are all favorable to binding SARS-CoV2-RBD. Vitamin D3 or its hydroxyderivatives with favorable binding to the interaction sites between ACE2 and SARS-CoV2-RBD – either on ACE2 or on SARS-CoV2-RBD (Figure 3(A, B)) – is likely to prevent SARS-CoV2-RBD binding ACE2 to constrain cellular entry of SARS-CoV-2. For TMPRSS2, vitamin D3 and its hydroxyderivatives all bind to the active site of TMPRSS2, which could prevent SARS-CoV-2 from infecting a cell (Figure 3(C)). The above findings are important because CYP11α1-derived 20S(OH)D3, 20S23S(OH)2D3 lack the calcemic effects, while 1α,20S(OH)2D3 has low calcemic activity, which contrasts with highly calcemic 1α,25(OH)2D3 (Chen et al., 2014; Slominski et al., 2010, 2013; Wang et al., 2012). It must be noted that CYP11-derived vitamin D3 hydroxymetabolites are non-toxic in preclinical studies (Chen et al., 2014; Slominski et al., 2010, 2013; Wang et al., 2012), are endogenously produced in the human body (Slominski et al., 2015, 2020a) and are detectable in natural products such as honey (Kim et al., 2020). This defines them as hormonal/biological regulators and/or natural products. However, they still require FDA approval to be considered as potential therapeutics for COVID-19 (Slominski et al., 2020c). Therefore, taking into consideration this constraint, we focused on the classical 1α,25(OH)2D3 for subsequent MD simulations.
Figure 3.
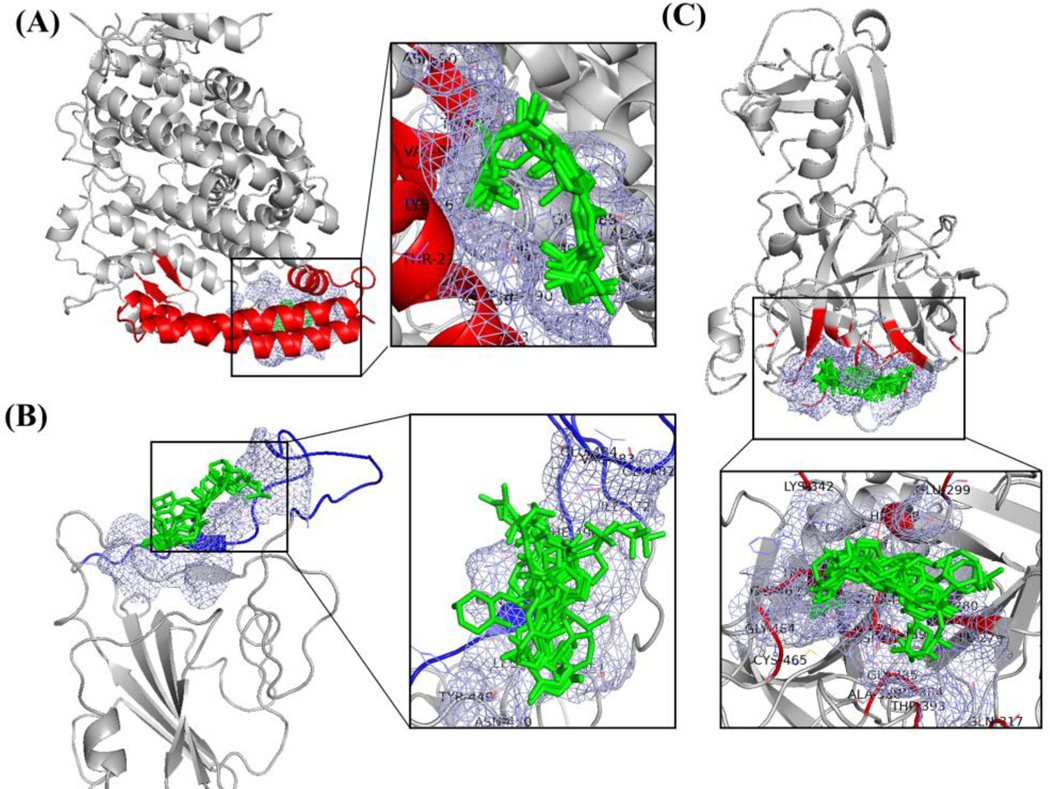
Binding mode for compounds listed in Table 1 in ligand binding mode of (A) ACE2, (B) SARS-CoV-2 RBD and (C) TMPRSS2. The light blue meshing areas shown in the figure is the binding area. All ligands listed in Table 1 are shown as green (image made with PyMOL (v2.4.0, https://pymol.org/2/)).
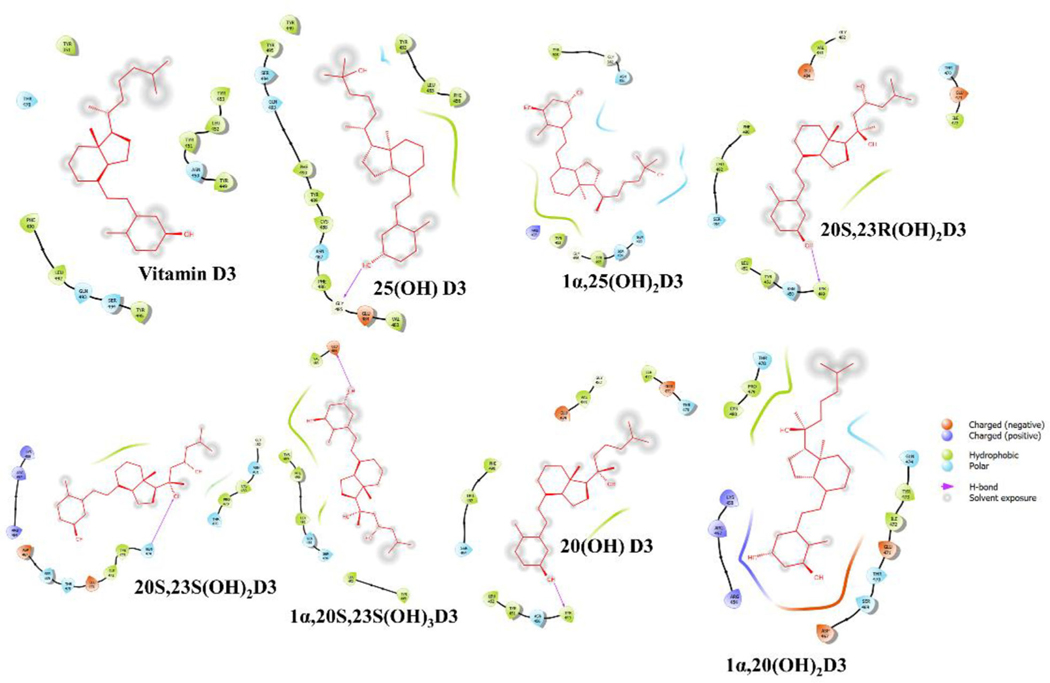
Binding interactions analysis between ACE2, SARS-CoV2-RBD or TMPRSS2 with vitamin D3 and its derivatives
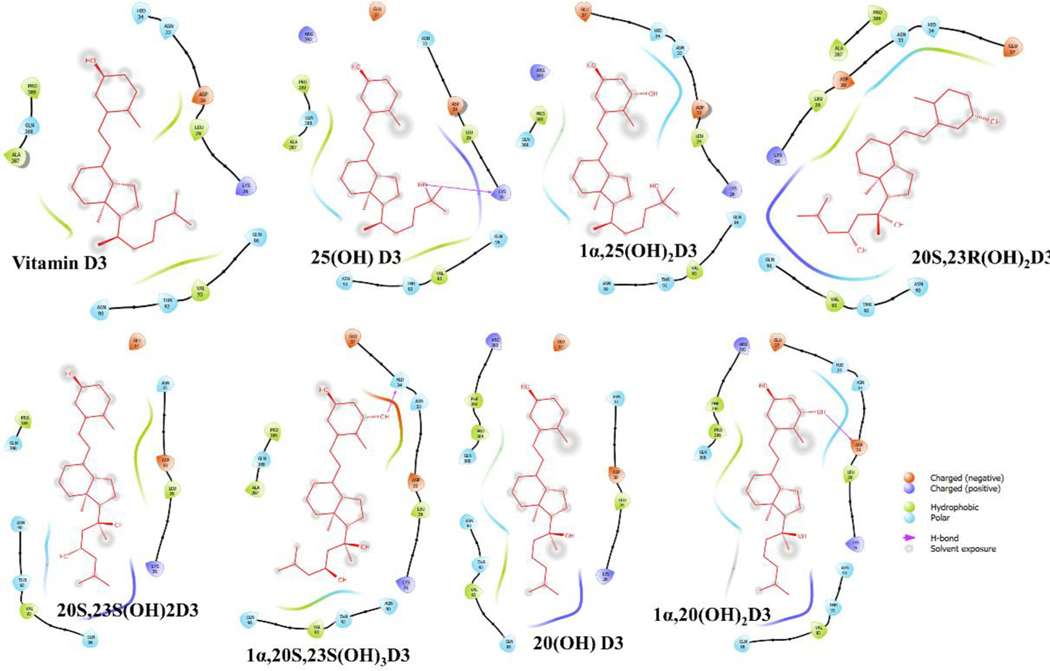
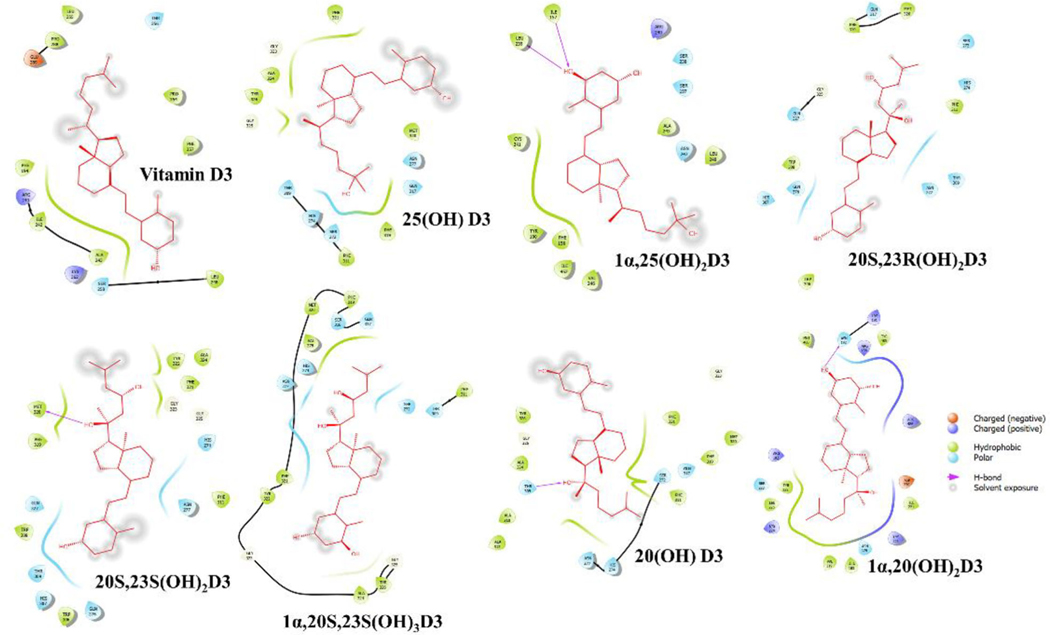
The binding interaction profile of the vitamin D3 and its hydroxyderivatives with ACE2, SARS-CoV2-RBD and TMPRSS2 was analyzed through the Maestro v12.4 (Figures 4–6). Several potential hydrogen bonds and non-bonding interactions were observed between vitamin D3 or its hydroxyderivatives and the binding site amino residues of ACE2, SARS-CoV2-RBD or TMPRSS2. 25(OH)D3’s hydroxyl group and 1α,20S,23S(OH)3D3’s hydroxyl group on C1α site were found to be critical to interact with Lys26 and Hid34 in ACE2 by forming one hydrogen bond, respectively (Figure 4). For complex of SARS-CoV2-RBD with vitamin D3 or its hydroxyderivatives, 20S,23R(OH)2D3 and 20(OH)D3’s hydroxyl group all formed one hydrogen bond with Tyr449, and 20S,23S(OH)2D3’s hydroxyl group on C20S site formed one hydrogen bond with Gln474 (Figure 5). For complex of TMPRSS2 with vitamin D3 or its hydroxyderivatives, 1α,25(OH)2D3 docked nicely into the active site of TMPRSS2, its hydroxyl group interacted with Leu239 and Ile157 through two hydrogen bonds, 20S,23S(OH)2D3, 20(OH)D3 and 1α,20(OH)2D3 formed one hydrogen bond with Met320, Thr309 and Asn192, respectively (Figure 6). Beyond above, several hydrophobic interactions and electrostatic interactions were observed between vitamin D3 or its hydroxyderivatives with ACE2, SARS-CoV2-RBD or TMPRSS2.
Figure 4.
2D interactions of vitamin D3 and its hydroxyderivatives with ACE2. The figure was generated with Maestro (v12.4, https://www.schrodinger.com/products/maestro).
Figure 6.
2D interactions of vitamin D3 and its hydroxyderivatives with TMPRSS2. The figure was generated with Maestro (v12.4, https://www.schrodinger.com/products/maestro).
Figure 5.
2D interactions of vitamin D3 and its hydroxyderivatives with SARS-CoV2-RBD. The figure was generated with Maestro (v12.4, https://www.schrodinger.com/products/maestro).
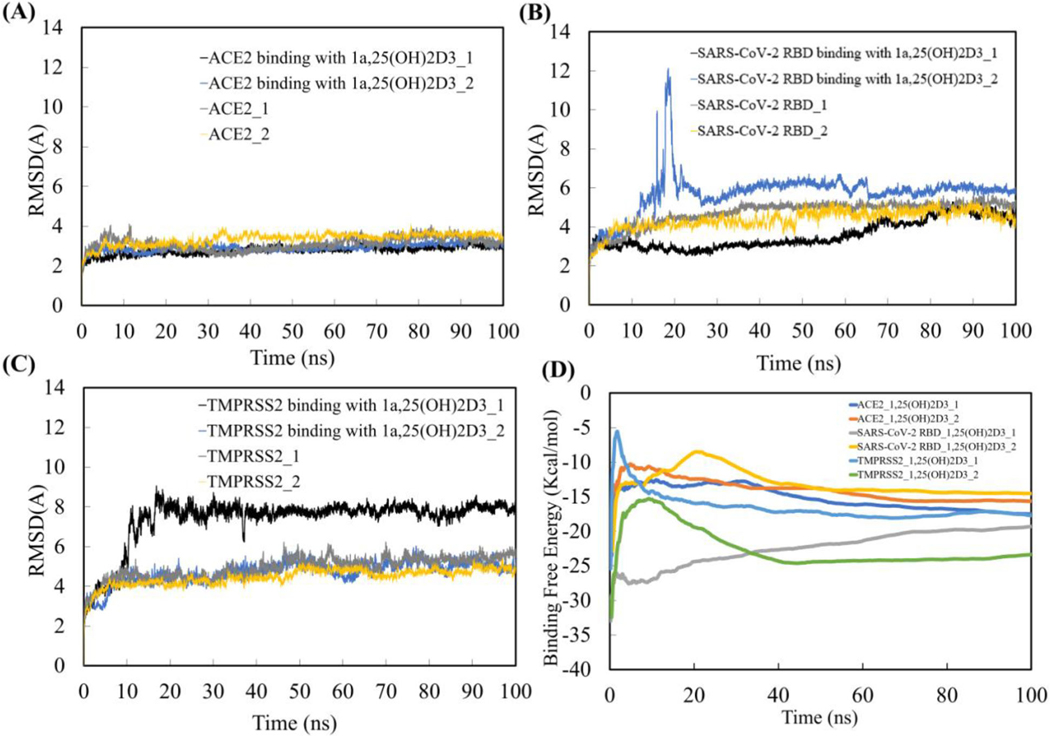
Equilibration of MD simulation systems
The RMSD of the backbone of ACE2, SARS-CoV-2 RBD and TMPRSS2 over 100 ns MD simulations (Figure 7(A, B & C)) reached initial equilibration after 30 ns MD simulations for all twelve systems with or without 1,25(OH)2D3 binding. The binding free energies for all the complexes as a function of cumulative time over the 100 ns MD simulation also showed that the complexes converged after the first 30 ns MD simulation (Figure 7(D)). The last 70 ns of equilibrated MD simulation trajectories for ACE2, SARS-CoV-2 RBD and TMPRSS2 with or without 1,25(OH)2D3 binding were used for the analyses of the binding thermodynamics of ACE2, SARS-CoV-2 RBD and TMPRSS2 with 1,25(OH)2D3, and for determining the structural, conformational and motion characteristics of ACE2, SARS-CoV-2 RBD and TMPRSS2 by binding with 1,25(OH)2D3.
Figure 7.
RMSD of ACE2 (A), SARS-coV-2 (B) and TMPRSS2 (C) with or without binding 1a,25(OH)2D3 (1,25(OH)2D3) over 100 ns MD simulations, and binding free energy of ACE2, SARS-coV-2 and TMPRSS2 with 1,25(OH)2D3 as a function of cumulative time over the 100 ns MD simulation (D).
Binding thermodynamics and ligand–residue interaction decomposition analysis of ACE2, SARS-CoV-2 RBD and TMPRSS2 with 1,25(OH)2D3 based on equilibrated MD simulation trajectories
Based on the equilibrated last 70 ns MD simulations trajectories performed in duplicate, we calculated the binding free energy of ACE2, SARS-CoV-2 and TMPRSS2 with 1,25(OH)2D3 (Table 2). Energy components of electrostatic energy (ΔEelectrostatic), van der Waals energy (ΔEvdW), polar solvation energy (ΔGpolar-solvation) and nonpolar solvation energy (ΔGnonpolar-solvation) that contributed to the binding free energy were also calculated. The means of the binding free energy and energy components were obtained by averaging over duplicate trajectories, and the standard deviations were calculated with the independent periods of the MD simulation trajectories, using the bootstrap statistics method described in the ‘Materials and Methods’ section as in our previous studies (Lee et al., 2008; Liu et al., 2008; Pan and Song, 2010; Song et al., 2005; Suever et al., 2008; Wang et al., 2014; Yan et al., 2010, 2011). Results in Table 2 show that the binding free energy of ACE2, SARS-CoV-2 and TMPRSS2 with 1,25(OH)2D3 are −18.55 ± 4.16, −16.97 ± 1.69 and −21.04 ± 1.53 kcal/mol separately, further confirming that ACE2, SARS-CoV-2 and TMPRSS2 are all favorable binding with 1,25(OH)2D3. Although 1,25(OH)2D3 display favorable binding with ACE2, SARS-CoV-2 and TMPRSS2, different components of binding energy such as van der Waals energy, electrostatics energy and polar solvation energy were observed between 1,25(OH)2D3 in complex with ACE2, SARS-CoV-2 and TMPRSS2. The different structure, conformation and atomic charge parameters for ACE2, SARS-CoV-2 and TMPRSS2 directly affect 1,25(OH)2D3 interactions with each receptor. These different interactions could result in the varied hydrogen bond formation, van der Waals interactions and electrostatic interactions, further contributing to the observed different energy components for binding free energy between different complexes of 1,25(OH)2D3 with ACE2, SARS-CoV-2 and TMPRSS2.
Table 2.
Binding free energy of ACE2, SARS-CoV-2 and TMPRSS2 with 1,25(OH)2D3 (kcal/mol).
| ACE2_1,25(OH)2D3 | SARS-CoV-2 RBD _1,25(OH)2D3 | TMPRSS2_ 1,25(OH)2D3 | |
|---|---|---|---|
|
| |||
| ΔEvdW | −26.44 ± 0.42 | −24.08 ± 1.13 | −38.99 ± 1.08 |
| ΔEelectrostatic | −38.99 ± 1.08 | −11.51 ± 1.08 | −12.03 ± 4.90 |
| ΔGnonpolar-solvation | −4.35 ± 0.04 | −4.20 ± 0.09 | −5.47 ± 0.08 |
| ΔGpolar-solvation | 22.37 ± 3.45 | 22.95 ± 1.12 | 35.52 ± 1.83 |
| ΔGbinding | −18.55 ± 4.16 | −16.97 ± 1.69 | −21.04 ± 1.53 |
All values in this table were expressed in term of kcal/mol. ΔEvdW, van der Waals energy; ΔEelectrostatic, electrostatic energy; ΔGnonpolar-solvation, nonpolar solvation energy; ΔGpolar-solvation, polar solvation energy; ΔGbingding, binding free energy for the complex.
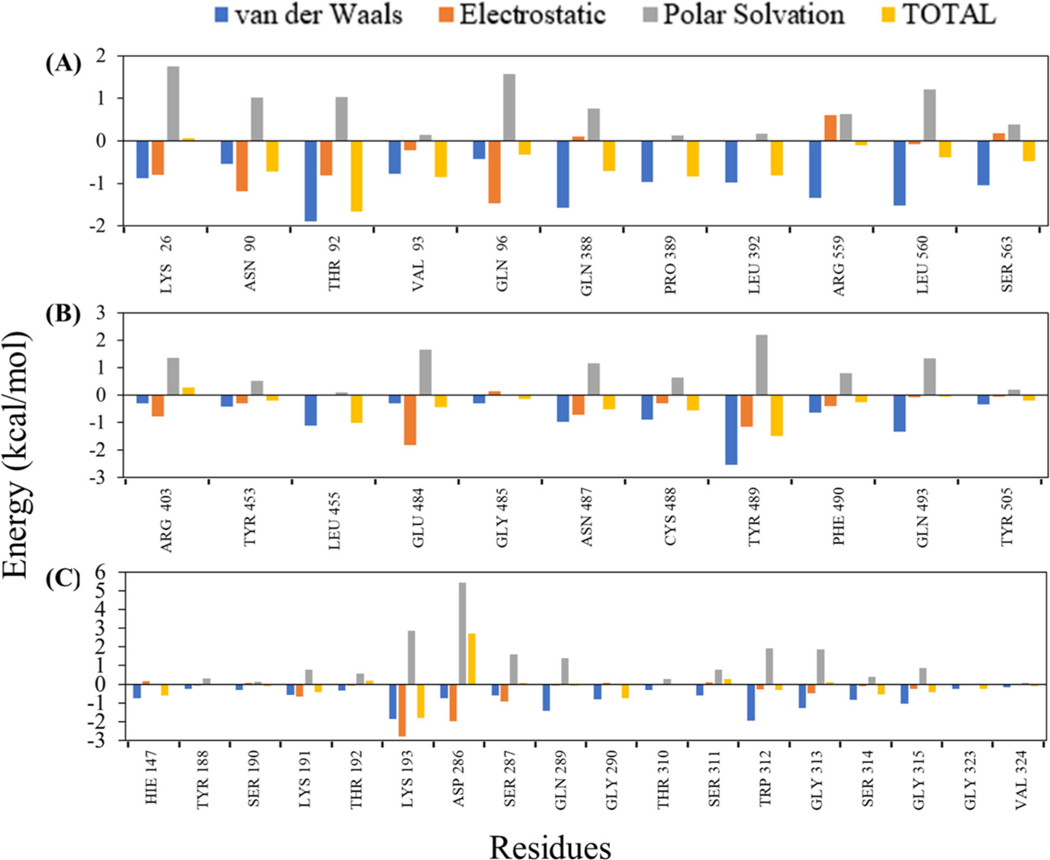
To further quantify the contribution of binding pocket residues to the molecular interaction of 1,25(OH)2D3 with ACE2, SARS-CoV-2 RBD and TMPRSS2, the energy decomposition per residue was performed (Figure 8). The plots of energy decomposition analyses show that the active site residues, Lys26, Asn90, Thr92, Val93, Gln96, Gln388, Pro389, Leu392, Arg559, Leu560 and Ser563 energetically favor the binding stability of 1,25(OH)2D3 to ACE2. Remarkably, Thr92 contributed the highest binding energy, ΔEvdW (−1.89kcal/mol), ΔEelectrostatic (−0.81kcal/mol) and ΔGbingding (−1.67kcal/mol), which indicated the favorable electrostatic and vdW interactions with 1,25(OH)2D3 (Figure 8(A)). The binding interaction with SARS-CoV-2 RBD shows that the amino acid Arg403, Tyr453, Leu455, Glu484, Gly485, Asn487, Cys488, Tyr489, Phe490, Gln493 and Tyr505 contributed the most to the total ΔGbingding of −16.97 kcal/mol (Figure 8(B)). Figure 8(C) shows the energy decomposition plot of TMPRSS2 indicates the substantial contribution of amino acids, Lys193, Asp286, Ser287, Gln289, Trp312 and Gly313 to hold 1,25(OH)2D3 at the binding pocket.
Figure 8.
Residue decomposition plots obtained from MM-PBSA that represent the binding energy contribution of active site residues in ACE2 (A), SARS-CoV-2 RBD (B) and TMPRSS2 (C) when binding with 1,25(OH)2D3 based on the equilibrated last 70 ns MD simulations trajectories.
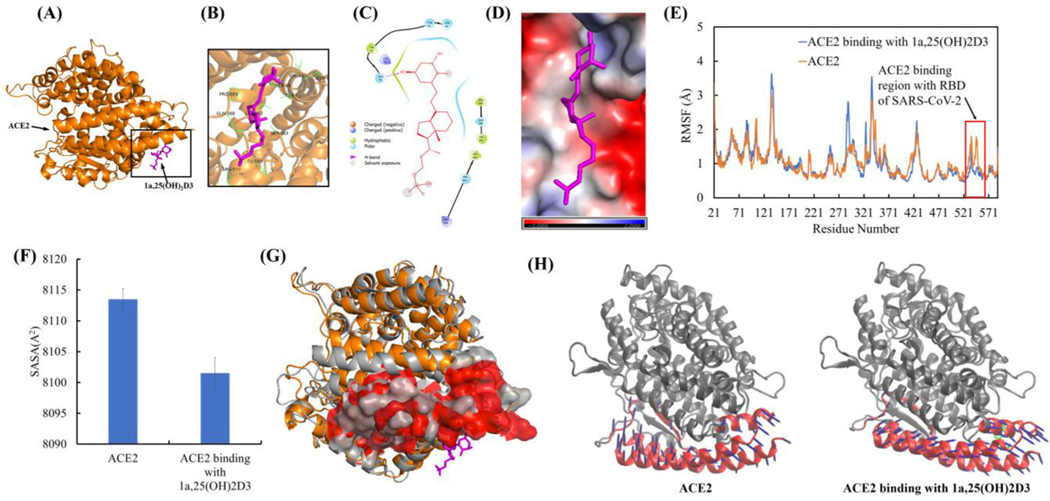
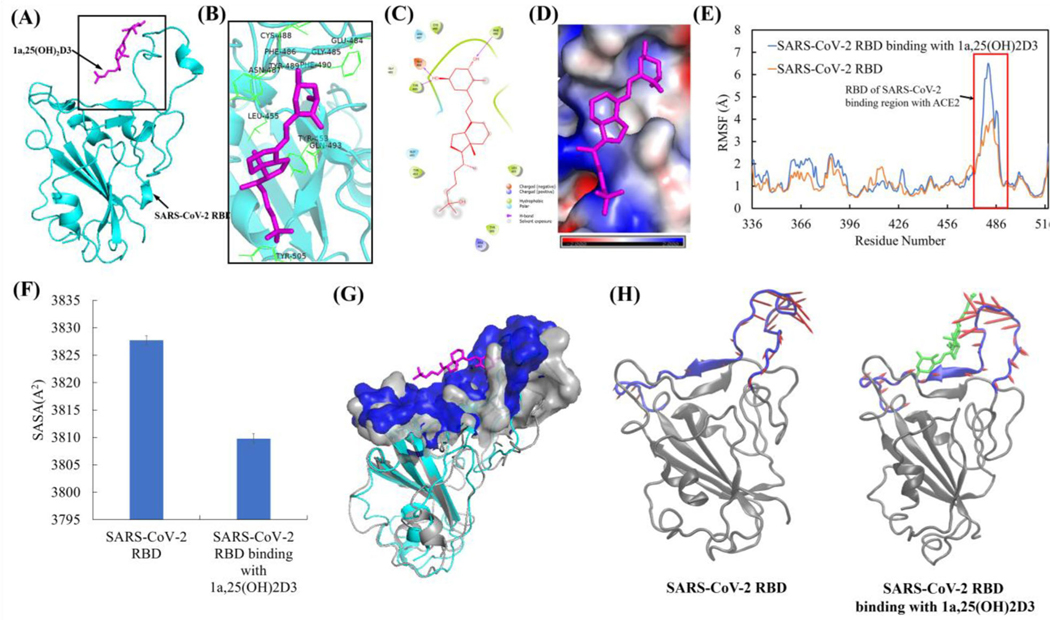
Interaction of 1,25(OH)2D3 with ACE2 and its effect on structure, conformation, and dynamical motion of ACE2 binding surface for SARS-CoV-2 RBD
The representative complex structure of 1,25(OH)2D3 with ACE2 in the equilibrated MD trajectory is shown in Figure 9(A). The interaction analyses showed that 1,25(OH)2D3 bound ACE2 through one hydrogen bond with Gln96 and hydrophobic interaction with Val93, Pro389, Leu392 and Leu560. The important residues confining the binding site were found to be Lys26, Gln96, Asn90, Thr92, Val93, Gln388, Pro389, Leu392, Leu560, Ser563 and Arg559 (Figure 9(B, C)). These residues are all belong to the ACE2 binding region of SARS-CoV-2 RBD, which means that the conformational changes within this region could preclude the binding to the SARS-CoV-2 RBD (Gross et al., 2020). The electrostatic potential analyses showed the strong negative electrostatic potential (in red) in the surrounding of ACE binding region due to the negatively charged residues of Glu22, Glu23 Asp30 and Glu564. The binding surface with strong negative electrostatic potential can steadily hold the 1,25(OH)2D3 polar groups that have partial positive charges through electrostatic interaction (Figure 9(D)). Based on the post-equilibrated MD simulation trajectories, conformational and secondary structural analyses showed that 1,25(OH)2D3 interaction with ACE2 resulted in the conformational fluctuation changes of ACE2, including the ACE2 binding region for SARS-CoV-2 RBD (res 521–571) (Figure 9(E)), but not significant structural and secondary structural changes (Table S2, Figures S2 and S3(A)). 1,25(OH)2D3 interaction with ACE2 resulted in the significantly decreased solvent accessible surface area of ACE2 binding region for SARS-CoV-2 RBD (Figure 9(F)). Overlay of the representative structure of ACE2 with and without 1,25(OH)2D3 binding from the equilibrated MD simulation trajectories further demonstrated the conformation changes of the ACE2 binding region for SARS-CoV-2 RBD (Figure 9(G)). PCA of ACE2 binding region for RBD of SARS-CoV-2 showed that 1,25(OH)2D3 binding ACE2 resulted in the changed dynamical motion of the ACE2 binding region for RBD of SARS-CoV-2 compared to ACE2 alone based on the first PCA mode (Figure 9(H)) and the second and the third PCA modes (Figure S4) that contributed most to the ACE2 dynamical motion. Dynamical motion change of ACE2 by binding 1,25(OH)2D3 supported the observed changes of the conformation and solvent accessible surface area of ACE2 binding region for RBD of SARS-CoV-2.
Figure 9.
1a,25(OH)2D3 (1,25(OH)2D3) interactions with ACE2 resulted in the conformational and dynamical motion changes of ACE2 binding surface for SARS-CoV-2 RBD. (A) The representative ACE2 binding mode with 1,25(OH)2D3 from equilibrated MD simulation (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (B) 3D representation of 1,25(OH)2D3 interaction with binding region of ACE2 (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (C) 2D representation of 1,25(OH)2D3 interaction with binding region of ACE2 (image made with Maestro (v12.4, https://www.schrodinger.com/products/maestro)). (D) Electrostatic potential of ACE2 at its binding region with 1,25(OH)2D3 (in magenta). Red: negative electrostatic potential; Blue: positive electrostatic potential and White: neutral electrostatic potential (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (E) RMSF of ACE2 with and without 1,25(OH)2D3 binding (Δuplicate and data shown in average). (F) Comparison of SASA of ACE2 binding region (residues 21–101 and 323–356) for SARS-CoV-2 RBD with and without dynamic interactions with 1,25(OH)2D3. (G) Overlay of ACE2 with and without dynamic interactions with 1,25(OH)2D3 (Before dynamic interactions with 1,25(OH)2D3: ACE2 shown in gray, the surface of ACE2 binding region for SARS-CoV-2 RBD shown in gray. After dynamic interactions with 1,25(OH)2D3: ACE2 shown in orange, the surface of ACE2 binding region with SARS-CoV-2 RBD shown in red). Residues 106–130 were used for two structures alignment. (H) PCA motions of ACE2 binding region for RBD of SARS-CoV-2 with and without binding 1,25(OH)2D3. ACE2 were shown as new cartoon in gray and its binding site for RBD of SARS-CoV-2 in red. 1,25(OH)2D3 shown as licorice in green. Arrows of principal dynamic motion shown as blue. The images were made with VMD (v1.9.2, http://www.ks.uiuc.edu/Research/vmd/).
Interaction of 1,25(OH)2D3 with SARS-CoV-2 RBD and its effect on conformation and dynamical motion of SARS-CoV-2 RBD binding surface for ACE2
The representative complex structure of 1,25(OH)2D3 with SARS-CoV-2 RBD in the equilibrated MD trajectory is shown in Figure 10(A). Interaction analyses showed that 1,25(OH)2D3 bound SARS-CoV-2 RBD through one hydrogen bond with Phe490 at C3 and two hydrogen bonds with Glu484 and Tyr489 at C1α site. 1,25(OH)2D3 bound SARS-CoV-2 RBD also through hydrophobic interaction with Tyr453, Leu455, Cys486, Tyr489 and Phe490. The important amino residues confining the binding site were found to be Arg403, Tyr453, Leu455, Glu484, Gly485, Asn487, Cys488, Tyr489, Phe490, Gln493 and Tyr505 (Figure 10(B, C)). Electrostatic potential analyses showed both positive (in blue) and negative electrostatic potential (in red) in SARS-CoV-2 RBD binding region for ACE2 (Figure 10(D)). Positive electrostatic potential due to positive charged residues of Arg403 and Lys417 can hold 1,25(OH)2D3 polar groups that have partial negative charges. In the meanwhile, negative charged Glu406 and Glu484 can hold 1,25(OH)2D3 polar groups that have partial positive charges through electrostatic interactions (Figure 10(D)). Based on the post-equilibrated MD simulation trajectories, conformational and secondary structural analyses showed that 1,25(OH)2D3 interaction with SARS-CoV-2 RBD resulted in the increased conformational fluctuation for SARS-CoV-2 RBD binding surface for ACE2 (res 470–504) (Figure 10(E)), but not significant structural and secondary structural changes (Table S2, Figures S2 and S3(B)). 1,25(OH)2D3 interaction with SARS-CoV-2 RBD resulted in the significantly decreased solvent accessible surface area of SARS-CoV-2 RBD binding region for ACE2 (Figure 10(F)). Overlay of the representative structure of SARS-CoV-2 RBD with and without 1,25(OH)2D3 binding from the equilibrated MD simulation trajectories further demonstrated the conformation changes of the SARS-CoV-2 RBD binding region for ACE2 (Figure 10(G)). Principal component analyses of the dynamical motion of SARS-CoV-2 RBD binding surface for ACE2 showed that 1,25(OH)2D3 binding SARS-CoV-2 RBD resulted in the changed dynamical motion of the SARS-CoV-2 RBD (Figure 10(H) and Figure S5), which support the observed changes of the conformation and solvent accessible surface area of SARS-CoV-2 RBD binding region for ACE2.
Figure 10.
1a,25(OH)2D3 (1,25(OH)2D3) interactions with SARS-CoV-2 RBD resulted in the conformation and dynamical motion changes of SARS-CoV-2 RBD binding surface for ACE2. (A) The representative SARS-CoV-2 RBD binding mode with 1,25(OH)2D3 from equilibrated MD simulation (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (B) 3D representation of 1,25(OH)2D3 interaction with binding region of SARS-CoV-2 RBD (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (C) 2D representation of 1,25(OH)2D3 interaction with binding region of SARS-CoV-2 RBD (image made with Maestro (v12.4, https://www.schrodinger.com/products/maestro)). (D) Electrostatic potential of SARS-CoV-2 RBD at its binding region with 1,25(OH)2D3 (in magenta). Red: negative electrostatic potential; Blue: positive electrostatic potential and white: neutral electrostatic potential (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (E) RMSF of SARS-CoV-2 RBD with and without dynamic interactions with 1,25(OH)2D3 binding (Δuplicate and data shown in average). (F) Comparison of SASA of SARS-CoV-2 RBD binding region (residues 470–504) for ACE2 with and without dynamic interactions with 1,25(OH)2D3. (G) Overlay of SARS-CoV-2 RBD with and without dynamic interactions with 1,25(OH)2D3 (without 1,25(OH)2D3 binding: SARS-CoV-2 RBD shown in gray, the surface of ACE2 binding region with RBD of SARS-CoV-2 shown in gray. without 1,25(OH)2D3 binding, SARS-CoV-2 RBD shown in cyan, the surface of ACE2 binding region with RBD of SARS-CoV-2 shown in blue) with the alignment using residues 506–518. (H) PCA motions of SARS-CoV-2 RBD binding region for ACE2 with and without dynamic interactions with 1,25(OH)2D3. SARS-CoV-2 RBD were shown as new cartoon in gray and its binding with RBD of SARS-CoV-2 in blue. 1,25(OH)2D3 shown as licorice in green. Arrows of principal dynamic motion shown as red. The images were made with VMD (v1.9.2, http://www.ks.uiuc.edu/Research/vmd/).
Interaction of 1,25(OH)2D3 with TMPRSS2 and its effect on conformation and dynamical motion of TMPRSS2
The representative complex structure of 1,25(OH)2D3 with TMPRSS2 in the equilibrated MD trajectory is shown as Figure 11(A). Interaction analyses showed that 1,25(OH)2D3 bound TMPRSS2 through hydrophobic interactions with Tyr337, Trp461, Val473 and Tyr474. The important amino residues confining the binding site were found to be Hie296, Tyr337, Ser339, Lys342, Asp435, Ser436, Gly439, Thr459, Ser460, Trp461, Gly462, Ser463, Gly464, Gly472, Gln438, Val473 and Tyr474 (Figure 11(B, C)). Electrostatic potential analyses showed the strong negative electrostatic potential (in red) in the binding region of TMPRSS2 due to the negatively charged residues of Glu299, Asp338, Asp345, Glu389, Asp435, Asp440 and Asp458. The binding surface with strong negative electrostatic potential can steadily hold the 1,25(OH)2D3 polar groups that have partial positive charges through electrostatic interaction (Figure 11(D)). Based on the post-equilibrated MD simulation trajectories, conformational and secondary structural analyses showed that 1,25(OH)2D3 interaction with TMPRSS2 resulted in the increased conformational fluctuation, particularly for residues between 210 and 240 that are near the N-terminal of TMPRSS2 (Figure 11(E)), but not significant structural and secondary structural change (Table S2, Figures S2 and S3(C)). To determine whether TMPRSS2 conformational changes is associated with dynamical motions changes of TMPRSS2, dynamic cross-correlation maps (ΔCCM) between all Ca atoms from the backbone of TMPRSS2 with and without dynamic interactions with 1,25(OH)2D3 were analyzed. DCCM results showed that 1,25(OH)2D3 interaction with TMPRSS2 caused overall increased both correlated motion (red) and anti-correlated motion (blue) compared to TMPRSS2 alone (Figure 11(F)).
Figure 11.
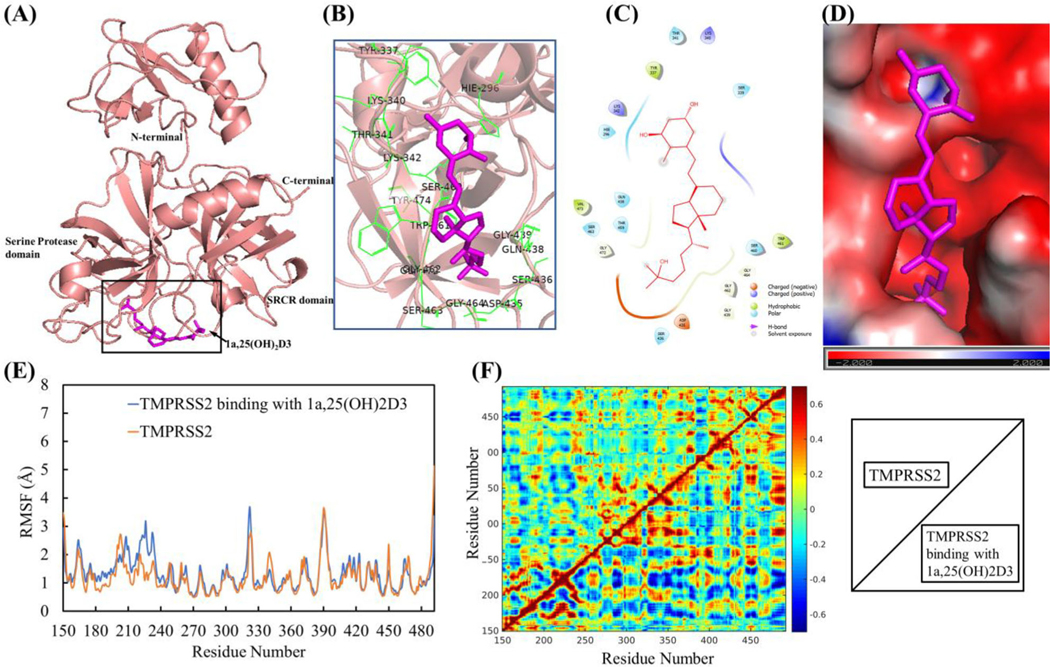
1a,25(OH)2D3 (1,25(OH)2D3) interactions with TMPRSS2 resulted in the conformational and dynamical motion changes. (A) The representative TMPRSS2 binding mode with 1,25(OH)2D3 from equilibrated MD simulation (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (B) 3D representation of 1,25(OH)2D3 interaction with binding region of TMPRSS2 (image made with PyMOL (v2.4.0, https://pymol.org/2/)). (C) 2D representation of 1,25(OH)2D3 interaction with binding region of TMPRSS2 (image made with Maestro (v12.4, https://www.schrodinger.com/products/maestro)). (D) Electrostatic potential of TMPRSS2 at its binding region with 1,25(OH)2D3 (in magenta). Red: negative electrostatic potential; Blue: positive electrostatic potential and White: neutral electrostatic potential. (image made with PyMOL (v2.4.0,https://pymol.org/2/)). (E) RMSF of TMPRSS2 with and without 1,25(OH)2D3 binding (Duplicate and data shown in average). (F) DCCM dynamical motion analyses of TMPRSS2 with and without binding with 1,25(OH)2D3 (correlated motion shown in read, anti-correlated motion shown in blue). The images were made with MATLAB (vR2015a, https://www.mathworks.com/products/matlab.html).
Conclusion
The epidemiologic correlations of SARS-CoV-2 infection and poor prognosis of disease with vitamin D deficiency are observed worldwide, however, their molecular mechanisms are not fully understood. In this study, we used combined molecular docking, MD simulations and binding free energy analyses to investigate the potentials of vitamin D3 and its hydroxyderivatives as TMPRSS2 inhibitor and to inhibit the SARS-CoV-2 RBD binding to ACE2, as well as to unveil molecular and structural basis of 1,25(OH)2D3 capability to inhibit ACE2 and SARS-CoV-2 RBD interactions. The results have shown that vitamin D3 and its hydroxyderivatives are favorable to bind active site of TMPRSS2 and the binding site(s) between ACE2 and SARS-CoV2-RBD, which indicate that vitamin D3 and its biologically active hydroxyderivatives can serve as TMPRSS2 inhibitors and can inhibit ACE2 binding of SARS-CoV-2 RBD to prevent SARS-CoV-2 entry. The interactions of classical active form of vitamin D3, 1,25(OH)2D3, with SARS-CoV-2 RBD and ACE2 resulted in the conformation and dynamical motion changes of the binding surfaces between SARS-CoV-2 RBD and ACE2 to interrupt the binding of SARS-CoV-2 RBD with ACE2. The interaction of 1,25(OH)2D3 with TMPRSS2 also caused the conformational and dynamical motion changes of TMPRSS2, which could affect TMPRSS2 to prime SARS-CoV-2 spike proteins. Therefore, we propose that vitamin D3 and its biologically active hydroxyderivatives are promising drugs or adjuvants in the treatment of COVID-19 or to use their backbones for further modification to develop efficient drugs through medicinal chemistry approach.
Supplementary Material
Acknowledgments
Funding
The study was supported by NIH grants 1R01αR073004-01α1 and R01αR071189-01α1 to ATS, R21 AI152047-01α1 to CR and ATS, pilot grant from Hugh Kaul Precision Medicine Institute at University of Alabama at Birmingham (UAB) (YHS). This work was enabled by COVID-19 High-Performance Computing (HPC) Consortium award (TGBIO200084 for YHS, ATS and YWS) with computational resources from the Extreme Science and Engineering Discovery Environment (XSEDE) and was also enabled by the high-performance computing recourses at UAB IT Research Computing.
Abbreviations:
- ACE2
angiotensin-converting enzyme 2
- COVID-19
coronavirus disease 2019
- RBD
receptor binding domain
- SARS-CoV-2
severe acute respiratory syndrome coronavirus
- SASA
solvent accessible surface area
- TMPRSS2
transmembrane serine protease 2
- MD
molecular dynamics
- MM-PBSA
molecular mechanics Poisson–Boltzmann surface area
- NPT
constant number–pressure–temperature
- NVT
constant number–volume–temperature
- RMSD
root mean square deviation
- RMSF
root mean square fluctuations
- SASA
solvent accessible surface area analyses
- DCCM
dynamic cross-correlation map
- PCA
principal component analysis
- dbscan
density-based spatial clustering of applications with noise
Footnotes
Supplemental data for this article can be accessed online at https://doi.org/10.1080/07391102.2021.1964601
Disclosure statement
The authors declare that there are no conflicts of interest.
Data availability statement
Force field of 1,25(OH)2D3, constructed TMPRSS2 structure, the docking complex structures and MD simulation trajectories are available upon request.
References
- Alipio M. (2020). Vitamin D supplementation could possibly improve clinical outcomes of patients infected with coronavirus-2019 (COVID-19). Available at SSRN 3571484. [Google Scholar]
- Ardizzone S, Cassinotti A, Trabattoni D, Manzionna G, Rainone V, Bevilacqua M, Massari A, Manes G, Maconi G, Clerici M, & Bianchi Porro G. (2009). Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: An in vitro study. International Journal of Immunopathology and Pharmacology, 22(1), 63–71. 10.1177/039463200902200108 [DOI] [PubMed] [Google Scholar]
- Aygun H. (2020). Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-Schmiedeberg’s Archives of Pharmacology, 393(7), 1157–1160. 10.1007/s00210-02001911-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey NTJ (1995). Statistical methods in biology (Vol. third edition ed.). Cambridge University Press. [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, & McCammon JA (2001). Electrostatics of nanosystems: Application to microtubules and the ribosome. Proceedings of the National Academy of Sciences of the United States of America, 98(18), 10037–10041. 10.1073/pnas.181342398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HJ, & Hayward S. (2000). Collective protein dynamics in relation to function. Current Opinion in Structural Biology, 10(2), 165–169. 10.1016/S0959-440X(00)00061-0 [DOI] [PubMed] [Google Scholar]
- Bikle DD (2008). Vitamin D receptor, UVR, and skin cancer: A potential protective mechanism. The Journal of Investigative Dermatology, 128(10), 2357–2361. 10.1038/jid.2008.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD (2010a). Vitamin D and the skin. Journal of Bone and Mineral Metabolism, 28(2), 117–130. 10.1007/s00774-009-0153-8 [DOI] [PubMed] [Google Scholar]
- Bikle DD (2010b). Vitamin D: Newly discovered actions require reconsideration of physiologic requirements. Trends in Endocrinology and Metabolism: TEM, 21(6), 375–384. 10.1016/j.tem.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD (2011). Vitamin D metabolism and function in the skin. Molecular and Cellular Endocrinology, 347(1–2), 80–89. 10.1016/j.mce.2011.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D, & Christakos S. (2020). New aspects of vitamin D metabolism and action – Addressing the skin as source and target. Nature Reviews Endocrinology, 16(4), 234–252. 10.1038/s41574-019-0312-5 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, & Mahon BD (2005). D-hormone and the immune system. The Journal of Rheumatology. Supplement, 76, 11–20. [PubMed] [Google Scholar]
- Case D, Ben-Shalom I, Brozell S, Cerutti D, Cheatham T III, Cruzeiro V, Darden T, Duke R, Ghoreishi D, & Gilson M. (2018). AMBER 2018. University of California. [Google Scholar]
- Chang JM, Di Tommaso P, & Notredame C. (2014). TCS: A new multiple sequence alignment reliability measure to estimate alignment accuracy and improve phylogenetic tree reconstruction. Molecular Biology and Evolution, 31(6), 1625–1637. 10.1093/molbev/msu117 [DOI] [PubMed] [Google Scholar]
- Chang JM, Di Tommaso P, Lefort V, Gascuel O, & Notredame C. (2015). TCS: A web server for multiple sequence alignment evaluation and phylogenetic reconstruction. Nucleic Acids Research, 43(W1), W3–W6. 10.1093/nar/gkv310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tang Z, Slominski AT, Li W, Zmijewski MA, Liu Y, &_ Chen J. (2020). Vitamin D and its analogs as anticancer and antiinflammatory agents. European Journal of Medicinal Chemistry, 207, 112738. 10.1016/j.ejmech.2020.112738 [DOI] [PubMed] [Google Scholar]
- Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, & Li W. (2014). Novel vitamin D analogs as potential therapeutics: Metabolism, toxicity profiling, and antiproliferative activity. Anticancer Research, 34(5), 2153–2163. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang J, Ge X, Du J, Deb DK, & Li YC (2013). Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase b protein. The Journal of Biological Chemistry, 288(27), 19450–19458. 10.1074/jbc.M113.467670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovos C, & Yeates TO (1993). Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Science: A Publication of the Protein Society, 2(9), 1511–1519. 10.1002/pro.5560020916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P, Ronconi G, Caraffa A, Gallenga C, Ross R, Frydas I, & Kritas S. (2020). Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Antiinflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34, 1. [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. (2020). The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology, 5, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolo A, Lucchini R, Keller F, & Cantu M. (2020). 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients, 12, 1359. 10.3390/nu12051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, & Notredame C. (2011). T-coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Research, 39(Web Server issue), W13–W17. 10.1093/nar/gkr245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, & Baker NA (2007). PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Research, 35(Web Server issue), W522–W525. 10.1093/nar/gkm276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky TJ, Nielsen JE, McCammon JA, & Baker NA (2004). PDB2PQR: An automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Research, 32(Web Server issue), W665–W667. 10.1093/nar/gkh381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Du H, & Gardner L. (2020). An interactive web-based dashboard to track COVID-19 in real time. The Lancet. Infectious Diseases, 20(5), 533–534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M, & Montano-Loza AJ (2020). Perspective: Improving vitamin D status in the management of COVID-19. European Journal of Clinical Nutrition, 74(6), 856–859. 10.1038/s41430-020-0661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, & Tibshirani RJ (1998). An introduction to the bootstrap. Chapman & Hall. [Google Scholar]
- Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Dıaz JF, LopezMiranda J, Bouillon R, & Quesada Gomez JM (2020). Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. The Journal of Steroid Biochemistry and Molecular Biology, 203, 105751. 10.1016/j.jsbmb.2020.105751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester M, Kriegel H-P, Sander J, & Xu X. (1996). A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of 2nd International Conference on Knowledge Discovery and Data Mining (pp. 226–231). [Google Scholar]
- Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, & Sali A. (2006). Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics Chapter 5, Unit–5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoth A, Friedman R, Nachliel E, & Gutman M. (2006). A molecular dynamics study and free energy analysis of complexes between the Mlc1p protein and two IQ motif peptides. Biophysical Journal, 91(7), 2436–2450. 10.1529/biophysj.106.085399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, & Bhattoa HP (2020). Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients, 12, 988. 10.3390/nu12123741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross LZF, Sacerdoti M, Piiper A, Zeuzem S, Leroux AE, & Biondi RM (2020). ACE2, the receptor that enables the infection by SARS-CoV-2: Biochemistry, structure, allostery and evaluation of the potential development of ACE2 modulators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S, Kitao A, & Go N. (1995). Harmonicity and anharmonicity in protein dynamics: A normal mode analysis and principal component analysis. Proteins, 23(2), 177–186. 10.1002/prot.340230207 [DOI] [PubMed] [Google Scholar]
- Herter S, Piper DE, Aaron W, Gabriele T, Cutler G, Cao P, Bhatt AS, Choe Y, Craik CS, Walker N, Meininger D, Hoey T, & Austin RJ (2005). Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-αnchored serine protease implicated in prostate and ovarian cancers. The Biochemical Journal, 390(Pt 1), 125–136. 10.1042/BJ20041955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, & Pohlmann S. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e28. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF (2003). Vitamin D: A millenium perspective. Journal of Cellular Biochemistry, 88(2), 296–307. 10.1002/jcb.10338 [DOI] [PubMed] [Google Scholar]
- Holick MF (2007). Vitamin D deficiency. The New England Journal of Medicine, 357(3), 266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Hooft RW, Vriend G, Sander C, & Abola EE (1996). Errors in protein structures. Nature, 381(6580), 272. 10.1038/381272a0 [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, … Cao B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, & Schulten K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics, 14(1), 33–38. 10.1016/0263-7855(96)00018-5 [DOI] [PubMed] [Google Scholar]
- Jakovac H. (2020). COVID-19 and vitamin D – Is there a link and an opportunity for intervention? American Journal of Physiology. Endocrinology and Metabolism, 318(5), E589. 10.1152/ajpendo.00138.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr., & Slominski AT (2010). 23-Dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. Journal of Cellular Physiology, 20, 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, & Slominski AT (2009). 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One, 4(6), e5988. 10.1371/journal.pone.0005988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson C. (2019). The vitamin D metabolome: An update on analysis and function. Cell Biochemistry and Function, 37(6), 408–423. 10.1002/cbf.3421 [DOI] [PubMed] [Google Scholar]
- Jurrus E, Engel D, Star K, Monson K, Brandi J, Felberg LE, Brookes DH, Wilson L, Chen J, Liles K, Chun M, Li P, Gohara DW, Dolinsky T, Konecny R, Koes DR, Nielsen JE, Head-Gordon T, Geng W, … Baker NA (2018). Improvements to the APBS biomolecular solvation software suite. Protein Science: A Publication of the Protein Society, 27(1), 112–128. 10.1002/pro.3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W, & Sander C. (1983). Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers, 22(12), 2577–2637. 10.1002/bip.360221211 [DOI] [PubMed] [Google Scholar]
- Kaufman HW, Niles JK, Kroll MH, Bi C, & Holick MF (2020). SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. Plos One, 15(9), e0239252. 10.1371/journal.pone.0239252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Atigadda V, Brzeminski P, Fabisiak A, Tang EKY, Tuckey RC, & Slominski AT (2020). Detection of 7-dehydrocholesterol and vitamin D3 derivatives in honey. Molecules, 25. 10.3390/molecules25112583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, Lee M, Lee T, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, & Cheatham TE (2000). Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Accounts of Chemical Research, 33(12), 889–897. 10.1021/ar000033j [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, & Thornton JM (1993). PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26(2), 283–291. 10.1107/S0021889892009944 [DOI] [Google Scholar]
- Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, & Thornton JM (1996). AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. Journal of Biomolecular NMR, 8(4), 477–486. 10.1007/BF00228148 [DOI] [PubMed] [Google Scholar]
- Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, & Greiffenstein P. (2020). Vitamin D insufficiency is prevalent in severe COVID-19. medRxiv. [Google Scholar]
- Lee SJ, Song Y, & Baker NA (2008). Molecular dynamics simulations of asymmetric NaCl and KCl solutions separated by phosphatidylcholine bilayers: Potential drops and structural changes induced by strong Na+-lipid interactions and finite size effects. Biophysical Journal, 94(9), 3565–3576. 10.1529/biophysj.107.116335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pan D, Bellis SL, & Song Y. (2008). Effect of altered glycosylation on the structure of the I-like domain of beta1 integrin: A molecular dynamics study. Proteins, 73(4), 989–1000. 10.1002/prot.22126 [DOI] [PubMed] [Google Scholar]
- MATLAB. (2015). (R2015a). The MathWorks Inc. [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, & Manson JJ (2020). COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet (London, England), 395(10229), 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, & Solway J. (2020). Association of Vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Network Open, 3(9), e2019722. 10.1001/jamanetworkopen.2020.19722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T, Cao H, Zhang H, Kang Z, Xu D, Gong H, Wang J, Li Z, Cui X, Xu H, Wei H, Pan X, Zhu R, Xiao J, Zhou W, Cheng L, & Liu J. (2020). The insert sequence in SARS-CoV-2 enhances spike protein cleavage by TMPRSS. bioRxiv. 2020.2002.2008.926006. [Google Scholar]
- Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, & Frenkel-Morgenstern M. (2020). Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. The FEBS Journal, 287(17), 3693–3702. 10.1111/febs.15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, & Olson AJ (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785–2791. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan Jothimani MP, Al-Sehemi AG, & Muthusamy K. (2021). Association of vitamin-D and VDR with ACE2 modulates the severity in COVID-19. EC Orthopaedics, 12, 87–92. [Google Scholar]
- Notredame C, Higgins DG, & Heringa J. (2000). T-coffee: A novel method for fast and accurate multiple sequence alignment. Journal of Molecular Biology, 302(1), 205–217. 10.1006/jmbi.2000.4042 [DOI] [PubMed] [Google Scholar]
- Pan D, & Song Y. (2010). Role of altered sialylation of the I-like domain of beta1 integrin in the binding of fibronectin to beta1 integrin: Thermodynamics and conformational analyses. Biophysical Journal, 99(1), 208–217. 10.1016/j.bpj.2010.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Yan Q, Chen Y, McDonald JM, & Song Y. (2011). Trifluoperazine regulation of calmodulin binding to Fas: A computational study. Proteins, 79(8), 2543–2556. 10.1002/prot.23081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta F, Matera MG, Cazzola M, & Bianco A. (2020). Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter? Respiratory Medicine, 168, 105996. 10.1016/j.rmed.2020.105996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum LA, & DeLuca HF (2010). Vitamin D, disease and therapeutic opportunities. Nature Reviews. Drug Discovery, 9(12), 941–955. 10.1038/nrd3318 [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, & Manolagas SC (1983). 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science (New York, N.Y.), 221(4616), 1181–1183. 10.1126/science.6310748 [DOI] [PubMed] [Google Scholar]
- PyMOL. (2020). The PyMOL Molecular Graphics System v. 2.4.0. Schrodinger, LLC https://pymol.org/2/. [Google Scholar]
- Quesada-Gomez JM, Entrenas-Castillo M, & Bouillon R. (2020). Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. The Journal of Steroid Biochemistry and Molecular Biology, 202, 105719. 10.1016/j.jsbmb.2020.105719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JM, Subramanian S, Laird E, Griffin G, & Kenny RA (2021). Perspective: Vitamin D deficiency and COVID-19 severity – Plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis (R1). Journal of Internal Medicine, 289(1), 97–115. 10.1111/joim.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, & Sitrin MD (2008). Vitamin D’s role in cell proliferation and differentiation. Nutrition Reviews, 66(10 Suppl 2), S116–S124. 10.1111/j.1753-4887.2008.00094.x [DOI] [PubMed] [Google Scholar]
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, & Koerfer R. (2006). Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. The American Journal of Clinical Nutrition, 83(4), 754–759. 10.1093/ajcn/83.4.754 [DOI] [PubMed] [Google Scholar]
- Schrödinger (2021). (2021–1) Maestro 12.4. Schrödinger, LLC. [Google Scholar]
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, & Li F. (2020). Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences of the United States of America, 117(21), 11727–11734. 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Chaiprasongsuk A, Janjetovic Z, Kim TK, Stefan J, Slominski RM, Hanumanthu VS, Raman C, Qayyum S, Song Y, Song Y, Panich U, Crossman DK, Athar M, Holick MF, Jetten AM, Zmijewski MA, Zmijewski J, & Tuckey RC (2020a). Photoprotective properties of vitamin D and lumisterol hydroxyderivatives. Cell Biochemistry and Biophysics, 78(2), 165–180. 10.1007/s12013-020-00913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, & Holick MF (2010). Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show antileukemia effects, having low or absent calcemic activity. PLoS One, 5(3), e9907. 10.1371/journal.pone.0009907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, Li W, Tuckey RC, & Jetten AM (2017). Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORa and RORc. The Journal of Steroid Biochemistry and Molecular Biology, 173, 42–56. 10.1016/j.jsbmb.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Janjetovic Z, Brozyna AA, Zmijewski MA, Xu H, Sutter TR, Tuckey RC, Jetten AM, & Crossman DK (2018). Differential and overlapping effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on gene expression in human epidermal keratinocytes: Identification of AhR as an alternative receptor for 20,23(OH)(2)D3. International Journal of Molecular Sciences, 19, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, & Tuckey RC (2015). Detection of novel CYP11α1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Scientific Reports, 5, 14875. 10.1038/srep14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, & Tuckey RC (2014a). The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. The Journal of Steroid Biochemistry and Molecular Biology, 144 Pt A, 28–39. 10.1016/j.jsbmb.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, & Tuckey RC (2012). In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 26(9), 3901–3915. 10.1096/fj.12-208975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, & Jetten AM (2014b). RORa and ROR c are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20hydroxy- and 20,23-dihydroxyvitamin D. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 28(7), 2775–2789. 10.1096/fj.13-242040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim T-K, Qayyum S, Song Y, Janjetovic Z, Oak ASW, Slominski RM, Raman C, Stefan J, Mier-αguilar CA, Atigadda V, Crossman DK, Golub A, Bilokin Y, Tang EKY, Chen JY, Tuckey RC, Jetten AM, & Song Y. (2021). Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Scientific Reports, 11(1), 8002. 10.1038/s41598-02187061-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Slominski RM, Goepfert PA, Kim TK, Holick MF, Jetten AM, & Raman C. (2020b). Reply to Jakovac and to Rocha et al.: Can vitamin D prevent or manage COVID-19 illness? American Journal of Physiology. Endocrinology and Metabolism, 319(2), E455–E457. 10.1152/ajpendo.00348.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, & Postlethwaite AE (2013). 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. The Journal of clinical endocrinology and metabolism, 98(2), E298–E303. 10.1210/jc.2012-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski RM, Stefan J, Athar M, Holick MF, Jetten AM, Raman C, & Slominski AT (2020c). COVID-19 and Vitamin D: A lesson from the skin. Experimental Dermatology, 29(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, & Slominski AT (2020d). Extra-αdrenal glucocorticoid biosynthesis: Implications for autoimmune and inflammatory disorders. Genes and Immunity, 21(3), 150–168. 10.1038/s41435-020-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Guallar V, & Baker NA (2005). Molecular dynamics simulations of salicylate effects on the micro- and mesoscopic properties of a dipalmitoylphosphatidylcholine bilayer. Biochemistry, 44(41), 13425–13438. 10.1021/bi0506829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suever JD, Chen Y, McDonald JM, & Song Y. (2008). Conformation and free energy analyses of the complex of calcium-bound calmodulin and the Fas death domain. Biophysical Journal, 95(12), 5913–5921. 10.1529/biophysj.108.130542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Luo F, Xing J-C, Zhang F, Xu J-z., & Zhang Z-h. (2018). 1,25(OH)2 D3 inhibited Th17 cells differentiation via regulating the NF-κB activity and expression of IL-17. Cell Proliferation, 51(5), e12461. 10.1111/cpr.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B, Ravindran J, Prasad S, Pandey MK, & Aggarwal BB (2010). Gossypol induces death receptor-5 through activation of the ROSERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. The Journal of Biological Chemistry, 285(46), 35418–35427. 10.1074/jbc.M110.172767 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Trott O, & Olson AJ (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461. 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukas CD, Provvedini DM, & Manolagas SC (1984). 1,25-Dihydroxyvitamin D3: A novel immunoregulatory hormone. Science (New York, N.Y.), 224(4656), 1438–1440. 10.1126/science.6427926 [DOI] [PubMed] [Google Scholar]
- Tuckey RC, Cheng CYS, & Slominski AT (2019). The serum vitamin D metabolome: What we know and what is still to discover. The Journal of Steroid Biochemistry and Molecular Biology, 186, 4–21. 10.1016/j.jsbmb.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, & Li W. (2012). 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Research, 32(3), 739–746. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang W, Kollman PA, & Case DA (2001). Antechamber: An accessory software package for molecular mechanical calculations. Journal of the American Chemical Society, 222, U403. [Google Scholar]
- Wang L, Murphy-Ullrich JE, & Song Y. (2014). Molecular insight into the effect of lipid bilayer environments on thrombospondin-1 and calreticulin interactions. Biochemistry, 53(40), 6309–6322. 10.1021/bi500662v [DOI] [PubMed] [Google Scholar]
- Wang L, Murphy-Ullrich JE, & Song Y. (2019). Multiscale simulation of the interaction of calreticulin–thrombospondin-1 complex with a model membrane microdomain. Journal of Biomolecular Structure & Dynamics, 37(3), 811–822. 10.1080/07391102.2018.1433065 [DOI] [PubMed] [Google Scholar]
- Wang L, Pan D, Yan Q, & Song Y. (2017). Activation mechanisms of aVb3 integrin by binding to fibronectin: A computational study. Protein Science: A Publication of the Protein Society, 26(6), 1124–1137. 10.1002/pro.3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lim WA, Jakalian A, Wang J, Wang J, Luo R, Bayly CI, & Kollman PA (2001). An analysis of the interactions between the Sem-5 SH3 domain and its ligands using molecular dynamics, free energy calculations, and sequence analysis. Journal of the American Chemical Society, 123(17), 3986–3994. 10.1021/ja003164o [DOI] [PubMed] [Google Scholar]
- Wilson S, Greer B, Hooper J, Zijlstra A, Walker B, Quigley J, & Hawthorne S. (2005). The membrane-αnchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. The Biochemical Journal, 388(Pt 3), 967–972. 10.1042/BJ20041066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Murphy-Ullrich JE, & Song YH (2010). Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulininduced focal adhesion disassembly. Biochemistry, 49(17), 3685–3694. 10.1021/bi902067f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Murphy-Ullrich JE, & Song YH (2011). Molecular and structural insight into the role of key residues of thrombospondin-1 and calreticulin in thrombospondin-1–calreticulin binding. Biochemistry, 50(4), 566–573. 10.1021/bi101639y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, & Zhou Q. (2020). Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (New York, N.Y.), 367(6485), 1444–1448. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, & Song Y. (2016). Structural insight for roles of DR5 death domain mutations on oligomerization of DR5 death domain-FADD complex in the death-inducing signaling complex formation: A computational study. Journal of Molecular Modeling, 22(4), 89. 10.1007/s00894-016-2941-0 [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, & Reiter RJ (2020a). COVID-19: Melatonin as a potential adjuvant treatment. Life Sciences, 250, 117583. 10.1016/j.lfs.2020.117583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, … Zhang S. (2020b). Coagulopathy and antiphospholipid antibodies in patients with Covid-19. The New England Journal of Medicine, 382(17), e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, & Carlberg C. (2020). Vitamin D receptor(s): In the nucleus but also at membranes? Experimental Dermatology, 29(9), 876–884. 10.1111/exd.14147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.