Abstract
Urinary concentrations of phenols, parabens and triclocarban have been extensively used as biomarkers of exposure. However, because these compounds are quickly metabolized and excreted in urine, characterizing participants’ long-term average exposure from a few spot samples is challenging. To examine variability of urinary concentrations of these compounds during pregnancy, we quantified four phenols, four parabens and triclocarban in 357 first morning voids (FMVs) and 203 pooled samples collected during the 2nd and 3rd trimesters of 173 pregnancies. We computed intraclass correlation coefficients (ICCs) by sample type (FMV, pool) across two trimesters and by the number of composite samples in pools, ranging from 2 to 4, within the same trimester. Among three compounds detected in more than 50% of the samples, ICCs across two trimesters were higher in pools (0.29-0.68) than FMVs (0.17-0.52) and the highest ICC within the same trimester was observed when pooling either two or three composites. Methyl paraben and propyl paraben primarily exposed via cosmetic use had approximately 2 to 3 times higher ICCs than bisphenol A primarily exposed via diet. Our findings support that within-subject pooling of biospecimens can increase reproducibility of pregnant women’s exposure to these compounds and thus could potentially minimize exposure misclassification.
Keywords: biospecimens, exposure misclassification, pooling, reproducibility, sample type
Graphical Abstract
1. Introduction
Phenols, parabens and triclocarban (TCC) are widely used in a variety of common consumer and personal care products.1, 2 Bisphenols such as bisphenol A (BPA) are used in the manufacture of polymer plastics. TCC and triclosan (TCS) with antibacterial properties are used in soaps, detergents, and toothpaste.2–4 Parabens are widely used as preservatives in cosmetics, shampoos, and various body care products.5 Because of their widespread use in consumer and personal care products,6, 7 exposures to these compounds are ubiquitous and they have been detected in urine of the general U.S. population, including infants, toddlers, and pregnant women.8–14 Phenols, parabens and TCC have also been detected in cord blood,15–19 suggesting that they can cross the placenta.
Gestational exposure to phenols, parabens and TCC is of interest because of their endocrine-disrupting potential in in vitro studies20 and toxicities in laboratory animal studies.21–25 In addition, higher exposure to some phenols and parabens during pregnancy was associated with adverse health outcomes in epidemiologic studies.26–29 For example, BPA and TCS are well-characterized thyroid hormone disruptors and can disrupt signaling pathways that are critical for brain development.30 Thus, prenatal phenol exposures have been associated with altered thyroid hormones8, 31, 32 and child behavioral outcomes.33–36 Higher pregnancy TCS exposures were associated with lower child cognitive scores measured at 8 years of age.37 Pregnancy urinary BPA concentrations were associated with externalizing behaviors in girls at 2 years of age but not among all children in the same age.34
Urinary concentrations of phenols, parabens and TCC have been extensively used as biomarkers of exposure to these compounds during pregnancy.6, 7 However, because they are rapidly metabolized and eliminated in urine with elimination half-lives on the order of hours38, 39 and exposures to these compounds tend to be episodic, concentrations of phenols, parabens and TCC measured in a single or few spot urine samples are only indicative of recent exposure.40 Several studies have reported moderate to high variability of urinary concentrations of these compounds during a long period of pregnancy (i.e., at least across two trimesters)41–52 and a short period of pregnancy (i.e., less than a few weeks),40, 53 indicating low to moderate reproducibility of pregnant women’s exposure to these compounds. Thus, poor characterization of average exposures to these compounds might have led to non-differential exposure misclassification in epidemiologic studies, resulting in decreased statistical power.
For epidemiologic studies with compounds having a short elimination half-life such as phenols, parabens and TCC, the approach of pooling multiple biospecimens collected from the same individual can reduce sample analysis cost and increase the number of subjects for statistical analyses.54–56 There are advantages and disadvantages when pooling multiple biospecimens and they differ for the three common urine collection methods (spot, 24-hour, and first morning void [FMV]). For example, pooling spot samples collected throughout the day may better represent an individual’s average exposure over a long period of time, but increases exposure misclassification.40 Pooling 24-hour samples (i.e., all samples collected over a 24-hour period) may minimize exposure misclassification but because of increased participant sampling burden, it is generally infeasible for large epidemiologic studies.57 Pooling FMV samples can increase reproducibility of exposure to short half-life compounds, although levels may not necessarily represent daily average exposure due to overnight fasting.57 To evaluate the impact of pooling biospecimens on variability of urinary concentrations of these compounds, two studies pooled multiple spot samples or 24-hour samples collected from pregnant women during a few specific weeks of pregnancy,51, 52 with one of them reporting higher reproducibility of exposure to phenols, parabens and TCC in daily or weekly pooled samples compared to spot samples.51 To our knowledge, no study has yet examined the degree to which pooling FMV samples can improve reproducibility of pregnant women’s exposure, compared to spot samples across multiple trimesters of pregnancy.
In the current study, we examined variability of urinary concentrations of four phenols, four parabens, and TCC using multiple FMVs collected during pregnancy in the MARBLES (Markers of Autism Risk in Babies – Learning Early Signs) study, which enrolls pregnant women in California who previously delivered a child with autism spectrum disorder (ASD). The MARBLES study collects multiple FMVs during each trimester of pregnancy to identify risk factors or markers of ASD, including exposure to our target compounds, and to reduce exposure misclassification. Prenatal exposure to our target compounds was not significantly associated with increased risk of child ASD in MARBLES but showed a significantly increased risk of non-typical development (non-TD).9 We used 357 FMV samples and 203 pooled samples collected during the the 2nd and 3rd trimesters of 173 unique pregnancies to evaluate variability of urinary concentrations of our target compounds within the same trimester and across trimesters and to evaluate how much within-subject pools can improve reproducibility of pregnant women’s exposure to our target compounds.
2. Methods
2.1. Study population
Began in 2006 in California, MARBLES is a prospective cohort study, following pregnant women who previously delivered a child with ASD58 and thus are at high risk (~20%) for delivering another child who develops ASD.59 Participants are recruited from the lists of children receiving services for ASD through the California Department of Developmental Services, by self- or other referrals and various clinics. Details of study design, recruitment, sample size, exposure data, eligibility criteria for inclusion, and developmental diagnosis are available elsewhere.58
For the current study, we selected 164 women who provided at least two FMVs within the same trimester of pregnancy. Among 164 women, 7 women participated in this study for two different pregnancies and 1 woman participated for three different pregnancies. All urine samples included in this study were collected from a total of 173 unique pregnancies from 164 women. This study was approved by the institutional review boards for the State of California and the University of California Davis (UC Davis). Participants provided informed consent prior to collection of data.
2.2. Urine sample collection
Details of urine sample collection are described elsewhere.57, 60 Briefly, as part of an effort to better characterize average exposure to environmental chemicals with short elimination half-lives such as phenols and parabens, women in the MARBLES study were instructed to collect three FMVs (taken one week apart) and one 24-hour urine sample (all samples collected over a 24-hour period) during each trimester of pregnancy. Urine samples that were collected and stored in a home freezer were picked up within one month; samples that were collected and stored in a refrigerator were picked up the same day. These home visits were conducted by study staff, who transported them to UC Davis under temperature-controlled conditions such as coolers. Samples were thawed, aliquoted, and stored at −80 °C at the UC Davis biorepository until analysis.
2.3. Sample preparation for chemical analysis
We selected 9 women who provided at least four (either FMV or 24-hour) samples for each of the 2nd and 3rd trimesters and analyzed their samples individually for graphical representation of concentration variability over the course of the 2nd and 3rd trimesters for two compounds with distinct exposure sources (i.e., BPA primarily exposed via diet and methyl paraben (MEPB) primarily exposed from the use of cosmetics or various personal care products). Then, to reduce sample analysis cost, for women who provided three or more samples within a trimester, we selected the first FMV as an individual sample and pooled all remaining samples for that trimester.57, 60 Although all women included in the current study (n = 164) provided urine samples during the 2nd and 3rd trimesters, only 60 of them provided 87 samples during the 1st trimester and only 9 of them had multiple samples after pooling. In other words, approximately 85% of samples collected from the participating women were collected during the 2nd and 3rd trimesters. Therefore, we only selected samples collected during the 2nd and 3rd trimesters in the current study. After pooling, 577 samples from 173 pregnancies remained for chemical analysis: 357 FMVs, 203 within-subject pools (approximately 79% of the pools were comprised of at least one 24-hour sample), and 17 24-hour samples (from 9 women whose samples were not pooled for graphical representation). The number of samples included in each of the 203 pools varied based on the number of samples each woman collected (Figure 1).
Figure 1.
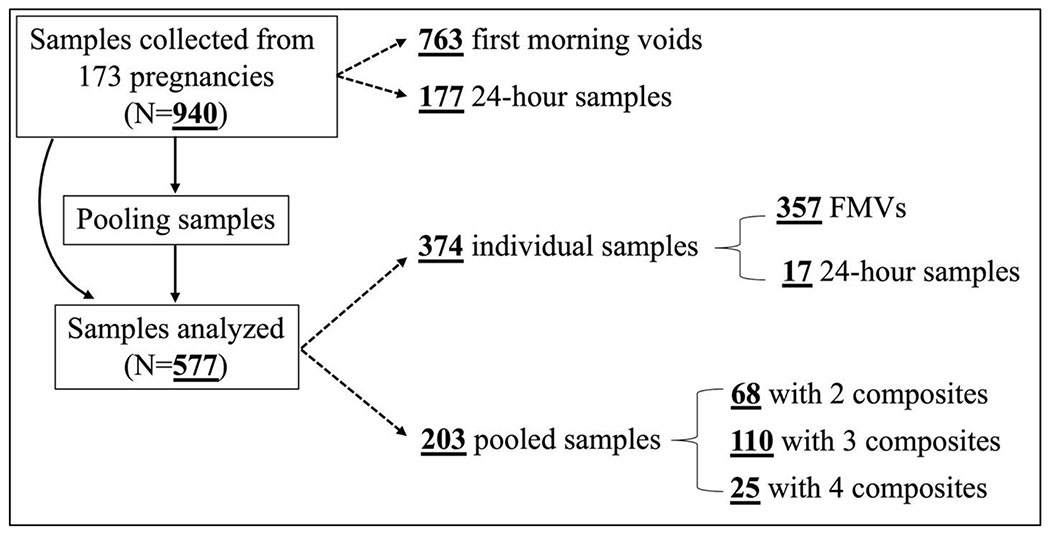
Type and number of 577 urine samples analyzed in this study from the 2nd (n = 253) and 3rd (n = 324) trimesters of 173 pregnancies. Theoretically, if all women were fully compliant with our sampling protocol (three FMVs and one 24-hour sample for each of the 2nd and 3rd trimesters), the total number of samples available for this present study should be 1,384. After selecting the first FMV as an individual sample and pooling the remaining samples, a total number of 692 samples should remain for analysis: 346 FMVs as an individual sample and 346 pools with 3 composites. Although all women were asked to collect four samples per trimester, many did not. In some cases, samples provided by women as the 2nd trimester sample were later determined as the 3rd trimester sample or vice versa. In other cases, women collected more samples than necessary. Thus, the number of samples included in each of the 203 pools varied based on the number of samples each woman collected.
2.4. Biomarker quantification
For biomarker quantification, we shipped the urine samples in a 1-mL aliquot to the Laboratory of Exposure Assessment and Development for Environmental Research (LEADER), Rollins School of Public Health, at Emory University. We quantified urinary concentrations for four phenols, four parabens and TCC using two liquid chromatographic-tandem mass spectrometric (LC-MS/MS) injections with different columns and mobile phases. Details of analytical methods are described elsewhere.9 The analytes included in this study were: BPA, bisphenol F (BPF), bisphenol S (BPS), TCC, TCS, butyl paraben (BUPB), ethyl paraben (ETPB), MEPB, and propyl paraben (PRPB).
In the current study, the average relative percent difference (RPD) of repeated measures of quality controls (QC) was below 11% (range: 6.7%-10.9%), depending on the analyte and QC concentration. The laboratory also analyzed 16 blind duplicates for quality assurance. Replicate analyses for individual pairs of duplicate samples exhibited good agreement. Among 8 pairs of duplicate samples, the average RPD was 15% (range: 9% to 22%, depending on the analyte). The limit of detection (LOD) varied between 0.5 and 15 nanograms per milliliter (ng/mL), depending on the analyte. For concentrations below the LOD, we assigned a value of the LOD divided by the square root of 2.61, 62
2.5. Correction for urinary dilution
To correct measured concentrations for urinary dilution, we measured specific gravity (SG) of each analyzed (individual or pooled) urine sample with a digital handheld refractometer (Atago Co., Ltd., Tokyo, Japan) at UC Davis. We then corrected measured concentrations for urinary dilution using the following formula:63 CSG = C[(1.012 – 1)/(SG-1)], where CSG is the SG-corrected urinary concentration (in ng/mL), C is the measured urinary concentration (in ng/mL), 1.012 is the median SG of all analyzed urine samples, and SG is the specific gravity of each sample.
2.6. Statistical analysis
We performed all statistical analyses using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). For all analyzed compounds, we provided summary statistics of SG-corrected urinary concentrations. For all other statistical analyses requiring sufficient detection frequency of the samples, we only included compounds detected in 60% or greater of the samples. To account for skewed distributions of urinary concentrations, we used ln-transformed SG-corrected concentrations.
To assess within-subject variability, we computed the intraclass correlation coefficient, ICC, defined as the ratio of between-subject variance to total variance (= within-subject variance + between-subject variance). ICC theoretically ranges from 0 (no reproducibility) to 1 (perfect reproducibility); ICC=1 means 100% of total variance is due to between-subject differences and ICC=0 means 100% of total variance is due to within-subject differences.64 ICCs and 95% confidence intervals (CI) were estimated using variance component estimates from a one-way analysis of variance (ANOVA). We used the ‘ICCest’ function in R for these calculations. To evaluate how sample type (FMV or pool) affects variability of target compound concentrations, we computed ICCs using two samples of the same urine type collected across the 2nd and 3rd trimesters: (1) two FMVs (n = 214 from 107 women) and (2) two pools (n = 112 from 56 women). Two samples using the same collection method (FMV or pooled) were used in ICC calculations and were collected approximately 3 months apart because of different trimesters. The proportion of obese women was similar in both the FMV and pooled sample groups (21.3% and 23.6%, respectively). The selected pooled samples were comprised of: FMV only (n = 24); a varying number of FMV plus one 24-hour sample (n = 84); or a varying number of FMV plus two 24-hour samples (n = 4). Because the majority (75%) of pooled samples had a varying number of FMV plus one 24-hour sample, we merged three groups into one. We did not compute ICCs for 24-hour samples due to a small sample size (n = 17). For the 8 women who participated in the study for multiple pregnancies, we treated their samples from each pregnancy independently.
Each pooled sample had a varying number of composites, ranging from 2 to 4. To examine how much pooled samples can improve reproducibility of individual’s exposure with an increasing number of composites, we also computed ICCs using two samples collected within the same trimester for the following four combinations: (1) two FMVs (n = 106), (2) one FMV and one pool with 2 composites (n = 130), (3) one FMV and one pool with 3 composites (n = 214), and (4) one FMV and one pool with 4 composites (n = 46). Two FMVs used in these ICC calculations were collected approximately 1 week apart. The last sample in the pool and the first FMV were approximately 2, 3, and 4 weeks apart depending on the number of composites in the pool. The number of individual and pooled samples used to compute ICCs varied by woman, because the number of samples each woman collected within a trimester varied. Using the same dataset above, we also calculated the concentration ratio of an FMV to a pool (CFMV/Cpool) to examine the impact of outliers on a pooled concentration. Lastly, we computed ICCs separately for each of the 2nd and 3rd trimesters to investigate trimester-specific variability of target compound concentrations.
3. Results
3.1. Population characteristics
The average age of the participating women at delivery was 34.3 years, ranging from 20.5 to 47.1 years (Table 1). They were 55% White, 23% Hispanic, and 23% other (5% Black, 14% Asian, and 3% multiracial). Approximately half of the women were either overweight (27%) or obese (23%). More than half of the women had a bachelor’s degree or a higher degree (53%). Other characteristics of our study population are shown in Table 1.
Table 1.
Characteristics of study population (n = 164 women from 173 unique pregnancies) included in the current study
| Characteristics a | n | % |
|---|---|---|
| Race/ethnicity | ||
| White (non-Hispanic) | 96 | 55% |
| Hispanic | 38 | 22% |
| Other b | 39 | 23% |
| Pre-pregnancy BMI | ||
| Normal/ underweight c | 86 | 50% |
| Overweight | 47 | 27% |
| Obese | 40 | 23% |
| Education | ||
| Less than bachelor’s degree | 82 | 47% |
| Bachelor’s degree | 69 | 36% |
| Graduate or professional degree | 29 | 17% |
| Age at delivery | ||
| < 35 years | 91 | 53% |
| ≥ 35 years | 82 | 47% |
| Homeownership | ||
| Yes | 62 | 36% |
| No | 107 | 62% |
| Missing | 4 | 2% |
| Parity | ||
| 1 | 69 | 40% |
| >1 | 100 | 58% |
| Missing | 4 | 2% |
Seven women participated in the study for two different pregnancies and one woman participated for three different pregnancies over our study period.
Includes Black (5%), Asian (14%), and multiracial (3%).
Only three women were underweight (1.7%).
3.2. Urinary concentrations of phenols, parabens and TCC
Among the nine target compounds included in this study, only three were detected above the LOD in 60% or greater of the samples: BPA (60%), MEPB (95%), and PRPB (73%) (Table 2). The other six compounds were detected in less than 50% of the samples. The highest median of SG-corrected concentrations was observed for MEPB (39.4 ng/mL), followed by PRPB (7.8 ng/mL) and BPA (1.0 ng/mL). The median BPA of pools (1.2 ng/mL) was higher than that of FMVs (0.9 ng/mL, p-value < 0.01). There was no statistically significant difference (p-value > 0.40) in the medians of MEPB and PRPB between FMVs versus pools. When comparing geometric means (GMs) of the three compounds between our pregnant women and the pregnant women (20-44 years of age) reported in the 2013-2014 National Health and Nutrition Examination Survey (NHANES),65 GMs of our pregnant women were approximately 44%, 89%, and 81% lower for BPA, MEPB, and PRPB, respectively. Spearman’s correlation coefficients between BPA and MEPB, between BPA and PRPB, and between MEPB and PRPB were 0.12, 0.11, and 0.76, respectively.
Table 2.
Distribution of SG-corrected concentrations [ng/mL] of phenols, parabens and TCC in 577 urine samples (357 FMVs, 203 pools and 17 24-hour samples) collected from 173 pregnancies during the period of 2007-2014.
| LOD [ng/mL] | % detect | All samples | FMV | Pool | 24-hr | FMV versus pools a | 2013-2014 GM b | NHANES GM c | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Percentiles | |||||||||||
| 25th | 50th | 75th | 50th | 50th | 50th | ||||||
| BPA | 0.8 | 60 | <LOD | 1.0 | 1.7 | 0.9 | 1.2 | 1.3 | <0.01 | 0.8 | 1.5 |
| BPF | 2.5 | 16 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | - | - | 0.5 |
| BPS | 0.5 | 13 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | - | - | 0.5 |
| TCC | 1.0 | 5 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | - | - | 0.3 |
| TCS | 15.0 | 33 | <LOD | <LOD | 47.1 | <LOD | <LOD | <LOD | - | - | 18.4 |
| BUPB | 1.5 | 7 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | - | - | 0.2 |
| ETPB | 0.5 | 47 | <LOD | <LOD | 3.9 | <LOD | <LOD | <LOD | - | - | 4.8 |
| MEPB | 0.5 | 95 | 13.6 | 39.4 | 115.3 | 38.0 | 42.7 | 51.1 | 0.43 | 18.9 | 174.4 |
| PRPB | 1.0 | 73 | <LOD | 7.8 | 25.0 | 7.1 | 8.4 | 23.5 | 0.62 | 4.6 | 24.6 |
Abbreviation: first morning void (FMV), geometric mean (GM), limit of detection (LOD), National Health and Nutrition Examination Survey (NHANES), specific gravity (SG), bisphenol A (BPA), bisphenol F (BPF), bisphenol S (BPS), triclocarban (TCC), triclosan (TCS), butyl paraben (BUPB), ethyl paraben (ETPB), methyl paraben (MEPB), propyl paraben (PRPB)
P-value from the Wilcoxon rank-sum test of the null hypothesis that two populations have the same distribution with the same median. Note that we did not compare medians of FMV versus 24-hour as well as pool versus 24-hour, because the sample size for 24-hour samples is small (n = 17)
Uncorrected GM concentrations [ng/mL] of the pregnant women included in the current study who provided FMVs during 2013-2014 (n = 63). For values below LOD, we assigned a value of the LOD divided by the square root of 2 for GM computation.
Uncorrected GM concentrations [ng/mL] of the pregnant women (20-44 years of age) reported in the 2013-2014 NHANES (n = 24).
3.3. Variability of urinary concentrations across the 2nd and 3rd trimesters
From the samples collected across the 2nd and 3rd trimesters, ICCs for BPA, MEPB and PRPB were 66%, 32%, and 26% higher, respectively, in pools (0.29-0.68) than FMVs (0.17-0.52) (Figure 2). Regardless of sample type, ICCs were low for BPA (0.17-0.29) for which diet is a primary exposure source, indicating low reproducibility (i.e., greater temporal variability), while ICCs were relatively high for MEPB (0.51-0.68) and PRPB (0.52-0.65) for which personal care products are a primary exposure source, indicating high reproducibility (i.e., lower temporal variability).
Figure 2.
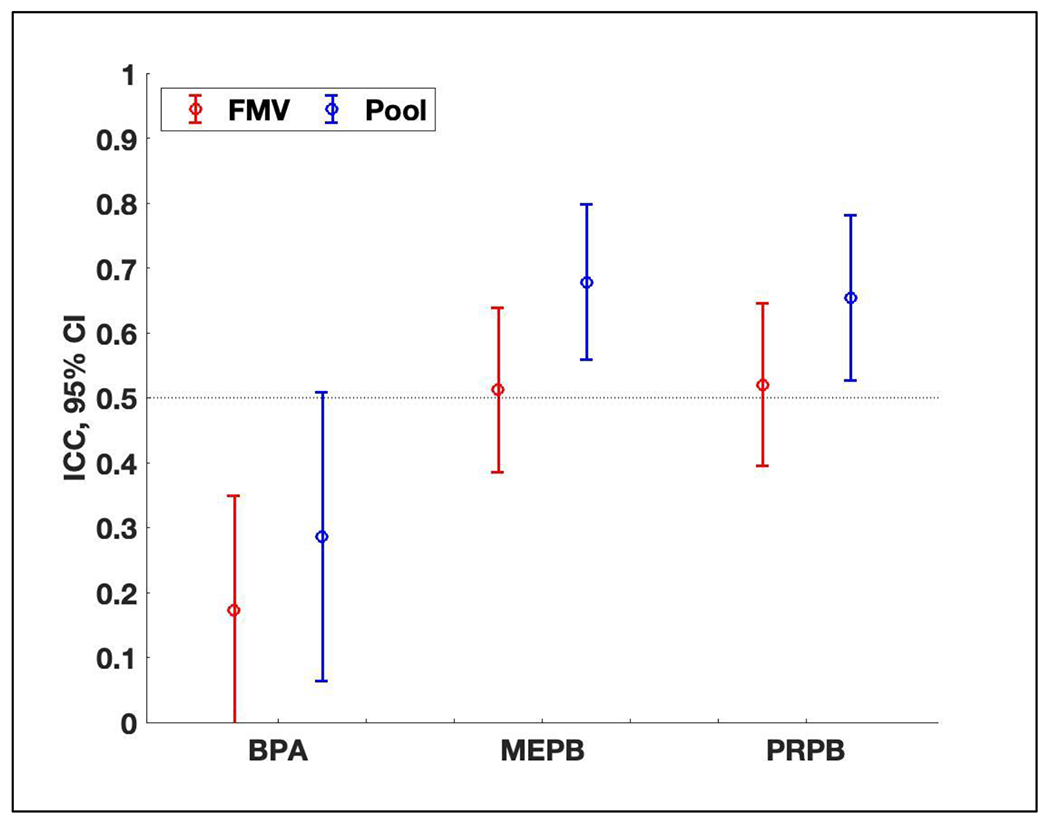
Intraclass correlation coefficients (ICC) and 95% confidence intervals (CI) of ln-transformed SG-corrected concentrations of bisphenol A (BPA), methyl paraben (MEPB), and propyl paraben (PRPB) using samples collected across the 2nd and 3rd trimesters of pregnancy: (1) two first morning voids (FMVs) (n = 214 from 107 women) and (2) two pools (n = 112 from 56 women). Two samples of the same urine type (FMV or pool) used in ICC calculations were collected approximately 3 months apart. The selected pools in this figure were comprised of FMVs only (n = 24), or a varying number of FMVs plus one 24-hour sample (n = 84), or a varying number of FMVs plus two 24-hour samples (n = 4). Refer to Table S1 for values of ICCs and 95% CIs.
From longitudinal samples of 9 women who provided at least four (either FMV or 24-hour) samples for each of the 2nd and 3rd trimesters (Figure 3), MEPB and BPA concentrations varied across sample collection times. Note that Spearman’s correlation coefficients between MEPB and PRPB was 0.76 in this study. For MEPB, 5 women showed relatively low within-subject variability with coefficient of variation (CV, defined as the ratio of the standard deviation to the mean) less than 0.65 (Figure 3A). On the other hand, 4 women showed relatively high within-subject variability with CV greater than 1.0 (Figure 3B). Urinary MEPB concentrations for subject 9 varied in almost three orders of magnitude during their sampling period. One of the 5 women who had low within-subject variability in MEPB (subject 1) showed high within-subject variability for BPA, with CV of 1.44 (Figure 3C). Three of the 4 women who had high within-subject variability in MEPB (subjects 7, 8, 9) showed low within-subject variability for BPA, with CV less than 0.67 (Figure 3D).
Figure 3.
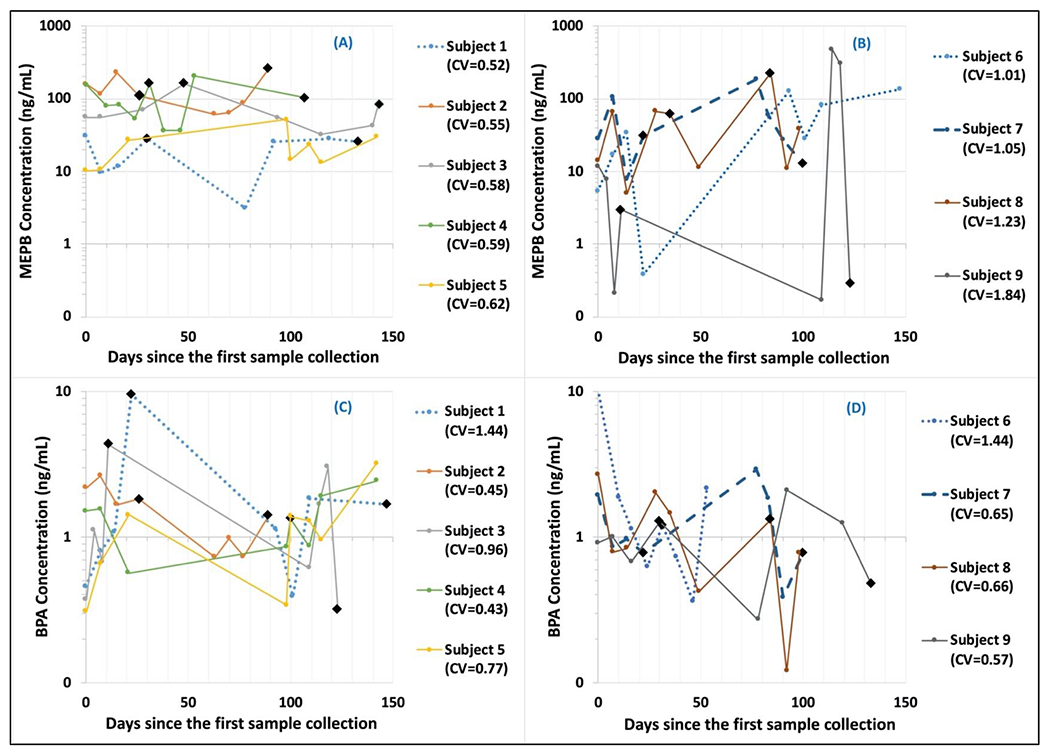
Variability of urinary methyl paraben (MEPB) and bisphenol A (BPA) concentrations [ng/mL] from longitudinal samples of 9 women, plotted versus the days since the first sample collection. First four or five samples were typically collected once a week during the 2nd trimester and the next four or five samples were typically collected once a week during the 3rd trimester. Circle points represent first morning voids (FMVs) and black diamond points represent 24-hour samples.
3.4. Variability of urinary concentrations within the same trimester
From the samples collected within the same trimester (2nd or 3rd), ICCs for MEPB and PRPB tended to be larger for women who provided three or more samples (from the second through fourth pairs in Figure 4) than those who provided only two samples (the first pair in Figure 4). For MEPB and PRPB, ICCs for one FMV and one pool with 2 composites (the second pair) were significantly higher than those for two FMVs (the first pair) at the 5% level. The ICC for MEPB appeared to be highest when the pooled sample group had 3 composites, and for PRPB it appeared to be highest when the pool had 2 composites, although they were not different (p-value > 0.10). Similar results were observed when calculating correlation coefficients (Table S2). From the same dataset as above, the medians of concentration ratios of an FMV to a pooled sample did not decrease or increase with an increasing number of samples (Figure S1).
Figure 4.
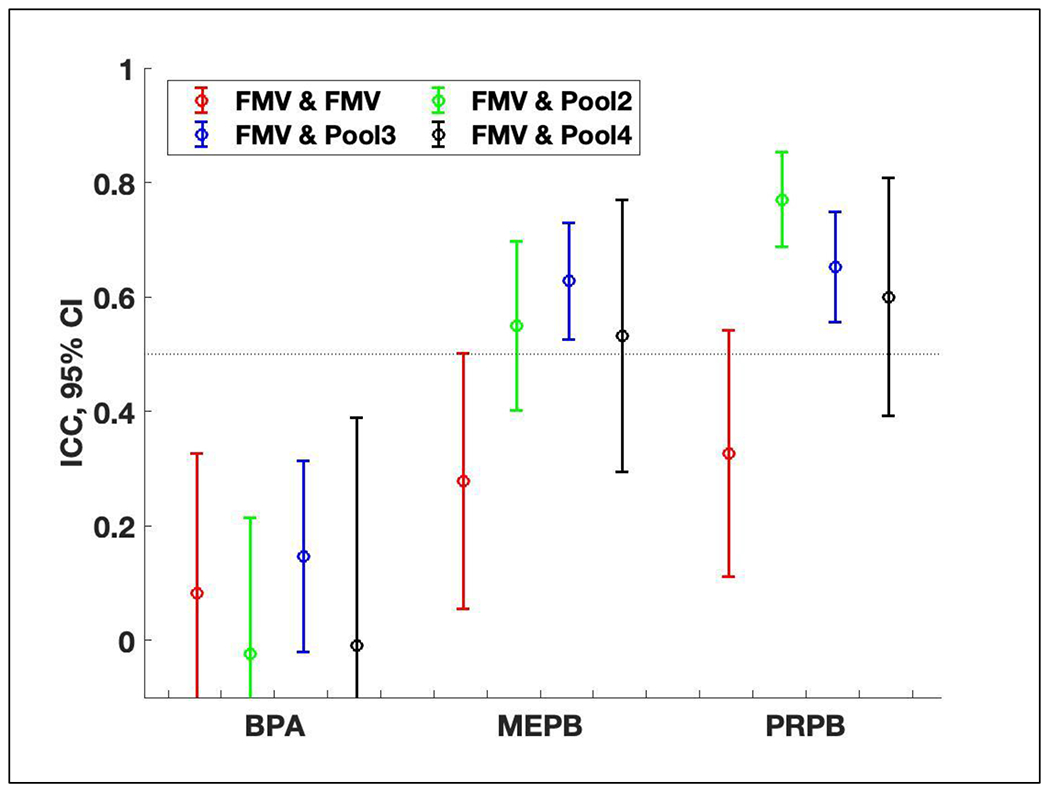
Intraclass correlation coefficients (ICC) and 95% confidence intervals (CI) of ln-transformed SG-corrected concentrations of bisphenol A (BPA), methyl paraben (MEPB), and propyl paraben (PRPB) using samples collected within the same (2nd or 3rd) trimester from 173 pregnancies: (1) two first FMVs (first morning voids) in case when only FMVs were available in each trimester (n = 106 from 53 women), (2) one FMV and one pool with 2 composites (n = 130 from 65 women), (3) one FMV and one pool with 3 composites (n = 214 from 107 women), and (4) one FMV and one pool with 4 composites (n = 46 from 23 women). Two FMVs used in ICC calculations were collected approximately 1 week apart and the last sample in the pool and the first FMV were approximately 2, 3, or 4 weeks apart depending on the number of composites in the pool. Pooled samples with a different number (2, 3, or 4) of composites were indicated in legend as Pool2, Pool3, and Pool4, respectively. In the selected pools, approximately 57%, 85%, and 76% of pools were comprised of at least one 24-hour sample in the pool with 2, 3, and 4 composites, respectively. Refer to Table S2 for values of ICCs and 95% CIs.
When computing trimester-specific ICCs (Figure S2), ICCs followed similar trends to those when the sample pairs from both trimesters were combined (Figure 4). For MEPB and PRPB, ICCs for one FMV and one pool with 3 composites (the third pair) were significantly higher than those for two FMVs (the first pair) at the 5% level, but confidence intervals became large due to the reduced sample size. ICCs for one FMV and one pool with 3 composites were consistently larger than those for two FMVs, regardless of the trimester. In constrast, ICCs for BPA followed no trend.
4. Discussion
In this current study, we used FMVs and pooled samples collected during the 2nd and 3rd trimesters of pregnancies in the MARBLES study and examined the degree to which pooling multiple FMV samples can improve reproducibility of pregnant women’s exposure to phenols, parabens, and TCC over spot samples. Among three compounds detected above the LOD in 60% or greater of the samples, we observed low reproducibility (measured by ICC) for BPA and relatively moderate reproducibility for MEPB and PRPB. From the samples collected across two trimesters, we observed higher ICCs in pools than FMVs for all three compounds. From the samples collected within the same trimester, we observed higher ICCs for MEPB and PRPB in women who provided three or more samples than those who provided only two samples, while there was no statistically significant difference in ICCs for BPA among four subsets with a varying number of samples (i.e., two FMVs, one FMV and one pool with 2 composites, one FMV and one pool with 3 composites, one FMV and one pool with 4 composites).
Our finding is consistent with previous studies reporting that variability of urinary BPA concentrations during a long period of pregnancy (i.e., samples collected during at least two trimesters) is high (mostly ICC of 0.25 or lower),43–49 regardless of urinary dilution correction methods (i.e., SG or creatinine). Findings from our study and other studies suggest that multiple measurements are needed to improve reproducibility of BPA exposure, rather than a single measurement.49 Compared to our pregnant women who provided mostly FMVs (ICCs for MEPB and PRPB were 0.51 and 0.52, respectively, using two FMVs), reproducibility for MEPB and PRPB in other pregnant women who provided spot samples was low (ICC of 0.40 or lower),42, 45, 49, 52 similar (ICC between 0.42 and 0.61 for both compounds)46, 50 or high (ICC of 0.82 and 0.79, respectively).47 This finding suggests that the number of measurements required to accurately characterize long-term average exposure to MEPB and PRPB during pregnancy can vary by study populations. Although previous studies used either SG or creatinine to correct for urinary dilution, ICCs for MEPB and PRPB were almost identical between two corrected concentrations,46 indicating that correction methods for urinary dilution may not affect ICCs of these two compounds.
In this study, we also learned that compared to using two individual samples, pooling multiple urine samples can improve reproducibility of exposure to BPA, MEPB and PRPB across the mid- to late pregnancy. Specifically, ICCs for BPA, MEPB and PRPB were higher in pools (0.29, 0.68, 0.65, respectively) than FMVs (0.17, 0.51, 0.52, respectively). Two previous studies also pooled spot or 24-hour samples collected during a few specific weeks of pregnancy and estimated ICCs.51, 52 One study pooled 24-hour samples for one week at 13, 23, and 32 weeks of pregnancy and reported higher reproducibility of exposure to BPA, MEPB and PRPB (i.e., between-week ICC of 0.59, 0.81, 0.86, respectively)51 than the current study. The other study pooled three spot samples (i.e., morning, dinner, and night) for one week collected twice approximately 14 weeks apart, but reproducibility for BPA, MEPB and PRPB was relatively low (i.e., ICC of 0.24, 0.38, 0.36, respectively),52 suggesting that a few spot samples collected during any time of a day may not increase exposure reproducibility, compared to FMVs used in the current study and 24-hour samples used in the other study.51
The main strength of this study is a rich dataset of urine samples collected during the 2nd and 3rd trimesters of pregnancies, including but not limited to: (1) 107 pairs of FMVs and 56 pairs of pools collected across two trimesters, (2) 53 pairs of FMVs and 196 pairs of one FMV and one pool with a varying number of composites collected within the same trimester (approximately within 2, 3, and 4 weeks depending on the number of composites). The first subset of our samples allowed us to evaluate long-term variability of urinary concentrations of our target compounds. Specifically, we observed that the relative variability of BPA, and MEPB and PRPB concentrations across two trimesters decreased up to 66%, 32%, and 26%, respectively, when using pools. The second subset enabled us to determine the number of composites to be pooled to achieve relatively high ICCs or a certain degree of reproducibility. In the current study, four FMV samples were required for MEPB to reach an ICC of 0.63 and only three FMV samples were required for PRPB to reach an ICC of 0.77 within a trimester. MEPB and PRPB have relatively similar and routine exposure sources, such as personal care products (Spearman’s rho between MEPB and PRPB = 0.76). On the other hand, BPA has variable exposure sources, originating primarily from diet, which tends to differ more from day to day than personal care products, which are often used daily or regularly without changes (Spearman’s rho between BPA and MEPB = 0.12, Spearman’s rho between BPA and PRPB = 0.11). Thus, the ICC of BPA did not increase with an increasing sample size within the same trimester; this suggests that more than 5 samples are required to reach an ICC of 0.5 or higher. In addition, from longitudinal samples of 9 women who provided at least 8 samples (FMVs or 24-hour) during the 2nd and 3rd trimesters, we observed that the magnitude of within-subject variability varied not only between subjects, but also by compounds within subject.
Some limitations should be noted for this study. First, the ICCs calculated from the participating women may not represent the reproducibility of pregnant women’s exposure sampled from the general population. Approximately 70% of the samples in this study were FMV collected after an average of 9 hours of sleep, prior to eating or use of any personal care product.66 This predominant sample collection method could explain the lower concentrations of the three compounds in MARBLES participants vs. NHANES participants, from whom spot samples were collected throughout the day. Because California is leading efforts to reduce exposure to our target compounds via advocacy campaigns or legislation, geographic variations in product use could also explain some differences between two populations.67–69 In addition, because our participants already had a child with ASD, they may have been more aware of/concerned about their use of sunscreens, cosmetics, and other personal care products. Thus, our results should be interpreted with caution.
From this study, we observed high concentration variability of BPA for which diet is a primary exposure source and moderate concentration variability of MEPB and PRPB for which personal care products are a primary exposure source. Nevertheless, we also learned that the urinary concentrations of MEPB and PRPB with relatively consistent daily exposure sources can vary considerably from day to day for some subjects. We observed similar trends in our previous study that urinary phthalate metabolites with relatively consistent exposure sources such as personal care products had higher ICCs than those with variable exposure sources such as diet.57 In addition, our findings support that pooling multiple biospecimens within the same subject can increase reproducibility of pregnant women’s exposure to BPA, MEPB and PRPB and thus could potentially minimize exposure misclassification and reduce the cost associated with sample analysis. Thus, future epidemiologic studies examining associations between prenatal exposure to these compounds and adverse health outcomes can increase statistical power by retaining the same analysis cost by pooling multiple specimens, though costs for multiple collections may increase. In addition, because our target compounds have various exposure sources, further studies may need to comprehensively examine their sources before urine collection.
Supplementary Material
Synopsis:
When characterizing exposure to chemicals detected in indoor and outdoor environments having short elimination half-lives, multiple pooled biospecimens improve reliability.
Acknowledgments
We would like to thank the MARBLES study participants for making this research possible. We would also like to acknowledge Grace Lee and Priya D’Souza for their contribution to laboratory analyses at Emory University’s LEADER. We also acknowledge Dr. Kelly K. Ferguson at the National Institute of Environmental Health Sciences (NIEHS) and Dr. John D. Meeker at the University of Michigan School of Public Health who kindly provided annual GM biomarker concentrations measured in LIFECODES and PROTECT studies, respectively. Lab and epidemiological data for the MARBLES cohort are hosted at the CHEAR Data Center Repository (https://cheardatacenter.mssm.edu/) under the following DOIs: 10.36043/CHEAR-2016-1449-UEP_Trim1, 10.36043/CHEAR-2016-1449-UEP_Trim2_3, 10.36043/CHEAR-2016-1449-Covars, 10.36043/CHEAR-2016-1449-Demo, 10.36043/CHEAR-2016-1449-Outcome,10.36043/CHEAR-2016-1449-Spec.
Funding
This research was supported by grants from the National Institutes of Health (R21-ES025551, R21-ES028131, R01-ES020392, R24-ES028533, P30-ES023513, P01-ES011269, U2C-ES026555, U2C-ES026560, P30-ES019776, U54-HD079125, UH3-OD023342), and the U.S. Environmental Protection Agency (83543201), and UC Daivs MIND Institute.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c04140.
ICC and 95% of ln-transformed SG-corrected urinary concentrations of BPA, MEPB, and PRPB using samples collected across the 2nd and 3rd trimesters (Table S1), ICC and 95% of ln-transformed SG-corrected urinary concentrations of BPA, MEPB, and PRPB using samples collected within the same trimester (Table S2), the ratio of the urinary BPA, MEPB, and PRPB concentrations of an FMV to another FMV or an FMV to a pooled sample (Figure S1), and trimester-specific ICC and 95% CI of ln-transformed SG-corrected urinary concentrations of BPA, MEPB, and PRPB (Figure S2).
Complete contact information is available at: https://pubs.acs.org/doi/10.1021/acs.est.1c04140.
Conflict of interest
The authors declare that they have no actual or potential competing financial interest.
Ethics approval and consent to participate
The MARBLES study protocol and this study were approved by the institutional review boards for the State of California, the University of California Davis (UC Davis), and the University of Texas Arlington (UT Arlington). Participants provided written informed consent before collection of any data.
Credit authorship contribution statement
Hyeong-Moo Shin: Conceptualization, Methodology, Writing - original draft. Jiwon Oh: Writing - review & editing. Kyunghoon Kim: Writing - review & editing. Stefanie A. Busgang: Methodology, Writing - review & editing. Dana Boyd Barr: Methodology, Funding acquisition, Writing - review & editing. Parinya Panuwet: Methodology, Writing - review & editing. Rebecca J. Schmidt: Funding acquisition, Writing - review & editing. Irva Hertz-Picciotto: Funding acquisition, Writing - review & editing, Methodology. Deborah Bennett: Writing - review & editing, Methodology, Funding acquisition.
References
- 1.Sanchis Y; Coscolla C; Corpas-Burgos F; Vento M; Gormaz M; Yusa V; Bettermilk, p., Biomonitoring of bisphenols A, F, S and parabens in urine of breastfeeding mothers: Exposure and risk assessment. Environ Res 2020, 185, 109481. [DOI] [PubMed] [Google Scholar]
- 2.Calafat AM; Valentin-Blasini L; Ye X, Trends in Exposure to Chemicals in Personal Care and Consumer Products. Curr Environ Health Rep 2015, 2, (4), 348–55. [DOI] [PubMed] [Google Scholar]
- 3.Liao C; Liu F; Kannan K, Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 2012, 46, (12), 6515–22. [DOI] [PubMed] [Google Scholar]
- 4.Bedoux G; Roig B; Thomas O; Dupont V; Le Bot B, Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res Int 2012, 19, (4), 1044–65. [DOI] [PubMed] [Google Scholar]
- 5.Soni MG; Carabin IG; Burdock GA, Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005, 43, (7), 985–1015. [DOI] [PubMed] [Google Scholar]
- 6.Polinski KJ; Dabelea D; Hamman RF; Adgate JL; Calafat AM; Ye X; Starling AP, Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res 2018, 162, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S; Lee S; Shin C; Lee J; Kim S; Lee A; Park J; Kho Y; Moos RK; Koch HM; Kim S; Choi K, Urinary parabens and triclosan concentrations and associated exposure characteristics in a Korean population-A comparison between night-time and first-morning urine. Int J Hyg Envir Heal 2018, 221, (4), 632–641. [DOI] [PubMed] [Google Scholar]
- 8.Aker AM; Johns L; McElrath TF; Cantonwine DE; Mukherjee B; Meeker JD, Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int 2018, 113, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkoski JM; Busgang SA; Bixby M; Bennett D; Schmidt RJ; Barr DB; Panuwet P; Gennings C; Hertz-Picciotto I, Prenatal phenol and paraben exposures in relation to child neurodevelopment including autism spectrum disorders in the MARBLES study. Environ Res 2019, 179, 108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger K; Coker E; Rauch S; Eskenazi B; Balmes J; Kogut K; Holland N; Calafat AM; Harley K, Prenatal phthalate, paraben, and phenol exposure and childhood allergic and respiratory outcomes: Evaluating exposure to chemical mixtures. Sci Total Environ 2020, 725, 138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson KK; Lan Z; Yu Y; Mukherjee B; McElrath TF; Meeker JD, Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates. Environ Int 2019, 131, 104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaKind JS; Naiman DQ, Temporal trends in bisphenol A exposure in the United States from 2003-2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation. Environ Res 2015, 142, 84–95. [DOI] [PubMed] [Google Scholar]
- 13.Mervish N; McGovern KJ; Teitelbaum SL; Pinney SM; Windham GC; Biro FM; Kushi LH; Silva MJ; Ye X; Calafat AM; Wolff MS; Bcerp, Dietary predictors of urinary environmental biomarkers in young girls, BCERP, 2004-7. Environ Res 2014, 133, 12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calafat AM; Weuve J; Ye X; Jia LT; Hu H; Ringer S; Huttner K; Hauser R, Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009, 117, (4), 639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geer LA; Pycke BFG; Waxenbaum J; Sherer DM; Abulafia O; Halden RU, Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater 2017, 323, (Pt A), 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pycke BF; Geer LA; Dalloul M; Abulafia O; Halden RU, Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ Int 2015, 84, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towers CV; Terry PD; Lewis D; Howard B; Chambers W; Armistead C; Weitz B; Porter S; Borman CJ; Kennedy RC; Chen J, Transplacental passage of antimicrobial paraben preservatives. J Expo Sci Environ Epidemiol 2015, 25, (6), 604–7. [DOI] [PubMed] [Google Scholar]
- 18.Pycke BF; Geer LA; Dalloul M; Abulafia O; Jenck AM; Halden RU, Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol 2014, 48, (15), 8831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei L; Qiao P; Shi Y; Ruan Y; Yin J; Wu Q; Shao B, Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clinica Chimica Acta 2017, 466, 133–137. [DOI] [PubMed] [Google Scholar]
- 20.Shin HM; Moschet C; Young TM; Bennett DH, Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air 2020, 30, (1), 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guignard D; Gayrard V; Lacroix MZ; Puel S; Picard-Hagen N; Viguie C, Evidence for bisphenol A-induced disruption of maternal thyroid homeostasis in the pregnant ewe at low level representative of human exposure. Chemosphere 2017, 182, 458–467. [DOI] [PubMed] [Google Scholar]
- 22.Stroheker T; Chagnon MC; Pinnert MF; Berges R; Canivenc-Lavier MC, Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: a comparative study. Reprod Toxicol 2003, 17, (4), 421–32. [DOI] [PubMed] [Google Scholar]
- 23.Ji K; Hong S; Kho Y; Choi K, Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol 2013, 47, (15), 8793–800. [DOI] [PubMed] [Google Scholar]
- 24.Peretz J; Vrooman L; Ricke WA; Hunt PA; Ehrlich S; Hauser R; Padmanabhan V; Taylor HS; Swan SH; VandeVoort CA; Flaws JA, Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ Health Perspect 2014, 122, (8), 775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siracusa JS; Yin L; Measel E; Liang S; Yu X, Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod Toxicol 2018, 79, 96–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philippat C; Mortamais M; Chevrier C; Petit C; Calafat AM; Ye X; Silva MJ; Brambilla C; Pin I; Charles MA; Cordier S; Slama R, Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 2012, 120, (3), 464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang R; Chen MJ; Ding GD; Chen XJ; Han XM; Zhou K; Chen LM; Xia YK; Tian Y; Wang XR, Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut 2013, 178, 115–20. [DOI] [PubMed] [Google Scholar]
- 28.Velez MP; Arbuckle TE; Fraser WD, Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril 2015, 103, (4), 1011–1020 e2. [DOI] [PubMed] [Google Scholar]
- 29.Buckley JP; Herring AH; Wolff MS; Calafat AM; Engel SM, Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children’s Environmental Health Study. Environ Int 2016, 91, 350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mustieles V; Perez-Lobato R; Olea N; Fernandez MF, Bisphenol A: Human exposure and neurobehavior. Neurotoxicology 2015, 49, 174–84. [DOI] [PubMed] [Google Scholar]
- 31.Berger K; Gunier RB; Chevrier J; Calafat AM; Ye X; Eskenazi B; Harley KG, Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ Res 2018, 165, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevrier J; Gunier RB; Bradman A; Holland NT; Calafat AM; Eskenazi B; Harley KG, Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect 2013, 121, (1), 138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perera F; Vishnevetsky J; Herbstman JB; Calafat AM; Xiong W; Rauh V; Wang S, Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect 2012, 120, (8), 1190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun JM; Yolton K; Dietrich KN; Hornung R; Ye X; Calafat AM; Lanphear BP, Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009, 117, (12), 1945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harley KG; Gunier RB; Kogut K; Johnson C; Bradman A; Calafat AM; Eskenazi B, Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res 2013, 126, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philippat C; Nakiwala D; Calafat AM; Botton J; De Agostini M; Heude B; Slama R; Group EM-CS, Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ Health Perspect 2017, 125, (9), 097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson-Browne MS; Papandonatos GD; Chen A; Calafat AM; Yolton K; Lanphear BP; Braun JM, Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children. Environ Health Perspect 2018, 126, (5), 057001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janjua NR; Frederiksen H; Skakkebaek NE; Wulf HC; Andersson AM, Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl 2008, 31, (2), 118–129. [DOI] [PubMed] [Google Scholar]
- 39.Teeguarden JG; Calafat AM; Ye XY; Doerge DR; Churchwell MI; Gunawan R; Graham MK, Twenty-Four Hour Human Urine and Serum Profiles of Bisphenol A during High-Dietary Exposure. Toxicol Sci 2011, 123, (1), 48–57. [DOI] [PubMed] [Google Scholar]
- 40.Ye XY; Wong LY; Bishop AM; Calafat AM, Variability of Urinary Concentrations of Bisphenol A in Spot Samples, First Morning Voids, and 24-Hour Collections. Environ Health Persp 2011, 119, (7), 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aung MT; Ferguson KK; Cantonwine DE; McElrath TF; Meeker JD, Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ Res 2019, 169, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang YQ; Zhao HZ; Xia W; Li YY; Liu HX; Hao K; Chen J; Sun XJ; Liu WY; Li JF; Peng Y; Hu C; Li CH; Zhang B; Lu S; Cai ZW; Xu SQ, Prenatal exposure to benzophenones, parabens and triclosan and neurocognitive development at 2 years. Environ Int 2019, 126, 413–421. [DOI] [PubMed] [Google Scholar]
- 43.Guidry VT; Longnecker MP; Aase H; Eggesbo M; Zeiner P; Reichborn-Kjennerud T; Knudsen GP; Bertelsen RJ; Ye XY; Calafat AM; Engel SM, Measurement of Total and Free Urinary Phenol and Paraben Concentrations over the Course of Pregnancy: Assessing Reliability and Contamination of Specimens in the Norwegian Mother and Child Cohort Study. Environ Health Persp 2015, 123, (7), 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun JM; Kalkbrenner AE; Calafat AM; Bernert JT; Ye X; Silva MJ; Barr DB; Sathyanarayana S; Lanphear BP, Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect 2011, 119, (1), 131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meeker JD; Cantonwine DE; Rivera-Gonzalez LO; Ferguson KK; Mukherjee B; Calafat AM; Ye X; Anzalota Del Toro LV; Crespo-Hernandez N; Jimenez-Velez B; Alshawabkeh AN; Cordero JF, Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol 2013, 47, (7), 3439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philippat C; Wolff MS; Calafat AM; Ye XY; Bausell R; Meadows M; Stone J; Slama R; Engel SM, Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environ Health Persp 2013, 121, (10), 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polinski KJ; Dabelea D; Hamman RF; Adgate JL; Calafat AM; Ye XY; Starling AP, Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res 2018, 162, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yazdy MM; Coull BA; Gardiner JC; Aguiar A; Calafat AM; Ye XY; Schantz SL; Korrick SA, A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J Expo Sci Env Epid 2018, 28, (5), 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashrap P; Watkins DJ; Calafat AM; Ye X; Rosario Z; Brown P; Velez-Vega CM; Alshawabkeh A; Cordero JF; Meeker JD, Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environ Int 2018, 121, (Pt 1), 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith KW; Braun JM; Williams PL; Ehrlich S; Correia KF; Calafat AM; Ye X; Ford J; Keller M; Meeker JD; Hauser R, Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect 2012, 120, (11), 1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vernet C; Philippat C; Calafat AM; Ye XY; Lyon-Caen S; Siroux V; Schisterman EF; Slama R, Within-Day, Between-Day, and Between-Week Variability of Urinary Concentrations of Phenol Biomarkers in Pregnant Women. Environ Health Persp 2018, 126, (3), 037005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casas M; Basagana X; Sakhi AK; Haug LS; Philippat C; Granum B; Manzano-Salgado CB; Brochot C; Zeman F; de Bont J; Andrusaityte S; Chatzi L; Donaire-Gonzalez D; Giorgis-Allemand L; Gonzalez JR; Gracia-Lavedan E; Grazuleviciene R; Kampouri M; Lyon-Caen S; Panella P; Petraviciene I; Robinson O; Urquiza J; Vafeiadi M; Vernet C; Waiblinger D; Wright J; Thomsen C; Slama R; Vrijheid M, Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ Int 2018, 121, 561–573. [DOI] [PubMed] [Google Scholar]
- 53.Fisher M; MacPherson S; Braun JM; Hauser R; Walker M; Feeley M; Mallick R; Berube R; Arbuckle TE, Paraben Concentrations in Maternal Urine and Breast Milk and Its Association with Personal Care Product Use. Environ Sci Technol 2017, 51, (7), 4009–4017. [DOI] [PubMed] [Google Scholar]
- 54.Calafat AM, Contemporary Issues in Exposure Assessment Using Biomonitoring. Curr Epidemiol Rep 2016, 3, (2), 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrier F; Giorgis-Allemand L; Slama R; Philippat C, Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology 2016, 27, (3), 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heffernan AL; Aylward LL; Toms LM; Sly PD; Macleod M; Mueller JF, Pooled biological specimens for human biomonitoring of environmental chemicals: opportunities and limitations. J Expo Sci Environ Epidemiol 2014, 24, (3), 225–32. [DOI] [PubMed] [Google Scholar]
- 57.Shin HM; Bennett DH; Barkoski J; Ye X; Calafat AM; Tancredi D; Hertz-Picciotto I, Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int 2019, 122, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hertz-Picciotto I; Schmidt RJ; Walker CK; Bennett DH; Oliver M; Shedd-Wise KM; LaSalle JM; Giulivi C; Puschner B; Thomas J; Roa DL; Pessah IN; Van de Water J; Tancredi DJ; Ozonoff S, A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study. Environ Health Perspect 2018, 126, (11), 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozonoff S; Young GS; Carter A; Messinger D; Yirmiya N; Zwaigenbaum L; Bryson S; Carver LJ; Constantino JN; Dobkins K; Hutman T; Iverson JM; Landa R; Rogers SJ; Sigman M; Stone WL, Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011, 128, (3), e488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin HM; Schmidt RJ; Tancredi D; Barkoski J; Ozonoff S; Bennett DH; Hertz-Picciotto I, Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health 2018, 17, (1), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Antweiler RC, Evaluation of Statistical Treatments of Left-Censored Environmental Data Using Coincident Uncensored Data Sets. II. Group Comparisons. Environ Sci Technol 2015, 49, (22), 13439–46. [DOI] [PubMed] [Google Scholar]
- 62.Hornung RW; Reed LD, Estimation of Average Concentration in the Presence of Nondetectable Values. App Occu Environ Hyg 1990, 5, (1), 46–51. [Google Scholar]
- 63.Hauser R; Meeker JD; Park S; Silva MJ; Calafat AM, Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 2004, 112, (17), 1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adibi JJ; Whyatt RM; Williams PL; Calafat AM; Camann D; Herrick R; Nelson H; Bhat HK; Perera FA; Silva MJ; Hauser R, Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect 2008, 116, (4), 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CDC Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2019); Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, 2019. [Google Scholar]
- 66.Wu XM; Bennett DH; Lee K; Cassady DL; Ritz B; Hertz-Picciotto I, Longitudinal variability of time-location/activity patterns of population at different ages: a longitudinal study in California. Environ Health 2011, 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zota AR; Singla V; Adamkiewicz G; Mitro SD; Dodson RE, Reducing chemical exposures at home: opportunities for action. J Epidemiol Commun H 2017, 71, (9), 937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.CSCA, California Safe Cosmetic Act (CSCA). Public Law 2005, https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/CSCP/Pages/CSCP.aspx. 2005 (accessed: Dec 25, 2020). In 2005.
- 69.Kim K; Shin HM; Busgang SA; Barr DB; Panuwet P; Schmidt RJ; Hertz-Picciotto I; Bennett DH, Temporal Trends of Phenol, Paraben, and Triclocarban Exposure in California Pregnant Women during 2007-2014. Environ Sci Technol 2021, 55, (16), 11155–11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


