Abstract
The coronavirus disease (COVID-19) pandemic has threatened millions of lives worldwide with severe systemic inflammation, organ dysfunction, and thromboembolic disease. Within our institution, many critically ill COVID-19-positive patients suffered major thrombotic events, prompting our clinicians to evaluate hypercoagulability outside of traditional coagulation testing. We determined the prevalence of fibrinolysis shutdown via rotational thromboelastometry (ROTEM, Instrumentation Laboratories, Bedford, Mass) in patients admitted to the intensive care unit over a period of 3 weeks. In 25 patients who had a ROTEM test, we found that 11 (44%) met criteria for fibrinolysis shutdown. Eight of 9 (73%) of the VTE patients met criteria for fibrinolysis shutdown. Given the high rate of fibrinolysis shutdown in these patients, our data support using viscoelastic testing to evaluate for the presence of impaired fibrinolysis. This may help identify patient subsets who might benefit from the administration of fibrinolytics.
Keywords: Adult, COVID-19, fibrinolysis, fibrinolytic shutdown, hypercoagulability, thrombosis
INTRODUCTION
Initial reports of the coronavirus infection (COVID-19) described a constellation of severe, systemic inflammation with acute respiratory failure and organ dysfunction (1). Subsequent accounts of significant thrombotic events, severe procoagulant profiles in non-survivors, and anticoagulation associated mortality benefits lead to the release of international recommendations for prophylactic anticoagulation in patients with COVID-19 (2–6). Nevertheless, venous thromboembolism (VTE) rates up to 69% continue to be reported (7). Reports of pervasively high VTE events amidst patients receiving prophylactic anticoagulation have led to additional exploration of thrombosis-associated pathology and the development of advanced management guidelines (6, 8).
The hypercoagulable profile of patients infected with COVID-19 has been well described and includes elevated fibrinogen, d-dimer levels, and platelet counts when compared with other severe respiratory infections (9, 10). Recently published data regarding viscoelastic testing in COVID-19 patients have not only confirmed this hypercoagulable profile, but additionally noted increased clot strength parameters (10, 11). Persistently elevated thrombotic events rates—despite initiation of prophylactic anticoagulation—have launched investigation into the presence of hypofibrinolysis in addition to known hypercoagulability in this patient population. The utilization of thromboelastography to identify and characterize any phenotypical derangements in fibrinolysis (such as hyperfibrinolysis, hypofibrinolysis, and acute fibrinolytic shutdown) and guide successive therapeutic management has been previously described in traumatic injury and oncological literature (12–14). The concept of “fibrinolysis shutdown”—referring to a severe variant of hypofibrinolysis in which the endogenous inhibition of the fibrinolytic system occurs during the early stages of sepsis—has been well described and may predict increased morbidity and mortality (15–17). Although recent international studies have described thromboelastography (TEG) and rotational thromboelastometry (ROTEM) patterns suggestive of decreased fibrinolysis associated with COVID-19, the utilization of ROTEM testing to evaluate for the presence and implications of “acute fibrinolytic shutdown” in this patient population has yet to be fully explored (15).
Within our institution, we observed a high rate of VTE in COVID-19 patients despite escalating prophylactic and therapeutic anticoagulation protocols. Our clinicians sought to better evaluate for the presence of severe hypofibrinolysis outside of traditional coagulation testing. ROTEM, Instrumentation Laboratories, Bedford, Mass became utilized in one of our intensive care units (ICU) dedicated to the care of COVID-19 patients to determine if there was evidence of acute fibrinolysis shutdown in COVID positive patients.
METHODS
This retrospective case review was approved by the Emory University IRB. Subjects were all critically ill, adult patients (≥18 yrs of age) with laboratory-confirmed COVID-19 RNA, admitted to a single ICU from April 9, 2020 through April 30, 2020 who had a ROTEM performed at least once during their ICU stay. All diagnostic testing and therapeutic interventions, including the decision to perform ROTEM testing, were performed at the discretion of the treating clinicians. We retrospectively gathered demographic, clinical, and diagnostic data obtained during each patient’s ICU admission.
The outcome of thromboembolic event was defined as deep venous thrombosis (DVT) pulmonary embolus (PE) and MI, diagnosed by ultrasound, computed tomography scan and ECG/Troponin respectively. ROTEM testing was performed in the hospital central laboratory utilizing the ROTEM-delta with EXTEM and FIBTEM reagents (Instrumentation Laboratories, Bedford, Mass). The maximum clot firmness (MCF) was recorded for each (reference range 52 mm–70mm for EXTEM and 7–24mm for FIBTEM). The clot time (CT; reference range 35 s–80 s) and maximum lysis (ML; reference range 3.5%–15%) were also recorded from the EXTEM tracing. INTEM was not utilized due to the universal presence of heparin. In patients who had multiple ROTEMs performed, only the first ROTEM demonstrating fibrinolysis shutdown was analyzed. Fibrinolysis shutdown was defined as having EXTEM maximum lysis of <3.5%, consistent with the definition provided for trauma patients by Gomez-Builes et al. (18)
Decision to obtain ROTEM was guided by patients who met criteria for being at higher risk for hypercoagulable state, defined as either “Tier 2 or 3” based on our healthcare system COVID-19 specific thromboembolic risk stratification and management guidelines. Our institution implemented the following three-tier approach, with Tier 1 consisting of conventional VTE prophylaxis medication and dosing. Tier 2 utilized more aggressive therapeutic anticoagulation measures such as a low standard heparin infusion. Tier 3 was reserved for those patients with suspected/known VTE or multisystem organ failure with concern for microvascular thrombi etiology and consisted of high-dose therapeutic anticoagulation. Tiers and anticoagulation therapy was escalated based on D-dimer of over 3,000 ng/mL and/or worsening clinical status, with an emphasis on decompensated cardiopulmonary status. For each respective tier, guidelines for AntiXa goals were as follows: 0.1 unit/mL to 0.3 unit/mL, 0.3 unit/mL to 0.6 unit/mL, and 0.6 unit/mL to 1.0 unit/mL.
Descriptive statistics including median, interquartile range, and percentages were used to summarize the data. Comparisons between patients meeting criteria for fibrinolysis shutdown and those who did not were evaluated using the Fischer exact test for categorical data and the Wilcoxon rank sum test for continuous data. All statistical calculations were performed using SAS 9.4 (SAS Institute, Carey, NC).
RESULTS
During the study time period, ROTEM testing was performed on 25 of 38 critically ill patients admitted to one intensive care unit. The median age of those in the cohort was 63 years old (interquartile range [IQR] 53–77). Thirty-seven percent (n = 14) of patients were female. None of the patients evaluated had a familial history of venous thromboembolic disease. The majority of patients had moderate to severe ARDS on admission. All patients received prophylactic anticoagulation on admission with either unfractionated or low molecular weight heparin (Figs. 1 and 2).
Fig. 1.
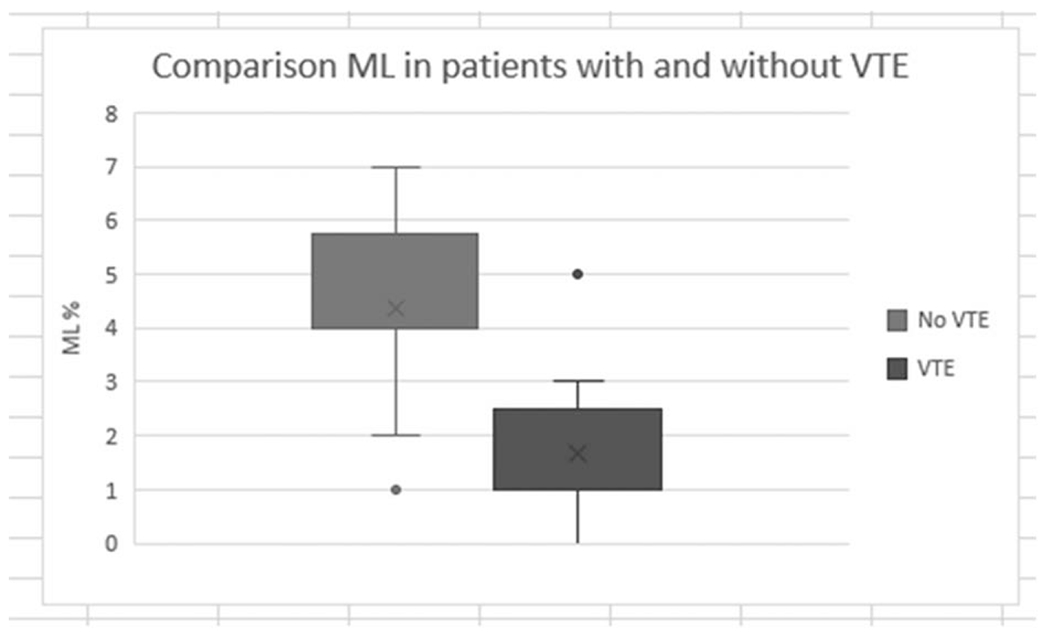
Comparison of ICU patient MLs in those diagnosed with and without thrombotic events. ICU indicates intensive care unit; ML, maximum lysis.
Fig. 2. ROTEM EXTEM tracings in ICU patients with and without fibrinolysis shutdown.
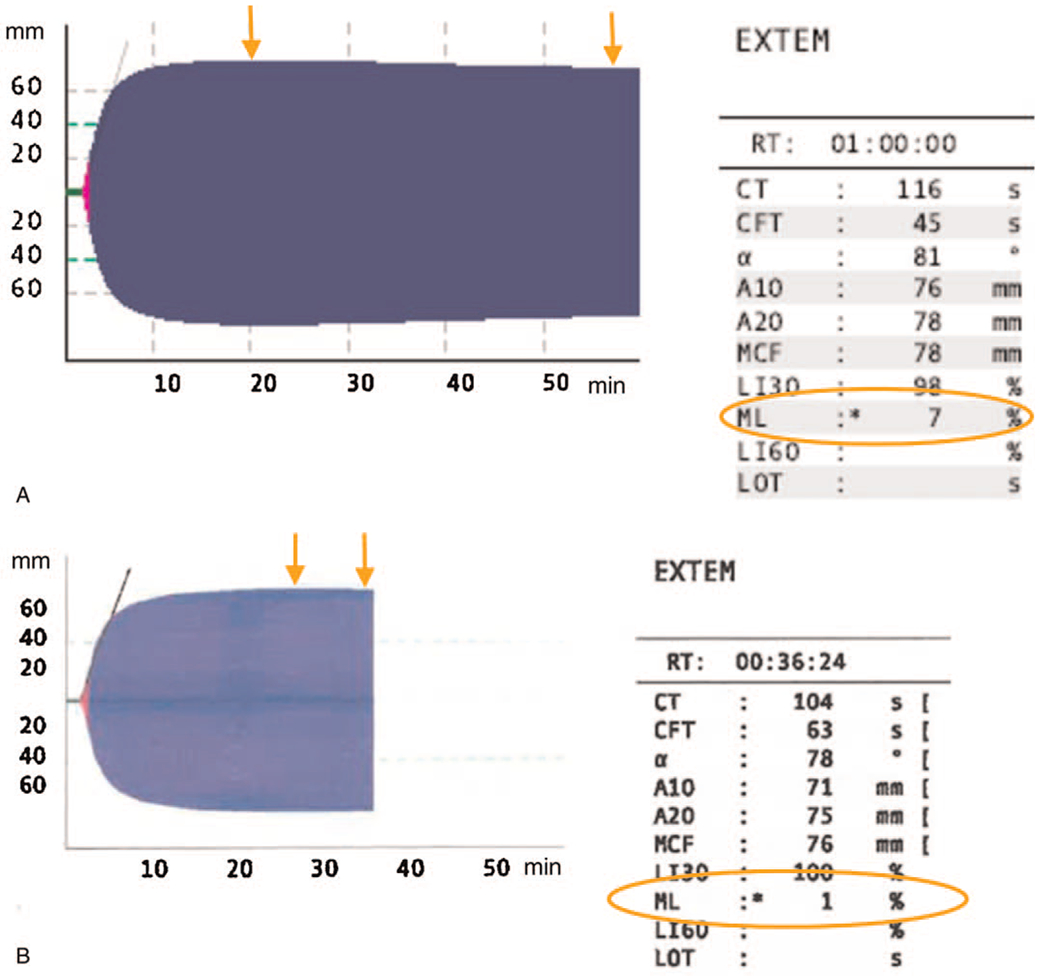
The above are examples of ROTEM EXTEM tracings. ML is calculated by taking the difference between MCF and the lowest amplitude following MCF and then dividing this by MCF. The orange arrows demonstrate the two points used for this ML calculation and the resultant ML (orange circle). A, It demonstrates an EXTEM from a patient with an ML of 7% (no evidence of Fibrinolysis Shutdown). B, It demonstrates an EXTEM from a patient with an ML of 1% (diagnostic of fibrinolysis shutdown) ML indicates maximum lysis; ROTEM, rotational thromboelastometry; MCF, maximum clot firmness.
Notable laboratory and diagnostics of these patients who had ROTEMs performed during their ICU hospitalization included elevations in median peak d-dimer 7287 ng/mL (IQR 4,939–23,912; P = 0.0008), median peak C-reactive protein (CRP) 276 mg/dL (IQR 229–326; P = 0.0801), and lower median P:F ratios of 124 (IQR 91–196; P = 0.0990). Demographics and diagnostics for the entire cohort are provided in Table 1.
Table 1.
ICU cohort demographics and comparison of disease severity
| All patients | Patients without ROTEM | Patients with ROTEM | P | |
|---|---|---|---|---|
| n | 38 | 13 | 25 | |
| Age (yrs) | 63 (53, 77) | 60 (44, 75) | 65 (53, 77) | 0.5722 |
| Female patients | 14 (37%) | 4 (31%) | 10 (40%) | 0.7281 |
| Admission P:F ratio | 135 (95, 251) | 250 (103, 330) | 124 (91, 196) | 0.0990 |
| Peak CRP (mg/dL) | 276 (168, 322) | 222 (93, 296) | 276 (229, 326) | 0.0801 |
| Peak d-dimer (ng/mL) | 5,758 (2,576, 21,991) | 2,064 (1,487, 5,521) | 7,287 (4,939, 23,912) | 0.0008 |
P values based upon Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Data presented as median (1st quartile, 3rd quartile) or number (%).
P:F ratio indicates partial pressure of oxygen on arterial blood gas divided by the fraction of oxygen inspired (PaO2/FiO2);
CRP, c-reactive protein.
Outcomes
Forty-four percent of patients (n = 11) in this cohort met criteria for fibrinolysis shutdown. Of the patients found to have fibrinolysis shutdown, 73% (n = 8) had a thrombotic event, while only 7% (n = 1) of the non-fibrinolysis shutdown group had an event. A comparison between ROTEM parameters in patients with and without fibrinolysis shutdown is provided in Table 2.
Table 2.
Comparison of viscoelastic parameters, labs, and incidence of VTE between patients who did and did not demonstrate fibrinolysis shutdown on ROTEM testing
| Fibrinolysis shutdown | Non-fibrinolysis shutdown | P | |
|---|---|---|---|
| n | 11 (44%) | 14 (56%) | |
| d-dimer level (ng/mL) | 5,215 (2,21, 6,925) | 1,431 (1,159, 1,429) | 0.1080 |
| Platelet count (103/mL) | 288 (217, 394) | 294 (231, 387) | 0.9568 |
| EXTEM CT (s) | 90 (63, 107) | 80 (66, 93) | 0.9567 |
| EXTEM MCF (mm) | 78 (72, 81) | 76.5 (74, 79) | 0.9783 |
| FIBTEM MCF (mm) | 45 (39, 52) | 43 (41, 50) | 0.9136 |
| EXTEM ML (%) | 1.0, (1.0, 2.0) | 4.5 (4.0, 6.0) | 0.0003 |
| Patients with VTE | 8 (73%) | 1 (7%) | 0.0021 |
P values based upon Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Data presented as median (1st quartile, 3rd quartile) or number (%).The d-dimer and platelet count values were those obtained immediately preceding the ROTEM by up to 24 h.
CT indicates clot time; MCF maximum clot firmness; ML, maximum lysis; VTE, venous thromboembolic.
Amongst the 73% (n = 8) of patients who met criteria for fibrinolytic shutdown in this series and were diagnosed with thrombotic events, DVT was diagnosed in seven patients, PE was concomitantly diagnosed in three patients, and myocardial infarction was diagnosed in one patient. Two patients amongst this group suffered cardiopulmonary arrest with acute cor pulmonale confirmed on echocardiography. PE was subsequently confirmed in one of the two patients on subsequent diagnostic imaging; the other did not survive and pathology is pending.
DISCUSSION
In this case series we diagnosed fibrinolytic shutdown in 44% of critically ill patients with COVID-19 and performed ROTEM testing. Amongst those who suffered thrombotic complications, 73% (n = 8) were diagnosed with fibrinolytic shutdown compared with 7% (n = 1) without fibrinolytic shutdown. Our findings not only contribute to the body of emerging data demonstrating that thromboembolic events are a result of multifactorial etiologies that extend beyond hypercoagulable states in critically ill patients with COVID-19, but additionally demonstrate the ability to identify the presence of acute fibrinolysis shutdown in this patient population via ROTEM. The implications of an early diagnosis of acute fibrinolytic shutdown and timely targeted management of associated thrombotic events due to this phenomena—in the context of already known increased morbidity, mortality, and healthcare burden in the baseline critically ill population—cannot be understated (19).
The mechanism of fibrinolysis shutdown is not well understood. Thrombin activatable fibrinolysis inhibitor and PAI-1 are thought to play important roles (20). Unfortunately, measuring these specific factors is not always readily available nor widely used in ICU clinical practice. Viscoelastic testing, which has gained popularity as a point-of-care coagulation test, has been used to diagnose fibrinolytic shutdown in trauma patients, and may also be useful in infectious etiologies as well (17, 20, 21). To date, most viscoelastic testing in COVID-19 patients has focused on parameters of clot strength. Our findings of elevated MCFs on EXTEM and FIBTEM were consistent with other studies. In a single-center study from Italy, Spiezia et al. (22) found increased MCFs on EXTEM and FIBTEM of 69±6mm and 31±9mm respectively. It is notable that they found an incidence of VTE of 23% in patients on anticoagulation and reported an EXTEM ML of 1±3 in COVID-19 patients, although fibrinolysis shutdown was not specifically defined. Similar profiles, without delineation of fibrinolytic shutdown, were additionally noted in Pavoni et al. evaluation of critically ill patients with severe COVID-19 pneumonia (23). Our utilization of ROTEM to diagnose the presence of fibrinolytic shutdown and associated VTE risk is consistent with cohort findings reported by Wright et al. (24) in which they utilized another form of TEG testing to identify the presence of fibrinolysis shutdown.
Our findings not only highlight the ability to diagnose the presence of severely impaired fibrinolysis rapidly at the bedside with ROTEM, but have important potential clinical implications for utilization of fibrinolytic agents in patients with severe COVID-19. Case series of fibrinolytic usage in patients with COVID-19-associated ARDS has recently been proposed to improve clinical outcomes (24–26). Identifying patients with fibrinolytic shutdown may provide improved identification of candidates that would most benefit from this targeted therapy. Additionally, the trends in median d-dimer, CRP elevation, and admission P:F ratios speak to the investigative potential of biomarkers in risk stratification of critically ill patients at risk for fibrinolytic shutdown and associated microvascular and macrovascular events.
A limitation to our study is that cut-off points for diagnosing fibrinolysis shutdown in non-trauma patients have not been well established. We utilized the EXTEM ML <3.5% cutoff proposed by Gomez-Builes et al. (18) which is based upon 550 trauma patients. However, whether tissue injury from infection influences viscoelastic testing differently from that caused by trauma is not known. Due to the retrospective nature of our study and the fact the decision to perform viscoelastic testing was based on clinicians’ judgement, there could be selection bias as to which patients had ROTEM testing performed, but our findings are still both novel and correlate with clinically significant hypercoagulable manifestations in COVID-positive patients.
In conclusion, we found a high rate of fibrinolysis shutdown via utilization of ROTEM in a cohort of critically ill COVID-19 ICU patients. Patients with fibrinolysis shutdown had a significantly higher rate of thromboembolic events than those without. In light of the high mortality rate associated with hypercoagulability in COVID-19, our data support evaluation for the presence of impaired fibrinolysis using viscoelastic testing and its potential role in identifying patients who may benefit from fibrinolytic therapy.
Key Points.
We found a high rate of fibrinolysis shutdown in a cohort of critically ill COVID-19 ICU patients; those with fibrinolysis shutdown had a significantly higher rate of VTE than those without.
Our data supports the usage of viscoelastic testing to evaluate for the presence of impaired fibrinolysis and potentially identify patient subsets who might benefit from the administration of fibrinolytics.
Acknowledgments
Dr RS receives research funding from Grifols and Cerus, as well as consulting fees from Octapharma.
Dr SCA is supported by a NIH/NIAID grant #K23 AI134182.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xang J, Wabg Y, Song B, Gu X, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schroder AS, et al. : Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. M20–2003, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S: Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol 7:e362–e363, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar A, Muller M, Bouman C, Beenan L, Kottee R, Heijmans J, et al. : Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 18:1995–2002, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z: Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18:1094–1099, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wang J, Liu P, Elalamy I, Wang C: Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost 120:937–948, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llitjos J, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K: High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 18:1743–1746, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, Thierry S: Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open 3:e2010478, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han H, Yang L, Liu R, Lui F, Wu KL, Li Jie, Liu XH, Zhu CL: Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 58:1116–1120, 2020. [DOI] [PubMed] [Google Scholar]
- 10.Yin S, Huang M, Li D, Tang N: Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis 2020;1–4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L: The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18:1747–1751, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore HB, Moore EE, Neal MD, Sheppard FR, Kornblith LZ, Draxler DF, Walsh M, Medcalf RL, Cohen MJ, Cotton BA, et al. : Fibrinolysis shutdown in trauma. Anesthesia Analgesia 129:762–773, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, Silliman CC: Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion 56:S110–S114, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh M, Moore EE, Moore H, Thomas S, Vande Lune S, Zimmer D, Dynako J, Hake D, Crowell Z, McCauley R, et al. : Use of viscoelastography in malignancy-associated coagulopathy and thrombosis: a review. Semin Thromb Hemost 45:354–372, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A: Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 18:1738–1742, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt FCF, Manolov V, Morgenstern J, Fleming T, Heitmeier S, Uhle F, Al-Saeed M, Hackert T, Bruckner T, Schochl H, et al. : Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care 9:19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Neal MD, Sheppard FR, Kornblith LZ, Draxler DF, Walsh M, Medcalf RL, Cohen MJ, Cotton BA, et al. : Fibrinolysis shutdown in trauma: historical review and clinical implications. Anesth Analg 129:762–773, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SB: Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg 127:840–849, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malato A, Dentali F, Siragusa S, Fabbiano F, Kagoma Y, Boddi M, Gensini GF, Peris A, Crowther M, Napolitano M: The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus 13:559–568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gando S: Role of fibrinolysis in sepsis. Semin Thromb Hemost 39:392–399, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Müller MC, Meijers JCM, Vroom MB, Juffermans NP: Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care 18:R30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, Navalesi P, Simioni P: COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 120:998–1000, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC: Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis 50:281–286, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright FL, Vogler TO, Moore EE, Moore H, Wohlauer M, Urban S, Nydam T, Moore P, McIntyre R: Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg 231:193–203.e1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore HB, Barrett CD, Moore EE, McIntyre R, Moore P, Talmor S, Moore FA, Yaffe MB: Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome? J Trauma Acute Care Surg 88:1–2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Hajizadeh N, Moore EE, McIntyre R, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD: Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 18:1752–1755, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


