Abstract
OBJECTIVES
Vascular rings are rare anomalies of congenital heart disease that cause respiratory and gastrointestinal symptoms. This study assessed the long-term outcomes of patients with vascular ring division.
METHODS
A multi-institution retrospective review of 371 patients with vascular rings undergoing surgical division at 3 paediatric cardiac institutions between November 2007 and October 2019 was performed.
RESULTS
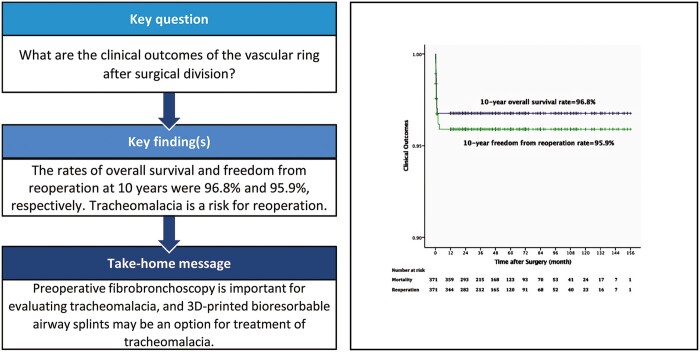
The complete vascular rings consisted of a double aortic arch (24.5%), right aortic arch with left ligamentum arteriosum (36.7%) and left aortic arch, with right ligamentum arteriosum (0.5%). The incomplete vascular rings consisted of a pulmonary artery sling (22.9%), left aortic arch with aberrant right subclavian artery (15.1%) and innominate artery compression syndrome (0.3%). Respiratory symptoms included stridor (71.4%), wheezing (49.1%), coughing (31.5%), gastrointestinal symptoms included choking (12.4%), dysphagia (3.2%) and emesis (1.9%). Only one patient died after discharge, yielding a late mortality rate of 0.3% (1/360). The 10-year overall survival rate was 96.8%. Postoperative complications were reported in 51 patients, 15 of whom required reoperation. The 10-year freedom from reoperation rate was 95.9%. Follow-up was completed in 95.4% (354/371) of patients, with a mean follow-up time of 4.3 ± 2.9 years (range from 1 to 13 years). Twenty patients (5.6%) experienced residual symptoms during long-term follow-up.
CONCLUSIONS
The outcomes of vascular ring division are excellent. A Kommerell diverticulum >1.5 times the aberrant left subclavian artery origin is an operative indication for primary resection. Tracheomalacia is a risk factor for reoperation and residual symptoms, and preoperative fibrobronchoscopy is important for evaluation.
Keywords: Vascular ring, Surgery, Kommerell diverticulum, Tracheomalacia, Tracheal ring
A vascular ring is a rare congenital cardiovascular anomaly that compresses the trachea and oesophagus and was first described by Robert Gross from Boston Children’s Hospital [1].
INTRODUCTION
A vascular ring is a rare congenital cardiovascular anomaly that compresses the trachea and oesophagus and was first described by Robert Gross from Boston Children’s Hospital [1]. Currently, the term vascular ring is used to refer to all congenital vascular anomalies of the aortic arch system that cause compression of the trachea and/or oesophagus. The primary symptoms of this compression are noisy breathing, cough, recurrent upper respiratory tract infections, wheezing, dyspnoea and dysphagia. With the improvement of imaging technology, computed tomography (CT) has become the preferred imaging method [2, 3], providing a precise surgical strategy [4]. This multi-institution study assessed the clinical characteristics of patients with vascular rings along with the long-term outcomes of patients undergoing vascular ring repair and the risk factors leading to reintervention.
MATERIALS AND METHODS
Definitions
Vascular ring classification was conducted as recommended by the International Congenital Heart Surgery Nomenclature and Database Committee [5, 6], including the classification of complete and incomplete vascular rings. Complete vascular rings occur as 4 major types: double aortic arch (DAA), right aortic arch (RAA) with left ligamentum arteriosum (LLA) and aberrant left subclavian artery (ALSCA), RAA with LLA and mirror-image branchings and left aortic arch (LAA), right-sided descending aorta and right ligamentum arteriosum. Incomplete vascular ring includes 3 major types: pulmonary artery sling (PAS), LAA with aberrant right subclavian artery (ARSCA) and innominate artery compression syndrome. Severe tracheomalacia is defined as the contract of the posterior wall with the anterior wall during expiration [7].
Patients
This multi-institution retrospective study was performed at 3 paediatric cardiac institutions: Children’s Hospital of Nanjing Medical University, Beijing Children’s Hospital and Children’s Hospital of Suqian. The human research ethics committees of all 3 institutional approved the present study. Patients diagnosed with vascular rings who underwent cardiac surgery between November 2007 and October 2019 were included in this study. A total of 371 patients were enrolled in the study. Clinical data were obtained by reviewing the medical records from initial admission until the last follow-up.
Operative techniques
DAA
Preoperative 3-dimensional (3D)-CT reconstruction was used to describe the development of the bilateral aortic arch. The approach was generally through the left fourth intercostal space to enter the chest; we preferred the left to the right fourth intercostal space as the RAA is smaller than the LAA. The mediastinal pleura was incised to expose the LAA and RAA, and then, the smaller aortic arch was divided to release the oppression, avoiding injury of the vagus, recurrent laryngeal and phrenic nerves. For patients with equally sized aortic arches, the division of the aortic arch was at the surgeon’s discretion.
RAA with LLA
The approach was through the left third or fourth intercostal space, and the mediastinal pleura was incised longitudinally along the oesophagus. The ligamentum arteriosum and left subclavian artery were fully freed, and care was taken to avoid injuring the vagus, recurrent laryngeal and phrenic nerves. Once the ligamentum arteriosum was divided, oxygenation was markedly increased. When the Kommerell diverticulum was >1.5 times the ALSCA and caused obvious compression of the trachea, it was resected, and the ALSCA was transferred to the left carotid artery [8].
PAS
The main pulmonary artery, left pulmonary artery, right pulmonary artery and ligamentum arteriosum were carefully dissected through median sternotomy. The ligamentum arteriosum was divided, and the left pulmonary artery was resected from the right pulmonary artery, transferred to the left of the trachea and then implanted in the left side of the main pulmonary artery. If patients had a long segmental complete tracheal ring (CTR), slide tracheoplasty was performed. The trachea was carefully evaluated with perioperative fibrobronchoscopy and cut off in the mid-portion. Longitudinal incisions were made in the upper and lower segments, and then, the 2 segmental tracheas were held together with running anastomoses. If patients had severe tracheomalacia as evaluated with preoperative fibrobronchoscopy, external tracheobronchial suspension was performed with a three-dimensional bioresorbable airway splint (polycaprolactone, Xi’an Jiaotong University State Key Laboratory of Manufacturing Systems Engineering, Supplementary Material, Fig. S1). The length of the malacic trachea was measured by fibrobronchoscopy, the splint was fixed on the tracheal cartilage, and the malacic trachea was suspended on the splint with interrupted anastomosis.
LAA with right-sided descending aorta and right ligamentum arteriosum
The ligamentum arteriosum was divided through the right third intercostal space as described above.
LAA with ARSCA
Patients with LAA and ARSCA were all had associated cardiac defects, so surgery was performed through a median sternotomy. The ligamentum arteriosum was divided as described above, and then, cardiopulmonary bypass (CPB) was initiated to repair the cardiac defect. The right subclavian artery was relocated to release compression on the oesophagus when patients had dysphagia.
Innominate artery compression syndrome
The innominate artery was suspended from the back of the sternum through a median sternotomy.
For patients with cardiac defects, the vascular ring was processed first, and then, the cardiac defect was repaired by CPB through a median sternotomy incision. The tracheal surgery was conducted at the same stage.
Data analysis
Statistical analysis was performed by IBM SPSS v22 (IBM Corp. Armonk, NY). Kaplan–Meier curves were used to analyse the overall survival rate and freedom from reoperation rate. Fisher’s exact test was used to analyse categorical variables, and the Mann–Whitney test was used to analyse continuous variables. Variables were assessed with the log-rank test, and each variable with P < 0.2 was included in the multivariable Cox proportional hazards regression analysis to identify risk factors for mortality, reoperation and residual symptoms. Statistical significance was set at P < 0.05.
RESULTS
Patient characteristics
A total of 371 vascular ring patients were enrolled. In this study, the median age at the time of operation was 10 months [interquartile range (IQR), 5–24 months]; 53.4% (198/371) of the patients were younger than 1 year, and 18.1% (67/371) were older than 3 years. The median weight at operation was 8.7 kg (IQR, 6.9–12.0 kg), and 232 patients (62.5%) were boys. The classification of vascular rings and population distribution are listed in Table 1. Kommerell diverticulum was identified in 49 patients.
Table 1:
Classification of vascular ring
| Vascular ring type | No. patients (N = 371) |
|---|---|
| Complete vascular ring, n (%) | 229 (61.7) |
| Double aortic arch | 91 |
| Right arch dominant | 65 |
| Left arch dominant | 10 |
| Codominant | 16 |
| Right aortic arch with left ligamentum arteriosum | 136 |
| Aberrant left subclavian artery | 95 |
| Mirror-image branching | 41 |
| Left aortic arch, right-sided descending aorta with right ductus arteriosus | 2 |
| Incomplete vascular ring, n (%) | 142 (38.3) |
| Pulmonary artery sling | 85 |
| Left aortic arch with aberrant right subclavian artery | 56 |
| Innominate artery compressionsyndrome | 1 |
Associated anomalies
Concomitant cardiac defects were found in 163 patients (43.9%), and the top 3 were ventricular septal defect (VSD) (71, 19.1%), atrial septal defect/patent foramen ovale (52, 14.0%) and tetralogy of Fallot (18, 4.9%). Patients with LAA and ARSCA all had cardiac defects. Genetic syndromes were found in 8 patients (2.2%) and included Down’s syndrome (5 patients), DiGeorge syndrome (2 patients) and Goldenhar syndrome (1 patient). Other extracardiac malformations were rarely found. The patient characteristics are described in detail in Supplementary Material, Table S1.
Clinical manifestations
Due to compression of the trachea and/or oesophagus, most patients with complete vascular rings present with respiratory or gastrointestinal symptoms. Some patients with incomplete vascular rings, especially LAA with ARSCA, present as asymptomatic and are diagnosed by an incidental finding with associated cardiac defects. At the time of admission, respiratory symptoms included stridor found in 71.4% of patients (265/371), wheezing found in 49.1% (182/371), coughing found in 31.5% (117/371), shortness of breath found in 15.1% (56/371), recurrent respiratory tract infections found in 13.2% (49/371) and apnoea found in 5.7% (21/371). Gastrointestinal symptoms were relatively uncommon and presented as choking in 12.4% of patients (46/371), dysphagia in 3.2% (12/371) and emesis in 1.9% (7/371). Respiratory and gastrointestinal symptoms are outlined in Table 2.
Table 2:
Patient symptoms at clinical presentation
| Symptoms | Preoperative no. patients |
Postoperative no. patients |
|---|---|---|
| (N = 371)a | (N = 360)b | |
| Respiratory, n (%) | ||
| Stridor | 265 (71.4%) | 15 (4.2%) |
| Wheezing | 182 (49.1%) | 1 (0.3%) |
| Coughing | 117 (31.5%) | 2 (0.6%) |
| Shortness of breath | 56 (15.1%) | 0 (%) |
| Recurrent respiratory tract infections | 49 (13.2%) | 1 (0.3%) |
| Apnoea | 21 (5.7%) | 0 (0%) |
| Gastrointestinal, n (%) | ||
| Choking | 46 (12.4%) | 1 (0.3%) |
| Dysphagia | 12 (3.2%) | 0 (0%) |
| Emesis | 7 (1.9%) | 0 (0%) |
More than 1 symptom may occur for each patient.
Patients with residual symptoms on long-term follow-up.
Diagnosis
Magnetic resonance imaging and cardiac catheterization are not routine diagnostic methods for vascular rings. Echocardiography and 3D-CT reconstruction were performed in all patients to depict the inner cardiac and extracardiac anatomy. Fibrobronchoscopy was performed preoperatively in 189 patients (50.9%), of whom 68 (18.3%) were diagnosed with tracheomalacia. Overall, in the PAS patients, fibrobronchoscopy was conducted prior to or during surgery, and CTR was observed in 61.2% (52/85) of these patients. Upper gastrointestinal radiography had been conducted previously in only 14 patients.
Surgical management
In patients with DAA, the median operation age was 10 months (IQR, 4–24 months) and the median weight was 9.0 kg (IQR, 7.5–12.0 kg). The left arch was divided in 85.7% (78/91) of patients, and the right arch was divided in 14.3% (13/91). RAA with LLA seems ‘looser’ than DAA. Therefore, RAA with LLA patients were older (12 months (IQR, 6–33 months), P < 0.05) and heavier (10.0 kg (IQR, 7.0–15.0 kg), P < 0.05) than DAA patients at the operation. In most cases, the vascular ring was released by dividing the ligamentum arteriosum. Kommerell diverticulum resection and ALSCA implantation in the left carotid artery was performed in 13 patients (26.5%).
Patients with incomplete vascular rings accompanied by cardiac defects generally present with symptoms earlier than patients with complete vascular rings. The age and weight of patients with PAS were 10 months (IQR, 5–16 months) and 8.5 kg (IQR, 6.6–11.0 kg) and those of patients with LAA with ARSCA were 6 months (IQR, 3–11 months) and 5.9 kg (IQR, 4.7–9.0 kg). Slide tracheoplasty was performed in 6 PAS patients with a long segmental CTR and 4tracheobronchial suspension was performed in 4 patients with tracheomalacia. ARSCA implantation was conducted in 2 patients.
Mortality
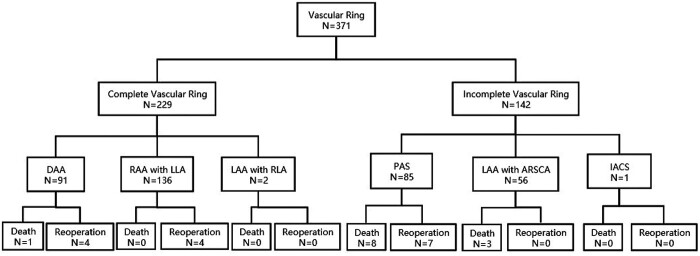
In this multi-institution study, there were 11 hospital deaths (3.0%, 11/371). Three patients with LAA and ARSCA, of whom 2 had total anomalous pulmonary venous connections and 1 had pulmonary atresia, died from low cardiac output and severe pulmonary infection after surgery. Seven patients with PAS accompanied by CTRs died from respiratory failure. A 1-month-old patient with DAA died from severe tracheomalacia and respiratory failure. There was 1 late death (0.3%, 1/360); a 10-month-old patient with PAS experienced cardiac arrest 1 month after discharge and died from multiple organ failure. The overall mortality rate was 3.2% (12/371). A CONSORT flow chart is illustrated in Fig. 1. Incomplete vascular rings, different types of vascular rings, CTR and concomitant cardiac defects were associated with an increased risk of mortality in the univariate analyses (Table 4). Complete vascular ring tended to predict mortality, yielding a hazard ratio of 0.10 (0.01–0.88, P = 0.038), in the multivariable Cox regression analysis (Table 5).
Figure 1:
The CONSORT diagram. ARSCA: aberrant right subclavian artery; DAA: double aortic arch; IACS: innominate artery compression syndrome; LAA: left aortic arch; LLA: left ligamentum arteriosum; PAS: pulmonary artery sling; RAA: right aortic arch; RLA: right ligamentum arteriosum.
Table 4:
Univariate analysis results identifying risk factors for mortality, reoperation and residual symptoms
| Variable | Mortality | P-value | Reoperation | P-value | Residual symptoms | P-value |
|---|---|---|---|---|---|---|
| (N = 371) | (N = 371) | (N = 360) | ||||
| Neonate | 0.59 | 0.37 | 0.008 | |||
| Yes | 1/18 | 0/18 | 2/18 | |||
| No | 11/353 | 15/353 | 18/342 | |||
| Male | 0.76 | 0.38 | 0.83 | |||
| Yes | 8/232 | 11/232 | 14/225 | |||
| No | 4/139 | 4/139 | 6/135 | |||
| Complete vascular ring | 0.005 | 0.45 | <0.001 | |||
| Yes | 1/229 | 8/229 | 10/228 | |||
| No | 11/142 | 7/142 | 10/132 | |||
| Tracheomalacia | 0.39 | <0.001 | 0.014 | |||
| Yes | 1/68 | 9/68 | 9/67 | |||
| No | 11/303 | 6/303 | 11/293 | |||
| Complete tracheal ring | <0.001 | 1.00 | 0.055 | |||
| Yes | 7/52 | 2/52 | 4/45 | |||
| No | 5/319 | 13/319 | 16/315 | |||
| Concomitant cardiac defect | 0.011 | 0.79 | 0.004 | |||
| Yes | 11/163 | 7/163 | 11/153 | |||
| No | 1/208 | 8/208 | 9/207 |
Table 5:
Multivariable analysis of risk factors for mortality, reoperation and residual symptoms
| Risk factor | HR (95% CI) | P-value | |
|---|---|---|---|
| Mortality | Complete tracheal ring | 3.51 (1.03–12.02) | 0.045 |
| Complete vascular ring | 0.24 (0.02–2.78) | 0.26 | |
| Concomitant cardiac defect | 4.51 (0.46–44.76) | 0.20 | |
| Reoperation | Tracheomalacia | 6.85 (2.44–19.25) | <0.001 |
| Residual symptoms | Tracheomalacia | 3.18 (1.29–7.88) | 0.012 |
| Neonate | 3.19 (0.60–17.15) | 0.18 | |
| Complete vascular ring | 0.34 (0.09–1.36) | 0.13 | |
| Complete tracheal ring | 1.63 (0.39–6.81) | 0.50 | |
| Concomitant cardiac defect | 2.01 (0.66–6.15) | 0.22 |
CI: confidence interval; HR: hazard ratio.
Complications
Vascular ring surgery relieved compression of the trachea; however, persistent tracheomalacia remained in 16 patients (4.3%, 2 patients with DAA, 5 patients with RAA with LLA and 9 patients with PAS). Due to injury to the thoracic duct and phrenic and recurrent laryngeal nerves during the operation, chylothorax, diaphragmatic paralysis and recurrent laryngeal nerve palsy were observed in 14 (3.8%), 7 (1.9%) and 2 (0.5%) patients, respectively. Other rare complications included wound indolence, observed in 2 (0.5%) patients, pneumothorax, observed in 3 (0.8%), pleural effusion, observed in 4 (1.1%), and pericardial effusion, observed in 2 (0.5%). Two of 6 patients undergoing slide tracheoplasty were observed through fibrobronchoscopy to have granulation tissue. The postoperative outcomes are summarized in Supplementary Material, Table S2.
Follow-up and reoperation
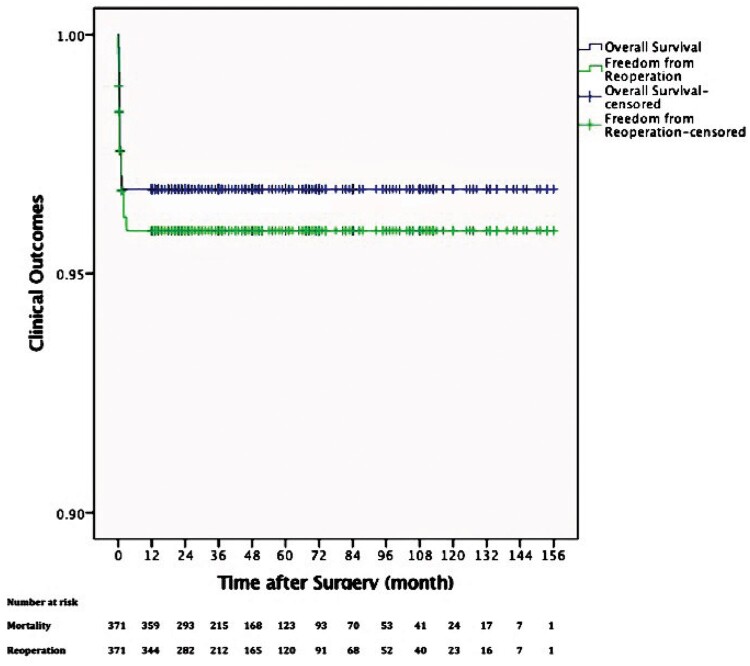
Five patients were lost to follow-up, and a total of 354 patients (95.4%) completed follow-up. The mean follow-up time was 4.3 ± 2.9 years (ranging from 1 year to 13 years). The overall survival rates at 1, 5 and 10 years were 96.8%, 96.8% and 96.8%, respectively (Fig. 2). A total of 15 patients underwent reoperation, including 4 patients with diaphragm plication, 2 with thoracic duct ligation, 7 with endotracheal stent implantation and 2 with interventional therapy under fibrobronchoscopy, as summarized in Table 3. The freedom from reoperation rates at 1, 5 and 10 years were 95.9%, 95.9% and 95.9%, respectively (Fig. 2). Residual symptoms were defined as persistent respiratory or gastrointestinal symptoms at the last follow-up, and 20 patients had residual symptoms, including respiratory symptoms in 19 patients such as stridor (15/360, 4.2%), coughing (2/360, 0.6%), wheezing (1/360, 0.3%), respiratory tract infection (1/360, 0.3%) and gastrointestinal symptom in 1 patient, namely choking (1/360, 0.3%). The univariate analyses and multivariable Cox regression analysis identified tracheomalacia as an independent risk factor for reoperation and residual symptoms (Tables 4 and 5).
Figure 2:
Rate of overall survival and freedom from reoperation related to vascular ring repair. Y-axis is truncated at 0.9.
Table 3:
Reinterventions by type of vascular ring
| Type of vascular ring | Time to reintervention | Type of reintervention |
|---|---|---|
| Double aortic arch | 10 days | Diaphragm plication |
| Pulmonary artery sling | 15 days | Diaphragm plication |
| Pulmonary artery sling | 3 days | Diaphragm plication |
| Double aortic arch | 5 days | Diaphragm plication |
| Double aortic arch | 7 days | Thoracic duct ligation |
| RAA, mirror-image branching, LLA | 15 days | Thoracic duct ligation |
| Pulmonary artery sling | 1 day | Endotracheal stent implantation |
| Pulmonary artery sling | 1 month | Endotracheal stent implantation |
| RAA-mirror-image branching, LLA | 2 months | Endotracheal stent implantation |
| Pulmonary artery sling | 7 days | Endotracheal stent implantation |
| RAA-mirror-image branching, LLA | 3 months | Endotracheal stent implantation |
| Double aortic arch | 1 month | Endotracheal stent implantation |
| RAA-ALSCA, LLA | 2 months | Endotracheal stent implantation |
| Pulmonary artery sling | 14 days | Interventional therapy under fibrobronchoscopy |
| Pulmonary artery sling | 1 month | Interventional therapy under fibrobronchoscopy |
ALSCA: aberrant left subclavian artery; RAA: right aortic arch; LLA: left ligamentum arteriosum.
DISCUSSION
Vascular ring development begins with the embryonic aortic arch system, which consists of a ventral and dorsal aorta connected by 6 pairs of primitive aortic arches [9]. Persistence, regression or involution of the arches determines the type of vascular ring, of which there are multiple, and results in varying degrees of tracheal and/or oesophageal compression and patient presentation with various symptoms.
The formation of DAA is due to the persistence of the LAA and RAA and dorsal aorta. Previous studies reported that the percentage of right arch dominance was 80%, which is consistent with our results (71.4%, 65/91) [6, 10]. Moreover, it is uncommon in DAA patients with cardiac defects [11], and only 7 ventricular septal defects and 1 transposition of great arteries were identified in our patients with DAA. In DAA, the ring consists of 2 aortic arches and seems relatively “tighter” than other types of vascular rings; accordingly, the symptoms of respiratory distress and feeding problems develop earlier. We believe that in all patients with DAA, the DAA should be repaired after diagnosis to avoid serious complications caused by long-term compression of the trachea and oesophagus. In the patients in this study, 3D-CT reconstruction was performed to diagnose DAA quickly with a low radiation dose and to provide information about the trachea and oesophagus; this approach allows precise planning of the surgical strategy. The approach is usually through the left fourth intercostal space for a dominant right arch and equal-sized arches, and we recommended dividing the left arch. The mediastinal pleura should be left open after the operation to avoid scar tissue regeneration and recurrent stenosis. Four patients continued to have slight wheezing after the operation, but none underwent reoperation. The vagus and recurrent laryngeal and phrenic nerves must be carefully identified and protected; otherwise, diaphragmatic paralysis can occur, as we found in 5 patients, and diaphragm plication was performed in 2 patients.
The persistence of the right fourth arch with interruption of the left arch results in RAA with LLA. This type of vascular ring seems ‘looser’ than DAA, and the symptoms manifest later. Echocardiography can comprehensively assess the intracardiac defect and reveal the vascular ring; however, it cannot depict the trachea and oesophagus. In the patients in this study, 3D-CT reconstruction was conducted and provided an excellent evaluation preoperatively. The approach to isolate RAA with LLA is through the left fourth intercostal space. Once a patient is diagnosed with a cardiac defect, the operation is performed through a median sternotomy incision with concomitant CPB. Division of the ligamentum arteriosum significantly relieved compression, especially in patients with RAA with mirror-image branching and LAA [12]. The dilated origin of ALSCA is a remnant of the fourth aortic arch, called the Kommerell diverticulum, which was first described in 1936 [13]. Backer et al. [8] found that Kommerell diverticulum is one of the 4 primary indications for reoperation in 26 patients. However, previous studies have reported that severe complications, such as aortic dissection and aorta rupture, can occur after Kommerell diverticulum resection [14, 15]. In our series of 49 patients with Kommerell diverticulum, we resected the Kommerell diverticulum and translocated the ALSCA to the left carotid artery in 13 patients [8]. The remaining patients had a simple division of the ligamentum arteriosum, and only 7 of them had slight or moderate respiratory symptoms. Therefore, primary resection of the Kommerell diverticulum and translocation of the ALSCA might achieve good outcomes in eliminating respiratory symptoms after the operation. However, considering the risk of severe complications during the procedure and the relief of respiratory symptoms after division of the ligamentum arteriosum (0/13 vs 7/36, P = 0.17) [2, 16], a Kommerell diverticulum >1.5 times the origin of the ALSCA with obvious compression of the trachea is an operative indication. For patients with a small Kommerell diverticulum, single division of the ligamentum arteriosum could simplify the operative procedure, reduce the severe operative complications and achieve desirable clinical effects.
PAS is a malformation in which the left pulmonary artery arises from the right pulmonary artery, crosses behind the trachea and in front of the oesophagus and reaches the left pulmonary hilum, causing compression of the trachea as a sling. Berdon [17] first proposed the ‘ring-sling complex’ and more than half of PAS cases occur in combination with a CTR [18]. Therefore, patients suffer respiratory symptoms owing to both artery compression and tracheal stenosis. Echocardiography could not only depict the intracardiac anatomy but also distinguish the anomalous origin of the left pulmonary artery. In addition, 3D-CT reconstruction and fibrobronchoscopy were performed to evaluate the trachea. In our series of patients with PAS, 61.2% (52/85) of patients had CTRs. Surgery was recommended when the patient was diagnosed with PAS. The left pulmonary artery was transected from its origin on the right pulmonary artery, pulled to the left side in front of the trachea and implanted in the anatomic origin of the left pulmonary artery in the main pulmonary artery through median sternotomy. Wilcox et al. [19] reported that an estimated 17% of children could be managed conservatively but not those with long segmental CTRs. A previous study revealed that the CTR grew at a faster-than-normal rate and reached a normal diameter by the age of 9 years [20]. Six patients underwent slide tracheoplasty, among them, granulation tissue was observed in 2 patients, 1 of whom died. The mortality (16.7%, 1/6) and incidence of granulation tissue (33.3%, 2/6) were consistent with Zhang et al.’s early-stage results [20]. Our study showed that CTR may be a risk factor for hospital mortality (7/52 vs 0/33, P = 0.040), while slide tracheoplasty did not increase hospital mortality (1/6 vs 6/46, P = 1.00). Therefore, we recommended slide tracheoplasty for patients with a long segmental CTR and stenosis more than half of the normal diameter, allowing simultaneous repair of the cardiac defect. Among the remaining 45 patients with CTR, only 4 patients presented with mild respiratory symptoms and showed slight tracheal stenosis with 3D-CT reconstruction (4/45 vs 0/32, P = 0.14), which indicated that the CTR has growth potential.
The respiratory symptoms of patients with LAA and ARSCA are not caused by compression of the trachea but by cardiac insufficiency. Occasionally, retroesophageal ARSCA might cause dysphagia. Diagnosis was made by accident with echocardiography or 3D-CT reconstruction. Cardiac defects were repaired by CPB, the ligamentum arteriosum was divided, and the patients usually had excellent outcomes. The rare vascular ring patients included 2 patients with LAA with right-sided descending aorta and RAA and 1 patient with innominate artery compression syndrome. Ligamentum arteriosum division and innominate artery suspension released compression of the trachea, and the outcome was satisfactory.
Tracheomalacia is often accompanied by a vascular ring, and after relief of the compression, the trachea can require several months to recover. Backer et al. [17] stated that tracheomalacia is one of the primary indications for reoperation, which is consistent with our results. In our series of vascular rings, there were 16 patients with persistent tracheomalacia after operation, 7 of whom underwent reoperation with endotracheal stent implantation. The remaining 9 patients continued to present with mild-to-moderate respiratory symptoms, such as stridor and wheezing. Tracheomalacia was a predictor of reoperation and residual symptoms, which adversely impact the long-term outcomes of vascular ring division. Therefore, preoperative fibrobronchoscopy evaluation of the degree of tracheomalacia is important for designing surgical strategies.
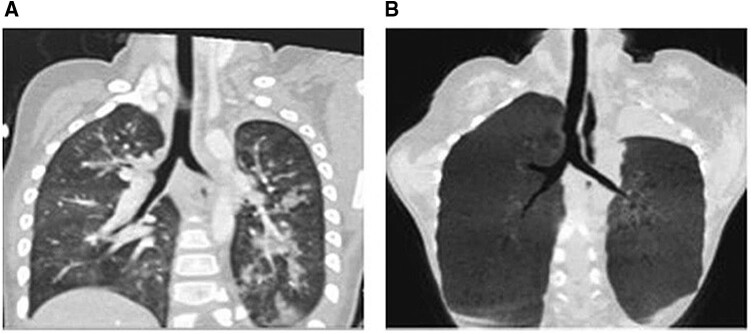
In 2013, the University of Michigan reported the first application of a 3D-printed bioresorbable airway splint, which was manufactured from polycaprolactone with a bellowed topology design to an infant with severe tracheomalacia, which provided resistance against collapse while simultaneously allowing flexion, extension and expansion with growth [21]. In a cohort of children with severe tracheomalacia, the 3D-printed bioresorbable airway splint was found safe and provided a desirable clinical outcome [22]. In the series in the present study, 4 patients with tracheomalacia were treated preoperatively with 3D-printed, patient-specific, bioresorbable airway splints, and all had excellent outcomes (Fig. 3). According to Serio et al.’s suggestion [23], we managed tracheomalacia and found that the tracheal lumen decreased by >70% during the operation. Therefore, we believe that in addition to endotracheal stent implantation, 3D-printed bioresorbable airway splints may be an option for patients with severe tracheomalacia.
Figure 3:
Patients with severe tracheomalacia treated with 3D-printed bioresorbable airway splints: (A) preoperatively and (B) 1 year postoperatively.
Limitations
This present study had the limitations of a retrospective study. As the study was a multi-institution study, surgeon experience and the level of perioperative management might have affected the results.
CONCLUSION
Surgical repair of the vascular ring carries a low risk of mortality and reoperation, with excellent outcomes. Primary resection of the Kommerell diverticulum and translocation of ALSCA should be considered to eliminate respiratory symptoms when the Kommerell diverticulum is >1.5 times the origin of the ALSCA and causes obvious compression of the trachea. Tracheomalacia is a risk factor for reoperation and residual symptoms; therefore, preoperative fibrobronchoscopy for the evaluation of tracheomalacia is important and 3D-printed bioresorbable airway splints may be an option for patients with severe tracheomalacia.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFC1308105 and 2016YFC1101001), Clinical Frontier Technology of Clinical Medicine of Jiangsu Provincial Science and Technology Department (BE2017608) and National Natural Science Foundation of China (81700288 and 81970265).
Conflict of interest: none declared.
Author contributions
Di Yu: Conceptualization; Data curation; Formal analysis; Writing—original draft. Zhangke Guo: Conceptualization; Data curation; Formal analysis; Writing—original draft. Xin You: Data curation; Formal analysis; Writing—original draft. Wei Peng: Conceptualization; Writing—original draft. Jirong Qi: Formal analysis; Methodology; Writing—original draft. Jian Sun: Methodology; Writing—original draft. Kaihong Wu: Data curation; Formal analysis; Writing—original draft. Xiaofeng Li: Conceptualization; Writing—review & editing. Xuming Mo: Conceptualization; Funding acquisition; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Carl Lewis Backer and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- 3D
Three-dimensional
- ALSCA
Aberrant left subclavian artery
- ARSCA
Aberrant right subclavian artery
- CPB
Cardiopulmonary bypass
- CT
Computed tomography
- CTR
Complete tracheal ring
- DAA
Double aortic arch
- IQR
Interquartile range
- LAA
Left aortic arch
- LLA
Left ligamentum arteriosum
- PAS
Pulmonary artery sling
- RAA
Right aortic arch
REFERENCES
- 1. Gross RE. Surgical relief for tracheal obstruction from a vascular ring. N Engl J Med 1945;233:586–90. [DOI] [PubMed] [Google Scholar]
- 2. Backer CL, Mavroudis C, Rigsby CK, Holinger LD.. Trends in vascular ring surgery. J Thorac Cardiovasc Surg 2005;129:1339–47. [DOI] [PubMed] [Google Scholar]
- 3. Lee EY, Boiselle PM, Shamberger RC.. Multidetector computed tomography and 3-dimensional imaging: preoperative evaluation of thoracic vascular and tracheobronchial anomalies and abnormalities in pediatric patients. J Pediatr Surg 2010;45:811–21. [DOI] [PubMed] [Google Scholar]
- 4. Lambert V, Sigal-Cinqualbre A, Belli E, Planché C, Roussin R, Serraf A. et al. Preoperative and postoperative evaluation of airways compression in pediatric patients with 3-dimensional multislice computed tomographic scanning: effect on surgical management. J Thorac Cardiovasc Surg 2005;129:1111–8. [DOI] [PubMed] [Google Scholar]
- 5. Backer CL, Mavroudis C.. Congenital Heart Surgery Nomenclature and Database Project: vascular rings, tracheal stenosis, pectus excavatum. Ann Thorac Surg 2000;69:S308–18. [DOI] [PubMed] [Google Scholar]
- 6. Yoshimura N, Fukahara K, Yamashita A, Doi T, Yamashita S, Homma T. et al. Congenital vascular ring. Surg Today 2020;50:1151–8. [DOI] [PubMed] [Google Scholar]
- 7. Nuutinen J. Acquired tracheobronchomalacia. A clinical study withbronchological correlations. Ann Clin Res 1977;9:350–5. [PubMed] [Google Scholar]
- 8. Backer CL, Monge MC, Russell HM, Popescu AR, Rastatter JC, Costello JM.. Reoperation after vascular ring repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2014;17:48–55. [DOI] [PubMed] [Google Scholar]
- 9. Edwards JE. Anomalies of the derivatives of the aortic arch system. Med Clin North Am 1948;32:925–49. [DOI] [PubMed] [Google Scholar]
- 10. Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW, Brown JW.. Follow-up of surgical correction of aortic arch anomalies causing tracheoesophageal compression: a 38-year single institution experience. J Pediatr Surg 2009;44:1328–32. [DOI] [PubMed] [Google Scholar]
- 11. Alsenaidi K, Gurofsky R, Karamlou T, Williams WG, McCrindle BW.. Management and outcomes of double aortic arch in 81 patients. Pediatrics 2006;118:e1336-41–e1341. [DOI] [PubMed] [Google Scholar]
- 12. Binsalamah ZM, Ibarra C, John R, Zea-Vera R, Adachi I, Imamura M. et al. Contemporary midterm outcomes in pediatric patients undergoing vascular ring repair. Ann Thorac Surg 2020;109:566–72. [DOI] [PubMed] [Google Scholar]
- 13. Naimo PS, Fricke TA, Donald JS, Sawan E, d'Udekem Y, Brizard CP. et al. Long-term outcomes of complete vascular ring division in children: a 36-year experience from a single institution. Interact CardioVasc Thorac Surg 2017;24:234–9. [DOI] [PubMed] [Google Scholar]
- 14. Braunberger E, Mercier F, Fornes P, Julia PL, Fabiani JN.. Aortic dissection of Kommerell's diverticulum in Marfan's syndrome. Ann Thorac Surg 1999;67:1160–2. [DOI] [PubMed] [Google Scholar]
- 15. Fisher RG, Whigham CJ, Trinh C.. Diverticula of Kommerell and aberrant subclavian arteries complicated by aneurysms. Cardiovasc Intervent Radiol 2005;28:553–60. [DOI] [PubMed] [Google Scholar]
- 16. Shinkawa T, Greenberg SB, Jaquiss RDB, Imamura M.. Primary translocation of aberrant left subclavian artery for children with symptomatic vascular ring. Ann Thorac Surg 2012;93:1262–5. [DOI] [PubMed] [Google Scholar]
- 17. Berdon WE, Baker DH, Wung JT, Chrispin A, Kozlowski K, de Silva M. et al. Complete cartilage-ring tracheal stenosis associated with anomalous left pulmonary artery: the ring-sling complex. Radiology 1984;152:57–64. [DOI] [PubMed] [Google Scholar]
- 18. Butler CR, Speggiorin S, Rijnberg FM, Roebuck DJ, Muthialu N, Hewitt RJ. et al. Outcomes of slide tracheoplasty in 101 children: a 17-year single-center experience. J Thorac Cardiovasc Surg 2014;147:1783–9. [DOI] [PubMed] [Google Scholar]
- 19. Wilcox LJ, Hart CK, de Alarcon A, Schweiger C, Peddireddy NS, Tabangin M. et al. Unrepaired complete tracheal rings: natural history and management considerations. Otolaryngol Head Neck Surg 2018;158:729–35. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, Wang S, Lu Z, Zhu L, Du X, Wang H. et al. Slide tracheoplasty in 81 children: improved outcomes with modified surgical technique and optimal surgical age. Medicine (Baltimore) 2017;96:e8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE.. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med 2013;368:2043–5. [DOI] [PubMed] [Google Scholar]
- 22. Les AS, Ohye RG, Filbrun AG, Ghadimi Mahani M, Flanagan CL, Daniels RC. et al. 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019;129:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serio P, Fainardi V, Leone R, Baggi R, Grisotto L, Biggeri A. et al. Tracheobronchial obstruction: follow-up study of 100 children treated with airway stenting. Eur J Cardiothorac Surg 2014;45:e100–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.