Abstract
Background
Data from phase 3 trials have demonstrated the efficacy and safety of benralizumab in patients with severe eosinophilic asthma (SEA). We conducted a real-world study examining the baseline characteristics of a large SEA population treated with benralizumab in clinical practice and assessed therapy effectiveness.
Methods
ANANKE is an Italian multi-center, retrospective cohort study including consecutive SEA patients who had started benralizumab therapy ≥ 3 months before enrolment (between December 2019 and July 2020), in a real-world setting. Data collection covered (1) key patient features at baseline, including blood eosinophil count (BEC), number and severity of exacerbations and oral corticosteroid (OCS) use; (2) clinical outcomes during benralizumab therapy. We also conducted two post-hoc analyses in patients grouped by body mass index and allergic status. Analyses were descriptive only.
Results
Of 218 patients with SEA enrolled in 21 Centers, 205 were evaluable (mean age, 55.8 ± 13.3 years, 61.5% females). At treatment start, the median BEC was 580 cells/mm3 (interquartile range [IQR]: 400–850); all patients were on high-dose inhaled controller therapy and 25.9% were on chronic OCS (median dose: 10 mg/die prednisone-equivalent [IQR: 5–25]); 92.9% experienced ≥ 1 exacerbation within the past 12 months (annualized exacerbation rate [AER] 4.03) and 40.3% reported ≥ 1 severe exacerbation (AER 1.10). During treatment (median duration: 9.8 months [IQR 6.1–13.9]; ≥ 12 months for 34.2% of patients), complete eosinophil depletion was observed; exacerbation-free patients increased to 81% and only 24.3% reported ≥ 1 severe event. AER decreased markedly to 0.27 for exacerbations of any severity (− 93.3%) and to 0.06 for severe exacerbations (− 94.5%). OCS therapy was interrupted in 43.2% of cases and the dose reduced by 56% (median: 4.4 mg/die prednisone-equivalent [IQR: 0.0–10.0]). Lung function and asthma control also improved. The effectiveness of benralizumab was independent of allergic status and body mass index.
Conclusions
We described the set of characteristics of a large cohort of patients with uncontrolled SEA receiving benralizumab in clinical practice, with a dramatic reduction in exacerbations and significant sparing of OCS. These findings support benralizumab as a key phenotype-specific therapeutic strategy that could help physicians in decision-making when prescribing biologics in patients with SEA.
Trial registration ClinicalTrials.gov Identifier: NCT04272463
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-01952-8.
Keywords: Benralizumab, Exacerbations, OCS, Real world, Severe eosinophilic asthma
Background
Severe asthma (SA) is defined by the inability to control symptoms despite treatment with high-dosage inhaled corticosteroids (ICS) plus a second controller and/or systemic corticosteroids [1]. SA affects up to 10% of the asthmatic population [1, 2] and, in approximately half [3], it presents with an eosinophilic phenotype (i.e., severe eosinophilic asthma, SEA) characterized by high blood and/or airway eosinophilia and persistent eosinophilic inflammation, recurrent exacerbations, lung function decline, and poor asthma control [4–6].
Albeit maintenance therapy with oral corticosteroids (OCS) has long been the mainstay to control symptoms and prevent exacerbations, their long-term use exposes patients to a significant dose-dependent risk of important sequelae, a higher risk of mortality and considerable costs [7–14].
The advent of monoclonal antibodies (mAbs) targeting the interleukin-5 (IL-5) pathway has provided a new phenotype-specific approach. Unlike the anti-IL-5 mAbs mepolizumab [15] and reslizumab [16], benralizumab [17] targets IL-5 receptor alpha (IL-5Rα), which is highly expressed by human eosinophils and eosinophil progenitors [6], and has the unique ability to induce rapid and complete depletion of blood and tissue eosinophils, and of other IL-5Rα + cells as well, via afucosylation-dependent Ab-dependent cell-mediated cytotoxicity (ADCC) [18, 19]. In the pivotal phase 3 trials, benralizumab as add-on maintenance treatment in patients with uncontrolled SEA decreased the annual exacerbation rate (AER) by up to 70%, improved lung function and asthma symptoms, and allowed a 75% reduction in the median dose of OCS (discontinuation rate: 52%), with a good safety and tolerability profile [20–22]. Notably, this benefit persisted for up to 5 years of continuous treatment [23–26].
Real-world evidence complements data from randomized controlled trials (RCTs) in a population fully representative of clinical practice [27]. However, the studies testing the effectiveness of benralizumab are small retrospective analyses and case reports [28–39], with the exception of two recent observational studies including 111 [39] and 130 patients [29], both reporting significant improvements in all outcome measures, including exacerbation rate and OCS consumption.
To expand the knowledge on the effectiveness of benralizumab in a large cohort of patients with SEA, we undertook the ANANKE study. Here, we describe the baseline characteristics of the patient population and the clinical outcomes observed after at least 3 months of treatment.
Methods
Study design
ANANKE (ClinicalTrials.gov Identifier: NCT04272463) is an Italian multi-center, observational retrospective cohort study including patients with SEA who had started benralizumab therapy as per clinical practice or within the Italian sampling program, activated after benralizumab approval in January 2018 and before reimbursement [17]. At the time of activation of the sampling program, clinical data on patients exposed to benralizumab were already available. This has permitted to collect data and meet the primary endpoint in a relatively short period of time after enrollment initiation.
Patients were enrolled consecutively during regular visits between December 2019 and July 2020 at 21 sites distributed across the country. The start date of benralizumab treatment was defined as the “index date”; the enrolment visit corresponded to the end of each patient’s retrospective observation window and occurred at least 3 months after the index date. Therefore, per protocol, data collection covered a period of > 15 months (i.e., 12 months before the index date) to retrieve a restricted set of clinical data plus at least 3 months between the index date and the enrolment visit (Additional file 1: Fig. S1).
ANANKE was performed in accordance with the principles of the Declaration of Helsinki and with the regulations and guidelines governing medical practice and ethics in Italy. Ethical approval was provided by the ethics committees/institutional review boards at each participating site (first approval: n 248/19Sept2019—Università Magna Graecia, Catanzaro, Italy).
Study population
Inclusion criteria were (1) age ≥ 18 years; (2) diagnosis of SEA requiring stable treatment with high doses of ICS and a long-acting β2-agonist (LABA) ± additional asthma controller; 3) start of benralizumab treatment at least 3 months before enrolment, either within the sampling program or as per routine practice, and at least one injection performed during this period (30 mg every 4 weeks for the first three doses, and then 30 mg every 8 weeks). Patients who temporarily or permanently interrupted benralizumab therapy before the enrolment visit may be included. Those who received benralizumab in a clinical experimental trial or participated in studies imposing a specific patient management strategy that did not correspond to the site’s normal clinical practice during the observation period were excluded.
All patients provided written informed consent before study entry.
Outcomes and variables
After each patient had signed the informed consent and privacy form, data were collected from each hospital’s medical charts according to clinical practice and were entered into the electronic case report form (eCRF) by the study investigators.
Primary endpoint
The primary endpoint was to describe the patients’ key features recorded at the index date. As already stated, data collection started 12 months before the index date and included the following information, in addition to demographics, life habits and physical examination:
Comorbidities such as rhinitis (allergic and non-allergic), gastroesophageal reflux, chronic obstructive pulmonary disease (COPD), sinusitis, chronic rhinosinusitis, nasal polyposis, atopic dermatitis, other eosinophilic conditions, conditions related to chronic OCS use (osteoporosis, cataract, etc.) and other conditions deemed as relevant by the treating physician at the index date;
Clinical assessments: total IgE and blood eosinophil count (BEC) at the index date;
Number and severity of asthma exacerbations, defined according to clinical judgement, that occurred in the 12 months before starting benralizumab. Severe exacerbations were defined as worsening of asthma that led to one of the following: i) use of systemic corticosteroids for ≥ 3 days or a temporary increase in a stable, background dosage of OCS; ii) an emergency department (ED) or urgent care visit (< 24 h) due to asthma that required systemic corticosteroids; or iii) admission to hospital (≥ 24 h) due to asthma. AER for any and severe exacerbations was calculated for these patients.
Previous asthma medications, including maintenance treatments at the index date and biologics during the 12 months before the index date;
Lung function assessments: forced expiratory volume in 1 s (FEV1), FEV1% of predicted, forced vital capacity (FVC), FEV1/FVC, fractional exhaled nitric oxide (FeNO)—both pre- and post-bronchodilator, if available, at the index date;
Patient-reported outcomes (PROs): asthma control test (ACT) questionnaire score [40, 41] and Asthma Quality of Life Questionnaire (AQLQ) score [42] at the index date;
Healthcare resource utilization, in terms of general practitioner (GP)/specialist visits, ED admissions, and hospitalizations in the 12 months before the index date.
Secondary endpoint
The secondary endpoint relied on the description of the outcomes recorded during benralizumab treatment between the index date and the enrolment visit; when available, data at 16, 24 and 48 weeks after the index date were retrieved:
Patients’ adherence to benralizumab therapy calculated as the ratio (as a percentage) between the number of actual injections received during the observation period and the number of expected injections, estimated based on the summary of product characteristics (SmPC), benralizumab discontinuation and the reasons leading to discontinuation and subsequent biologic treatments during the observation period;
BEC and IgE levels and changes over time with respect to the start of benralizumab treatment;
Exacerbations of any severity, severe exacerbations and their AER during the observation period;
ICS and OCS reduction during the observation period;
Lung function parameters and changes over time;
PROs and changes over time;
Healthcare resource utilization (i.e., GP/specialist visits, ED admissions and hospitalizations) during the observation period.
Post-hoc analyses
A post-hoc analysis of the SIROCCO and CALIMA studies showed that elevated BEC only, but not serum IgE levels, do increase the risk of exacerbations in patients with SEA [43]. We therefore conducted a post-hoc analysis to evaluate the performance of benralizumab according to the patients’ allergic status (i.e., positivity or not to the skin prick test for perennial allergens). Moreover, we performed a post-hoc analysis of obese vs overweight vs underweight/normal body mass index (BMI) patients. Indeed, a higher BMI is a risk factor for SA and, in particular, obesity is considered a chronic inflammatory disease with a negative effect on pulmonary ventilation and quality of life [44].
Outcome measures were OCS use, the median (interquartile range [IQR]) OCS dose reduction, and the AER of any/severe exacerbations before and after benralizumab treatment.
Statistical analyses
Sample size was defined based on feasibility considerations: indeed, according to the volume of patients managed by the sites involved, inclusion of 200 participants (10 patients/site on average) meeting the inclusion/exclusion criteria was deemed as reasonable during the planned 8-month enrolment period. An evaluation of the possible achievable precision of the estimates for the primary analysis was performed considering the primary objective of the study and the literature data, when available. Assuming that 20% of enrolled patients could be non-eligible and/or non-evaluable for the primary analysis, the total number of evaluable patients would have been 160. With a sample size of 160 patients, an observed frequency of comorbidities and asthma treatments > 21% would have had an acceptable precision (i.e., relative error < 30%).
As all objectives were descriptive in nature, analyses were descriptive only, and were carried out using the mean, standard deviation (SD), median, IQR, range, and absolute and relative frequencies. No statistical method was applied to check for normality of data distribution. The Median and IQR were preferred over mean (SD) in case of high variability in data distribution (i.e., high SD or high difference between mean and median). Per protocol, evaluable patients with missing data for certain variables were not excluded from the study and their data were not replaced, although they were not considered for the analyses including those variables. The frequency of missing data was given for all the variables analyzed. No formal hypotheses were prespecified. The analyses were performed using SAS software (SAS Institute, Cary, NC, USA).
Results
Patient disposition
Between December 2019 and July 2020, 218 patients were enrolled in 21 centers across all regions in the country: 211 were fully eligible whereas 7 were excluded because benralizumab was started < 3 months before enrolment (N = 2), no LABA treatments (either in the 12 months before starting benralizumab, or during the observation period; N = 1), or incomplete/inconsistent data entry in the eCRF for relevant fields (N = 4). Six more patients were excluded because of the lack of information regarding BEC at the start of benralizumab treatment. Thus, the complete evaluable set comprised 205 (94.0%) patients with SEA, which fulfils the required sample size.
Baseline characteristics
Patient demographics and clinical characteristics recorded at the index date or during the previous 12 months are presented in Tables 1 and 2.
Table 1.
Patient characteristics recorded before the start of benralizumab therapy (i.e., at the index date or during the 12 months prior to the index date)
| Characteristics | Evaluable population N = 205 |
|---|---|
| Age at the index date, yrs | 55.8 ± 13.3 |
| Female sex | 126 (61.5%) |
| BMI at the index date, kg/m2 (N = 182) | |
| Under/Normal weight | 70 (38.5%) |
| Overweight | 79 (43.4%) |
| Obese | 33 (18.1%) |
| Smoking status at the index date (N = 195) | |
| Non-smoker | 139 (71.3%) |
| Previous smoker | 50 (25.6%) |
| Current smoker | 6 (3.1%) |
| Age at asthma diagnosis, yrs (N = 203) | 38.9 ± 16.7 |
| Asthma duration at the index date, yrs (N = 203) | 12.4 (6.3–24.6) |
| SEA duration at the index date, yrs (N = 203) | 1.6 (1.0–3.1) |
| Atopy at the index date | 85 (41.5%) |
| Comorbidities at the index date | |
| ≥ 1 current asthma-related condition | 103 (50.2%) |
| Chronic rhinosinusitis | 50 (24.4%) |
| GERD | 43 (21%) |
| Allergic conjunctivitis | 28 (13.7%) |
| Allergic rhinitis | 45 (22%) |
| Other (atopic dermatitis, urticaria, etc.) | 17 (8.3%) |
| ≥ 1 current OCS-related condition | 77 (37.6%) |
| Hypertension | 46 (22.4%) |
| Osteoporosis | 23 (11.2%) |
| Cataract | 12 (5.9%) |
| Anxiety/Depression | 11 (5.3%) |
| Type 2 Diabetes Mellitus | 10 (4.9%) |
| Obstructive sleep apnoea | 10 (4.9%) |
| Cardiovascular disease | 7 (3.4%) |
| Other OCS-related ongoing comorbidities | 19 (9.3%) |
| ≥ 1 other ongoing comorbidities | 35 (17.1%) |
| Thyroid disorders | 8 (3.9%) |
| Bronchiectasis | 6 (2.9%) |
| Blood eosinophil count at the index date, cells/mm3 | 580 (400–850) |
| Total serum IgE at the index date, IU/mL (N = 123) | 289 (85–573) |
| Exacerbations during the 12 months prior to the index date (N = 196) | |
| ≥ 1, any severity | 182 (92.9%) |
| AER | 4.03 |
| ≥ 1 mild | 101 (51.5%) |
| ≥ 1 moderate | 121 (61.7%) |
| ≥ 1 severe | 79 (40.3%) |
| AER | 1.10 |
Data are N (%), mean ± SD, or median (IQR). Unless otherwise stated, the evaluable population included 205 patients
yrs years, BMI body mass index, SEA severe eosinophilic asthma, GERD gastroesophageal reflux disease, OCS oral corticosteroids, AER annual exacerbation rate
Table 2.
Data on prior asthma medication, lung function, patient-recorded outcomes and healthcare utilization recorded before the start of benralizumab therapy (i.e., at the index date or during the 12 months prior to the index date)
| Characteristics | Evaluable population N = 205 |
|---|---|
| Corticosteroid use at the index date | |
| ICS/LABA or ICS + LABA | |
| Any ICS dose | 205 (100%) |
| Low ICS dose | 3 (1.5%) |
| Low/Medium ICS dose | 16 (7.8%) |
| Medium ICS dose | 6 (2.9%) |
| High ICS dose | 179 (87.3%) |
| Data unknown | 1 (0.5%) |
| LAMA | 104 (50.7%) |
| Other* | 88 (42.9%) |
| OCS | 53 (25.9%) |
| OCS dosage among users, prednisone-equivalent, mg/die (N = 48) | 10 (5–25) |
| Biologic use during the 12 months prior to the index date | |
| Yes, any biologic | 58 (28.3%) |
| Omalizumab | 34 (16.6%) |
| Mepolizumab | 19 (9.3%) |
| Omalizumab > Mepolizumab | 5 (2.4%) |
| No | 147 (71.7%) |
| Lung function at the index date | |
| Pre-bronchodilator FEV1, L (N = 154) | 2.0 ± 0.8 |
| Post-bronchodilator FEV1, L (N = 92) | 2.1 ± 0.9 |
| Pre-bronchodilator FEV1, % predicted (N = 159) | 70.6 ± 21.6 |
| Post-bronchodilator FEV1, % predicted (N = 90) | 75.3 ± 22.9 |
| Pre-bronchodilator FVC, L (N = 148) | 3.0 ± 1.0 |
| FeNO, ppb (N = 66) | 46.7 ± 34.6 |
| ACT score at the index date (N = 161) | 14.7 ± 4.7 |
| AQLQ score at the index date (N = 32) | 3.7 ± 1.2 |
| Healthcare resource utilization for asthma per patient during the 12 months prior to the index date (N = 189) | |
| Primary care physician/GP office visits | 1.0 ± 2.0 |
| Specialist visits | 2.4 ± 3.0 |
| ED admissions | 0.2 ± 0.5 |
| Hospitalizations | 0.1 ± 0.4 |
Data are N (%), mean ± SD, or median (IQR). Unless otherwise stated, the evaluable population included 205 patients
ICS inhaled corticosteroid, LABA long-acting β2-agonist, LAMA long-acting muscarinic receptor antagonist, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, FeNO fractioned exhaled nitric oxide ACT, asthma control test, AQLQ asthma quality of life questionnaire, GP general practitioner, ED emergency department
Patients (mean age, 55.8 ± 13.3 years, 126 [61.5%] females) were mostly overweight/obese (112/182, 61.5%) and non-smokers (139/195, 71.3%); 101 (49.3%) subjects were positive for ≥ 1 seasonal/perennial allergen and 85 (41.5%) for ≥ 1 perennial allergens, and, in particular, 63 (30.7%) to house dust mite (D. Pteronyssinus), 20 (9.8%) to cat dander, 16 (7.8%) to dog dander, 16 (7.8%) to Aspergillus, and 5 (2.4%) to mould mix. The median duration of asthma and SEA at the index date was 12.4 years (IQR 6.3–24.6) and 1.6 (IQR 1.0–3.1), respectively.
Of 175 (85.4%) patients with relevant comorbidities, 110 (53.7%) had current or past positive history for nasal polyposis, 103 (50.2%) had current asthma-related conditions (chronic rhinosinusitis in 50 [24.4%], allergic rhinitis in 45 [22.0%], and gastroesophageal reflux in 43 [21.0%]), and 77 (37.6%) had OCS-related conditions (hypertension in 46 [22.4%] and osteoporosis in 23 [11.2%]).
Before the start of benralizumab treatment, the median BEC was 580 cells/mm3 (IQR 400–850) and the median level of IgE was 289 IU/mL (IQR 85–573). All patients were on maintenance treatment with ICS/LABA or ICS + LABA (the dose of ICS was high in most cases), and 53 (25.9%) reported chronic OCS use, with a median dose of 10 mg/die prednisone-equivalent (IQR: 5–25). Nonetheless, lung function was suboptimal and asthma control and quality of life were poor, and of 196 patients with available data, only 14 (7.1%) were exacerbation-free at the index date,1 whereas 182 (92.9%) had experienced ≥ 1 exacerbation of any severity with an AER of 4.03, and 79 (40.3%) had experienced ≥ 1 severe exacerbation with an AER of 1.10 (Table 1 and Fig. 1).
Fig. 1.
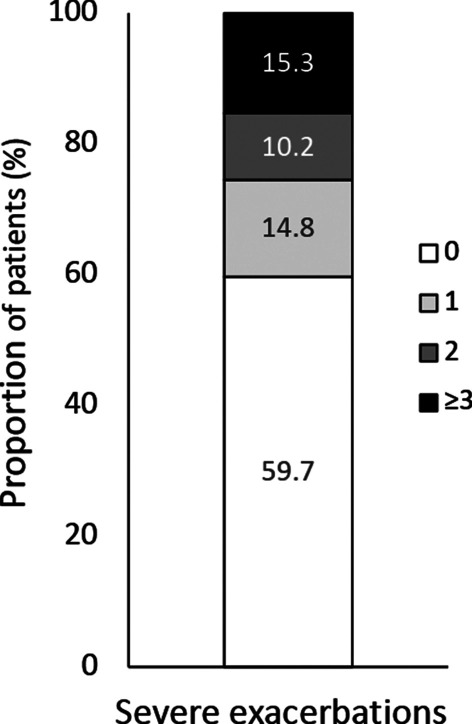
Distribution of patients according to the number of severe exacerbations experienced in the 12 months prior to the index date. Evaluable patients with information on previous exacerbations were 196
Last, in the 12 months prior to the index date, 53 (25.9%) patients had received one biologic (median time gap between previous biologic end and benralizumab start: 2.9 months [IQR 1.4–9.5]), which was omalizumab in most cases, and 5 (2.4%) had received two different biologics, omalizumab followed by mepolizumab; however, because of uncontrolled disease, these patients were switched to benralizumab. A post-hoc analysis will be provided on this subgroup of patients.
Parameters recorded during benralizumab treatment
Benralizumab exposure, adherence, and persistence on treatment
Among 202 evaluable patients, 198 (98.0%) were still on treatment with benralizumab at enrolment; the median (IQR) duration of exposure was 9.8 months (IQR 6.1–13.9); 69 (34.2%) patients received therapy for at least 12 months.
The median number of actual injections administered per patient was 7.0 (IQR 5.0–9.0); deviations from the SmPC were recorded for 24/190 (12.6%) evaluable patients; the median level of patients’ adherence was 100% (range 66.7–100%).
Only 4 patients discontinued therapy permanently (after a median of 5 months [IQR 1.4–11.9], due to patient decision (N = 2, 1.0%), lack of clinical efficacy (N = 1, 0.5%) or physician decision (N = 1, 0.5%). Because this study is retrospective, reasons for patient or physician decisions for discontinuation were not collected. Additional file 2: Fig. S2 shows persistence on benralizumab treatment.
After discontinuing benralizumab, 1 patient switched to another biologic (omalizumab) because of inadequate clinical response in terms of exacerbations, FEV1 deterioration and symptom recurrence.
Finally, of 194 patients evaluable with the dose of ICS available at the index date, 179 (92.3%) did not reduce the dose of ICS during treatment with benralizumab, while 15 (7.7%) did (any reduction of any extent).
Eosinophil count and IgE level
During benralizumab treatment, compared with the index date, the BEC fell to 0 (IQR 0.0–0.0) at week 16 and remained low thereafter (Fig. 2). As for the level of IgE, the limited number of patients with data available at the prespecified time points (N = 9 at 16 weeks; N = 12 at 24 weeks; N = 4 at 48 weeks; N = 1 at enrolment) did not allow the extent of variation over time to be evaluated with sufficient precision.
Fig. 2.
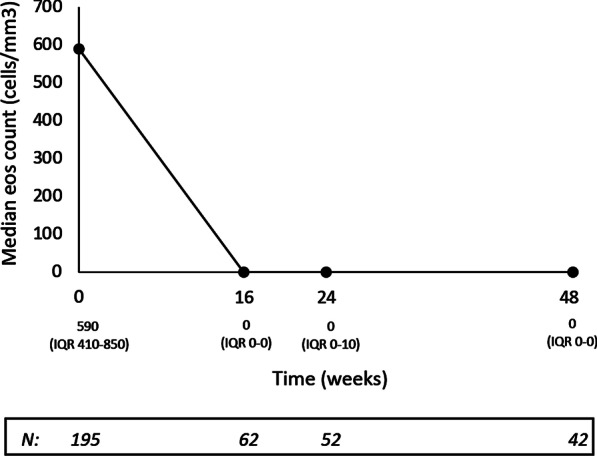
Median blood eosinophil count (cells/mm3) recorded at the index date (week 0) and during benralizumab treatment, up to week 48
Exacerbations
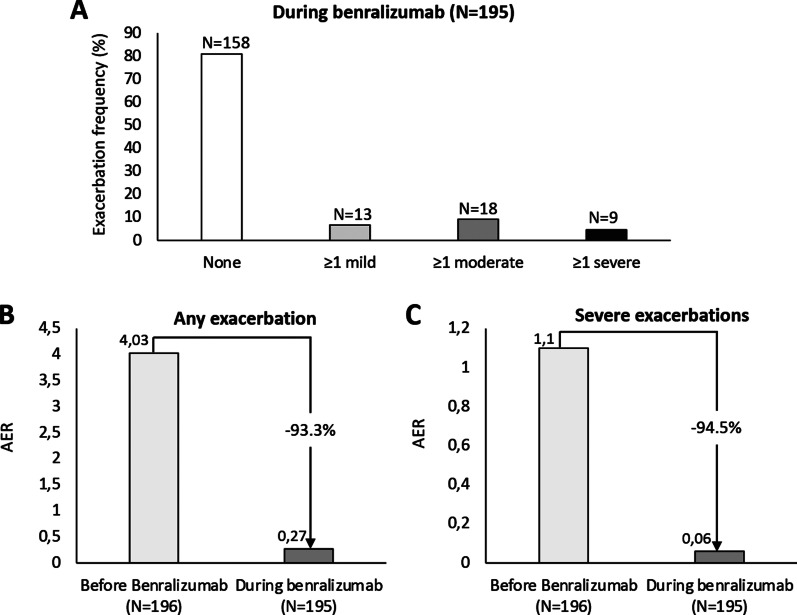
During benralizumab treatment, as much as 158/195 (81%) patients reported zero exacerbations; of the 37 who had at least 1 exacerbation, only 9 (24.3%) experienced a severe event (8 patients reported 1 severe exacerbation and 1 reported 2 severe exacerbations) (Fig. 3A): 2 occurred between the index date and week 2, 5 by week 16, 1 at week 24, and 2 at week 48. All severe exacerbations resolved and benralizumab therapy was continued thereafter.
Fig. 3.
Data on exacerbations during benralizumab treatment. A Exacerbation occurrence. B Any exacerbation annual exacerbation rate (AER) variations C Severe exacerbation AER variations. Week 0 corresponds to the index date
Moreover, compared with the index date, a marked decrease in AER was recorded for both any and severe exacerbations: from 4.03 to 0.27 for any, − 93.3%; from 1.1 to 0.06 for severe exacerbations, − 94.5% (Fig. 3B).
OCS use
Of 53 OCS users at baseline, 9 patients maintained their dose and 44 had data documenting dose variations during benralizumab treatment. Therapy with OCS was reduced in as much as 22 of 44 patients (50%) and interrupted in 19 (43.2%), with similar rates between patients who had started on a dose ≤ 5 mg/die vs > 5 mg/die prednisone-equivalent (Table 3). The overall dose reduction was of 56%, from a median OCS dose of 10.0 mg/die prednisone-equivalent (IQR 5.0–25.0) at benralizumab start to 4.4 mg/die prednisone-equivalent (IQR 0.0–10.0) during treatment (Fig. 4). Dose reduction was independent of eosinophil count at the index date, age at asthma onset, IgE level and number of exacerbations recorded in the previous 12 months (data not shown).
Table 3.
Changes in OCS use (dosage expressed as prednisone-equivalent) during treatment with benralizumab
| Variable | Evaluable N = 44 | Starting dose ≤ 5 mg/die (N = 14) | Starting dose > 5 mg/die (N = 30) |
|---|---|---|---|
| Any reduction | 22 (50%) | 8 (57.1%) | 14 (46.7%) |
| Reduction from baseline | |||
| 100% | 19 (43.2%) | 6 (42.9%) | 13 (43.3%) |
| ≥ 90% | 19 (43.2%) | 6 (42.9%) | 13 (43.3%) |
| ≥ 75% | 20 (45.5%) | 6 (42.9%) | 14 (46.7%) |
| ≥ 25% | 21 (47.7%) | 7 (50%) | 14 (46.7%) |
| No reduction | 22 (50%) | 6 (42.9%) | 16 (53.3%) |
Data are expressed as frequencies (N [%])
Fig. 4.
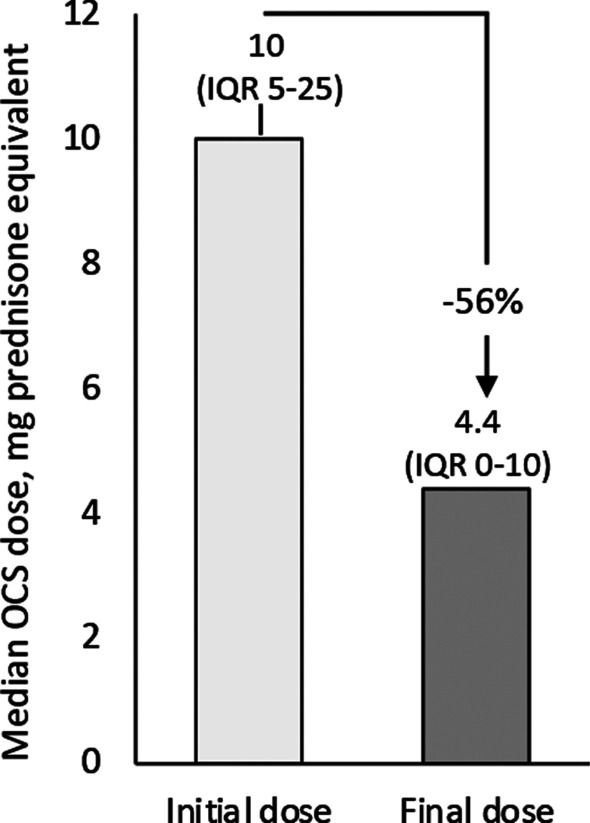
OCS dosage reduction during benralizumab treatment. Data are expressed as median in mg prednisone equivalent
Lung function and asthma control
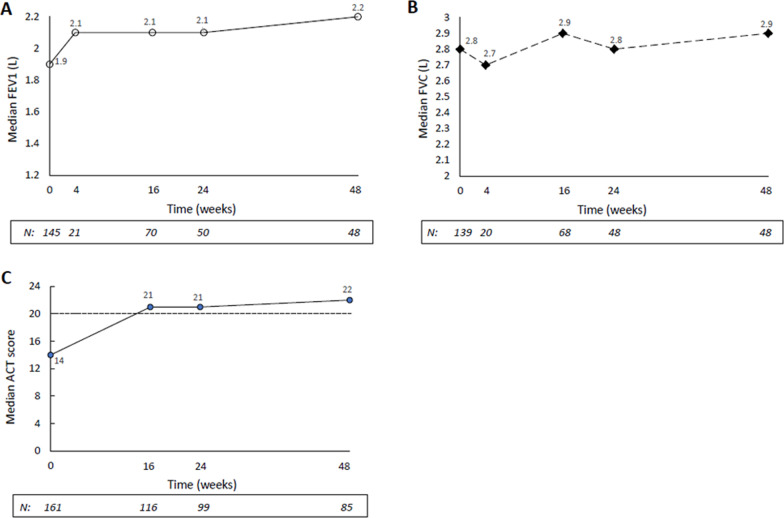
During treatment with benralizumab, we observed an improvement in lung function (at week 48 vs the index date: median FEV1, + 300 mL and median FVC, + 100 mL; Fig. 5A, B), and in asthma control (median ACT: from 14 at the index date to 22 at week 48; Fig. 5C). In particular, for patients with available data, the proportion of patients with well-controlled asthma (i.e., ACT score ≥ 20) increased over time from 16.8% (N = 27) at week 0 (index date) to 65.5% (N = 76) at week 16, 70.7% (N = 70) at week 24 and 81.2% (N = 69) at week 48, and, at every evaluation during benralizumab treatment, ≥ 75% of the patients with completed questionnaires achieved the minimum important difference from baseline. No evaluation could be made for the AQLQ score, due to incomplete questionnaires as filled out by patients in actual clinical practice.
Fig. 5.
Outcome measures recorded at the index date (week 0) and during benralizumab treatment, up to week 48. A FEV1 (L); B FVC (L); C ACT score
Health care resource utilization
During the observation period (mean: 10.3 ± 5 months), 190 (97.4%) patients had no ED admissions and 191 (97.9%) had no hospitalizations. Variations recorded during benralizumab therapy are reported in Table 4.
Table 4.
Variations of healthcare resource utilization recorded during benralizumab therapy
| Item | After benralizumab |
|---|---|
| Primary care physician/GP office visits for asthma per patient |
0.42 − 58% |
| Specialist visits for asthma per patient |
2.35 − 2.1% |
| ED admissions for asthma per patient |
0.025 − 87.5% |
| Hospitalizations for asthma per patient |
0.021 − 79% |
Data are expressed as mean and % of variation vs index date. Evaluable patients, N = 195
GP general practitioner, ED emergency department
Post-hoc analyses
Allergic vs non-allergic patients
Allergic patients and non-allergic were 85/205 (41.5%) and 120/205 (58.5%), respectively. The main characteristics recorded at the index date and in the 12 months before are reported in Additional file 5: Table S1. The total duration of benralizumab exposure was 10.3 ± 4.5 months for allergic patients and 10.3 ± 5.3 months for non-allergic patients.
Data on the use of OCS are summarized in Additional file 6: Table S2. Among the evaluable allergic patients (N = 14), the rate of OCS reduction during benralizumab treatment was (42.9%, N = 6), and the rateof discontinuation was 35.7% (N = 5), with a 56% OCS dosage reduction. In evaluable non-allergic patients (N = 30), the corresponding rates were 53.3% (N = 16) and 46.7% (N = 14), with a 77% OCS dosage reduction.
In both groups, a remarkable decline in the AER of any and of severe exacerbations was observed: for any exacerbation, by − 91.8% in allergic patients and − 94.3% in non-allergic patients; for severe exacerbations, by − 91.9% and − 97%, respectively (Additional file 3: Fig. S3).
Obese vs overweight vs underweight/normal BMI patients
The main features recorded at the index date and in the 12 months before are reported in Additional file 7: Table S3. The total duration of benralizumab exposure was 10.4 ± 4.8 months for obese patients, 10.1 ± 4.6 months for overweight patients and 10.2 ± 5.4 months for underweight/normal BMI patients.
During benralizumab treatment, the AER of any exacerbation and of severe exacerbations decreased considerably in all groups: for any exacerbation, by − 90.9% in obese patients, − 92.0% in overweight and − 96.0% in underweight/normal BMI patients; for severe exacerbations, by − 92.9%, − 91.1% and − 97.0%, respectively (Additional file 4: Fig. S4). Data on OCS use in these subgroups of patients were too small to be reported and analyzed.
Discussion
ANANKE describes the baseline characteristics of a large cohort of 205 patients with uncontrolled SEA receiving benralizumab in clinical practice. Moreover, it further supports the effectiveness of benralizumab in terms of exacerbation reduction, OCS sparing, lung function improvement and asthma control. To our knowledge, this is the largest cohort published so far.
Five biologics targeting the IL-5, IgE and IL-4/IL-13 pathways are currently available for the treatment of patients with SEA. No direct comparison exists and the differences in the trial populations hamper any indirect comparisons. According to the treatment algorithm provided in the 2020 European Academy of Allergy and Clinical Immunology biologicals guidelines, the decision relies on the combination of phenotypic traits, biomarkers and clinically relevant asthma-related end points [45]. A strong recommendation on the use of all biologicals has been issued with regard to the decrease in SA exacerbations, and on the use of benralizumab (if BEC > 150/μL) and mepolizumab for decreased use of withdrawal of OCS.
Recent analyses have identified BEC ≥ 300 cells/μL, the presence of nasal polyposis, adult-onset asthma, OCS use, compromised lung function, and a history of ≥ 3 exacerbations/year at baseline as predictors of enhanced response to benralizumab, particularly for reduction in exacerbations and improvement in lung function [43, 46–48]. The phenotype of the ANANKE population (median BEC: 589 [IQR 400–850]; nasal polyposis: 53.7%; mean age at asthma diagnosis: 38.9 ± 16.7 years; OCS use: 25.9%; FEV1: 70.6% ± 21.6% of predicted; ≥ 3 exacerbations/year: 15.3%) likely accounts for the observed effectiveness of benralizumab.
The unique mechanism of action of this mAb (i.e., anti-IL-5Rα and ADCC) explains the rapid (as early as 24 h after the start of treatment) [37] and complete depletion of eosinophils observed in treated patients with SEA. Eosinophils play a central role in asthma pathogenesis by inducing structural remodeling of small airways and airflow limitations; moreover, high eosinophil counts are important predictors of the risk of exacerbations [43]. In keeping with these data, compared with baseline, we observed complete eosinophil suppression, which was sustained throughout week 48, together with an impressive drop of > 93% in the AER of any and severe exacerbations, and a remarkable increase in the proportion of any exacerbation-free patients, from 7.1 to 81%. Importantly, severe exacerbations decreased from 79 to 9 events, and those reported during treatment resolved without interruption of therapy. In the observational study by Pelaia et al. [39], a significant reduction in the rate of exacerbations was observed after 24 weeks of treatment, from 4 (3–6) to 0 (0–0). Moreover, Kavanagh et al. documented a 72.8% reduction in AER (from 4.92 ± 3.35 to 1.34 ± 1.71, P < 0.001) after 48 weeks of benralizumab therapy [29].
Albeit the use of OCS remains a matter of concern, a considerable proportion of patients with SA still rely on OCS therapy, often at high doses [49, 50]. In the present study, 25% of SEA patients used OCS regularly (and 37.6% had OCS-related conditions at baseline), a proportion lower than that reported by the Severe Asthma Network in Italy (SANI, N = 437) registry (62% to 64.1%) [7, 8] and by other real-world experiences [29, 32, 50] but similar to the recently published Italian registry on Severe Asthma (IRSA) [38, 51]. These discrepancies may depend on to the different definitions of OCS usage, e.g., OCS burst vs maintenance use vs chronic use. Regardless, in ANANKE, as much as 43.2% of patients discontinued OCS therapy and 50% reduced the dosage, with an overall dose reduction from baseline of 56%. These findings further support the OCS-sparing effect of benralizumab observed even in real-world settings [21, 25, 29, 39]. Indeed, Pelaia et al. [39] demonstrated a significant reduction in the dose, from 5 mg (IQR 0–12.5) to 0 mg (IQR 0–0), and in the proportion of patients on OCS, from 72 to 20%; Kavanagh et al. [29] documented a 100% reduction in the median dose of OCS, with a discontinuation rate of 51.4%.
During benralizumab treatment, we observed amelioration of lung function and asthma control, in line with previous studies. In ANANKE, the median FEV1 increased already at week 4, reaching a + 300 mL change at week 48. Pelaia et al. [39] observed a progressive, significant increase in FEV1 from month 1 (+ 390 mL) to month 6 (+ 530 mL), and Kavanagh et al. [29] reported a change in FEV1 of + 140 mL at 48 weeks.
All patients received benralizumab for at least 3 months and 34.2% for at least 12 months; treatment adherence was very high (likely favored by the ease of administration), with only 2% of patients permanently discontinuing therapy; this is important to ensure the rapid and sustained effects of the drug [29, 32, 36, 46].
Finally, the post-hoc analyses testing the performance of benralizumab according to the patients’ allergic status (allergic vs non-allergic) and BMI (obese vs overweight vs underweight/normal), demonstrated a benefit on both exacerbations (> 91% reduction in AER of any and severe exacerbations) and OCS use regardless of the presence of allergy, in line with previous reports [28, 32, 39, 52]. As for BMI, at the end of observation, a consistent AER reduction of > 90% was observed for both any and severe exacerbations, in all subgroups analyzed. These trends deserve further investigations in larger cohorts.
The main limitations of this study are linked to the retrospective, real-world study design. However, in our opinion, this also represents a strength, because the large sample size, the high number of centers involved throughout Italy and the comprehensive set of data collected make these results generalizable to a population of patients with SEA. We acknowledge that no sensitivity analyses have been performed to help identifying selection and center bias for the results. However, we think that the risk of inhomogeneity is minimized by the fact that benralizumab was prescribed according to the therapeutic plan issued by the Italian Medicines Agency in all the centers involved in this study.
Real-world evidence (RWE) studies are of increasing importance to both regulatory agencies and reimbursement authorities [53]. For these reasons too, our extensive real-life study can provide important and solid information not only for clinicians but also for decision-makers.
Conclusion
In clinical practice, defining the patient profile is important to maximize treatment efficacy. Here, we describe in a large cohort of patients with uncontrolled SEA the set of characteristics identifying the patients treated with benralizumab in clinical practice and confirm that these patients attain a remarkable response in terms of exacerbations, OCS use, lung function and asthma control. Altogether, these findings contribute to make benralizumab a key phenotype-specific strategy and should help physicians in decision-making.
ANANKE will end in 2022 and, as part of a large program in other European countries, its results will contribute to a pan-European and Canadian 2-year evaluation of benralizumab treatment in clinical practice.
Supplementary Information
Additional file 1: Fig. S1. Study design.
Additional file 2: Fig. S2. Persistence on benralizumab treatment: Kaplan-Meier survival analysis (eligible patientswith consistent data). Time from index date to treatment discontinuation (weeks) is the time elapsed between index date and date of benralizumab discontinuation (in case of patients permanently discontinuing treatment) or date of enrolment visit (in case of patients not permanently discontinuing treatment). The event is defined as the permanent discontinuation of the treatment. The patients who didn’t discontinue treatment during observation period were censored at date of enrolment visit.
Additional file 3: Fig. S3. Variations in the annualized exacerbation rate (AER) of (A) any exacerbation and (B) of severe exacerbations during benralizumab treatment, in allergic vs non-allergic patients.
Additional file 4: Fig. S4. Variations in the annualized exacerbation rate (AER) of (A) any exacerbation and of (B) severe exacerbations during benralizumab treatment, in obese vs overweight vs underweight/normal BMI patients.
Additional file 5: Table S1. Patient characteristics recorded before the start of benralizumab therapy. Data are N (%), mean±SD, or median (IQR). Unless otherwise specified, the evaluable populations included 85 allergic and 120 non-allergic patients.
Additional file 6: Table S2. Evaluable patients with data on OCS use at the index date and at enrolment are 14 for allergic and 30 for non-allergic subjects. OCS dose is indicated as a median (IQR).
Additional file 7: Table S3. Patient characteristics recorded before the start of benralizumab therapy. Data are N (%), mean±SD, or median (IQR). Unless otherwise specified, the evaluable populations included 33 obese, 79 overweight and 70 underweight/normal BMI patients.
Acknowledgements
We thank all the patients and physicians who participated in this study This study was sponsored by AstraZeneca. We are grateful to Fabio Ferri, Claudio Marchese, Sara Rizzoli, Barbara Roncari, Alessandro Zullo and the entire MediNeos team for the support during the design, management and statistical analysis of the data. Medical writing support and editorial assistance was provided by Clara Ricci, PhD (EDRA SpA, Milan, Italy) and funded by AstraZeneca.
Abbreviations
- ACT
Asthma control test
- ADCC
Afucosylation-dependent Ab-dependent cell-mediated cytotoxicity
- AER
Annual exacerbation rate
- AQLQ
Asthma Quality of Life Questionnaire
- BEC
Blood eosinophil count
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- eCRF
Electronic case report form
- FDA
Food and Drug Administration
- FeNO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- GP
General practitioner
- ICS
Inhaled corticosteroids
- IL-5
Interleukin-5
- IL-5Rα
IL-5 receptor alpha
- IQR
Interquartile range
- IRSA
Italian registry on Severe Asthma
- LABA
Long-acting β2-agonist
- mAbs
Monoclonal antibodies
- OCS
Oral corticosteroids
- PROs
Patient reported outcomes
- RCTs
Randomized controlled trials
- RWE
Real-world evidence
- SA
Severe asthma
- SANI
Severe Asthma Network in Italy
- SD
Standard deviation
- SEA
Severe eosinophilic asthma
Authors’ contributions
FM: Investigation, Resources, Writing—review & editing, EB: Investigation, Resources, Writing—review & editing. MA: Investigation, Resources, Writing—review & editing. PB: Investigation, Resources, Writing—review & editing. LB: Investigation, Resources, Writing—review & editing. MFC: Investigation, Resources, Writing—review & editing. CC: Investigation, Resources, Writing—review & editing. SC: Investigation, Resources, Writing—review & editing. MDA: Investigation, Resources, Writing—review & editing. SDG: Investigation, Resources, Writing—review & editing. FDM: Investigation, Resources, Writing—review & editing. FDM: Investigation, Resources, Writing—review & editing. EAP: Investigation, Resources, Writing—review & editing. GP: Investigation, Resources, Writing—review & editing. PR: Investigation, Resources, Writing—review & editing. MR: Investigation, Resources, Writing—review & editing. PS: Investigation, Resources, Writing—review & editing. GS: Investigation, Resources, Writing—review & editing. AV: Investigation, Resources, Writing—review & editing. AZ: Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing—review & editing. SB: Conceptualization, Writing—original draft, Writing—review & editing, Project administration. GV: Conceptualization, Writing—original draft, Writing—review & editing, Visualization. GWC: Investigation, Resources, Writing—review & editing. All authors read and approved the final manuscript.
Funding
Financial support for the preparation of the article was provided by AstraZeneca SpA Italy, which had a role in the study design and in the collection and analysis of data; it had no role in the interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles laid down in the Declaration of Helsinki and to the regulations and guidelines governing medical practice and ethics in Italy. All patients provided informed consent before being enrolled in the study. Ethical approval was provided by the ethics committees/institutional review boards at each participating site.
Consent for publication
Not applicable.
Competing interests
FM declares research fundings as Principal investigator by AstraZeneca, Chiesi Farmaceutici, Novartis, Sanofi; fees as speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Novartis, Sanofi; EB has nothing to declare; MA has nothing to declare; PB has nothing to declare; LB has nothing to declare; MFC has nothing to declare; CC has nothing to declare; SC declares grants and/or personal fees from AstraZeneca, Boheringer Ingelheim, Chiesi, Glaxo Smith Kline, Guidotti, Menarini, Novartis, Valeas; MDA has nothing to declare; SDG received grants and/or personal fees from AstraZeneca, Chiesi, Glaxo Smith Kline, Menarini, Novartis; FDM has nothing to declare; FDM has received lectures fees at national and international meetings and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompe, Guidotti/Malesci, GlaxoSmithKline, Menarini, Novartis, and Zambon; EAP has nothing to declare; GP has received lecture fees and consultancy fees from Alfasigma, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis, Sanofi, Zambon; PR has participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, her department has received funding from Almirall, Boehringer Ingelheim, Chiesi, Novartis, and Zambon; MR declares grants and personal fees from Boehringer Ingelheim, Roche, AstraZeneca, Novartis, Chiesi, GSK, Menarini, Guidotti, AlfaSigma, Zambon; PS has nothing to declare; GS has nothing to declare; AV received payment for lectures and consultant arrangements from Novartis, GlaxoSmithKline, Teva, AstraZeneca; LS and AO are a employees of MediNeos Observational Research; SB and GV are AstraZeneca employees; EA has nothing to declare; GWC has received grant/research support from Boehringer Ingelheim, ALK, and Stallergenes, and honoraria or consultation fees from Menarini, GSK, Sanofi, Teva, Hal, AstraZeneca, and Novartis.
Footnotes
Benralizumab is reimbursed by the Italian healthcare system in adult patients with refractory SEA in the following situations: i) if they had ≥ 300 BEC in the absence of systemic steroid treatment; ii) if, despite maximum ICS therapy, they experienced in the previous 12 months ≥ 2 exacerbations treated with systemic steroids or requiring hospitalization OR if, in the previous 12 months, they received chronic systemic steroids, on top of the maximum ICS therapy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.Hekking P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Maio S, Baldacci S, Bresciani M, Simoni M, Latorre M, Murgia N, et al. RItA: the Italian severe/uncontrolled asthma registry. Allergy. 2018;73:683–695. doi: 10.1111/all.13342. [DOI] [PubMed] [Google Scholar]
- 4.de Groot JC, Ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1. [DOI] [PMC free article] [PubMed]
- 5.Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8:1375. doi: 10.3390/jcm8091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matucci A, Maggi E, Vultaggio A. Eosinophils, the IL-5/IL-5Rα axis, and the biologic effects of benralizumab in severe asthma. Respir Med. 2019;160:105819. doi: 10.1016/j.rmed.2019.105819. [DOI] [PubMed] [Google Scholar]
- 7.Heffler E, Blasi F, Latorre M, Menzella F, Paggiaro P, Pelaia G, et al. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019;7:1462–1468. doi: 10.1016/j.jaip.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Canonica GW, Colombo GL, Bruno GM, Di Matteo S, Martinotti C, Blasi F, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12:100007. doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefebvre P, Duh MS, Lafeuille M-H, Gozalo L, Desai U, Robitaille M-N, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1488–1495. doi: 10.1016/j.jaci.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110–116.e7. doi: 10.1016/j.jaci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourdin A, Molinari N, Vachier I, Pahus L, Suehs C, Chanez P. Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J. 2017;50:1701486. doi: 10.1183/13993003.01486-2017. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Ryu J, Nam E, Chung SJ, Yeo Y, Park DW, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54:1900804. doi: 10.1183/13993003.00804-2019. [DOI] [PubMed] [Google Scholar]
- 15.nucala-epar-product-information_it.pdf [Internet]. [cited 2021 May 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_it.pdf.
- 16.cinqaero-epar-product-information_en.pdf [Internet]. [cited 2021 May 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/cinqaero-epar-product-information_en.pdf.
- 17.fasenra-epar-product-information_en.pdf [Internet]. [cited 2019 Dec 6]. Available from: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf.
- 18.Caminati M, Bagnasco D, Vaia R, Senna G. New horizons for the treatment of severe, eosinophilic asthma: benralizumab, a novel precision biologic. Biologics. 2019;13:89–95. doi: 10.2147/BTT.S157183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelaia C, Calabrese C, Vatrella A, Busceti MT, Garofalo E, Lombardo N, et al. Benralizumab: from the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. Biomed Res Int. 2018;2018:4839230. doi: 10.1155/2018/4839230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 21.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 22.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 23.Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7:46–59. doi: 10.1016/S2213-2600(18)30406-5. [DOI] [PubMed] [Google Scholar]
- 24.FitzGerald JM, Bleecker ER, Bourdin A, Busse WW, Ferguson GT, Brooks L, et al. Two-year integrated efficacy and safety analysis of benralizumab in severe asthma. J Asthma Allergy. 2019;12:401–413. doi: 10.2147/JAA.S227170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourdin A, Shaw D, Menzies-Gow A, FitzGerald JM, Bleecker ER, Busse WW, et al. Two-year integrated steroid-sparing analysis and safety of benralizumab for severe asthma. J Asthma. 2019;1–9. [DOI] [PubMed]
- 26.Bourdin A, Korn S, Chupp G l., Cosio B, Arbetter D, Shah M, et al. Integrated safety and efficacy among patients with severe asthma receiving benralizumab for up to five years. D7 D007 Advances in Asthma Therapies [Internet]. 2021; A1205–A1205. 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A1205.
- 27.Brown T, Jones T, Gove K, Barber C, Elliott S, Chauhan A, et al. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52:1801444. doi: 10.1183/13993003.01444-2018. [DOI] [PubMed] [Google Scholar]
- 28.Jackson D, Roxas C, Thompson L, Fernandes M, Green L, Kavanagh J, et al. Real-world oral glucocorticoid-sparing effect of benralizumab in severe asthma. Eur Respir J [Internet]. 2019 [cited 2019 Dec 6];54. Available from: https://erj.ersjournals.com/content/54/suppl_63/PA2527.
- 29.Kavanagh JE, Hearn AP, Dhariwal J, d’Ancona G, Douiri A, Roxas C, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2020; [DOI] [PubMed]
- 30.Pelaia C, Busceti MT, Vatrella A, Ciriolo M, Garofalo E, Crimi C, et al. Effects of the first three doses of benralizumab on symptom control, lung function, blood eosinophils, oral corticosteroid intake, and nasal polyps in a patient with severe allergic asthma. SAGE Open Med Case Rep. 2020;8:2050313X20906963. doi: 10.1177/2050313X20906963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelaia C, Busceti MT, Vatrella A, Rago GF, Crimi C, Terracciano R, et al. Real-life rapidity of benralizumab effects in patients with severe allergic eosinophilic asthma: assessment of blood eosinophils, symptom control, lung function and oral corticosteroid intake after the first drug dose. Pulm Pharmacol Ther. 2019;58:101830. doi: 10.1016/j.pupt.2019.101830. [DOI] [PubMed] [Google Scholar]
- 32.Pelaia C, Busceti MT, Crimi C, Carpagnano GE, Lombardo N, Terracciano R, et al. Real-Life effects of benralizumab on exacerbation number and lung hyperinflation in atopic patients with severe eosinophilic asthma. Biomed Pharmacother. 2020;129:110444. doi: 10.1016/j.biopha.2020.110444. [DOI] [PubMed] [Google Scholar]
- 33.Menzella F, Ruggiero P, Galeone C, Scelfo C, Bagnasco D, Facciolongo N. Significant improvement in lung function and asthma control after benralizumab treatment for seve. Pulm Pharmacol Ther. 2020;64:101966. doi: 10.1016/j.pupt.2020.101966. [DOI] [PubMed] [Google Scholar]
- 34.Di Bona D, Minenna E, Albanesi M, Nettis E, Caiaffa MF, Macchia L. Benralizumab improves patient reported outcomes and functional parameters in difficult-to-treat patients with severe asthma: data from a real-life cohort. Pulm Pharmacol Ther. 2020;64:101974. doi: 10.1016/j.pupt.2020.101974. [DOI] [PubMed] [Google Scholar]
- 35.Bagnasco D, Brussino L, Bonavia M, Calzolari E, Caminati M, Caruso C, et al. Efficacy of Benralizumab in severe asthma in real life and focus on nasal polyposis. Respir Med. 2020;171:106080. doi: 10.1016/j.rmed.2020.106080. [DOI] [PubMed] [Google Scholar]
- 36.Menzella F, Bonavia M, Bonini M, D’Amato M, Lombardo S, Murgia N, et al. Real-world experience with benralizumab in patients with severe eosinophilic asthma: a case series. J Asthma Allergy. 2021;14:149–161. doi: 10.2147/JAA.S295676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padilla-Galo A, Levy-Abitbol RC, Olveira C, Valencia Azcona B, Pérez Morales M, Rivas-Ruiz F, et al. Real-life experience with benralizumab during 6 months. BMC Pulm Med. 2020;20:184. doi: 10.1186/s12890-020-01220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renner A, Marth K, Patocka K, Idzko M, Pohl W. Benralizumab rapidly improves asthma control in Austrian real-life severe eosinophilic asthmatics. Allergy. 2020;75:3272–3275. doi: 10.1111/all.14441. [DOI] [PubMed] [Google Scholar]
- 39.Pelaia C, Crimi C, Benfante A, Caiaffa MF, Calabrese C, Carpagnano GE, et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: real-life evaluation correlated with allergic and non-allergic phenotype expression. J Asthma Allergy. 2021;14:163–173. doi: 10.2147/JAA.S297273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 43.Jackson DJ, Humbert M, Hirsch I, Newbold P, Garcia GE. Ability of serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Adv Ther. 2020;37:718–729. doi: 10.1007/s12325-019-01191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agache I, Akdis CA, Akdis M, Canonica GW, Casale T, Chivato T, et al. EAACI biologicals guidelines-recommendations for severe asthma. Allergy. 2021;76:14–44. doi: 10.1111/all.14425. [DOI] [PubMed] [Google Scholar]
- 46.Harrison TW, Chanez P, Menzella F, Canonica GW, Louis R, Cosio BG, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med. 2021;9:260–274. doi: 10.1016/S2213-2600(20)30414-8. [DOI] [PubMed] [Google Scholar]
- 47.Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52. [DOI] [PMC free article] [PubMed]
- 48.FitzGerald JM, Bleecker ER, Menzies-Gow A, Zangrilli JG, Hirsch I, Metcalfe P, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6:51–64. doi: 10.1016/S2213-2600(17)30344-2. [DOI] [PubMed] [Google Scholar]
- 49.van Bragt JJMH, Adcock IM, Bel EHD, Braunstahl G-J, Ten Brinke A, Busby J, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J. 2020;55. [DOI] [PubMed]
- 50.Vetrano DL, Zucchelli A, Bianchini E, Marconi E, Lombardo FP, Cricelli C, et al. Patterns of oral corticosteroids use in primary care patients with severe asthma. Respir Med. 2020;166:105946. doi: 10.1016/j.rmed.2020.105946. [DOI] [PubMed] [Google Scholar]
- 51.Bilò MB, Antonicelli L, Carone M, De Michele F, Menzella F, Musarra A, et al. Severe asthma management in the era of biologics: insights of the Italian Registry on Severe Asthma (IRSA) Eur Ann Allergy Clin Immunol. 2021;53:103–114. doi: 10.23822/EurAnnACI.1764-1489.196. [DOI] [PubMed] [Google Scholar]
- 52.Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120:504–511.e4. doi: 10.1016/j.anai.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 53.Commissioner O of the. Real-World Evidence [Internet]. FDA. FDA; 2021 [cited 2021 Jul 30]. Available from: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Study design.
Additional file 2: Fig. S2. Persistence on benralizumab treatment: Kaplan-Meier survival analysis (eligible patientswith consistent data). Time from index date to treatment discontinuation (weeks) is the time elapsed between index date and date of benralizumab discontinuation (in case of patients permanently discontinuing treatment) or date of enrolment visit (in case of patients not permanently discontinuing treatment). The event is defined as the permanent discontinuation of the treatment. The patients who didn’t discontinue treatment during observation period were censored at date of enrolment visit.
Additional file 3: Fig. S3. Variations in the annualized exacerbation rate (AER) of (A) any exacerbation and (B) of severe exacerbations during benralizumab treatment, in allergic vs non-allergic patients.
Additional file 4: Fig. S4. Variations in the annualized exacerbation rate (AER) of (A) any exacerbation and of (B) severe exacerbations during benralizumab treatment, in obese vs overweight vs underweight/normal BMI patients.
Additional file 5: Table S1. Patient characteristics recorded before the start of benralizumab therapy. Data are N (%), mean±SD, or median (IQR). Unless otherwise specified, the evaluable populations included 85 allergic and 120 non-allergic patients.
Additional file 6: Table S2. Evaluable patients with data on OCS use at the index date and at enrolment are 14 for allergic and 30 for non-allergic subjects. OCS dose is indicated as a median (IQR).
Additional file 7: Table S3. Patient characteristics recorded before the start of benralizumab therapy. Data are N (%), mean±SD, or median (IQR). Unless otherwise specified, the evaluable populations included 33 obese, 79 overweight and 70 underweight/normal BMI patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.