Abstract
Mycoplasma genitalium (MG) infection, a sexually transmitted infection (STI), causes cervicitis and may cause reproductive sequelae and adverse pregnancy outcomes. Some MG-infected women report dysuria, a symptom frequently attributed to urinary tract infection (UTI). Given potential MG-associated morbidity and the likelihood that UTI treatment would be ineffective in eradicating MG, an improved understanding of MG infection frequency and clinical significance in young women reporting dysuria is needed. We conducted MG testing on stored urogenital specimens collected in a pilot study on frequency of STIs in young women presenting to an emergency department for dysuria evaluation and performed a literature review on MG infection frequency in women reporting dysuria. Among 25 women presenting for dysuria evaluation in our pilot study, 6 (24.0%) had MG detected and one third had co-infection with chlamydia and one-third with trichomoniasis; half with MG detected did not receive an antibiotic with known efficacy against MG, while the other half received azithromycin. In 5 studies identified in the literature review, dysuria was reported by 7%−19% of women and MG detected in 5%−22%. MG infection is common in young women with dysuria and empiric UTI treatment may not be effective against MG. Studies evaluating the clinical significance of MG infection in women reporting dysuria are needed.
Keywords: Mycoplasma genitalium, dysuria, women, review
Introduction
Mycoplasma genitalium (MG) is a sexually transmitted bacteria that causes infection of the urogenital, anorectal, and oropharyngeal sites.1,2 MG infection may be a significant health burden in women, in whom it can cause cervicitis and has been associated with reproductive sequelae (e.g., pelvic inflammatory disease and infertility) and adverse pregnancy outcomes (e.g., preterm birth) in limited studies.3 MG infection prevalence is low in the general female population (0.8%−2%),4–6 with rates lower than those reported for another important sexually transmitted infection (STI), chlamydia.4–6 However, MG prevalence is much higher in clinic-based female populations, ranging from about 10%−16%,7–10 and can be higher than the chlamydia prevalence among these female populations.7,9 Although antibiotic resistance has not been a concern with treating chlamydia, which is commonly managed using doxycycline or azithromycin, antibiotic resistance, including macrolide resistance, is a major challenge in managing MG infection and there are currently limited available effective therapies to treat resistant MG strains.11
The majority of MG infections are asymptomatic,4,5 however symptoms that have been reported in MG-infected women include vaginal discharge and odor, dysuria, pain during sex, pelvic/abdominal pain, and bleeding between menstrual periods.4,5,12,13 The most common cause of dysuria (pain on urination) in young women is either urinary tract infection (UTI) or STI.14 Dysuria in young women is most frequently attributed to a UTI,15–17 which merely requires a history and urinalysis for diagnosis; urine culture is not recommended in young women with a suspected uncomplicated UTI.14 The overall lack of diagnostic rigor in evaluating women with dysuria has potentially led to the overdiagnosis of UTI and underdiagnosis of STIs in both adolescent and young adult female populations.15–17 Because commonly prescribed antibiotics for uncomplicated UTIs may not adequately treat STIs, the lack of STI testing in young women with dysuria can lead to a missed opportunity to diagnose and treat an STI; this incomplete patient management could put women at risk for developing reproductive sequelae of STIs.18 Previous studies that have performed testing for STIs (chlamydia and gonorrhea, with or without trichomoniasis) in young women presenting with urinary symptoms (dysuria, frequency, and/or urgency) have reported one or more STIs diagnosed in 9%−28.0%.15–18 We previously conducted a retrospective medical record review study to investigate how often women diagnosed with a UTI in a university-affiliated emergency department (ED) had chlamydia testing done and the positivity and treatment rate.19 We found that only 20% of women with dysuria had chlamydia testing done and among these women, 21% tested positive; however, only 42% were prescribed a recommended treatment for chlamydia at that visit. Thus, because STIs, such as chlamydia, are a common cause of dysuria in young women, differentiating a UTI from STIs is crucial to ensure effective treatment is provided.14
There are sparse data on presence of MG infection in young women with dysuria,4,5,12,20–26 and to our knowledge, there have been no previous studies that have investigated MG infection in women presenting for evaluation of a chief complaint of dysuria, a population most likely to be diagnosed with a UTI. Currently, there are no recommendations for MG testing in women with dysuria. Given the potential contribution of MG infection to reproductive sequelae and adverse pregnancy outcomes3 and the likelihood that treatment prescribed for a UTI would be ineffective in eradicating MG, an improved understanding of the frequency of MG infection in young women with dysuria and its clinical significance is needed. To begin to address this need, we performed MG testing on stored urogenital specimens that had been collected in a prospective pilot study on the frequency of STIs in young women presenting with a chief complaint of dysuria to the same ED as our previous retrospective study.19 In addition to performing MG testing on specimens from this study cohort from the ED, we conducted a literature review on the frequency of MG infection in women reporting dysuria to determine if the MG prevalence we found in this cohort reflected the published data on MG prevalence in women reporting dysuria.
Methods
Pilot study and MG testing
Study design, population, and procedures.
We performed MG testing on stored urogenital specimens and analyzed stored clinical data that were previously obtained in a pilot study whose aim was to evaluate the prevalence of chlamydia in women presenting with dysuria and to determine the clinical and laboratory characteristics that distinguish chlamydia versus UTI as the cause for dysuria. The study enrolled women 16–29 years of age who presented to a university-affiliated ED in a U.S. city for evaluation of a chief complaint of dysuria. The study excluded women who were pregnant, had a prior hysterectomy, had HIV infection, diabetes, or another immunocompromising medical condition, who were on immunosuppressive therapy, who received antibiotics with anti-chlamydia activity within the prior one month, or who reported never having been sexually active. All eligible patients provided written informed consent before participating in the study. At enrollment, participants were interviewed and information was collected on demographics and medical and sexual history. Participants self-collected a vaginal swab for NAAT testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis (Aptima, Hologic Inc., San Diego, CA) and a midstream clean-catch urine specimen for urinalysis and urine culture. Participants who tested positive for an STI or UTI were provided treatment if they did not already receive adequate treatment as part of their routine ED visit. The study began enrolling in July 2017 and there were 30 participants enrolled through February 2020, of whom 25 consented for future use of their urogenital specimens for STI research and were tested for MG in this current study. For this study, residual vaginal swab and urine specimens stored at −20° C were thawed and MG testing was performed using the Aptima MG assay (Hologic, Inc.) per the manufacturer’s instructions. The study was approved by the university’s Institutional Review Board.
Statistical analyses.
Analyses were conducted on Stata (version 14.0, StataCorp, College Station, TX). Demographical, clinical, and laboratory data were summarized by descriptive statistics (frequencies, median, and range). Associations of MG with these data were evaluated by Fisher’s exact and Wilcoxon rank sum tests for categorical and continuous variables, respectively.
Literature review
Data source and searches.
A review of the literature from 1981 to April 2020 was conducted using the new PubMed computerized database of the US National Library of Medicine. Multiple literature searches were conducted using the following major subject heading [MeSH] terms: “Mycoplasma genitalium” and “dysuria”, “Mycoplasma genitalium” and “urethritis”, “Mycoplasma genitalium” and “cervicitis”, “Mycoplasma genitalium” and “urinary tract infection”, “Mycoplasma genitalium” and “female urethral syndrome”, and “Mycoplasma genitalium” and “symptoms”. The searches were limited to females and human subjects. Duplicate articles were eliminated.
Study selection.
Inclusion criteria for initial screening of studies included dysuria as a documented reported symptom in females. Studies were then screened for inclusion based on whether they included MG testing results; they were excluded if they pre-selected study groups based on MG infection status. Studies meeting inclusion criteria after this initial screening were then assessed for eligibility based upon if: 1) dysuria was stratified by gender, 2) dysuria was stratified from other symptoms, and 3) MG results in women with dysuria were separated from results of other STI testing. Thus, to address our objective of summarizing the reported frequency of MG in women reporting dysuria, studies in which the frequency of MG in women reporting dysuria could be determined were eligible and those in which it could not be determined were excluded. The literature review was not intended to evaluate for association of MG infection with dysuria. The selection process is outlined in Figure 1 in a 4-phase flow diagram per the PRISMA Statement.27
Figure 1.
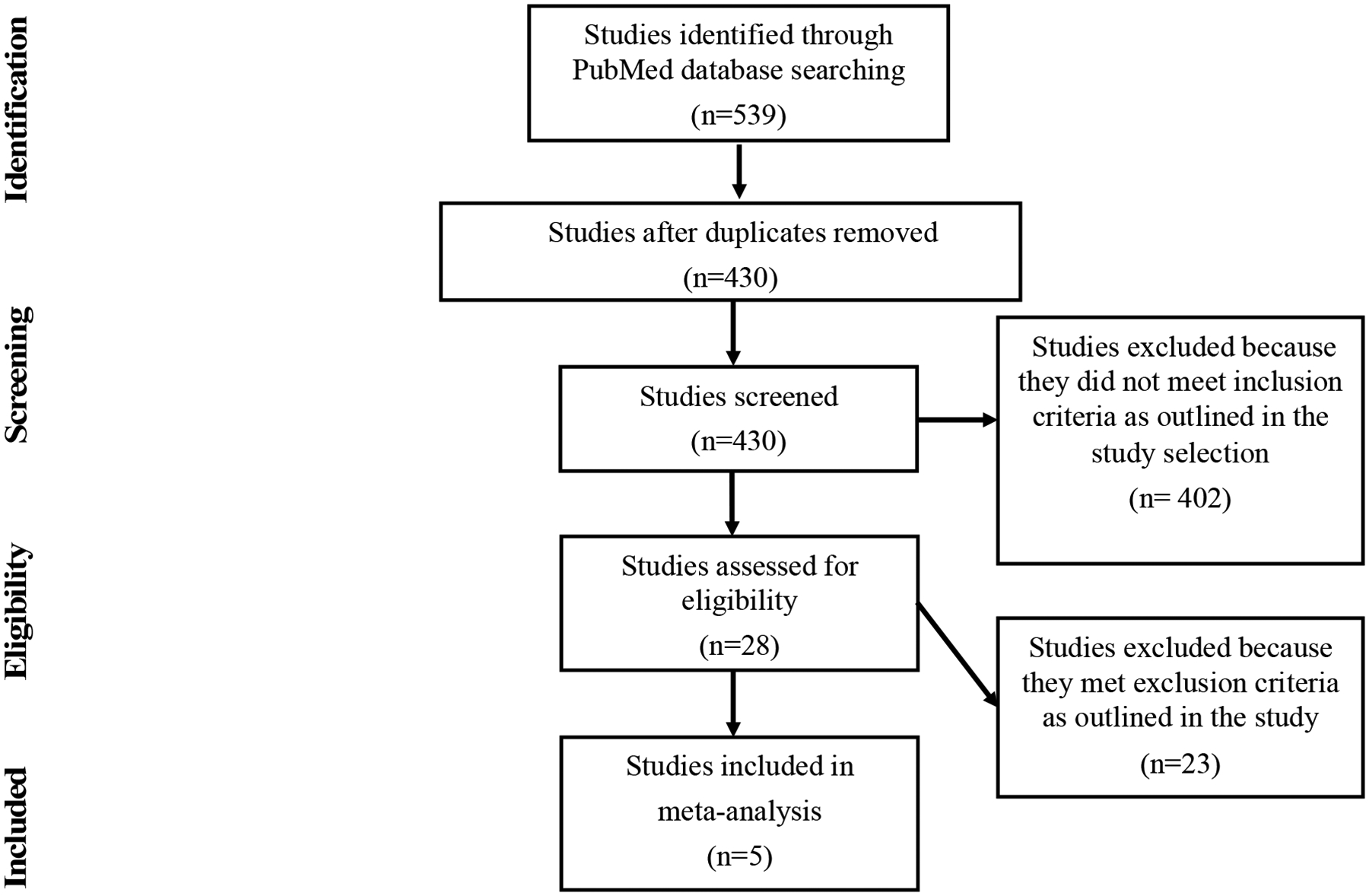
Study selection process for the literature review on Mycoplasma genitalium infection frequency in women reporting dysuria
Data extraction and synthesis.
A review matrix was used to assess articles and extract the following data items: author, title, journal, publication year, methodological design, clinical setting and study population, study location, female sample size, age, the proportion of women with dysuria, and the frequency of MG infection among women with dysuria. The matrix was used to identify which studies met eligibility criteria.
Results
MG positivity in the pilot study participants
The demographic, behavioral, and clinical characteristics of the 25 participants evaluated, stratified by MG test result (positive vs. negative), are shown in Table 1. The median age was 22 years (range 17–29). Most women (96.0%) reported being sexually active in the last 3 months, with a median sexual partner number of 1 (range 1–4) and median number of days since last sexual contact of 3 (range 0–30). The majority (64.0%) were non-Hispanic black, with 28.0% Non-Hispanic white, 4.0% non-Hispanic multi-race, and 4.0% Hispanic other race. In addition to all women reporting dysuria, the majority reported one or more other urogenital symptoms, with the most common being urinary urgency (80.0%), pelvic bladder fullness (64.0%), pelvic pain (76.0%), back pain (64.0%), and vaginal discharge (52.0%). Most women (88.0%) reported a prior UTI diagnosis and 52.0% reported prior chlamydia.
Table 1.
Comparison of characteristics between women with dysuria who tested positive versus negative for Mycoplasma genitalium (MG)
| Characteristics | Total (n = 25) |
MG positive (n = 6) |
MG negative (n = 19) |
p-valuesa |
|---|---|---|---|---|
| Age, median (range) | 22 (17–29) | 21 (17–24) | 22 (18–29) | 0.249 |
| African American, N (%) | 17 (68.0%) | 5 (83.3%) | 12 (63.2%) | 0.624 |
| Hispanic, N (%) | 1 (4.0%) | 0 (0.0%) | 1 (5.3%) | 1.000 |
| Sexually active in last 3 months, N (%)b | 24 (96.0%) | 6 (100.0%) | 18 (94.7) | 1.000 |
| Sexual partner number last 3 months, median (range)b | 1 (1–4) | 1(1–4) | 1 (1–2) | 0.181 |
| Number of days since last sex with any partner, median (range)b | 3 (0–30) | 1.5 (1–30) | 3 (0–21) | 0.613 |
| Hormonal contraceptive use, N (%) | 4 (16.0%) | 1 (16.7%) | 3 (15.8%) | 1.000 |
| Self-reported symptoms | ||||
| Urinary urgency | 20 (80.0%) | 5 (83.3%) | 15 (79.0%) | 1.000 |
| Pelvic bladder fullness | 16 (64.0%) | 5 (83.3%) | 11 (57.9%) | 0.364 |
| Pelvic pain | 19 (76.0%) | 3 (50.0%) | 16 (84.2%) | 0.125 |
| Back pain | 16 (64.0%) | 6 (100.0%) | 10 (52.6%) | 0.057 |
| Vaginal discharge | 13 (52.0%) | 6 (100.0%) | 7 (36.8%) | 0.015 |
| Irregular vaginal bleeding | 5 (20.0%) | 2 (33.3%) | 3 (15.8%) | 0.562 |
| Painful intercourse | 9 (36.0%) | 3 (50.0%) | 6 (31.6%) | 0.630 |
| Self-reported lifetime history | ||||
| Chlamydia | 13 (52.0%) | 5 (83.3%) | 8 (42.1%) | 0.160 |
| UTI | 22 (88.0%) | 6 (100.0%) | 16 (84.2%) | 0.554 |
| Gonorrhea | 4 (16.0%) | 1 (16.7%) | 3 (15.8%) | 1.000 |
| Trichomoniasis | 6 (24.0%) | 2 (33.3%) | 4 (21.1%) | 0.606 |
| Vaginal candidiasis | 3 (12.0%) | 2 (33.3%) | 1 (5.3%) | 0.133 |
| Syphilis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Genital herpes | 1 (4.0%) | 1 (16.7%) | 0 (0.0%) | 0.240 |
| Bacterial vaginosis | 5 (20.0%) | 1 (16.7%) | 4 (21.1%) | 1.000 |
| Genital warts | 2 (8.0%) | 1 (16.7%) | 1 (5.3%) | 0.430 |
| Laboratory Testing | ||||
| Positive urine leukocytes | 16 (64.0%) | 3 (50.0%) | 13 (68.4%) | 0.630 |
| Positive urine culturec | 10 (40%) | 2 (33.3%) | 8 (42.11%) | 1.000 |
| Positive C. trachomatis NAAT, N (%) | 5 (20.0%) | 2 (33.3%) | 3 (15.79%) | 0.562 |
| Positive N. gonorrhoeae NAAT, N (%) | 2 (8.0%) | 0 (0.0%) | 2 (10.53%) | 1.000 |
| Positive T. vaginalis NAAT, N (%) | 3 (12.0%) | 2 (33.3%) | 1 (5.26%) | 0.133 |
NAAT: nucleic acid amplification test
p-value determined for the following variables using Wilcoxon rank sum tests: age, sexual partner number last 3 months, and number of days since last sex with any partner; p-values for all other variables were determined with Fisher’s exact test
N=1 reporting no sexual activity in the last 3 months (in the MG negative group), resulting in N = 1 missing for the following variables: number of partners in last 3 months and number of days since last sex with any partner
only includes known uropathogens at ≥102 CFU/mL
There were 6 (24.0%) women who tested positive for MG, 4 by both vaginal swab and urine specimens and 2 by vaginal swab specimens only. Of those testing positive for MG, 33.3% were co-infected with chlamydia, 33.3% with trichomoniasis, and none with gonorrhea; also, 33.3% had a urine culture positive for a known UTI pathogen at ≥102 CFU/mL. Among the demographic, behavioral, and clinical characteristics analyzed, MG positivity was significantly associated with a reported symptom of vaginal discharge (p = 0.015); there was also a trend towards MG positivity being associated with reported back pain (p = 0.057). There were no MG positive tests in women who denied having sex within 3 months prior to enrollment. Half of the women who tested positive for MG did not receive an antibiotic that would be effective against MG, while the other half received azithromycin.
Literature review findings
The search for studies on MG infection in women reporting dysuria in which the MeSH term “Mycoplasma genitalium” was combined with different MeSH terms that encompassed dysuria returned a total of 539 studies (Figure 1). There were 9 studies found using the MeSH term “dysuria”, 61 using “urethritis”, 96 using “cervicitis”, 14 using “urinary tract infections”, 9 using “female urethral syndrome”, and 350 using “symptoms”. The total number of studies was reduced to 430 after accounting for duplicates. The remaining full-text articles were screened based on eligibility criteria. A total of 5 studies met the eligibility criteria (Table 2).12,20,21,23,24
Table 2.
Literature review findings for studies on Mycoplasma genitalium (MG) infection frequency in women reporting dysuria.
| Reference | Pub.year | Methodological design | Clinical setting and population | Location | Female subjects | Age | Dysuria | Dysuria, MG+ |
|---|---|---|---|---|---|---|---|---|
| n | n (%) | n (%) | ||||||
| 20 | 2012 | cross-sectional case control | women involved in commercial sex work or employed in entertainment facilities were recruited via community engagement for screening in a general care clinic | Kampala, Uganda | 972 | median 26 (IQR 22–30) | 110 (11) | 24 (22) |
| 21 | 2008 | cross-sectional | sexually active females attending gynecological evaluations in private clinics | Zulia, Venezuela | 172 | mean 31 (range 20–48) | 20 (12) | 1 (5) |
| 23 | 2008 | cross-sectional case control | sexually active female adolescents with genitourinary symptoms or at risk for STI were recruited from an urban Teen Health Center or ED | Cincinatti, USA | 331 | mean 18 (range 14–21) | 63 (19) | 9 (14) |
| 24 | 2005 | cross-sectional | patients attending an STD clinic due to STI symptoms or for a check up | Falun, Sweden | 443 | median 25 (range 14–55) | 70 (16) | 7 (10) |
| 12 | 2020 | prospective case control | sexually active persons with or without genitourinary STI symptoms seen at participating clinical sites | Multi-city, USA | 1737 | median 29 (range 15–74) | 125 (7) | 18 (14) |
The clinical settings of the 5 identified studies included community and private clinics, EDs, an adolescent clinic (teen health center), STD clinics, family planning clinics, and clinical research centers (Table 2).12,20,21,23,24 Two studies were based in the US,12,23 one in Sweden,24 one in Uganda,20 and one in Venezuela.21 The populations were heterogeneous, including sex workers recruited via community engagement, women presenting with or without gynecological or genitourinary symptoms of varying acuity, sexually active adolescents, and women presenting for check-ups.12,20,21,23,24 Study population sizes ranged from 172–1737 women and the mean or median age ranged from 18 to 31. Most studies did not find significant associations between MG infection and sociodemographic factors, with the exception of Manhart et al.,12 who found that women who were black, non-Hispanic, or seeking care in ED or STI clinic settings were more likely to have MG infection. Most studies showed that MG infection was more prevalent among younger women, although one study showed the highest prevalence was observed in women ages 31–40.21 One study found that MG-infected women less often reported hormonal contraception use at the time of their last sexual encounter.23 Multiple studies concluded that MG infection was more prevalent among women with specific sexual risk behaviors, including, but not limited to, shorter interval since last sexual contact, sexual contact with a MG positive partner(s), increasing number of sexual partners, and being co-infected with other STIs.20,23,24
In contrast to our pilot study which only included women reporting dysuria, the proportion of women reporting dysuria in the 5 identified studies ranged from 7%−19%; the proportion of women with dysuria who had MG detected in urogenital specimens ranged from 5%−22%.12,20,21,23,24 Two studies reported MG infection was significantly associated with genitourinary symptoms, including vaginal discharge, vaginal odor, and dysuria;12,20 one study reported a negative association of dysuria with MG infection.23
Discussion
Our MG prevalence study is the first to our knowledge to evaluate the frequency of urogenital MG infection in young women specifically presenting for evaluation of a chief complaint of dysuria, a symptom in young women which is usually attributed to a UTI.15–17 We found MG infection was common in women presenting to the ED with dysuria, being detected in about one out of four women, which was higher than the frequency of MG positivity among women reporting dysuria in the 5 studies identified in our literature review (5%−22%).12,20,21,23,24 While this may in part reflect differences in STI risk behaviors in the populations (about 50% of our study participants reported prior chlamydia), it also likely reflects differences in sensitivities of the MG NAAT used. Most of the studies from the literature review used a research-based MG NAAT, while our study used the Hologic Aptima MG assay (now U.S. FDA-cleared), which has been shown to have a much higher sensitivity than several other research-based MG NAATs.28–32 Consistent with prior studies on performance of MG NAATs, we found a greater number of MG infections detected in vaginal swabs vs. urine.32
Another important finding from our study was that STIs other than MG were also common in women presenting for evaluation of dysuria. One or more STIs were detected in 12 of the 25 women evaluated, with MG being the most common (24%), followed by chlamydia (20%), trichomoniasis (12%), and gonorrhea (8%). This high frequency of STIs in women with dysuria is consistent with earlier studies that reported chlamydia, gonorrhea, or trichomoniasis were detected in 9%−28% of young women presenting with urinary symptoms.15–18 Co-infections were also common in our pilot study cohort. Among the MG-infected women, one-third were co-infected with chlamydia and one-third with trichomoniasis. These findings are consistent with a study by Getman et al that found 31% of MG-infected women (symptomatic or asymptomatic women seen at multiple clinical sites) were co-infected with chlamydia, gonorrhea, or trichomoniasis.7
An important clinical implication of high STI rates in women presenting for evaluation of dysuria is that many will have an STI but will be misdiagnosed as having a UTI. This puts them at risk for reproductive complications from their STI if the UTI treatment prescribed is ineffective against the STI, the latter which is highly likely since the first line recommended treatments for uncomplicated UTI (trimethoprim sulfamethoxazole, nitrofurantoin, and fosfomycin)33 are not recommended STI treatments.34 In our previous retrospective medical record review study of women seen in an ED who had dysuria and a UTI diagnosis, we found that in a subset of women who were tested for chlamydia and had a positive chlamydia test, only 42% received a prescription for an antibiotic treatment that would be effective against chlamydia.19 Even if women evaluated for dysuria are given empiric treatment for chlamydia or gonorrhea, the treatment is likely to be ineffective against MG infection. Ceftriaxone has no activity against MG, doxycycline has a very low efficacy against MG,11 and a meta-analysis reported a pooled azithromycin cure rate of 67% for MG infection from studies conducted since 2009.35 The declining azithromycin cure rates for MG infection reflect an increase in macrolide-resistant strains,11 which two studies in the U.S. reported about half of MG-infected women had macrolide-resistant strains.7,8 In our study, half the MG infected women did not receive a treatment with known MG activity and the other half received azithromycin, thus at least half or more would not have been effectively treated. In the U.S., moxifloxacin is the recommended antibiotic for treating macrolide-resistant MG infection.11,34 The clinical significance and natural history of MG infection in women with dysuria is unknown, and such knowledge will be important in guiding whether to test for MG in women with dysuria, the timing of such testing (i.e., initial presentation vs. a repeat visit with persisting dysuria), and in the decision whether to treat for MG.
Our study had some limitations. One main limitation was the small sample size of women enrolled, which may have affected our ability to detect associations of participant characteristics with MG infection and the accuracy of the MG infection frequency and associations detected. The small number of women enrolled was in part due to availability of research staff but also a much lower number of eligible women than expected. Because it was a convenience sample, this could affect the representativeness of the study sample relative to the population from which they were recruited. It is possible our study findings may not be generalizable to women seen for dysuria in other EDs or other clinical settings (e.g., primary care). Since our study is the first to report MG infection frequency in only women evaluated for a primary complaint of dysuria, its MG infection frequency and association of vaginal discharge with MG infection in this population will need to be verified in other comparable cohorts. Another main limitation was that our study and the accompanying literature review only focused on MG prevalence in women presenting with dysuria and did not assess a negative control group of women without dysuria. In the absence of a negative control group, one is not able to evaluate the potential causality/contribution of MG in the dysuria syndrome in women and asymptomatic MG infection rates may be high in some female populations; we previously reported an MG prevalence of 14.8% in women 16–29 years of age without urogenital symptoms who were seen a community-based ED in the same U.S. city as this current study.36 Case control study and prospective cohort study designs with a negative control group have been used to firmly establish MG as a cause of urethritis in men,1 but data from such study designs addressing MG urethritis in women is insufficient. Thus, studies addressing the clinical significance of MG infection in women with dysuria are needed.
In conclusion, we found that MG was detected in about one-fourth of young sexually active women presenting to an ED for evaluation of dysuria, and co-infection with chlamydia or trichomoniasis was common. We also found in a literature review on frequency of MG detection in women reporting dysuria that MG detection rates ranged from 5%−22% across 5 studies identified in the review and study populations were heterogeneous;12,20,21,23,24 none of the studies from the literature review solely evaluated women presenting for evaluation of dysuria. Because STIs are common in young sexually active women presenting for dysuria and empiric UTI treatment may not be effective against STIs, testing for STIs should be considered in this population of women. However, because the clinical significance and natural history of MG infection in women with dysuria is unknown, further studies on this are needed to help guide MG testing and treatment decisions in women with dysuria.
Acknowledgments
We thank staff of the University of Alabama at Birmingham Center for Injury Science Research Assistant Program, Joel Rodgers, and Kristal Aaron for their valuable contributions to the study.
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BVDP reports receiving grant support from Abbott, Becton, Dickinson, binx health, Cepheid, Hologic, Rheonix, Roche and SpeeDx. WMG reports receiving grant support and consulting fees from Hologic.
Funding
The authors disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by grant number 1K24AI125685 (to WMG) from the National Institute of Allergy and Infectious Diseases at the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health.
References
- 1.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24: 498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latimer RL, Vodstrcil L, De Petra V, et al. Extragenital Mycoplasma genitalium infections among men who have sex with men. Sex Transm Infect 2020; 96: 10–18. [DOI] [PubMed] [Google Scholar]
- 3.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61: 418–426. [DOI] [PubMed] [Google Scholar]
- 4.Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health 2007; 97: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnenberg P, Ison CA, Clifton S, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol 2015; 44: 1982–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen B, Sokolowski I, Østergaard L, Kjølseth Møller J, Olesen F, Jensen JS. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sex Transm Infect 2007; 83: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium: prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54: 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao L, Waites KB, Van Der Pol B, Aaron KJ, Hook EW 3rd, Geisler WM. Mycoplasma genitalium infections with macrolide and fluoroquinolone resistance-associated mutations in heterosexual African American couples in Alabama. Sex Transm Dis 2019; 46: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munson E, Bykowski H, Munson KL, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-mediated amplification using primary female urogenital specimens. J Clin Microbiol 2016; 54: 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaydos CA, Manhart LE, Taylor SN, et al. Molecular testing for Mycoplasma genitalium in the United States: results from the AMES prospective multicenter clinical study. J Clin Microbiol 2019;57:e01125–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw CS, Jensen JS, Waites KB. New horizons in Mycoplasma genitalium treatment. J Infect Dis 2017; 216(suppl_2): S412–S419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manhart LE, Gaydos CA, Taylor SN, et al. Characteristics of Mycoplasma genitalium urogenital infections in a diverse patient sample from the United States; results from the Aptima Mycoplasma genitalium Evaluation Study (AMES). J Clin Microbiol 2020; 58: e00165–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol 2016; 30:1650–1656. [DOI] [PubMed] [Google Scholar]
- 14.Michels TC, Sands JE. Dysuria: evaluation and differential diagnosis in adults. Am Fam Physician 2015; 92: 778–786. [PubMed] [Google Scholar]
- 15.Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53: 2686–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro T, Dalton M, Hammock J, et al. The prevalence of urinary tract infections and sexually transmitted disease in women with symptoms of a simple urinary tract infection stratified by low colony count criteria. Acad Emerg Med 2005; 12: 38–44. [DOI] [PubMed] [Google Scholar]
- 17.Prentiss KA, Newby PK, Vinci RJ. Adolescent female with urinary symptoms: a diagnostic challenge for the pediatrician. Pediatr Emerg Care 2011; 27: 789–794. [DOI] [PubMed] [Google Scholar]
- 18.Musacchio NS, Gehani S, Garofalo R. Emergency department management of adolescents with urinary complaints: missed opportunities. J Adolesc Health 2009; 44: 81–83. [DOI] [PubMed] [Google Scholar]
- 19.Wilbanks MD, Galbraith JW, Geisler WM. Dysuria in the emergency department: missed diagnosis of Chlamydia trachomatis. West J Emerg Med 2014; 15: 227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandepitte J, Bukenya J, Hughes P, et al. Clinical characteristics associated with Mycoplasma genitalium infection among women at high risk of HIV and other STI in Uganda. Sex Transm Dis 2012; 39: 487–491. [DOI] [PubMed] [Google Scholar]
- 21.Arráiz RN, Colina Ch S, Marcucci JR, et al. Mycoplasma genitalium detection and correlation with clinical manifestations in population of the Zulia State, Venezuela. Rev Chilena Infectol 2008; 25: 256–261. [PubMed] [Google Scholar]
- 22.Bjartling C, Osser S, Persson K. Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. Am J Obstet Gynecol 2012; 206: 476.e1–8. [DOI] [PubMed] [Google Scholar]
- 23.Huppert JS, Mortensen JE, Reed JL, et al. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anagrius C, Loré B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect 2005; 81: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heytens S, De Sutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017; 23: 647–652. [DOI] [PubMed] [Google Scholar]
- 26.Carne CA, Gibbs J, Delaney A, et al. Prevalence, clinical features and quantification of genital non-viral infections. Int J STD AIDS 2013; 24: 273–277. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 28.de Salazar A, Espadafor B, Fuentes-López A, et al. Comparison between Aptima assays (Hologic) and the Allplex STI Essential Assay (Seegene) for the diagnosis of sexually transmitted infections. PLoS ONE 2019; 14: e0222439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unemo M, Salado-Rasmussen K, Hansen M, et al. Clinical and analytical evaluation of the new Aptima Mycoplasma genitalium assay, with data on M. genitalium prevalence and antimicrobial resistance in M. genitalium in Denmark, Norway and Sweden in 2016. Clin Microbiol Infect 2018; 24: 533–539. [DOI] [PubMed] [Google Scholar]
- 30.Chernesky M, Jang D, Martin I, et al. Mycoplasma genitalium, Chlamydia trachomatis, and Neisseria gonorrhoeae detected with Aptima assays performed on self-obtained vaginal swabs and urine collected at home and in a clinic. Sex Transm Dis 2019; 46: e87–e89. [DOI] [PubMed] [Google Scholar]
- 31.Roy CL, Pereyre S, Hénin N, Bébéar C. French prospective clinical evaluation of the Aptima Mycoplasma genitalium CE-IVD assay and macrolide resistance detection using three distinct assays. J Clin Microbiol 2017;55(11):3194–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Der Pol B, Waites KB, Xiao L, et al. Mycoplasma genitalium detection in urogenital specimens from symptomatic and asymptomatic men and women by use of the cobas TV/MG Test. J Clin Microbiol 2020; 58: e02124–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–e120. [DOI] [PubMed] [Google Scholar]
- 34.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03): 1–137. [PMC free article] [PubMed] [Google Scholar]
- 35.Lau A, Bradshaw CS, Lewis D, et al. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis 2015; 61: 1389–1399. [DOI] [PubMed] [Google Scholar]
- 36.Gragg SD, Gupta KA, Olson KM, et al. Mycoplasma genitalium infection in young women without urogenital symptoms presenting to a community-based emergency department in Birmingham, Alabama. Sex Transm Dis 2021; 48: e27–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]


