Abstract
Purpose:
Medications with anticholinergic and sedative properties are widely used among older adults despite strong evidence of harm. The drug burden index (DBI), a pharmacological screening tool, measures these properties across drug classes, and higher DBI drug exposure (DBI > 1) has been associated with certain physical function-related adverse events. Our aim was to quantify mean daily DBI drug exposure among older adults in the United States (US).
Methods:
We screened medications for DBI properties and operationalized the DBI for US Medicare claims. We then conducted a retrospective cohort study of a 20% random, nationwide sample of 4 137 384 fee-for-service Medicare beneficiaries aged 66+ years (134 757 039 person-months) from January 2013 to December 2016. We measured the monthly distribution based on mean daily DBI, categorized as (a) >0 vs 0 (any use) and (b) 0, 0 < DBI ≤ 1, 1 < DBI ≤ 2, and DBI > 2, and examined temporal trends. We described patient-level factors (eg, demographics, healthcare use) associated with high (>2) vs low (0 < DBI≤1) DBI drug exposure.
Results:
The distribution of the mean daily DBI, aggregated at the month-level, was: 58.1% DBI = 0, 29.0% 0 < DBI≤1, 9.3% 1 < DBI≤2, and 3.7% DBI > 2. Predictors of high monthly DBI drug exposure (DBI > 2) included certain indicators of increased healthcare use (eg, high number of drug claims), white race, younger age, frailty, and a psychosis diagnosis code.
Conclusions:
The predictors of high DBI drug exposure can inform discussions between patients and providers about medication appropriateness and potential deprescribing. Future Medicare-based studies should assess the association between the DBI and adverse events.
Keywords: aging, cholinergic antagonists, drug burden index, drug utilization, hypnotics and sedatives, inappropriate prescribing, pharmacoepidemiology
1 |. INTRODUCTION
Medication management in older adults is challenging because aging alters the body’s immune response and capacity to metabolize drugs, leading to an increased sensitivity to intended and unintended drug effects.1 Furthermore, older adults often are treated with multidrug regimens, which may include medications with anticholinergic and sedating properties.2 These medications are commonly used among older adults despite strong evidence of their harms.3–7 Traditional anticholinergics include certain antidepressants, antihistamines, and antipsychotics.8 However, many medications not typically recognized as anticholinergic (eg, promethazine, meclizine) also have anticholinergic properties that contribute to a patient’s overall anticholinergic burden.9 Medications with anticholinergic properties can have unintended effects on the peripheral (eg, blurred vision, tachyarrhythmia) and central (eg, confusion, delirium, drowsiness) nervous systems.10,11 Older adults are particularly susceptible because aging is associated with reduced muscarinic receptor density and activity, which increases risk for side effects including cognitive impairment and falls.9,12,13 Medications with sedating properties include benzodiazepines, opioids, and antipsychotics.14 Use of medications with sedative-hypnotic properties is cautioned against in older adults due to associations with cognitive impairment, delirium, and falls.15–17
Multiple medications with anticholinergic and sedating properties continue to be prescribed to older adults.3–5 This may in part be due to difficulty in identifying anticholinergic and sedating properties, as there is no international consensus on how much anticholinergic or sedating activity warrants classifying a medication as an “anticholinergic” or “sedative.”14,18 While medications with anticholinergic properties share a common mechanism (ie, blocking the binding of acetylcholine to cholinergic receptors), they often have additional mechanisms of action. Medications with sedating properties have diverse chemical structures and mechanisms, and span many drug classes, making them more difficult to identify.14,19 Moreover, there is no standardized definition of sedating effects,14 which compounds the challenge of measuring the cumulative burden or load that these medications pose. Numerous tools have been proposed to quantify cumulative anticholinergic and sedating loads separately.14,18
The drug burden index (DBI) is a pharmacological screening tool that quantifies both anticholinergic and sedative properties cumulatively, and therefore, may be particularly relevant for medication management interventions.20 The DBI assigns each patient a composite score based on the dose of drugs with anticholinergic and sedative properties taken as well as a country-specific minimum recommended daily dose.20 Increases in the DBI score have generally been associated with falls18,21–28 and mortality22,29–31 in European and Australian cohorts. However, the DBI has not been applied in a large, general cohort of older adults in the United States (US), where the medications available, patterns of use, and the minimum recommended daily dose likely vary from other settings.32 Recently, the DBI was adapted for implementation using healthcare databases in New Zealand, Finland, Ireland, and the Netherlands.29,30,33,34 Our study sought to expand DBI accessibility for future claims-based research quantifying anticholinergic and sedating drug dispensing in the US. We developed an updated US-based DBI drug list and operationalized the DBI for Medicare claims, and used this framework to (a) measure the monthly distribution based on mean daily DBI, (b) identify patient-level predictors of high mean daily DBI exposure, and (c) describe temporal trends in the prevalence of several DBI thresholds and the proportion of commonly dispensed medications.
2 |. METHODS
2.1 |. Data sources
We utilized a 20% nationwide, random sample of Medicare beneficiaries. Data were obtained through a data use agreement between the Centers for Medicaid and Medicare Services (CMS) and the University of North Carolina at Chapel Hill (UNC-CH). This research was approved by the Institutional Review Board of the UNC-CH (Study #18–2999).
2.2 |. Study population and design
We conducted a retrospective cohort study of Medicare beneficiaries during 2013 to 2016. This study period was selected because benzodiazepines (BZDs), a major class of medications with sedating properties, were not covered by Medicare Part D from 2006 to 2012.35 Eligibility criteria differed for the monthly and annual analyses. However, for both, beneficiaries were required to have continuous enrollment in Medicare fee-for-service (Parts A, B) and Part D for the 12 months prior to exposure assessment. This time period was used to define baseline covariates (eg, comorbidities, healthcare utilization, prior medication use). Fee-for-service and Part D coverage were also required during the exposure assessment window (ie, month or calendar year of interest) (Figure S1). Beneficiaries needed to be ≥66 years at the start of the calendar year of interest; beneficiaries <65 years are a unique subgroup who receive coverage based on disability or advanced disease. For all analyses, we required at least one insurance claim (medical care or pharmaceutical) during that year to restrict to persons utilizing Medicare benefits.
2.3 |. The drug burden index (DBI)
The DBI is a screening tool that was designed by Hilmer and colleagues in 2007 to assess anticholinergic and sedative drug burden.20 The DBI contribution of a single drug with anticholinergic and/or sedating properties incorporates the daily dose (D) and country-specific minimum recommended daily dose (δ) as follows:
| (1) |
Each DBI drug contribution can range from zero to one.36 To compute a patient’s total drug burden (TDB), all DBI drug contributions are summed.37 Operationalizing the DBI for a US-based claims implementation required that we develop an updated US-based DBI drug list and estimate each patient’s TDB using prescription claims.
2.4 |. US-based DBI drug list
To develop a list of DBI drugs dispensed during the study period (Table S1), two geriatric pharmacists (MJP and JCB) reviewed the unique drug generic names listed in the Part D claims. Drugs were classified as having anticholinergic or sedating effects, both, or neither. Sedating drugs were defined based on pharmacological classification as those that depress the central nervous system and consisted of the following pharmacological classes: BZDs, nonbenzodiazepine BZD receptor agonist hypnotics, antidepressants, antipsychotics, anticonvulsants, antihistamines, skeletal muscle relaxants, and opioid analgesics. Anticholinergic drugs included those classified as anticholinergics and those with strong anticholinergic properties, as defined by the 2019 Beers Criteria38 and scoring 2 or 3 on the Anticholinergic Cognitive Burden Scale.39 For combination drugs, the active ingredient(s) possessing these properties were labeled. Formulations unlikely to have systemic effects (creams, ointments, gels, waxes, lotions, liniments, paints, pastes, drops, irrigations, shampoos, and mouthwashes) were excluded.
2.5 |. Total drug burden (TDB)
To estimate the TDB, we (a) calculated the minimum recommended daily dose (δ) for each DBI drug, (b) estimated the daily dose (D) for each DBI drug dispensed in our data, (c) calculated the daily DBI contribution of each drug, using both δ and D, and (d) estimated the TDB at the day level by summing across all daily DBI contributions for each calendar day in the study period.
To calculate the minimum recommended daily dose (δ) for each drug, we used the lowest on-label daily dose available for any nonadjunct indication by formulation as listed in IBM’s Micromedex Solutions software (Truven Health Analytics, Greenwood Village, CO) as described in Table S2. For combination drugs, for each active ingredient with anticholinergic and/or sedating properties, we assigned the minimum dose based on the most commonly dispensed combination drug with that ingredient. We then estimated the daily dose (D) for each DBI drug dispensed as follows:
| (2) |
Quantity dispensed, days supply, and strength for each dispensed prescription were extracted from the Part D file. Prescriptions dispensed in 2012 with days supply carrying over into the study period (2013–2016) were included. We then identified combination and noncombination drugs based on the generic name and strength fields, and estimated drug strength based on the information contained in the drug strength field as described in Table S3.
Next, we identified distinct periods of continuous use for each dispensed DBI prescription by daily dose, not allowing for any gaps between prescription fills. Using these periods of use, we noted any periods of overlap, and adjusted the start and end date for each daily dose accordingly to account for potential forward stockpiling. For each beneficiary, we used the daily dose (D) and minimum recommended daily dose (δ) to compute the daily DBI contribution of each drug (Formula (1)).
We then estimated each individual’s TDB at the day level by summing across all the daily DBI drug contributions for each calendar day in the study period. This daily DBI drug exposure was averaged at the month and calendar year level to obtain monthly mean daily DBI and annual mean daily DBI estimates. Hereinafter, simply “monthly” and “annual DBI.” These steps were repeated to estimate the individual components (DBI-Anticholinergic and DBI-Sedating, respectively) separately.
We categorized the DBI as (a) > 0 vs 0 (any use vs none) for comparability with the previous longitudinal claims-based studies, and also as (b) 0 (none), 0 < DBI ≤ 1 (low exposure, for example, up to two drugs at the minimum recommended daily dose), 1 < DBI ≤ 2 (medium exposure), and DBI > 2 (high exposure, eg, more than four drugs at or above the minimum recommended daily dose).30,33
2.6 |. Covariate assessment
Several covariates were examined using data extracted from the Part A, B, and D claim files and assessed during the 12-month period prior to exposure assessment. Demographic characteristics included age, race (Black, white, Asian, Hispanic, Native American, other, unknown), and sex (male, female). We used the following dichotomous (any, none) proxy measures for socioeconomic status relating to Part D coverage: Partial Low-Income Subsidy, Full Low-Income Subsidy, and State Buy-In Parts A and B coverage. We estimated a proxy measure for frailty using the validated Faurot Medicare claims-based algorithm that incorporated predictors of activities of daily living dependency.40 Beneficiaries’ predicted probabilities of being frail were categorized as: low (0%-< 10%), low/intermediate (10%-< 20%), intermediate/high (20%-< 50%), and high (≥50%).41 We considered the component comorbidities that comprise the Gagne Combined Comorbidity Score, a validated tool designed for predicting mortality in Medicare claims.42 We also examined several healthcare utilization indicators: number of outpatient visits, number of emergency department visits, number of hospital admissions, and number of unique dispensed medications (count by generic name). Codes are provided in Table S4.
Since CMS transitioned to using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) on 1 October 2015, we used an updated version of the Faurot frailty algorithm with ICD-10-CM mapping developed by Eavey and colleagues in the Kaiser Permanente group (J Eavey, written personal communication, January 2018). Likewise, we used the ICD-10-CM adapted and validated Gagne Score developed by Sun and colleagues.43
2.7 |. Statistical analysis
We described the monthly and annual DBI score distributions graphically and examined the patient-level characteristics outlined earlier stratified by several DBI score categories (henceforth, DBI thresholds): 0, 0 < DBI≤1, 1 < DBI≤2, and DBI > 2. Next, we estimated associations between these characteristics and medium (1 < DBI≤2) and high (DBI > 2) vs low (0 < DBI≤1) monthly DBI drug exposure. For each characteristic, we used modified Poisson regression models with robust variance44 to estimate crude and multivariable adjusted prevalence ratios (PRs) with corresponding 95% confidence intervals (CIs). To facilitate model convergence, we excluded person-months with missing sex from all analyses (n = 23).
Finally, we examined temporal trends in DBI drug dispensing. We first described how the proportion of the most commonly dispensed DBI drugs changed over time. We then considered whether the DBI threshold prevalence changed over time. To do so, we determined specific eligibility for each prevalence window (ie, for each month and calendar year included). Using the monthly DBI we computed earlier, we estimated the monthly point prevalence and 95% confidence intervals for each month of the study period as follows:
Similarly, using the annual DBI we computed earlier, we estimated annual period prevalence in each year of the study period as follows:
We used the same DBI thresholds, and for consistency with the two previous annual prevalence estimates, also considered >0 vs 0 (any use vs none).30,33 Statistical analyses were conducted using SAS Statistical Software, version 9.3 (Cary, NC) and graphs were generated using R Statistical Software (version 3.6.0).
2.8 |. Sensitivity analyses
For consistency with Medicare quality measures estimation,45 we conducted a sensitivity analysis in which we further required ≥1 prescriptions to be filled in the time period in which prevalence was estimated. We also graphed the monthly point prevalence of DBI ≥1 vs DBI <1 because this is commonly reported in the literature.23,24,26,31
3 |. RESULTS
3.1 |. DBI drug identification
We identified 187 distinct active ingredients with DBI properties (Table S1). Of these, one had only anticholinergic properties, 118 had only sedating properties, and 68 had both properties.
3.2 |. Demographic characteristics
The analysis population included 134 757 039 person-months from 4 137 384 Medicare beneficiaries during 2013 to 2016 (Table 1). On average, these individuals were 76.3 years old, 61.5% were women, and 86.0% identified as white.
TABLE 1.
Descriptive characteristics of the analysis population by monthly DBI drug exposure, Medicare Beneficiaries 2013–2016
| Monthly DBI | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics (%) | DBI = 0 N = 78 243 433a (3 263 474 beneficiaries) | 0 < DBI ≤ 1 N = 39 103 243 (2 989 271 beneficiaries) | 1 < DBI ≤ 2 N = 12 481 590 (1 168 044 beneficiaries) | DBI > 2 N = 4 928 773 (423 313 beneficiaries) | Total N = 134 757 039 (4 137 384 beneficiaries) |
| Demographics | |||||
| Age, mean (SD) | 76.0 (7.39) | 77.1 (7.84) | 76.5 (7.69) | 74.7 (7.06) | 76.3 (7.56) |
| Sex, Male | 34 247 490 (43.8%) | 12 696 675 (32.5%) | 3 639 152 (29.2%) | 1 354 272 (27.5%) | 51 937 589 (38.5%) |
| Race | |||||
| White | 66 254 781 (84.7%) | 34 093 092 (87.2%) | 11 085 067 (88.8%) | 4 431 913 (89.9%) | 115 864 876 (86.0%) |
| Black | 5 951 231 (7.6%) | 2 710 955 (6.9%) | 812 133 (6.5%) | 298 812 (6.1%) | 9 773 131 (7.3%) |
| Other | 1 479 800 (1.9%) | 438 033 (1.1%) | 104 573 (0.8%) | 37 185 (0.8%) | 2 059 591 (1.5%) |
| Asian | 2 117 352 (2.7%) | 719 222 (1.8%) | 138 279 (1.1%) | 32 547 (0.7%) | 3 007 400 (2.2%) |
| Hispanic | 1 429 719 (1.8%) | 748 555 (1.9%) | 221 867 (1.8%) | 80 383 (1.6%) | 2 480 524 (1.8%) |
| Native American | 272 055 (0.3%) | 145 474 (0.4%) | 53 952 (0.4%) | 25 485 (0.5%) | 496 966 (0.4%) |
| Unknown | 738 495 (0.9%) | 247 912 (0.6%) | 65 719 (0.5%) | 22 448 (0.5%) | 1 074 574 (0.8%) |
| Healthcare Utilization | |||||
| No. of outpatient office visits, mean (SD) | 7.2 (6.36) | 9.9 (7.85) | 11.3 (8.89) | 12.5 (10.16) | 8.6 (7.44) |
| No. of Emergency Dept. visits, mean (SD) | 0.4 (1.09) | 0.8 (1.61) | 1.1 (1.96) | 1.4 (2.47) | 0.6 (1.45) |
| No. of hospital admissions, mean (SD) | 0.2 (0.78) | 0.5 (1.17) | 0.6 (1.38) | 0.8 (1.60) | 0.4 (1.03) |
| No. of Rx fills in month, mean (SD) | 1.9 (2.32) | 4.0 (3.48) | 6.2 (4.66) | 9.0 (6.07) | 3.2 (3.64) |
| Polypharmacy (≥5 fills /month) | 9 534 312 (12.2%) | 13 812 190 (35.3%) | 7 251 696 (58.1%) | 3 854 176 (78.2%) | 34 452 374 (25.6%) |
| Any months of Parts A and B State Buy-in, mean (SD) | 11 541 835 (14.8%) | 8 193 246 (21.0%) | 3 625 059 (29.0%) | 1 964 523 (39.9%) | 25 324 686 (18.8%) |
| Any months of Partial Low-Income Subsidy | 12 407 613 (15.9%) | 9 122 695 (23.3%) | 4 123 277 (33.0%) | 2 253 964 (45.7%) | 27 907 572 (20.7%) |
| Economic status proxy | |||||
| Any months of Parts A and B State Buy-in, mean (SD) | 11 541 835 (14.8%) | 8 193 246 (21.0%) | 3 625 059 (29.0%) | 1 964 523 (39.9%) | 25 324 686 (18.8%) |
| Any Months of Full Low-Income Subsidy | 2 305 163 (2.9%) | 1 326 256 (3.4%) | 481 781 (3.9%) | 205 086 (4.2%) | 4 318 286 (3.2%) |
| Any Months of Full Low-Income Subsidy | 2 305 163 (2.9%) | 1 326 256 (3.4%) | 481 781 (3.9%) | 205 086 (4.2%) | 4 318 286 (3.2%) |
| Health status indicators | |||||
| Frailty Probability | |||||
| Low (0%- < 10%) | 70 481 308 (90.1%) | 29 392 245 (75.2%) | 7 751 476 (62.1%) | 2 463 546 (50.0%) | 110 088 598 (81.7%) |
| Low/intermediate (10%- < 20%) | 3 867 149 (4.9%) | 4 201 439 (10.7%) | 1 839 245 (14.7%) | 856 602 (17.4%) | 10 764 435 (8.0%) |
| Intermediate/high (20%- < 50%) | 2 544 739 (3.3%) | 3 436 911 (8.8%) | 1 741 840 (14.0%) | 929 167 (18.9%) | 8 652 657 (6.4%) |
| High (≥50%) | 1 350 237 (1.7%) | 2 072 648 (5.3%) | 1 149 029 (9.2%) | 679 458 (13.8%) | 5 251 372 (3.9%) |
| Gagne Comorbidity Score, mean (SD) | 0.5 (1.40) | 0.8 (1.77) | 1.0 (1.85) | 1.2 (1.87) | 0.7 (1.61) |
| Comorbiditiesb | |||||
| Metastatic cancer | 1 078 213 (1.4%) | 889 899 (2.3%) | 294 962 (2.4%) | 112 479 (2.3%) | 2 375 553 (1.8%) |
| Congestive heart failure | 10 040 545 (12.8%) | 8 015 722 (20.5%) | 3 068 456 (24.6%) | 1 345 860 (27.3%) | 22 470 583 (16.7%) |
| Dementia | 3 047 259 (3.9%) | 3 970 720 (10.2%) | 1 915 372 (15.3%) | 973 857 (19.8%) | 9 907 208 (7.4%) |
| Renal failure | 9 148 167 (11.7%) | 6 716 997 (17.2%) | 2 438 763 (19.5%) | 992 290 (20.1%) | 19 296 217 (14.3%) |
| Weight loss | 1 298 734 (1.7%) | 1 312 908 (3.4%) | 546 911 (4.4%) | 269 131 (5.5%) | 3 427 684 (2.5%) |
| Hemiplegia | 553 974 (0.7%) | 656 081 (1.7%) | 343 920 (2.8%) | 198 008 (4.0%) | 1 751 983 (1.3%) |
| Alcohol abuse | 340 994 (0.4%) | 287 574 (0.7%) | 136 981 (1.1%) | 79 286 (1.6%) | 844 835 (0.6%) |
| Any tumor | 11 045 270 (14.1%) | 6 360 337 (16.3%) | 1 931 969 (15.5%) | 699 684 (14.2%) | 20 037 260 (14.9%) |
| Cardiac arrhythmias | 15 990 540 (20.4%) | 10 722 580 (27.4%) | 3 587 030 (28.7%) | 1 405 031 (28.5%) | 31 705 181 (23.5%) |
| Chronic pulmonary disease | 13 586 480 (17.4%) | 10 676 894 (27.3%) | 4 234 840 (33.9%) | 2 007 386 (40.7%) | 30 505 600 (22.6%) |
| Coagulopathy | 2 676 091 (3.4%) | 1 986 515 (5.1%) | 708 233 (5.7%) | 305 403 (6.2%) | 5 676 242 (4.2%) |
| Complicated diabetes | 8 131 523 (10.4%) | 6 107 307 (15.6%) | 2 380 047 (19.1%) | 1 034 282 (21.0%) | 17 653 159 (13.1%) |
| Deficiency anemias | 14 049 886 (18.0%) | 10 897 027 (27.9%) | 4 216 314 (33.8%) | 1 920 237 (39.0%) | 31 083 476 (23.1%) |
| Fluid and electrolyte disorders | 8 127 737 (10.4%) | 7 167 041 (18.3%) | 2 895 840 (23.2%) | 1 392 725 (28.3%) | 19 583 343 (14.5%) |
| Liver disease | 1 993 897 (2.5%) | 1 410 225 (3.6%) | 514 493 (4.1%) | 233 187 (4.7%) | 4 151 802 (3.1%) |
| Peripheral vascular disorder | 11 637 332 (14.9%) | 9 044 815 (23.1%) | 3 454 730 (27.7%) | 1 521 298 (30.9%) | 25 658 187 (19.0%) |
| Psychosis | 2 136 477 (2.7%) | 5 221 458 (13.4%) | 3 398 743 (27.2%) | 2 212 286 (44.9%) | 12 968 964 (9.6%) |
| Pulmonary circulation disorders | 2 137 796 (2.7%) | 1 757 940 (4.5%) | 637 984 (5.1%) | 261 252 (5.3%) | 4 794 972 (3.6%) |
| HIV/AIDS | 77 943 (0.1%) | 49 147 (0.1%) | 20 991 (0.2%) | 12 571 (0.3%) | 160 652 (0.1%) |
| Hypertension | 55 328 180 (70.7%) | 31 906 224 (81.6%) | 10 443 794 (83.7%) | 4 138 398 (84.0%) | 101 816 619 (75.6%) |
This total refers to the number of patient-months included.
These comorbidities are defined based on the Gagne Comorbidity Score.
3.3 |. Drug burden index
The daily DBI distribution was very right skewed, with a mean (±SD) of 0.38 (±0.69) over the study period (Figure 1A). After restricting to beneficiaries with some DBI exposure (ie, non-zero daily DBI scores), the mean (±SD) was 1.06 (±0.79) (Figure 1A). The daily DBI distribution remained skewed after averaging at the month and year levels (Figure 1B). There was no notable seasonal or temporal variation in the DBI distribution (Figure S2). The distribution of the monthly DBI was 58.1% DBI = 0, 29.0% 0 < DBI≤1, 9.3% 1 < DBI≤2, and 3.7% DBI > 2. (Table 1).
FIGURE 1.
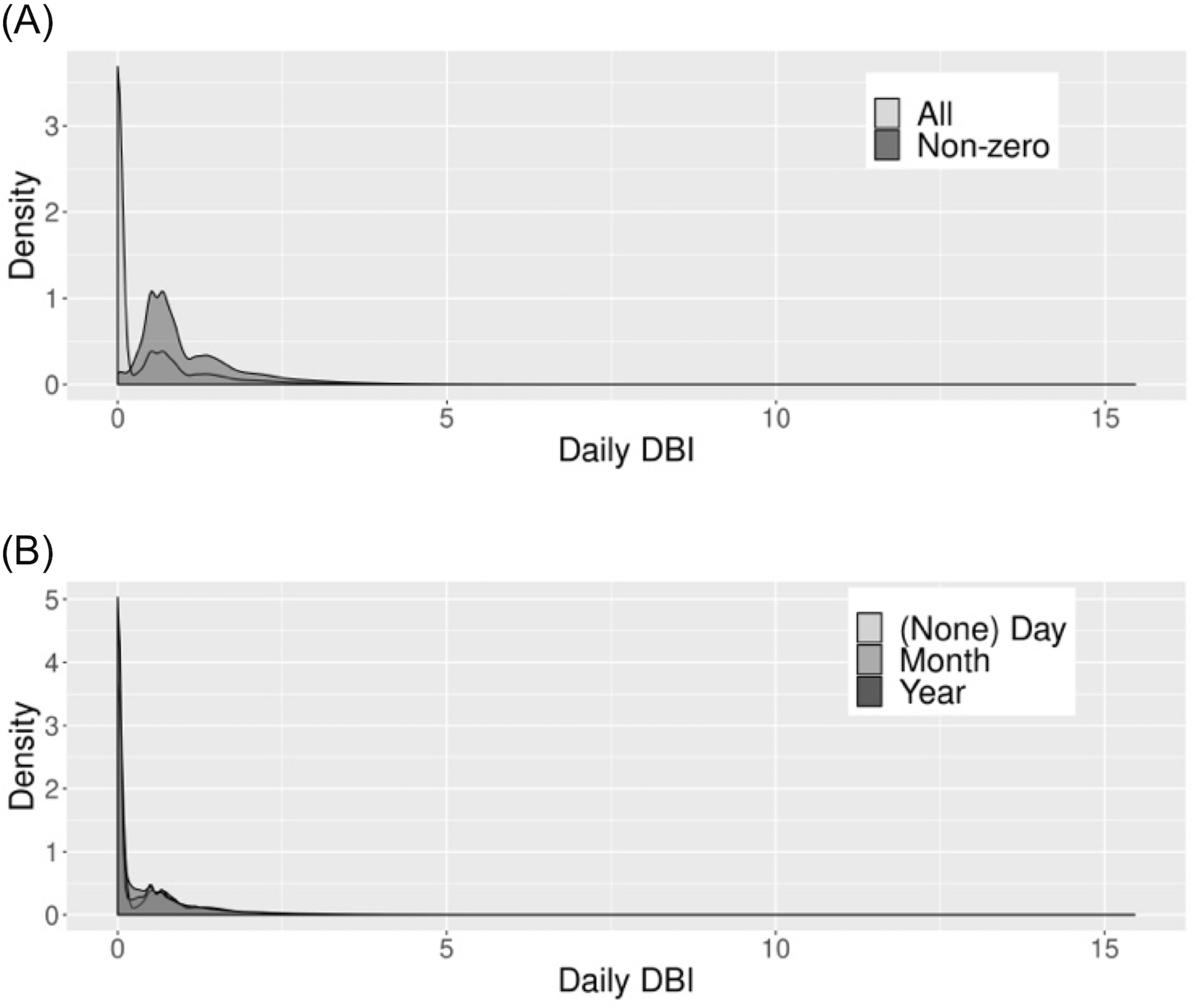
Daily drug burden index (DBI) distribution among Medicare Beneficiaries, 2013–2016. A, Daily DBI-total distribution. B, Daily DBI-total distribution for the study period, stratified by level (eg, (none) day, month, year) at which the DBI was averaged
3.4 |. Factors associated with high (>2) monthly DBI
Among the demographic characteristics examined, the strongest predictors of high DBI drug dispensation (>2), as compared to low DBI drug dispensation (0 < DBI ≤ 1), were younger age, female sex, and white race (Table 2). Of the healthcare utilization indicators examined, having a high number of prescription fills was strongly positively associated with high DBI drug exposure (adjusted PR for 10+ prescriptions vs 1–2 prescriptions: 9.96 (95% CI: 9.87–10.06)) (Table 2). Additionally, as frailty probability increased (from low/intermediate to high), the PR estimate moved further from the null (eg, adjusted PRs for low/intermediate and high frailty, respectively: 1.47 (95% CI: 1.46–1.48) and 1.80 (95% CI: 1.78–1.82)) (Table 2). While most of the component comorbidities had no or weak associations with high DBI drug exposure, there was a strong positive association with psychosis (adjusted PR: 2.30 (95% CI: 2.29–2.32)) (Table 2).
TABLE 2.
Factors associated with medium (1 < DBI≤2) and high (DBI >2), as compared to low (0 < DBI≤1), monthly drug burden index (DBI) drug exposure
| Medium DBI Exposure (1 < DBI≤2) |
High DBI Exposure (>2) |
||||||
|---|---|---|---|---|---|---|---|
| Factor | Level | Na | %b | Adjusted Prevalence Ratio (PR)c | Na | %b | Adjusted Prevalence Ratio (PR)c |
|
| |||||||
| Demographics | |||||||
| Age | 66–69 | 10 358 460 | 26.24 | REF | 9 058 525 | 15.65 | REF |
| 70–74 | 12 957 557 | 24.90 | 0.93 (0.92–0.93) | 11 174 874 | 12.91 | 0.84 (0.84–0.85) | |
| 75–79 | 10 268 026 | 23.89 | 0.84 (0.84–0.85) | 8 720 635 | 10.38 | 0.65 (0.64–0.66) | |
| 80–84 | 7 981 277 | 23.22 | 0.77 (0.77–0.78) | 6 714 208 | 8.73 | 0.50 (0.50–0.51) | |
| 85+ | 10 019 513 | 22.27 | 0.68 (0.67–0.68) | 8 363 774 | 6.89 | 0.34 (0.34–0.35) | |
| Sex | Male | 16 335 827 | 22.28 | REF | 14 050 947 | 9.64 | REF |
| Female | 35 249 006 | 25.09 | 1.09 (1.09–1.10) | 29 981 069 | 11.92 | 1.14 (1.13–1.14) | |
| Race | White | 45 178 159 | 24.54 | REF | 38 525 005 | 11.50 | REF |
| Black | 3 523 088 | 23.05 | 0.78 (0.78–0.79) | 3 009 767 | 9.93 | 0.64 (0.63–0.65) | |
| Other | 542 606 | 19.27 | 0.71 (0.69–0.72) | 475 218 | 7.82 | 0.59 (0.57–0.62) | |
| Asian | 857 501 | 16.13 | 0.55 (0.54–0.56) | 751 769 | 4.33 | 0.31 (0.30–0.32) | |
| Hispanic | 970 422 | 22.86 | 0.77 (0.76–0.78) | 828 938 | 9.70 | 0.64 (0.63–0.66) | |
| Native American | 199 426 | 27.05 | 0.86 (0.84–0.88) | 170 959 | 14.91 | 0.79 (0.76–0.83) | |
| Unknown | 313 631 | 20.95 | 0.80 (0.78–0.82) | 270 360 | 8.30 | 0.64 (0.61–0.67) | |
| Healthcare Utilization | |||||||
| No. of outpatient office visits, mean (SD) | None | 2 632 436 | 30.64 | REF | 2 252 742 | 18.95 | REF |
| 1–6 | 16 882 109 | 20.37 | 0.97 (0.96–0.98) | 14 601 216 | 7.93 | 0.87 (0.86–0.88) | |
| 7–12 | 16 209 285 | 22.69 | 1.10 (1.09–1.11) | 13 756 530 | 8.91 | 1.03 (1.02–1.04) | |
| 13+ | 15 857 942 | 28.74 | 1.28 (1.27–1.29) | 13 418 826 | 15.78 | 1.41 (1.39–1.42) | |
| No. of Emergency Dept. visits, mean (SD) | None | 31 061 250 | 21.90 | REF | 26 585 281 | 8.75 | REF |
| 1 | 10 263 707 | 25.58 | 1.03 (1.02–1.03) | 8 696 867 | 12.17 | 1.05 (1.05–1.06) | |
| 2–5 | 9 089 492 | 29.17 | 1.04 (1.04–1.04) | 7 716 460 | 16.57 | 1.09 (1.09–1.10) | |
| 6+ | 1 167 323 | 34.50 | 1.04 (1.04–1.05) | 1 030 706 | 25.81 | 1.13 (1.12–1.15) | |
| No. of hospital admissions, mean (SD) | None | 39 295 073 | 22.79 | REF | 33 577 886 | 9.65 | REF |
| 1 | 5 805 513 | 26.86 | 0.99 (0.98–0.99) | 4 936 932 | 13.99 | 0.99 (0.99–1.00) | |
| 2 | 3 424 927 | 29.16 | 0.94 (0.94–0.95) | 2 900 626 | 16.36 | 0.92 (0.92–0.93) | |
| 3+ | 3 056 259 | 31.61 | 0.88 (0.88–0.89) | 2 613 870 | 20.03 | 0.85 (0.84–0.85) | |
| No. of Rx fills in month, mean (SD) | None | 4 517 761 | 12.16 | 0.82 (0.82–0.83) | 4 057 264 | 2.19 | 0.75 (0.74–0.76) |
| 1–2 | 13 062 903 | 14.82 | REF | 11 459 380 | 2.90 | REF | |
| 3–4 | 12 940 283 | 21.21 | 1.41 (1.41–1.42) | 10 849 006 | 6.02 | 2.06 (2.04–2.07) | |
| 5–9 | 16 136 334 | 30.75 | 1.99 (1.98–2.00) | 13 148 444 | 15.01 | 4.71 (4.67–4.75) | |
| 10+ | 4 927 552 | 46.47 | 2.81 (2.80–2.82) | 4 517 922 | 41.62 | 9.96 (9.87–10.06) | |
| Economic Status Proxy | |||||||
| Months of Parts A and B State Buy-In | None | 39 766 528 | 22.27 | REF | 33 874 247 | 8.75 | REF |
| Any | 11 818 305 | 30.67 | 1.00 (1.00–1.01) | 10 157 769 | 19.34 | 0.98 (0.97–0.99) | |
| Months with Partial Low-Income Subsidy | None | 38 338 861 | 21.80 | REF | 32 655 357 | 8.19 | REF |
| Any | 13 245 972 | 31.13 | 1.12 (1.11–1.13) | 11 376 659 | 19.81 | 1.26 (1.24–1.27) | |
| Months with Partial Low-Income Subsidy | |||||||
| Any | 1 808 037 | 26.65 | 1.10 (1.09–1.11) | 1 531 342 | 13.39 | 1.20 (1.18–1.22) | |
| Health status indicators | |||||||
| Frailty Probability | Low (0%-<10%) | 37 143 721 | 20.87 | REF | 31 855 791 | 7.73 | REF |
| Low/intermediate (10%-<20%) | 6 040 684 | 30.45 | 1.26 (1.26–1.27) | 5 058 041 | 16.94 | 1.47 (1.46–1.48) | |
| Intermediate/high (20%-<50%) | 5 178 751 | 33.63 | 1.34 (1.33–1.34) | 4 366 078 | 21.28 | 1.64 (1.63–1.65) | |
| High (≥50%) | 3 221 677 | 35.67 | 1.41 (1.40–1.42) | 2 752 106 | 24.69 | 1.80 (1.78–1.82) | |
| Comorbiditiesd | |||||||
| Metastatic cancer | Yes | 1 184 861 | 24.89 | 0.97 (0.96–0.98) | 1 002 378 | 11.22 | 0.92 (0.91–0.94) |
| No | 50 399 972 | 24.18 | REF | 43 029 638 | 11.19 | REF | |
| Congestive heart failure | Yes | 11 084 178 | 27.68 | 0.92 (0.92–0.92) | 9 361 582 | 14.38 | 0.85 (0.84–0.85) |
| No | 40 500 655 | 23.24 | REF | 34 670 434 | 10.33 | REF | |
| Dementia | Yes | 5 886 092 | 32.54 | 1.02 (1.01–1.02) | 4 944 577 | 19.70 | 1.05 (1.04–1.06) |
| No | 45 698 741 | 23.12 | REF | 39 087 439 | 10.12 | REF | |
| Renal failure | Yes | 9 155 760 | 26.64 | 0.97 (0.96–0.97) | 7 709 287 | 17.01 | 0.91 (0.90–0.91) |
| No | 42 429 073 | 23.67 | REF | 363,22 729 | 10.98 | REF | |
| Weight loss | Yes | 1 859 819 | 29.41 | 0.98 (0.98–0.99) | 1 582 039 | 23.18 | 0.98 (0.97–0.99) |
| No | 49 725 014 | 24.00 | REF | 42 449 977 | 10.96 | REF | |
| Hemiplegia | Yes | 1 000 001 | 34.39 | 1.01 (1.00–1.02) | 854 089 | 21.61 | 0.99 (0.98–1.00) |
| No | 50 584 832 | 23.99 | REF | 43 177 927 | 11.11 | REF | |
| Alcohol abuse | Yes | 424 555 | 32.26 | 1.10 (1.08–1.11) | 366 860 | 9.91 | 1.13 (1.11–1.14) |
| No | 51 160 278 | 24.13 | REF | 43 665 156 | 11.44 | REF | |
| Any tumor | Yes | 8 292 306 | 23.30 | 0.95 (0.94–0.95) | 7 060 021 | 11.59 | 0.89 (0.89–0.90) |
| No | 43 292 527 | 24.37 | REF | 36 971 995 | 11.04 | REF | |
| Cardiac arrhythmias | Yes | 14 309 610 | 25.07 | 0.89 (0.89–0.90) | 12 127 611 | 15.83 | 0.81 (0.81–0.82) |
| No | 37 275 223 | 23.86 | REF | 31 904 405 | 9.32 | REF | |
| Chronic pulmonary disease | Yes | 14 911 734 | 28.40 | 1.04 (1.04–1.05) | 12 684 280 | 13.33 | 1.06 (1.06–1.07) |
| No | 36 673 099 | 22.49 | REF | 31 347 736 | 11.08 | REF | |
| Coagulopathy | Yes | 2 694 748 | 26.28 | 0.95 (0.94–0.95) | 2 291 918 | 14.48 | 0.91 (0.90–0.92) |
| No | 48 890 085 | 24.08 | REF | 41 740 098 | 10.56 | REF | |
| Complicated Diabetes | Yes | 8 487 354 | 28.04 | 0.96 (0.96–0.96) | 7 141 589 | 14.98 | 0.85 (0.85–0.86) |
| No | 43 097 479 | 23.44 | REF | 36 890 427 | 9.64 | REF | |
| Deficiency anemias | Yes | 15 113 341 | 27.90 | 1.04 (1.04–1.04) | 12 817 264 | 16.27 | 1.07 (1.06–1.07) |
| No | 36 471 492 | 22.66 | REF | 31 214 752 | 9.97 | REF | |
| Fluid and electrolyte disorders | Yes | 10 062 881 | 28.78 | 0.99 (0.99–1.00) | 8 559 766 | 14.19 | 1.00 (1.00–1.01) |
| No | 41 521 952 | 23.09 | REF | 35 472 250 | 11.08 | REF | |
| Liver disease | Yes | 1 924 718 | 26.73 | 0.96 (0.96–0.97) | 1 643 412 | 14.40 | 0.92 (0.91–0.93) |
| No | 49 660 115 | 24.10 | REF | 42 388 604 | 10.18 | REF | |
| No | 39 085 288 | 23.10 | REF | 33 465 903 | 7.42 | REF | |
| Psychosis | Yes | 8 620 201 | 39.43 | 1.57 (1.56–1.57) | 7 433 744 | 12.94 | 2.30 (2.29–2.32) |
| No | 42 964 632 | 21.14 | REF | 36 598 272 | 11.11 | REF | |
| Pulmonary circulation disorders | Yes | 2 395 924 | 26.63 | 0.92 (0.91–0.93) | 2 019 192 | 20.37 | 0.86 (0.85–0.87) |
| No | 49 188 909 | 24.08 | REF | 42 012 824 | 11.18 | REF | |
| HIV/AIDS | Yes | 70 138 | 29.93 | 0.94 (0.90–0.97) | 61 718 | 11.48 | 0.92 (0.87–0.97) |
| No | 51 514 695 | 24.19 | REF | 43 970 298 | 9.90 | REF | |
| Hypertension | Yes | 42 350 018 | 24.66 | 0.92 (0.91–0.92) | 36 044 622 | 17.01 | 0.79 (0.79–0.80) |
| No | 9 234 815 | 22.07 | REF | 7 987 394 | 10.98 | REF | |
| Temporal | |||||||
| Calendar Year | 2013 | 11 328 384 | 24.32 | REF | 9 673 400 | 11.38 | REF |
| 2014 | 12 909 214 | 24.23 | 1.03 (1.03–1.03) | 11 030 384 | 11.33 | 1.05 (1.05–1.05) | |
| 2015 | 13 282 273 | 24.07 | 1.03 (1.02–1.03) | 11 341 317 | 11.08 | 1.04 (1.04–1.05) | |
| 2016 | 14 064 962 | 24.17 | 0.98 (0.98–0.98) | 11 986 915 | 11.03 | 0.94 (0.94–0.95) | |
This refers to the total number of patient-months included (ie, the denominator).
Percentage of person-months with given exposure level.
Reference group is low DBI drug exposure (0 < DBI≤1), and adjustment set includes the other factors listed in the table.
These comorbidities are defined based on the Gagne Comorbidity Score.
3.5 |. Prevalence of anticholinergic and sedative drug exposure
Overall, the highest proportions of specific DBI medications (out of all DBI drug claims) in our study population were all for sedatives (hydrocodone (0.10), gabapentin (0.07), tramadol (0.06)). Notably, there was a decline in hydrocodone dispensing (from 0.11 to 0.08) and a simultaneous increase in gabapentin dispensing (from 0.06 to 0.08) from 2013 to 2016 (Figure 2).
FIGURE 2.
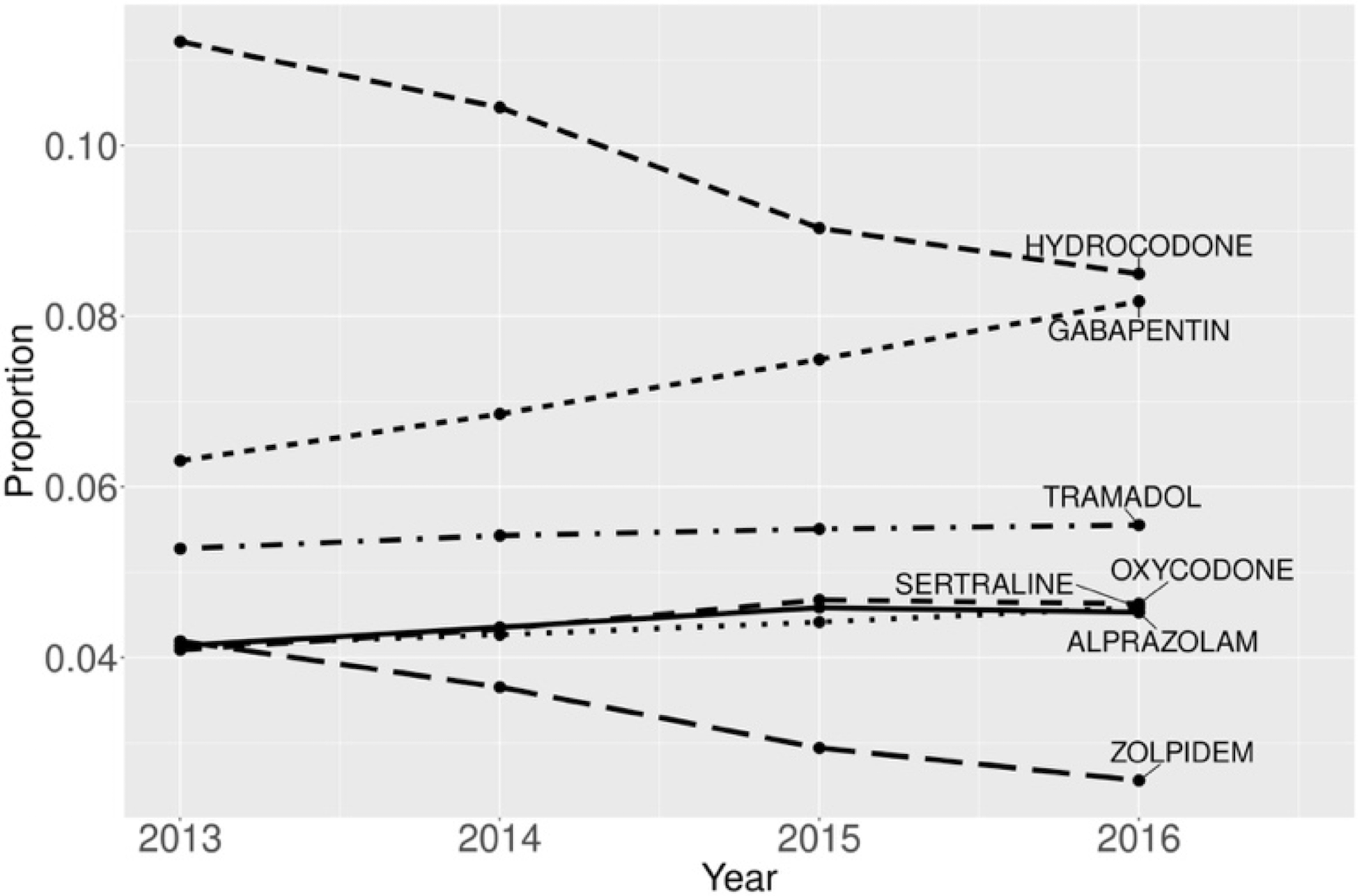
Temporal changes in the proportions of the most common prescriptions (among all DBI prescriptions) among Medicare Beneficiaries, 2013–2016
The annual period prevalence of any anticholinergic/sedative drug exposure decreased slightly, with 62.13% (95% CI: 62.07%−62.20%) of the population dispensed one or more DBI drugs in 2013 and 59.23% (95% CI: 59.18%−59.29%) dispensed in 2016 (Figure 3A). The monthly point prevalence was stable over the study period (overall: 41.99% (95% CI: 41.93%−42.04%)) (Figure 3B). When we examined DBI thresholds, we observed that the estimates were stable over the study period (Figure 3A,B). When stratifying by DBI components (DBI-Anticholinergic and DBI-Sedating), we saw similarly stable monthly point prevalence trends, with a higher prevalence for DBI-Sedating >0 than for DBI-Anticholinergic >0, since the majority of drugs were sedating only (Figure S3).
FIGURE 3.
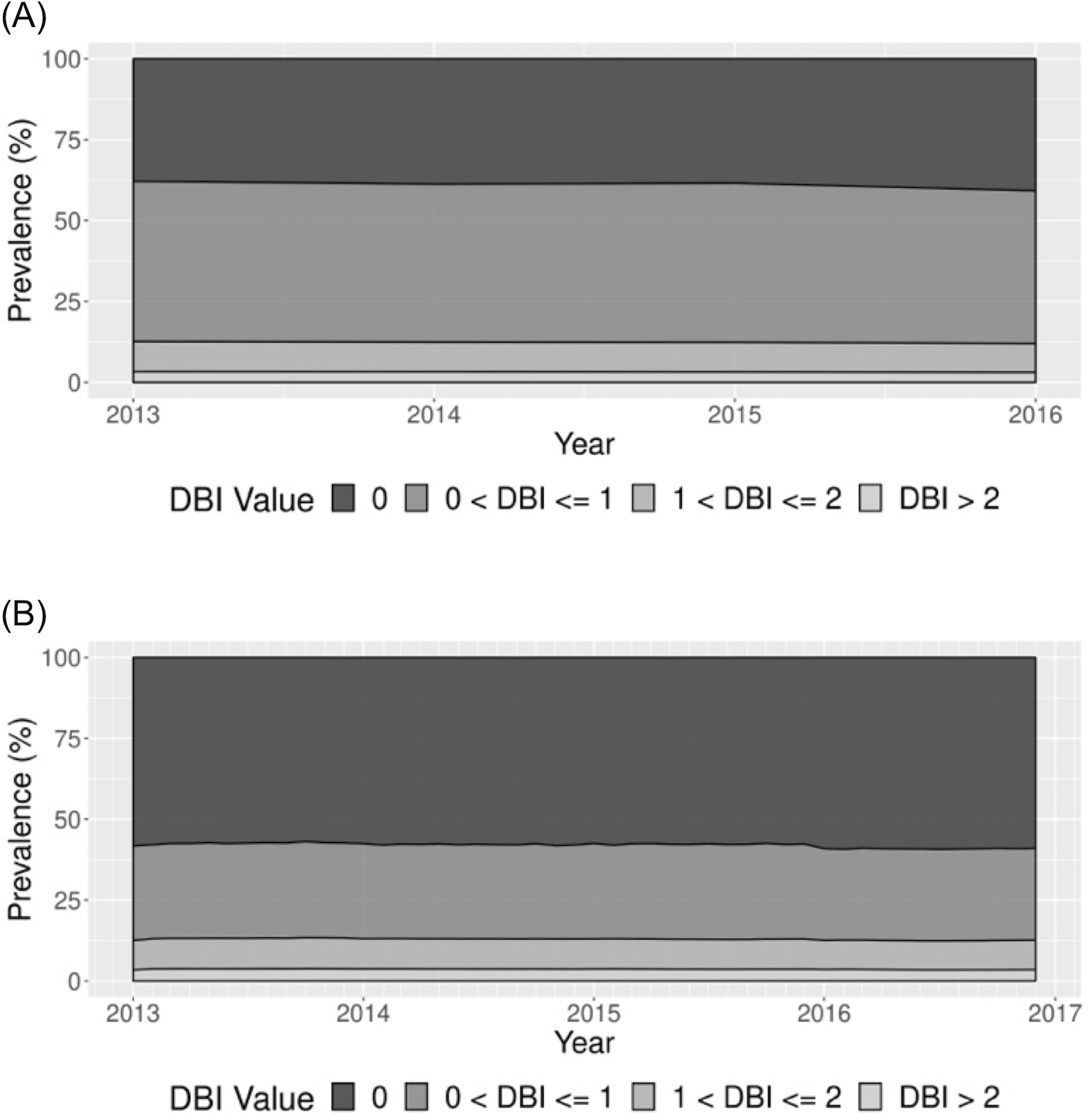
Prevalence of drug burden index (DBI) threshold exposure among Medicare Beneficiaries during 2013–2016. A, Annual period prevalence. B, Monthly point prevalence
3.6 |. Sensitivity analyses
When we restricted to beneficiaries dispensed at least one prescription during the time period in which prevalence was estimated, the prevalence estimates (for cutoffs of 0 and 1) were slightly higher for both point and period analyses, but the trends were consistent (Figure S4).
4 |. DISCUSSION
This study quantified total anticholinergic and sedating drug burden using the DBI, a pharmacological screening tool, in a large, US-based healthcare database. While we did not observe substantial temporal changes in the DBI scores over the study period (2013–2016), there were changes in the anticholinergic and sedating drugs dispensed. Consistent with the literature, and coinciding with the hydrocodone scheduling change in 2014, we noticed that as hydrocodone dispensing declined, gabapentin dispensing increased.46,47 The strongest predictors of high monthly DBI drug exposure (DBI > 2) included high number of drug claims, white race, younger age, frailty, and a psychosis diagnosis code.
While a medication count is an easy screening method, medication prescribing is complex and more refined tools such as the DBI may help better flag patients taking medications whose harms may outweigh their benefits.14 The DBI can support clinical decision-making and help monitor quality of care system-wide across multiple patients and healthcare providers.37 It is intended to promote good clinical judgement, rather than supersede it, by alerting providers to possible harms and reminding them to consider alternative treatments.37
The DBI was developed in a prospective cohort study of Medicare beneficiaries,20 the Health, Aging, and Body Composition (Health ABC) study, which recruited 3075 participants from two geographic sites during 1997–1998.20 However, to our knowledge, it has not been applied in a large, more representative US-based older adult population since then. In their original publication, Hilmer and colleagues reported a mean DBI score of 0.18 (±0.35), which is lower than our reported mean daily DBI score of 0.38 (±0.69) over the study period. This difference may be explained in part by differences in study population and exposure assessment. The Health ABC study participants were described as well-functioning, older adults aged 70–79, whereas our population was more diverse in terms of health status and age. Additionally, in the original DBI study, exposure was estimated based on a single medication inventory assessment designed to capture medication use in the past two weeks, whereas ours was based on longitudinal, pharmaceutical dispensing claims. In a longitudinal analysis incorporating three timepoints across five years, the percentage of Health ABC participants with nonzero DBI scores reported was 34%, 26%, and 29%.48 As anticipated, these are substantially lower than our annual period prevalence estimates, and more similar to our monthly point prevalence estimates. The high and sustained estimates of monthly point prevalence of any anticholinergic/sedative drug exposure we report over the study period suggest that the existing clinical guidelines (eg, the Beers Criteria38) are not reducing DBI drug use sufficiently and specific interventions for deprescribing may be warranted.
Four prior pharmacy claims-based DBI implementations have been reported.29,30,33,34 Two described short-term exposure (1-month or 4-month) and two described annual exposure. Of the short-term exposure studies, the Finnish study had a matched design in which patients with an Alzheimer’s disease diagnosis were matched to those without, rather than a general population, making its results difficult to compare.29 However, the results of a Dutch study, which reported on nationwide DBI drug exposure during November 2016, can be compared to our monthly exposure analyses.34 The proportion of older adults exposed to any anticholinergic/sedative drug in the Netherlands was 31.92% (766 174 / 2 400 000), while we found a point prevalence of any monthly anticholinergic/sedative drug exposure of 40.95% (95% CI: 40.90%−41.01%) for any use during November 2016 among US Medicare Beneficiaries.34 Similarly, our estimates during that month for 1 < DBI≤2 and DBI >2 (9.09% (95% CI: 9.06%−9.12%) and 3.50% (3.48%−3.52%), respectively) are higher than theirs for DBI >1 (8.7%).34
With respect to annual exposure studies, a New Zealand-based study reported a mean DBI score of 0.177 during 2011,30 and an Irish study reported a median DBI score of 0.52 during 2016,33 which is more comparable to our result. The New Zealand study reported an annual prevalence of any anticholinergic/sedative drug exposure of 43.22% (95% CI: 43.09–43.35),30 while the Irish study reported a prevalence of 66%,33 again more similar to our findings. Country-specific and temporal differences such as differences in drug availability, prescribing practices, and, in the case of the DBI score, dosing recommendations from regulatory authorities may help explain why our study results differ more from those of the New Zealand study. Interestingly, as in the Irish study, we found that the most commonly dispensed drug was an opioid (codeine and hydrocodone, respectively), and tramadol and alprazolam were also frequently dispensed. To calculate the DBI, both prior studies multiplied the TDB by the days dispensed and then divided by 365. In our study, however, we considered distinct periods of continuous use by dose and adjusted the start and end date for each daily dose accordingly to account for potential forward stockpiling. Another key difference is that we examined patient-level factors (assessed with a 12-month lookback window) associated with high (>2) DBI drug exposure, whereas the Irish study considered factors (measured during the study period) associated with any exposure (vs none). However, like our study, the Irish study also reported positive adjusted associations between DBI drug exposure and each of female sex, younger age, and high medication count.
The finding of an association with younger age is surprising since we thought that younger beneficiaries would be healthier on average and have lower DBI drug exposure. Given that higher frailty probability was also a predictor of high DBI exposure in our study, perhaps frailty probability is more indicative of underlying health status than a chronological measure such as age. Additionally, all of the reported associations are independent (ie, multivariable); therefore, some of the effect of age is accounted for by adjustment for frailty and comorbidity, which are strongly correlated with age. However, the association with a psychosis diagnosis was expected since antipsychotics can have both DBI properties. Other studies also identified comorbidities which were not examined (eg, Parkinson’s disease49) as predictors of high anticholinergic exposure.
Our study has several limitations. Prescription drug claims are considered a high quality measure of drug exposure since they are audited and undergo multiple validity checks to ensure their accuracy,50 however, Part D claims only capture outpatient prescription medication dispensed; over-the-counter medications and those administered in a hospital setting are not included. Because Part D claims arise from financial transactions, they do not confirm that a patient took a medication.51 Nonetheless, claims are one step closer to the patient actually taking a drug (as compared to prescribing data). Claims do not indicate whether medications should be used regularly or on an as-needed basis. If intended for as-needed use, patients may stretch their medication supply over a longer period of time, which can result in measurement error in prevalence estimates. Additionally, prescription information can be lost when patients have additional drug coverage.50 Finally, we could not access Medicare Part C (Medicare Advantage) data, and so our results may not be generalizable to those beneficiaries. However, as of 2016, the majority (69%) of Medicare beneficiaries were not enrolled in Part C.52
Our study has several important strengths. This was the first US-based claims implementation of the DBI, a measure that quantifies cumulative drug burden of medications with anticholinergic and sedating properties. Our analysis population was comprised of a large, nationwide random sample of Medicare beneficiaries. Using the Part D claims, we could describe the raw daily DBI and the mean daily DBI (at month and year levels) score distributions. Additionally, this data source enabled us to provide detailed temporal descriptions of both the daily DBI threshold distribution and the prevalence of anticholinergic/sedative drug dispensation. Recent studies of national trends in anticholinergic and sedative-hypnotic use among US-based older adults were conducted among patients seen in psychiatric and primary care settings using survey data that only captures up to 10 prescribed medications per patient.3,4 Additionally, since primary non-adherence (patients not filling new prescriptions) is common, a measure of medication dispensing (such as that offered by prescription claims) likely better reflects actual medication use.
5 |. CONCLUSIONS
Our study offers several key insights for both providers and researchers. For US-based geriatric healthcare providers, it highlights patient-level factors associated with high DBI drug exposure that may guide discussions regarding medication appropriateness with patients. It also describes temporal dispensing of medications with properties known to be harmful.17 For researchers, it facilitates future DBI operationalization in Medicare claims, by supplying an updated US-based DBI drug list with estimated minimum daily doses, and demonstrates practical considerations that arise (eg, handling combination drugs, and assigning drug strength) and potential solutions. Studies examining the association between Medicare claims-based DBI exposure measures and important adverse events are needed to further validate this tool’s ability to identify US-based older adults at high risk.
Supplementary Material
KEY POINTS.
This study highlights patient-level factors (eg, high number of drug claims, white race, younger age, frailty, and a psychosis diagnosis code) associated with high DBI drug exposure.
No substantial changes in the prevalence of any anticholinergic and/or sedative drug exposure were observed over the study period (2013–2016), however, there were changes in the types of anticholinergic and/or sedative drugs dispensed.
This study provides a framework to facilitate Medicare claims-based studies of the DBI and identifies predictors of high DBI drug exposure that can help inform discussions between older adults and their healthcare providers regarding medication appropriateness.
ACKNOWLEDGEMENTS
We would like to thank Magdalene M. Assimon, Christine D. Hsu, and Michael Webster-Clark for their pharmacological expertise. This work was supported by the following sources. The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL200811.0010); the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical and Translational Science Award (UL1TR002489); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine. SS received tuition and stipend support from the UNC Graduate School Dissertation Completion Fellowship during the conduct of the submitted work. She is currently supported by the PhRMA Foundation Postdoctoral Fellowship in Health Outcomes. DG is funded by the Australian National Health and Medical Research Council (NHMRC) Dementia Leadership Fellow.
CONFLICT OF INTEREST
SS is a consultant for CERobs Consulting, LLC on projects unrelated to the submitted work. TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG056479) from the National Institute on Aging (NIA), and as Co-Investigator (R01 HL118255, R01MD011680), National Institutes of Health (NIH). He also receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Takeda, AbbVie, Boehringer Ingelheim), from pharmaceutical companies (Novo Nordisk), and from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill. TS does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. JLL receives research support from the Center for Pharmacoepidemiology (members: GlaxoSmithKline, UCB BioSciences Inc, AbbVie, Boehringer Ingelheim, Takeda and Merck [past member]). JLL also receives salary support from AbbVie, Inc. for an unrelated research study. JLL’s spouse is a full-time, paid employee of and owns stock in GlaxoSmithKline. No potential conflicts of interest were disclosed by the other authors.
Funding information
Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults, Grant/Award Number: GIL200811.0010; The CER Strategic Initiative of UNC’s Clinical and Translational Science Award, Grant/Award Number: UL1TR002489; The UNC Graduate School Dissertation Completion Fellowship; The PhRMA Foundation Postdoctoral Fellowship in Health Outcomes; The Australian National Health and Medical Research Council (NHMRC) Dementia Leadership Fellowship
Footnotes
This work was presented as a poster at International Conference on Pharmacoepidemiology (ICPE) All Access on September 16–17, 2020, and was accepted for future poster presentation at the Society of Epidemiologic Research (SER) virtual conference to be held on December 15–18, 2020.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Davies EA, O’Mahony MS. Adverse drug reactions in special populations - the elderly. Br J Clin Pharmacol. 2015;80(4):796–807. 10.1111/bcp.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–2874. 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 3.Maust DT, Blow FC, Wiechers IR, Kales HC, Marcus SC. National Trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363–e371. 10.4088/JCP.16m10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee TG, Choi YC, Ouellet GM, Ross JS. National prescribing trends for high-risk anticholinergic medications in older adults. J Am Geriatr Soc. 2018;66(7):1382–1387. 10.1111/jgs.15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niznik J, Zhao X, Jiang T, et al. Anticholinergic prescribing in Medicare part D beneficiaries residing in nursing homes: results from a retrospective cross-sectional analysis of Medicare data. Drugs Aging. 2017;34(12):925–939. 10.1007/s40266-017-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084–1093. 10.1001/jamainternmed.2019.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(06):869–870. 10.4088/JCP.11ac07093. [DOI] [PubMed] [Google Scholar]
- 9.Doraiswamy PM, Husain MM. Anticholinergic drugs and elderly people: a no brainer? Lancet Neurol. 2006;5(5):379–380. 10.1016/S1474-4422(06)70421-5. [DOI] [PubMed] [Google Scholar]
- 10.Wawruch M, Macugova A, Kostkova L, et al. The use of medications with anticholinergic properties and risk factors for their use in hospitalised elderly patients. Pharmacoepidemiol Drug Saf. 2012;21(2): 170–176. 10.1002/pds.2169. [DOI] [PubMed] [Google Scholar]
- 11.Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209–220. 10.1111/bcp.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowie MW, Slattum PW. Pharmacodynamics in older adults: a review. Am J Geriatr Pharmacother. 2007;5(3):263–303. 10.1016/j.amjopharm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Zia A, Kamaruzzaman S, Myint PK, Tan MP. Anticholinergic burden is associated with recurrent and injurious falls in older individuals. Maturitas. 2016;84:32–37. 10.1016/j.maturitas.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Taipale HT, Hartikainen S, Bell JS. A comparison of four methods to quantify the cumulative effect of taking multiple drugs with sedative properties. Am J Geriatr Pharmacother. 2010;8(5):460–471. 10.1016/j.amjopharm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 16.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639–658. 10.2165/11633250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.American Geriatrics Society AGS. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 18.Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63(1):85–90. 10.1111/jgs.13206. [DOI] [PubMed] [Google Scholar]
- 19.Trevor AJ. Sedative-hypnotic drugs. In: Katzung BG, ed. Basic and Clinical Pharmacology. New York, NY: McGraw-Hill Education; 2017:14e http://accessmedicine.mhmedical.com/content.aspx?aid=1148435875. [Google Scholar]
- 20.Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–787. 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson HA, Nishtala PS, Scrase R, et al. Drug burden and its association with falls among older adults in New Zealand: a National Population Cross-Sectional Study. Drugs Aging. 2018;35(1):73–81. 10.1007/s40266-017-0511-5. [DOI] [PubMed] [Google Scholar]
- 22.Dauphinot V, Faure R, Omrani S, et al. Exposure to anticholinergic and sedative drugs, risk of falls, and mortality. J Clin Psychopharmacol. 2014;34(5):565–570. 10.1097/JCP.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 23.Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc. 2011;59(5):875–880. 10.1111/j.1532-5415.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 24.Best O, Gnjidic D, Hilmer SN, Naganathan V, McLachlan AJ. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43(8):912–918. 10.1111/imj.12203. [DOI] [PubMed] [Google Scholar]
- 25.Mayer T, Meid AD, Saum K-U, et al. Comparison of nine instruments to calculate anticholinergic load in a large cohort of older outpatients: association with cognitive and functional decline, falls, and use of laxatives. Am J Geriatr Psychiatry. 2017;25(5):531–540. 10.1016/j.jagp.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Jean-Bart E, Moutet C, Dauphinot V, Krolak-Salmon P, Mouchoux C. Exposure to anticholinergic and sedative medicines as indicators of high-risk prescriptions in the elderly. Int J Clin Pharmacol. 2017;39: 1237–1247. 10.1007/s11096-017-0533-4. [DOI] [PubMed] [Google Scholar]
- 27.Phillips A, Strobl R, Grill E, Laux G. Anticholinergic and sedative medications and the risk of vertigo or dizziness in the German primary care setting-a matched case-control study from the CONTENT registry. Pharmacoepidemiol Drug Saf. 2018;27:912–920. 10.1002/pds.4575. [DOI] [PubMed] [Google Scholar]
- 28.Byrne CJ, Walsh C, Cahir C, Bennett K. Impact of drug burden index on adverse health outcomes in Irish community-dwelling older people: a cohort study. BMC Geriatr. 2019;19(1):121. 10.1186/s12877-019-1138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnjidic D, Hilmer SN, Hartikainen S, et al. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer’s disease: a National Population Cohort Study. PLoS One. 2014;9(1):e83224. 10.1371/journal.pone.0083224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–758. 10.1002/pds.3624. [DOI] [PubMed] [Google Scholar]
- 31.Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and mortality in older people in residential aged care facilities. Drugs Aging. 2012;29(2):157–165. 10.2165/11598570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Wouters H, van der Meer H, Taxis K. Quantification of anticholinergic and sedative drug load with the drug burden index: a review of outcomes and methodological quality of studies. Eur J Clin Pharmacol. 2017;73(3):257–266. 10.1007/s00228-016-2162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne CJ, Walsh C, Cahir C, Ryan C, Williams DJ, Bennett K. Anticholinergic and sedative drug burden in community-dwelling older people: a national database study. BMJ Open. 2018;8(7):e022500. 10.1136/bmjopen-2018-022500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Meer HG, Taxis K, Teichert M, Griens F, Pont LG, Wouters H. Anticholinergic and sedative medication use in older community-dwelling people: a national population study in The Netherlands. Pharmacoepidemiol Drug Saf. 2019;28(3):315–321. 10.1002/pds.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong MK, Xu H, Zhang L, Azocar F, Ettner SL. Effect of medicare part D benzodiazepine exclusion on psychotropic use in benzodiazepine users. J Am Geriatr Soc. 2012;60(7):1292–1297. 10.1111/j.1532-5415.2012.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilmer SN. Calculating and using the drug burden index score in research and practice. Expert Rev Clin Pharmacol. 2018;11:1053–1055. 10.1080/17512433.2018.1528145. [DOI] [PubMed] [Google Scholar]
- 37.Kouladjian L, Gnjidic D, Chen TF, Mangoni AA, Hilmer SN. Drug burden index in older adults: theoretical and practical issues. Clin Interv Aging. 2014;9:1503–1515. 10.2147/CIA.S66660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.By the 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 39.Campbell N, Maidment I, Fox C, Khan B, Boustani M. The 2012 update to the anticholinergic cognitive burden scale. J Am Geriatr Soc. 2013;61(S1):142–143. 10.1111/jgs.2013.61.issue-s1. [DOI] [Google Scholar]
- 40.Cuthbertson CC, Kucharska-Newton A, Faurot KR, et al. Controlling for frailty in pharmacoepidemiologic studies of older adults. Epidemiology. 2018;1:556–561. 10.1097/EDE.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lund JL, Sanoff HK, Peacock Hinton S, Muss HB, Pate V, Stürmer T. Potential medication-related problems in older breast, colon, and lung cancer patients in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(1):41–49. 10.1158/1055-9965.EPI-17-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun JW, Rogers JR, Her Q, et al. Adaptation and validation of the combined comorbidity score for ICD-10-CM. Med Care. 2017;55(12): 1046–1051. 10.1097/MLR.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 44.Spiegelman D Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 45.CMS. What is a Quality Measure. 2017. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/What-is-a-Quality-Measure-SubPage.html. Accessed October 11, 2018.
- 46.Goodman CW, Brett AS. Gabapentin and Pregabalin for pain — is increased prescribing a cause for concern? N Engl J Med. 2017;377(5): 411–414. 10.1056/NEJMp1704633. [DOI] [PubMed] [Google Scholar]
- 47.2014–Final Rule: Rescheduling of Hydrocodone Combination Products From Schedule III to Schedule II. https://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0822.htm. Accessed January 13, 2020.
- 48.Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med. 2009;122(12): 1142–1149. 10.1016/j.amjmed.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joung K, Shin JY, Cho S. Features of anticholinergic prescriptions and predictors of high use in the elderly: population-based study. Pharmacoepidemiol Drug Saf. 2019;28(12):1591–1600. 10.1002/pds.4902. [DOI] [PubMed] [Google Scholar]
- 50.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013;22(8):899–906. 10.1002/pds.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savitz ST, Stearns SC, Zhou L, et al. A comparison of self-reported medication adherence to concordance between part D claims and medication possession. Med Care. 2017;55(5):500–505. 10.1097/MLR.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An Overview of Medicare j The Henry J. Kaiser Family Foundation https://www.kff.org/medicare/issue-brief/an-overview-of-medicare/. Accessed November 19, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


