Abstract
Background
Present work was aimed to gather accessible evidence on the eradication rates and related postoperative complications of antibiotic-loaded calcium sulfate (CS) as an implant in the treatment of chronic osteomyelitis (COM).
Methods
Databases including PubMed, EMBASE, Medline, Ovid and Cochrane library were searched from their dates of initiation until November 2021. Two independent authors scrutinized the relevant studies based on the effectiveness of radical debridement combined with antibiotic-loaded CS for COM; data extraction and quality assessment of the Methodological Index for Non-Randomized Studies (MINORS) criteria were also performed by the authors. In addition, clinical efficacy mainly depended on the evaluation of eradication rates and complications, and all the extracted data are pooled and analyzed by STATA 16.0.
Results
A total of 16 studies with 917 patients (920 locations) were recruited, with an overall eradication rate of 92%. Moreover, the overall reoperation rate, overall refracture rate, overall delayed wound healing rate, and the rate of aseptic wound leakage were 9.0%, 2.0%, 20.0%, and 12.0%, respectively. Moreover, the choice of tobramycin-loaded CS or vancomycin combined with gentamicin-loaded CS did not affect the eradication rate, and the incidence of postoperative complications in COM patients (all ). The general quality of the included studies was fair.
Conclusions
Our meta-analysis indicated that the overall eradication rate of COM treated with antibiotic-loaded CS was 92%. Delayed healing is the most common postoperative complication. The choice of tobramycin-loaded CS or vancomycin combined with gentamicin-loaded CS did not affect the eradication rate and the incidence of postoperative complications in COM patients.
Keywords: Chronic osteomyelitis, Calcium sulfate, Tobramycin, Gentamicin, Vancomycin, Meta-analysis
Introduction
Chronic osteomyelitis (COM) is defined as a significant and long-standing infection of bone tissue, which lasting more than several months or even years and involved in any bone including periosteum, bone marrow and surrounding tissue. Most infections occur after trauma, surgery or secondary to vascular and neurologic insufficiency (e.g. diabetic foot ulcers), characterized by persistent bacteria, low-grade inflammation, and the prevalence of fistula and dead bone [1, 2]. With the improvement of diagnosis and population aging, COM incidence has apparently increased [3, 4]. In 2004, nearly 600,000 artificial joint replacements and 2 million internal fixation of fracture caused more than 110,000 infections finally in the USA [5]. According to related research estimation, the treatment of implant-related COM will cost $1.62 billion by 2020 in US hospitals, and impose a substantial financial burden on patients and society [6]. Furthermore, infected chronic diabetic foot ulcers may lead to diabetic foot osteomyelitis, which will increase the mortality and risk of amputation [7, 8]. The recurrent and persistent infection is a challenging condition for both the physician and the patient.
The mainstays of COM treatment are radical debridement, then supplemented with targeted antimicrobial therapy (local and/or systemic) for long time. A recent study suggested that the combination of antibiotics and surgery seems to be more effective than any single method [9]; despite these measures, infection recurred in 20% patients [10]. Due to the vascular injury in infected bone, it is difficult to achieve effective local antibiotic concentration by oral or intravenous antibiotic treatment under local ischemia; and the limited biofilm penetration makes the treatment of COM more difficult [1, 11, 12]. Therefore, the focus of antibiotic therapy is to use the local drug delivery system to avoid the potential toxicity caused by systemic administration, while providing a sustained high concentration at the infection site [13]. It is very important for the long-term treatment of osteomyelitis, especially for the relief of symptoms [14].
Calcium sulfate (CS) is a kind of biodegradable material with low immunoreactivity, easy reabsorption and good tolerance, which has been proved to be effective and safe as an antibiotic carrier in the past two decades [15–18]. CS is as effective as polymethyl methacrylate (PMMA) as an antibiotic carrier in bone infections [19]. However, Chang et al. [20] compared the efficacy of debridement plus CS combined with tobramycin and simple debridement, the results showed that the curative effect was not obvious, and the success rate of bone osteomyelitis was 80% and 60%, respectively. In terms of complications, several experiments indicated that CS products have transient cytotoxicity, leading to inflammation, within the first 60 days after implantation, CS causes inflammation in the surrounding tissue. After 60 days, the inflammation in the affected bone subsided, but the inflammation in the surrounding soft tissue did not subside, and the problem of surgical wound healing followed [21]. Due to the inconsistency on the properties of CS materials and surgical procedures, aseptic wound leakage and re-fracture have gradually got increasing attention, which may become the causes of reoperation or infection recurrence [22]. Jiang et al. [23] found that the incidence of aseptic wound leakage after antibiotic-loaded CS implantation treated COM was very high. Therefore, in this review, we comprehensively evaluate the efficacy and complications of CS loaded with multiple antibiotics in the treatment of COM (including delayed healing, reoperation, refracture and aseptic wound leakage). Another controversial issue is the efficacy of different antibiotic-loaded CS. The most common antibiotics used with CS are tobramycin and vancomycin, the former effectively reduces the chance of prosthetic infection and prevents biofilm formation and colonization of bacteria such as Methicillin-resistant Staphylococcus aureus [24]; the latter combined with gentamicin can cover a broad spectrum of both gram-positive and gram-negative bacteria [25]. At present, a variety of products have been sold in the market [26], and a number of clinical studies have been conducted; but it is still unclear whether there are differences in the efficacy and complications of different antibiotics in the treatment of COM.
The major purpose of this meta-analysis was to investigate the eradication rates and local complications of antibiotic-loaded CS in treating COM patients. Second, we explored difference of curative effect and related postoperative complications between two antibiotic regimens. To provide reference for orthopedic surgeons using antibiotic-loaded CS in the treatment of COM.
Material and methods
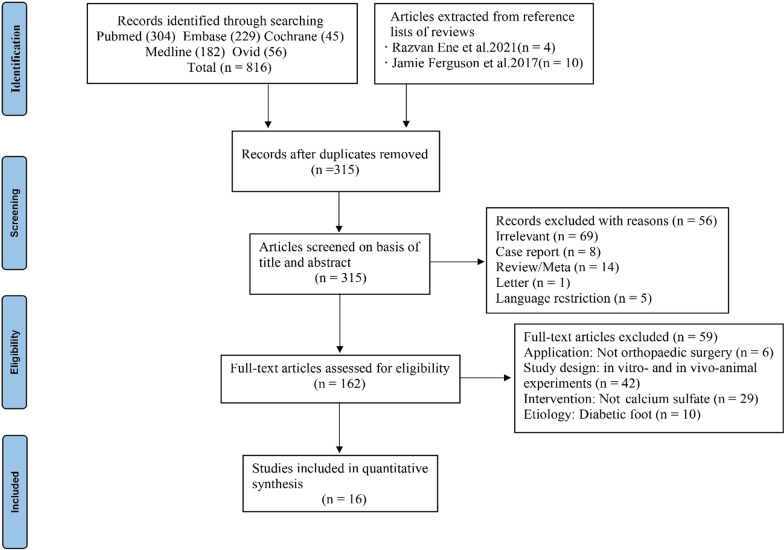
This study was presented in accordance with the PRISMA (Preferred Reporting Items for Meta-Analyses and Systematic Reviews) [27]. The PRISMA checklist is in the additional file, and the flow diagram of literature screening process is shown in Fig. 1. Ethical approval was not required.
Fig. 1.
PRISMA flow diagram of the study selection process
Search strategy
Online databases were used to identify eligible studies, we searched all articles in PubMed, EMBASE, Medline, Ovid and Cochrane Library up to November 2021. We also performed a supplementary search in the Ovid database to avoid missing articles. In addition, a manual search of references and similar documents of identified articles was also performed to determine potential relevance. Medical subject heading (MeSH) and Embase Tree tool (EMTREE) were used to guide the choice of appropriate search terms in all databases. We performed a search using the strategy (“osteomyelitis” OR “osteitis” OR “bone infection” OR “osteoarticular infection”) AND (“calcium sulfate” OR “calcium sulphate”). The last search was performed on November 15, 2021.
Eligibility criteria
The study selection criteria were established in accordance with the PICOS strategy (Patients, Intervention, Comparison, Outcomes, Study design): population—Patients with COM who underwent radical debridement and antibiotic-loaded CS; intervention—Operation with antibiotic-loaded calcium sulfate(including tobramycin, vancomycin and gentamicin); comparison—none; outcomes—Eradication rate and complications of failure, including reoperation, refracture, and delayed healing; study design—consecutive case–control studies or case series. Several eligibility criteria were applied in the present study. Only case series and consecutive case–control studies regarding the clinical application of antibiotic-loaded CS in the treatment of COM were eligible for inclusion, case reports, review articles, meta-analysis, animal researches, or unpublished studies were excluded. Articles written in a language other than English or German were excluded. Publication, studies involving patients with COM due to any causative mechanism except diabetic ulcer were eligible for inclusion. In order to be included, each eligible report had to contain at least one of the outcomes of interest. The main outcome of interest was eradication rates of infection, but studies that described postoperative local complications (wound-healing problems, aseptic wound leakage, etc.) were also eligible for inclusion. Patient selection was not restricted by age, gender, or other personal characteristics.
Data collection
All the identified studies were selected according to the title and abstract by two independent authors (XW.S. and YP.W.). Extracted data were performed by 2 other authors independently (NH.N. and MJ.L.) and they screened articles with potential relevance through overall assessment based on the inclusion and exclusion criteria. The extracted data was summarized into a table, divided into three parts: (1) specific information of eligibility studies, including the authors’ names, year of publication, region of research, and study design; (2) basic information of each case, including age, total number of cases and gender, follow-ups, treatment, bacterial culture and application of antibiotics; (3) postoperative condition of each patient including failure, refracture, reoperation, delayed wound healing, local complications and other adverse events. For studies with incomplete or unclear data, we attempted to contact the authors for details. If any disagreement existed, a third reviewer participated and reached consensus.
Quality assessment
The Methodological Index for Non-Randomized Studies (MINORS) criteria was used to evaluate the quality of the included studies independently by two authors, which was established to assess the quality of comparative and noncomparative studies [28]. The highest score is 24 for comparative studies and 16 for noncomparative studies. Specific rating criteria are as follows: in noncomparative studies, scores of 0–4 showed to very low quality, 5–7 showed to low quality, 8–12 showed to fair quality, and ≥ 13 showed to high quality; in comparative studies, scores of 0–6 showed to very low quality, 7–10 showed to low quality, 11–15 showed to fair quality, and ≥ 16 showed to high quality.
Statistical analysis
Stata 16.0 was used to pool the all results of the included studies were for the meta-analysis. A chi-squared-based Q statistical test was used to estimate the statistical heterogeneity. The degree of heterogeneity for each included study was quantified using the I2 statistic. When P > 0.1 and/or I2 < 50%, the heterogeneity was evaluated to be low, and a fixed-effects model was used for the meta-analysis. Otherwise, a random-effects model was used. We calculated the postoperative eradication rates and incidences of complications in COM patients, as well as its 95% confidence interval (CI) for each study. Further, the pooled rates were calculated and publication bias was evaluated with a funnel plot. All results are presented in the form of forest plots and tables, and P < 0.05 was considered statistically significant.
Results
Search results
Initially, 816 articles were identified by searching, and two authors selected 162 studies by reading the title and abstract. Finally, through scrutinizing the full text and performing a manual search, a total of 16 articles conforming to requirements were included in our study. The implants used in one study [25] were self-configured. After careful research and discussion, we decided to include this study because it had a large sample size and a long follow-up period, which provided specific information on the configuration of antibiotics-loaded CS. In addition, there were two studies [29, 30] from the same medical center at different times, but after careful comparison with the inclusive information of patients, we found that duplicate patients were not included, so we considered including these studies. We also contacted the author for more detailed patient information for comparison.
Study characteristics
All the included 16 studies were published between 2002 and 2021 [15, 23, 25, 29–41]. Of the included studies, three consecutive case–control studies were retrospective in nature; the remaining were case series, including 2 prospective studies and 11 retrospective studies. Of the 16 publications, 7 were from China, 2 were from USA, 2 was from UK, and Germany, France, Spain, Italy, and Egypt each account for one case. The implants used in these studies included vancomycin, gentamicin-impregnated CS and tobramycin-impregnated CS. After the antibiotics-loaded CS was implanted, different surgical methods were performed according to the situation, including external fixation, skin or muscle flap transplantation, and skin grafting. Moreover, all the COM patients met the diagnostic criteria: obvious local symptoms, changes in imaging examination, and elevated inflammatory markers. Table 1 lists the COM patients’ characteristics in this meta-analysi s.
Table 1.
Characteristics of included studies
| Study cohort | Study region | Study Design | Patients(I/C) | Sex(M/F) | Age (years) | Follow-up (months) | Location | Culture Results | Intervention | Local complications | Other Adverse Events | Outcomes | MINORS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Humm, 2014 [31] | UK | Retrospective outcome study | 21/NA | 18/3 | 49 (28–88) | 16 (6–25) | 21 tibia | 4 Staphylococcus aureus, 4 Coagulase-negative Staphylococci, 4 Polymicrobial, 3 Negative, 6 Other organisms | Debridement, tobramycin-impregnated calcium sulfate | 7 aseptic wound leakage, 5 pin-tract infection | a transient acute kidney injury | Eradication rate, reoperation rate, delayed healing rate, rate of aseptic wound leakage | 8 |
| Andreacchio, 2019 [32] | France | Retrospective outcome study | 12/NA | 8/4 | 10.3 (2–15) | 24–72 | 3 tibia,4 femur, 2 humerus,1 clavicle,1radius,1 IV Metatarsal | 3 Methicillin-resistant Staphylococcus aureus, 7 Negative (other NS) | Debridement, tobramycin-impregnated calcium sulfate | None | None | Eradication rate, reoperation rate, refracture rate, rate of aseptic wound leakage | 9 |
| Ferguson, 2014 [33] | UK | Retrospective outcome study | 193(195 locations)/NA | 150/43 | 46.1 (16.1–82) | 44.44 (15.6–85.2) | 88 tibia, 73 femur, 10 humerus, 6 ankle, 5 radius, 4 knee fusion, 4 pelvis, 3 calcaneum, 1 ulna, 1 forefoot | 49 Methicillin-sensitive Staphylococcus aureus, 10 Coagulase-negative staphylococci, 7 Methicillin resistant Staphylococcus aureus, 4 Escherichia coli, 4 Enterobacter cloacae (other NS) | Debridement, tobramycin-impregnated calcium sulfate | 30 aseptic wound leakage, 9 collection of fluid and 9 refracture | 7 death (other reason) | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 13 |
| McKee, 2002 [15] | Canada | Prospective outcome study | 25/NA | 15/10 | 43 (27–69) | 28 (20–38) | 8 tibia, 6 femur, 3 ulna, 1 humerus | 9 Staphylococcus aureus, 4 Staphylococcus epidermidis, 4 Pseudomonas aeruginosa, 2 Enterobacter cloacae, other polymicrobial infections | Debridement, tobramycin-impregnated calcium sulfate | 8 aseptic wound leakage, 3 refracture, 2 persistent nonunion, 1 superficial wound necrosis, 1 hypertrophic nonunion | NR | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 9 |
| Gitelis, 2002 [34] | USA | Retrospective outcome study | 6/NA | 3/3 | 50 (26–85) | 28 (18–40) | 3 tibia, 3 femur | 5 Staphylococcus aureus, 1 Polymicrobial infections | Debridement, tobramycin-impregnated calcium sulfate | None | NR | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 8 |
| Qin, 2020 [30] | China | Retrospective outcome study | 33/NA | 26/7 | 44.5 (17–67) | 35.9 (12–75) | 33 calcaneum | 8 Staphylococcus aureus,6 Pseudomonas aeruginosa, 2 Enterococcus faecalis, 2 Proteus mirabilis,2 Enterobacter cloacae,10 negative (other NS) | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 13 aseptic wound leakage | 1 death (cardiovascular disease) | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 7 |
| Ferrando, 2017 [35] | Spain | Retrospective comparative study | 13/12 | 9/4 | 48 (17–67) | 22 (16–29) | 6 tibia, 4 calcaneum, 2 femur, 1 humerus | 5 methicillin-sensitive Staphylococcus aureus, 2 Methicillin-resistant S.aureus, 1 Pseudomonas aeruginosa, 1 Enterobacter cloacae, 1 Streptococcus agalactis,1 Escherichia coli,1 Polymicrobial infections | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 1 hematoma,1 mild seroma | None | Eradication rate, reoperation rate, delayed healing rate, rate of aseptic wound leakage | 15 |
| Badie, 2019 [36] | Egypt | Prospective outcome study | 30/NA | 25/5 | 26.2 (17–53) | > 12 | 14 tibia, 11 femur, 2 radius, 2 humerus, 1 ulna | 15 S. aureus, 3 methicillin resistant S. aureus, 2 Klebsiella Pneumoniae, 2 Escherichia coli, 2 Proteus mirabilis, 1 Salmonella, 1 Streptococcus, 2 polymicrobial infections, 2 negative | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 1 refracture | NR | Eradication rate, reoperation rate, refracture rate, delayed healing rate | 11 |
| Jiang, 2020 [23] | China | Retrospective outcome study | 34/NA | 27/7 | 41 (3–67) | 26 (12–68) | 34 calcaneum | 5 Pseudomonas aeruginosa, 2 Enterobacter cloacae, 2 Staphylococcus aureus (other NS) | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 11 aseptic wound leakage | 1 death (other reason) | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 9 |
| Zhou, 2020 [37] | China | Retrospective outcome study | 42(43 locations)/NA | 24/18 | 43.7 (23–74) | 42.8 (12.8–77.5) | 24 left tibia, 19 right tibia | 11 Staphylococcus aureus, 3 Pseudomonas aeruginosa, 1 Polymicrobial infections | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 13 paseptic wound leakage, 4 slight pain after a long-distance walk, 4 limb weakness or discomfort, 1 slight claudication | NR | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 8 |
| Qin, 2018 [29] | China | Retrospective comparative study | 35/NA | 26/9 | 38 (18–60) | 33.7 (25 ~ 41) | 35 tibia | 15 Staphylococcus aureus, 5 Escherichia Coli, 3 Pseudomonas Aeruginosa, 2 Serratia Marcescens, 2 Acinetobacter Baumannii, 2 Klebsiella Pneumoniae, other negative | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 8 pin-tract infection, 3 knee stiffness | None | Eradication rate, reoperation rate, delayed healing rate, rate of aseptic wound leakage | 7 |
| Gramlich, 2017 [38] | Gemany | Retrospective outcome study | 93/NA | 59/34 | 62 (11–84) | 11 (6–22) | 35 femur, 28 tibia, 7 fibula, 5 humerus, 5 hip joint, 4 Radius, 3 talus,, 3 pelvis (other NS) | 27 Staphylococcus aureus, 19 Staphylococcus epidermidis, 8 Pseudomonas aeruginosa, 5 Escherichia coli, 3 Klebsiella Pneumoniae (other NS) | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | NR | NR | Eradication rate | 6 |
| Ruan, 2021 [39] | China | Retrospective outcome study | 35/NA | 25/10 | 54 (34–82) | 24–60 | 35 tibia | 4 Staphylococcus aureus, 2 Klebsiella Pneumoniae, 2 Streptococcus | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | 5 anterolateral numbness of the iliac thigh, 2 relapse, 2 hematocele in the iliac bone area, 1 nonunion, 1 aseptic exudate | NR | Eradication rate, reoperation rate, refracture rate, delayed healing rate, rate of aseptic wound leakage | 10 |
| Gauland, 2011 [25] | USA | Retrospective outcome study | 323/NA | NR | NR | 60 | Lower-Extremity (NS) | NR | Debridement, vancomycin and gentamicin-impregnated calcium sulfate | NR | NR | Eradication rate, reoperation rate, delayed healing rate, rate of aseptic wound leakage | 8 |
| Sun, 2017 [40] | China | Retrospective outcome study | 12/NA | 7/5 | 54 (16–72) | 10.8 (6–18) | 12 jaw | 3 Staphylococcus aureus, 2 β-hemolytic streptococcu, 1 Escherichia coli, 1 Streptococcus viridans (other NS) | Debridement, vancomycin-impregnated calcium sulfate | 2 aseptic wound leakage | None | Eradication rate, reoperation rate, delayed healing rate, rate of aseptic wound leakage | 7 |
| Zhao, 2020 [41] | China | Retrospective comparative study | 10/21 | 10/0 | 48 (28.98–67.42) | 21.7 (15.8–27.6) | 5 femur, 5 tibia | 4 Staphylococcus aureus, 4 Negative (other NS) | Debridement, vancomycin-impregnated calcium sulfate | 3 aseptic wound leakage | None | Eradication rate, reoperation rate, delayed healing rate, rate of aseptic wound leakage | 14 |
NR, not reported; NS, not specified
Quality assessment
The data were extracted and evaluated independently by two authors from the included studies. For 8 noncomparative studies, the results showed that average MINORS score was 8.5 (range from 6 to 13), suggesting fair quality. The remaining two comparative studies had scores of 14 and 15, both suggesting fair quality. Overall, the methodological quality of the included studies was moderate (Table 2).
Table 2.
Methodological quality of included studies
| Items methodological items for non-randomized studies | Humm, 2014 [31] | Andreacchio, 2019 [32] | Ferguson, 2014 [33] | McKee, 2002 [15] | Gitelis, 2002 [34] | Qin, 2020 [30] | Ferrando, 2017 [35] | Badie, 2019 [36] | Jiang, 2020 [23] | Zhou, 2020 [37] | Qin, 2018 [29] | Gramlich, 2017 [38] | Ruan, 2021 [39] | Gauland, 2011 [25] | Sun, 2017 [40] | Zhao, 2020 [41] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. A clearly stated aim: the question addressed should be precise and relevant in the light of available literature | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| 2. Inclusion of consecutive patients: all patients potentially fit for inclusion (satisfying the criteria for inclusion) have been included in the study during the study period (no exclusion or details about the reasons for exclusion) | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3. Prospective collection of data: data were collected according to a protocol established before the beginning of the study | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4. Endpoints appropriate to the aim of the study: unambiguous explanation of the criteria used to evaluate the main outcome which should be in accordance with the question addressed by the study. Also, the endpoints should be assessed on an intention-to-treat basis | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 |
| 5. Unbiased assessment of the study endpoint: blind evaluation of objective endpoints and double-blind evaluation of subjective endpoints. Otherwise the reasons for not blinding should be stated | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| 6. Follow-up period appropriate to the aim of the study: the follow-up should be sufficiently long to allow the assessment of the main endpoint and possible adverse events | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 |
| 7. Loss to follow up less than 5%: all patients should be included in the follow up. Otherwise, the proportion lost to follow up should not exceed the proportion experiencing the major endpoint | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 8. Prospective calculation of the study size: information of the size of detectable difference of interest with a calculation of 95% confidence interval, according to the expected incidence of the outcome event, and information about the level for statistical significance and estimates of power when comparing the outcomes | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Additional criteria in the case of comparative study | ||||||||||||||||
| 9. An adequate control group: having a gold standard diagnostic test or therapeutic intervention recognized as the optimal intervention according to the available published data | 0 | 0 | ||||||||||||||
| 10. Contemporary groups: control and studied group should be managed during the same time period (no historical comparison) | 2 | 2 | ||||||||||||||
| 11. Baseline equivalence of groups: the groups should be similar regarding the criteria other than the studied endpoints. Absence of confounding factors that could bias the interpretation of the results | 2 | 2 | ||||||||||||||
| 12. Adequate statistical analyses: whether the statistics were in accordance with the type of study with calculation of confidence intervals or relative risk | 2 | 2 | ||||||||||||||
| Total | 8 | 9 | 13 | 9 | 8 | 7 | 15 | 11 | 9 | 8 | 7 | 6 | 10 | 8 | 7 | 14 |
Rates of infectious eradication and complications
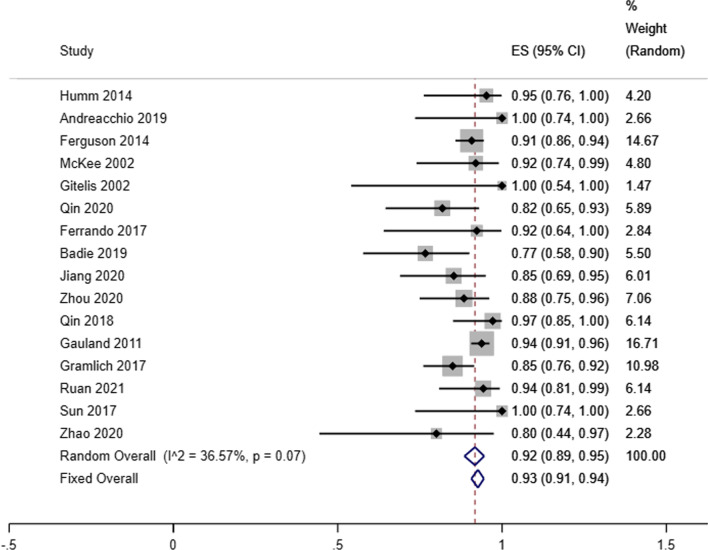
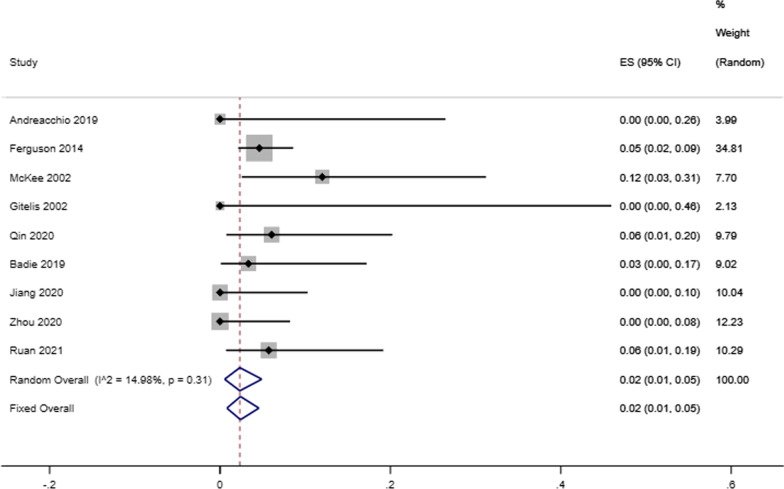
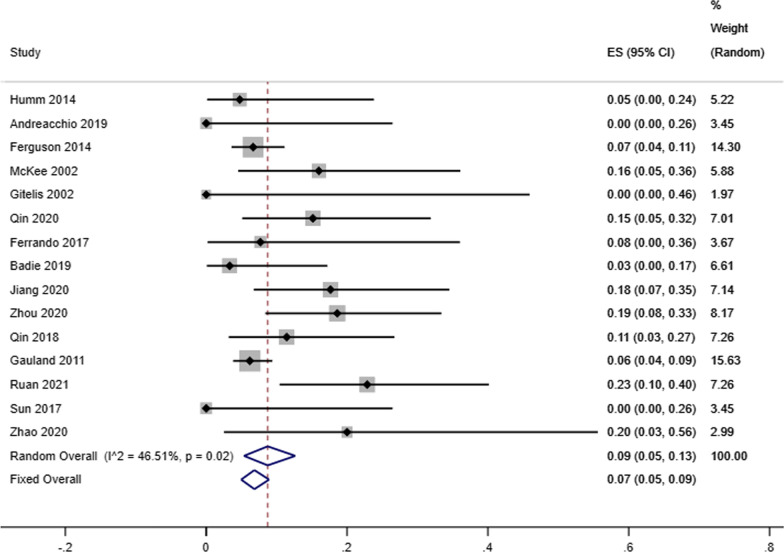
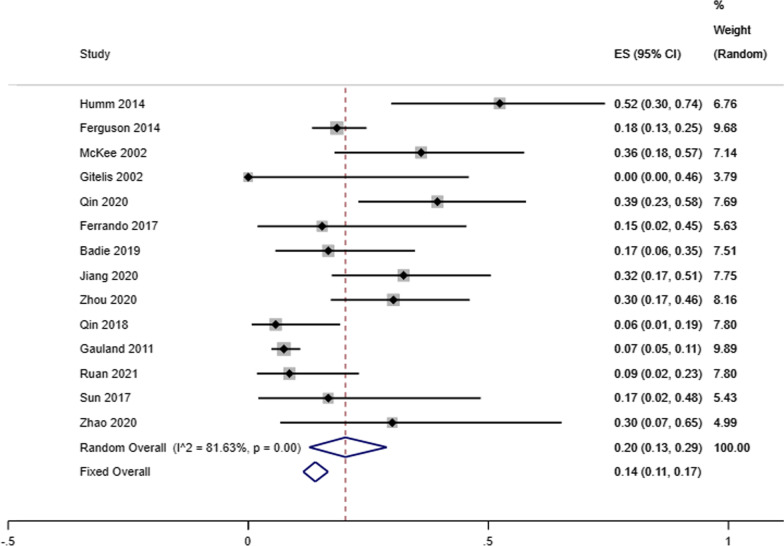
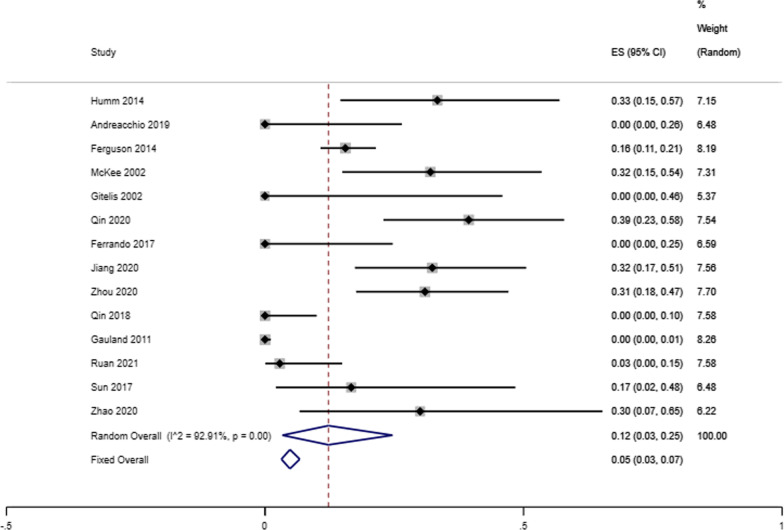
Our analysis showed that the infectious eradication rate among 717 patients (720 locations) receiving antibiotic-loaded calcium sulfate implantation was 92% reported by 16 studies (95% CI 0.89–0.95; P = 0.07), a random-effects model was used due to the low level of heterogeneity (I2 = 36.57%) (Fig. 2). A fixed effects model was used due to no heterogeneity (I2 = 23.96%), and the refracture rate was 2% in 9 studies (95% CI 0.00–0.04; P = 0.23) (Fig. 3). A random-effects model was used due to the low level of heterogeneity (I2 = 46.51%), and the reoperation rate was 9% reported by 15 studies that enrolled 827 cases4 (95% CI 0.05–0.13; P = 0.02) (Fig. 4). A random-effects model was used due to the high level of heterogeneity (I2 = 81.63%), and the delayed healing rate was 20% reported by 14 studies that enrolled 815 cases (95% CI 0.13–0.29; P = 0.001) (Fig. 5). A random-effects model was used due to the high level of heterogeneity (I2 = 92.91%), and the incidence of aseptic mouth leakage was 12% reported by 14 studies that enrolled 794 cases (95% CI 0.03–0.25; P = 0.001) (Fig. 6).
Fig. 2.
The overall eradication rate in COM patients with antibiotic-loaded calcium sulfate
Fig. 3.
The overall refracture rate in COM patients with antibiotic-loaded calcium sulfate
Fig. 4.
The overall reoperation rate in COM patients with antibiotic-loaded calcium sulfate
Fig. 5.
The overall rate of delayed healing in COM patients with antibiotic-loaded calcium sulfate
Fig. 6.
The overall rate of aseptic wound leakage in COM patients with antibiotic-loaded calcium sulfate
Among the 16 articles we included, we excluded one study that did not specify the location of COM [25]. The tibia was the most commonly affected part of COM and was detected in 46.73% of patients with COM (279/597). The second and third most common locations of COM were femur (139/597, 23.28%) and calcaneum (74/597, 12.40%), respectively. The remaining locations of COM included humerus (21/597, 3.52%), radius (12/597, 2.01%), pelvis (7/597,1.17%), fibula (7/597, 1.17%), ankle (6/597, 1.01%), hip joint (5/597, 0.84%), ulna (5/597, 0.84%), knee (4/597, 0.67%), talus (3/597, 0.50%), clavicle, forefoot, and fourth metatarsal each account for one case (1/597, 0.17%). A several COM patients had two involved locations [33, 37].
Effects and complications of subgroup
In order to further explore the difference between the two kinds of antibiotics in the treatment of COM, the eradication rate and complications were summarized. However, the results showed that the eradication rates of tobramycin-loaded CS group and vancomycin combined with gentamicin-loaded CS group were 92% (95% CI 0.88–0.95; P = 0.9) and 90% (95% CI 0.86–0.94; P = 0.03), respectively, and there was no significant difference (P = 0.96). In terms of the incidence of complications, the reoperation rates of tobramycin-loaded CS and vancomycin combined with gentamicin-loaded CS were 7% (95% CI 0.04–0.10; P = 0.3) and 11% (95% CI 0.86–0.94; P = 0.03), the aseptic wound leakage rates were 24% (95% CI 0.11–0.38; P = 0.06) and 26% (95% CI 0.05–0.46; P = 0.001), the bone fracture rates were 5% (95% CI 0.02–0.08; P = 0.5) and 5% (95% CI 0.01–0.09%; P = 0.9), the incidence of delayed healing rates were 34% (95% CI 0.14–0.28; P = 0.001) and 17% (95% CI 0.13–0.55; P = 0.001), respectively. The results showed no significant difference (all P > 0.05) (Table 3).
Table 3.
Summary of complications and efficacy outcomes in the included studies
| Tobramycin (95% CI) | Vancomycin and gentamicin (95% CI) | Sig (significant difference) | |
|---|---|---|---|
| Eradication rate | 0.92 (0.88, 0.95) | 0.90 (0.86, 0.94) | 0.373 |
| Reoperation rate | 0.07 (0.04, 0.10) | 0.11 (0.06, 0.15) | 0.497 |
| Refracture rate | 0.05 (0.02, 0.08) | 0.05 (0.01, 0.09) | 0.800 |
| Delayed healing rate | 0.34 (0.13, 0.55) | 0.17 (0.10, 0.25) | 0.327 |
| Rate of aseptic wound leakage | 0.24 (0.11, 0.38) | 0.26 (0.05, 0.46) | 0.857 |
Publication bias
A funnel chart would reflect whether there was publication bias in this study, and the symmetry of the funnel chart meant that there was no publication bias (Fig. 7). Furthermore, the symmetry test of the above graph showed that P = 0.882 > 0.05, which meant that the funnel graph was symmetrical. Therefore, it can be judged that there was no publication bias in current study.
Fig. 7.
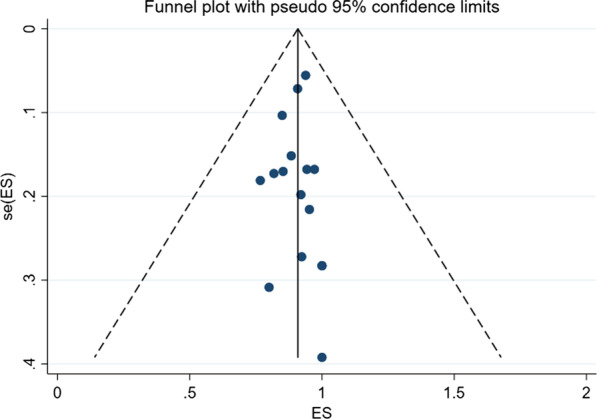
Funnel plot for assessing publication bias
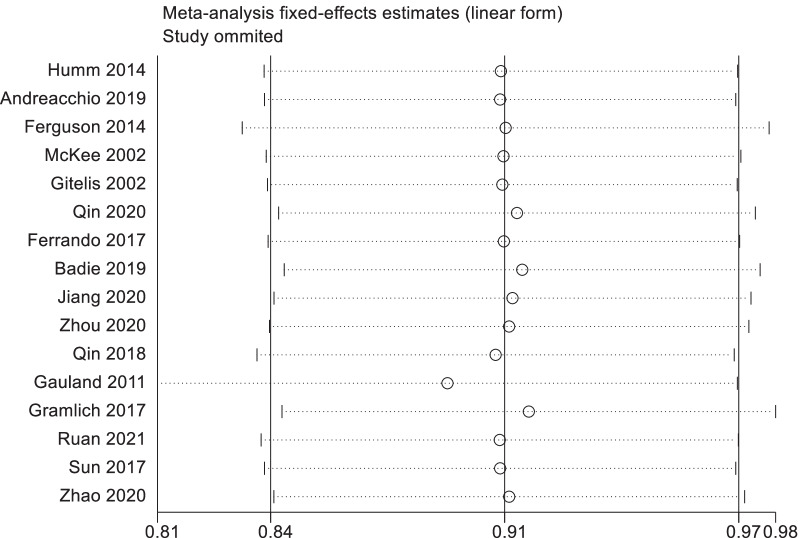
Sensitivity analysis
We eliminated each included study one by one, and summarized and analyzed the remaining studies to assess whether the individual study had an impact on the results of the meta-analysis. None of the studies had an excessive impact on the results of the meta-analysis, indicating that the results of the remaining studies were stable and reliable (Fig. 8).
Fig. 8.
The results of sensitivity analysis
Discussion
CS was discovered in 1970 until it was used clinically in the past two decades, and achieved good results in Europe [15]. However, no research reported the specific efficacy of CS antibiotic delivery system in the treatment of patients with COM. In the current study, 16 studies published from 2000 to 2021 were included, with a total of 717 patients (720 locations) with COM. To the best of our knowledge, this was the first study to investigate the eradication rate in patients with COM, and we concluded that the overall rate of eradication in COM patients was 92%. The eradication rate was also similar to those reported for treatment with antibiotic-loaded PMMA beads in the literature, which showed 60% and 100% in between [42, 43].
The successful eradication of COM remains a challenge for clinicians. Except for a study on chronic jaw osteomyelitis that did not specify the use of antibiotics [1], the treatment regimen in all 15 studies involving the antibiotics-loaded CS included implantation after radical debridement followed by adjuvant systemic administration of antibiotics. Statistically, the tibia is the most common site for COM to occur [44], This is similar to our summary study. Of the 597 patients with COM included, nearly half were tibial osteomyelitis, because of its poor blood supply (especially inferior third of tibia), higher risk of injuries, and of course, the inappropriate surgical managements. Unfortunately, even standard treatment protocols have been strictly implemented, the recurrent rate of chronic tibial osteomyelitis remains as high as 20–30% [45]. Although we included 279 patients with tibial osteomyelitis, the recurrence of infection in each study was unable to determine whether it was on tibial or not, therefore made it difficult to calculate the recurrence rate of tibial osteomyelitis.
While infection elimination had shown encouraging results, associated local complications were also of concern. Delayed wound healing was the most frequent complication in our study, with a relatively high incidence of 20%. Bibbo et al. [46] reported that the delayed healing rate of calcium sulfate loaded with vancomycin in the treatment of calcaneal fractures was 15%, which was similar to our summary results. Kallala et al. [47] reported that aseptic wound leakage is a common complication of degradation of CS after implantation, which is related to the composition of CS itself, with an incidence of 4.2%, this incidence was variant with each individual, mainly depending on the size of implanted CS and the abundance of soft tissues coverage. Romano et al. [48] found that the leakage rate of aseptic wound was as high as 27%, while our total leakage rate of aseptic wound was 12%. This may be explained that some of the studies we included used different treatment methods after implantation of CS according to different conditions of patients, such as muscle flap coverage [39] or external fixation [29]. On the other hand, it may also be attributed to the fact that synthetic CS contains no impurities compared with mined and refined CS. At present, there is no reliable large-sample comparative study to confirm the side effects of calcium sulfate-induced leakage of sterile wounds. In addition to the wound leakage, local CS implantation may also bring other complications, such as heterotopic ossification [47] and even hypercalcemia [49]. Therefore we also pooled the refracture rate and the reoperation rate, which are 2% and 9%, respectively.
Antibiotic-loaded CS delivery system had been put into clinical application in the past 20 years, loaded with several of the most common antibiotics which made into a variety of products, such as vancomycin, gentamicin and tobramycin. However, the efficacy of calcium sulfate-loaded antibiotics in the treatment of COM is not clear. In an in vitro experiment, vancomycin and tobramycin impregnated materials had similar germicidal properties and elution efficiency [15]. A systematic review shows that there may be no significant difference between CS and other products except for degradation time [50]. The same was true for our subgroup results, we found that there was no significant difference in eradication rate between tobramycin loaded and vancomycin loaded with gentamicin calcium sulfate. However, there was no significant difference in the incidence of complications, including delayed healing rate, aseptic wound leakage rate, bone fracture rate and reoperation rate. That might be explained by different risk factors and patient individual variations.
Our study has some limitations. First, the sample size is not big enough and the summary of patient population characteristics is incomplete, which may hinder the interpretation of research results. Therefore, the results should be interpreted cautiously, and more patients should be enrolled in future studies to draw more precise conclusions. Second, we did not analyze the risk factors of infection relapse and complications because of most included studies’ retrospective design. To better identify potential risk factors, a good comparison of these antibiotics-loaded materials requires large-scale prospective clinical trials, especially multi-center joint studies; crucially, the potential risk of aseptic wound leakage after local CS implantation should be fully considered when patients receive this treatment. Future studies may focus on the risk factors of local complication and infection relapse following local CS implantation, as well as the efficacy of other substitute materials. Third, we all know that the release time and concentration of such antibiotic sustained-release systems are critical to the treatment of COM, but the studies we included lack a detailed description of the pharmacokinetics, which is not conducive to further compare the efficacy of several types of antibiotics. We should pay more attention to the clinical research on the species and drug resistance of COM bacteria in the future [51]. Fourth, the studies included different sites of infection (femur, tibia, humerus, radius, ulna, pelvis, and even calcaneus) and four types of Cierny-Mader staging. Therefore, our research inevitably lacks an in-depth discussion of a single type and location of COM. More detailed data is useful for evaluation of the efficacy of antibiotic CS on different parts of COM in future.
Conclusion
In conclusion, this meta-analysis revealed that the eradication rate of implantation of antibiotic-loaded CS in the treatment of COM was as high as 92%, and the incidence of complications including delayed healing rate, aseptic wound leakage rate, refracture rate and reoperation rate were relatively low. Although there is no significant difference in efficacy and complications between the two antibiotic-loaded CS regimens in the treatment of COM. The clinical efficacy of antibiotic-loaded CS in the treatment of COM needs to be confirmed by further study.
Acknowledgements
Not applicable.
Abbreviations
- COM
Chronic osteomyelitis
- CS
Calcium sulfate
- PMMA
Polymethyl methacrylate
- PICOS
Population, intervention, comparison, outcomes, and study
- MINORS
Methodological items for non-randomized studies
- CI
Confidence interval
Authors’ contributions
XS, YW, and HN conceived and coordinated the study; designed, performed, and analyzed the experiments; and wrote the paper. Mingjun Li, Chaoqun Zhang, Baochuang Qi, Mingjie Wei, Teng Wang and Yongqing Xu carried out the data collection and data analysis and revised the paper. All authors reviewed the results and approved the final version of the manuscript. The authors read and approved the final manuscript.
Funding
This study was funded by National Natural Science Foundation of China: the MIF-based WNT/p38 MAPK/NF-κB Signaling Loop Regulates Differentiation of Osteoblasts, Osteoclasts, and Coupling Under Inflammatory Conditions (81772367); on the Role and Mechanism of Bap/avβ/TLR-4 Signal Regulating Osteogenesis by Osteoblast in Osteomyelitis (82072392); Yunnan Traumatology and Orthopedics Clinical Medical Center (ZX20191001).
Availability of data and materials
As a meta-analysis, all raw data of this study are extracted from ten included studies. The datasets supporting the conclusions of this article are available in the 16 included studies.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Xiangwen Shi, Yipeng Wu, and Haonan Ni contributed equally to this work
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–79. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 2.Nasser A, Azimi T, Ostadmohammadi S, Ostadmohammadi S. A comprehensive review of bacterial osteomyelitis with emphasis on Staphylococcus aureus. Microb Pathog. 2020;148:104431. doi: 10.1016/j.micpath.2020.104431. [DOI] [PubMed] [Google Scholar]
- 3.Pollard TC, Newman JE, Barlow NJ, Price JD, Willett KM. Deep wound infection after proximal femoral fracture: consequences and costs. J Hosp Infect. 2006;63(2):133–9. doi: 10.1016/j.jhin.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37(Suppl 2):S59–66. doi: 10.1016/j.injury.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61-5.e1. 10.1016/j.arth.2012.02.022. [DOI] [PubMed]
- 7.Demetriou M, Papanas N, Panopoulou M, Papatheodorou K, Bounovas A, Maltezos E. Tissue and swab culture in diabetic foot infections: neuropathic versus neuroischemic ulcers. Int J Low Extrem Wounds. 2013;12(2):87–93. doi: 10.1177/1534734613481975. [DOI] [PubMed] [Google Scholar]
- 8.Panagopoulos P, Drosos G, Maltezos E, Papanas N. Local antibiotic delivery systems in diabetic foot osteomyelitis: time for one step beyond? Int J Low Extrem Wounds. 2015;14(1):87–91. doi: 10.1177/1534734614566937. [DOI] [PubMed] [Google Scholar]
- 9.Aicale R, Cipollaro L, Esposito S, Maffulli N. An evidence based narrative review on treatment of diabetic foot osteomyelitis. Surgeon. 2020;18(5):311–20. doi: 10.1016/j.surge.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Conterno LO, Turchi MD. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev. 2013;9:Cd004439. doi: 10.1002/14651858.CD004439.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier GS, Roth KE, Andereya S, Birnbaum K, Niedhart C, Lühmann M, et al. In vitro elution characteristics of gentamicin and vancomycin from synthetic bone graft substitutes. Open Orthop J. 2013;7:624–9. doi: 10.2174/1874325001307010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobb LH, McCabe EM, Priddy LB. Therapeutics and delivery vehicles for local treatment of osteomyelitis. J Orthop Res. 2020;38(10):2091–103. doi: 10.1002/jor.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Jia W, Gu Y, Xiao W, Liu X, Wang D, et al. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials. 2010;31(22):5865–74. doi: 10.1016/j.biomaterials.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Ahluwalia R, Lázaro-Martínez JL, Reichert I, Maffulli N. Advances in pharmacotherapy for diabetic foot osteomyelitis. Expert Opin Pharmacother. 2021;22(16):2281–91. doi: 10.1080/14656566.2021.1954159. [DOI] [PubMed] [Google Scholar]
- 15.McKee MD, Wild LM, Schemitsch EH, Waddell JP. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma. 2002;16(9):622–7. doi: 10.1097/00005131-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DB, Brooks DE, Bice TG, DeJong ES, Lonergan KT, Wenke JC. Tobramycin-impregnated calcium sulfate prevents infection in contaminated wounds. Clin Orthop Relat Res. 2005;441:366–71. doi: 10.1097/01.blo.0000181144.01306.b0. [DOI] [PubMed] [Google Scholar]
- 17.Beardmore AA, Brooks DE, Wenke JC, Thomas DB. Effectiveness of local antibiotic delivery with an osteoinductive and osteoconductive bone-graft substitute. J Bone Joint Surg Am. 2005;87(1):107–12. doi: 10.2106/JBJS.C.01670. [DOI] [PubMed] [Google Scholar]
- 18.Nelson CL, McLaren SG, Skinner RA, Smeltzer MS, Thomas JR, Olsen KM. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J Orthop Res. 2002;20(4):643–7. doi: 10.1016/S0736-0266(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 19.McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res B Appl Biomater. 2015;103(4):870–7. doi: 10.1002/jbm.b.33247. [DOI] [PubMed] [Google Scholar]
- 20.Chang W, Colangeli M, Colangeli S, Di Bella C, Gozzi E, Donati D. Adult osteomyelitis: debridement versus debridement plus Osteoset T pellets. Acta Orthop Belg. 2007;73(2):238–243. [PubMed] [Google Scholar]
- 21.Robinson D, Alk D, Sandbank J, Farber R, Halperin N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transplant. 1999;4(3–4):91–97. [PubMed] [Google Scholar]
- 22.Lee GH, Khoury JG, Bell JE, Buckwalter JA. Adverse reactions to OsteoSet bone graft substitute, the incidence in a consecutive series. Iowa Orthop J. 2002;22:35–38. [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang N, Zhao XQ, Wang L, Lin QR, Hu YJ, Yu B. Single-stage debridement with implantation of antibiotic-loaded calcium sulphate in 34 cases of localized calcaneal osteomyelitis. Acta Orthop. 2020;91(3):353–9. doi: 10.1080/17453674.2020.1745423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandi SK, Bandyopadhyay S, Das P, Samanta I, Mukherjee P, Roy S, et al. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv. 2016;34(8):1305–17. doi: 10.1016/j.biotechadv.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Gauland C. Managing lower-extremity osteomyelitis locally with surgical debridement and synthetic calcium sulfate antibiotic tablets. Adv Skin Wound Care. 2011;24(11):515–23. doi: 10.1097/01.ASW.0000407647.12832.6c. [DOI] [PubMed] [Google Scholar]
- 26.Calhoun JH, Mader JT. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthop Relat Res. 1997;341:206–214. doi: 10.1097/00003086-199708000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 29.Qin C, Xu L, Liao J, Fang J, Hu Y. Management of osteomyelitis-induced massive tibial bone defect by monolateral external fixator combined with antibiotics-impregnated calcium sulphate: a retrospective study. Biomed Res Int. 2018;2018:9070216. doi: 10.1155/2018/9070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin CH, Zhou CH, Ren Y, Cheng GY, Zhang HA, Fang J, et al. Extensive eggshell-like debridement technique plus antibiotic-loaded calcium sulphate for one-stage treatment of chronic calcaneal osteomyelitis. Foot Ankle Surg. 2020;26(6):644–9. doi: 10.1016/j.fas.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Humm G, Noor S, Bridgeman P, David M, Bose D. Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET(®)-T: a review of 21 patients in a regional trauma centre. Strategies Trauma Limb Reconstr. 2014;9(3):157–61. doi: 10.1007/s11751-014-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreacchio A, Alberghina F, Paonessa M, Cravino M, De Rosa V, Canavese F. Tobramycin-impregnated calcium sulfate pellets for the treatment of chronic osteomyelitis in children and adolescents. J Pediatr Orthop B. 2019;28(3):189–95. doi: 10.1097/BPB.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J. 2014 doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 34.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong). 2002;10(1):53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 35.Ferrando A, Part J, Baeza J. Treatment of cavitary bone defects in chronic osteomyelitis: biogactive glass S53P4 vs. calcium sulphate antibiotic beads. J Bone Jt Infect. 2017;2(4):194–201. doi: 10.7150/jbji.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badie AA, Arafa MS. One-stage surgery for adult chronic osteomyelitis: concomitant use of antibiotic-loaded calcium sulphate and bone marrow aspirate. Int Orthop. 2019;43(5):1061–70. doi: 10.1007/s00264-018-4063-z. [DOI] [PubMed] [Google Scholar]
- 37.Zhou CH, Ren Y, Ali A, Meng XQ, Zhang HA, Fang J, et al. Single-stage treatment of chronic localized tibial osteomyelitis with local debridement and antibiotic-loaded calcium sulfate implantation: a retrospective study of 42 patients. J Orthop Surg Res. 2020;15(1):201. doi: 10.1186/s13018-020-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gramlich Y, Walter G, Gils J, Hoffmann R. Early results of adjuvant topical treatment of recurrent osteomyelitis with absorbable antibiotic carriers. Z Orthop Unfall. 2017;155(1):35–44. doi: 10.1055/s-0042-112228. [DOI] [PubMed] [Google Scholar]
- 39.Ruan W, Li M, Guo Q, Lin B. Gastrocnemius muscle flap with vancomycin/gentamicin-calcium sulfate and autogenous iliac bone graft for the phase I treatment of localized osteomyelitis after tibial plateau fracture surgery. J Orthop Surg Res. 2021;16(1):341. doi: 10.1186/s13018-021-02496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun HJ, Xue L, Wu CB, Zhou Q. Use of vancomycin-impregnated calcium sulfate in the treatment of osteomyelitis of the jaw. J Oral Maxillofac Surg. 2017;75(1):119–28. doi: 10.1016/j.joms.2016.06.178. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Z, Wang G, Zhang Y, Luo W, Liu S, Liu Y, et al. The effect of calcium sulfate/calcium phosphate composite for the treatment of chronic osteomyelitis compared with calcium sulfate. Ann Palliat Med. 2020;9(4):1821–33. doi: 10.21037/apm.2020.03.23. [DOI] [PubMed] [Google Scholar]
- 42.Blaha JD, Calhoun JH, Nelson CL, Henry SL, Seligson D, Esterhai JL, Jr, et al. Comparison of the clinical efficacy and tolerance of gentamicin PMMA beads on surgical wire versus combined and systemic therapy for osteomyelitis. Clin Orthop Relat Res. 1993;295:8–12. doi: 10.1097/00003086-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Walenkamp GH, Kleijn LL, de Leeuw M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthop Scand. 1998;69(5):518–22. doi: 10.3109/17453679808997790. [DOI] [PubMed] [Google Scholar]
- 44.Yikemu X, Tuxun A, Nuermaimaiti M, Abudukeyimu A, Shayiti A. Effects of vacuum sealing drainage combined with ilizarov bone transport technique in the treatment of tibial traumatic osteomyelitis. Med Sci Monit. 2019;25:6864–71. doi: 10.12659/MSM.915450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhattacharya R, Kundu B, Nandi SK, Basu D. Systematic approach to treat chronic osteomyelitis through localized drug delivery system: bench to bed side. Mater Sci Eng C Mater Biol Appl. 2013;33(7):3986–93. doi: 10.1016/j.msec.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 46.Bibbo C, Patel DV. The effect of demineralized bone matrix-calcium sulfate with vancomycin on calcaneal fracture healing and infection rates: a prospective study. Foot Ankle Int. 2006;27(7):487–93. doi: 10.1177/107110070602700702. [DOI] [PubMed] [Google Scholar]
- 47.Kallala R, Harris WE, Ibrahim M, Dipane M, McPherson E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: Safety profile and complication rates. Bone Joint Res. 2018;7(10):570–9. doi: 10.1302/2046-3758.710.BJR-2017-0319.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romanò CL, Logoluso N, Meani E, Romanò D, De Vecchi E, Vassena C, et al. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: a retrospective comparative study. Bone Joint J. 2014 doi: 10.1302/0301-620X.96B6.33014. [DOI] [PubMed] [Google Scholar]
- 49.Kallala R, Haddad FS. Hypercalcaemia following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for infection. Bone Joint J. 2015;97-b(9):1237–1241. doi: 10.1302/0301-620X.97B9.34532. [DOI] [PubMed] [Google Scholar]
- 50.van Vugt TA, Geurts J, Arts JJ. Clinical application of antimicrobial bone graft substitute in osteomyelitis treatment: a systematic review of different bone graft substitutes available in clinical treatment of osteomyelitis. Biomed Res Int. 2016;2016:6984656. doi: 10.1155/2016/6984656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maffulli N, Papalia R, Zampogna B, Torre G, Albo E, Denaro V. The management of osteomyelitis in the adult. Surgeon. 2016;14(6):345–60. doi: 10.1016/j.surge.2015.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As a meta-analysis, all raw data of this study are extracted from ten included studies. The datasets supporting the conclusions of this article are available in the 16 included studies.
Not applicable.