Abstract
Cystic fibrosis (CF) is a genetic disease caused by mutations of the gene encoding a cAMP-activated Cl− channel, the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR modulator therapies consist of small-molecule drugs that rescue mutant CFTR. Regimens of single or combinations of CFTR modulators still rely on endogenous levels of cAMP to regulate CFTR activity. We investigated CFTR activation by the natural mediator prostaglandin E2 (PGE2) and lubiprostone (a Food and Drug Administration-approved drug known to target prostaglandin receptors) and tested the hypothesis that receptor-mediated CFTR activators can be used in combination with currently available CFTR modulators to increase function of mutant CFTR. Primary-cultured airway epithelia were assayed in Ussing chambers. Experimental CFTR activators and established CFTR modulators were applied for 24 h and/or acutely and analyzed for their effect on CFTR activity as measured by changes in short-circuit current (ISC). In non-CF airway epithelia, acute application of lubiprostone and PGE2 activated CFTR to the levels comparable to forskolin (Fsk). Pretreatment (24 h) with antagonists to prostaglandin receptors EP2 and EP4 abolished the ability of lubiprostone to acutely activate CFTR. In F508del homozygous airway epithelia pretreated with the triple combination of elexacaftor, tezacaftor, and ivacaftor (ELEXA/TEZ/IVA; i.e., Trikafta), acute application of lubiprostone was able to maximally activate CFTR. Prolonged (24 h) cotreatment of F508del homozygous epithelia with ELEXA/TEZ/IVA and lubiprostone increased acute CFTR activation by ∼60% compared with the treatment with ELEXA/TEZ/IVA alone. This work establishes the feasibility of targeting prostaglandin receptors to activate CFTR on the airway epithelia and demonstrates that cotreatment with lubiprostone can further restore modulator-rescued CFTR.
Keywords: CFTR modulator, cystic fibrosis, prostaglandin, Trikafta
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic disease caused by mutations of the gene encoding a cAMP-activated Cl− channel, the cystic fibrosis transmembrane conductance regulator (CFTR) (1). In the lung, CFTR-mediated Cl− secretion is critical for maintaining balanced airway surface fluid and facilitating proper mucociliary clearance and lung function. Dysfunctional CFTR leads to a dehydrated lung surface that is unable to properly clear mucosal secretions, causing chronic infection, inflammation, and progressive lung disease.
Recent progress in the treatment of CF rests on the discovery of small-molecule drugs to aid in folding and trafficking (“correctors,” “enhancers,” and “amplifiers”) and channel gating (“potentiators”) of mutant CFTR (2–5). The most effective combination of CFTR modulators, the triple combination of tezacaftor, ivacaftor, and elexacaftor (marketed in the United States as Trikafta), has had remarkable clinical success, yet still only partially restores lung function and airway CFTR activity in individuals with the most common mutant of CFTR (F508del) (3, 5). Pharmacological “activators” of CFTR such as forskolin (Fsk) or 3-isobutyl-1-methylxanthine (IBMX), which achieve maximal CFTR activity by raising intracellular cAMP levels are highly effective at increasing CFTR activity in vitro, yet are not clinically safe for use in vivo. Treatments with currently available CFTR modulators still rely on endogenous levels of intracellular cAMP to regulate CFTR activity. Some G protein-coupled receptors (GPCRs) have been investigated for their ability to activate CFTR (6), but no GPCR activation pathway has shown much promise as an addition to CFTR modulator treatment.
Here, we sought to investigate a new class of CFTR modulators, prostones (bicyclic fatty acids derived from prostaglandins), that could serve as in vivo CFTR activators. Our lead compound in this study was lubiprostone (Amitiza), which is a Food and Drug Administration-approved drug originally developed as a chloride channel 2 (CLCN2) activator to treat constipation by increasing intestinal fluid transport (7, 8). Since its discovery, the CLCN2-mediated mechanism of action of lubiprostone in the intestine and airway has been disputed (9). Whereas some studies have reported evidence that lubiprostone increases CLCN2-mediated, CFTR-independent ion transport (10–14), others have indicated that lubiprostone may have off-target effects on intestinal CFTR or that CFTR is the sole target of lubiprostone altogether (9, 15–19). In these latter works, it is suggested that lubiprostone binds to a G protein-coupled prostaglandin E2 (PGE2) receptor 4 (EP4) on the lumenal intestine surface, which stimulates adenylate cyclase and increases intracellular cAMP, thereby activating CFTR and increasing intestinal fluid transport.
The activating action of lubiprostone on CFTR has been interpreted as having little to no relevance in treating CF because it could not aid in correcting the structural and functional deficiencies of mutant CFTR which underlie CF (17). We interpret the potential CFTR-mediated action of lubiprostone differently; our rationale for the present study was that if experimental CFTR activators (such as forskolin) can increase CFTR-mediated ion transport to maximal levels in vitro, then perhaps there is a class of CFTR activator (such as prostones) that could achieve the same result in vivo. We tested the hypothesis that lubiprostone, when used in combination with currently available structure-correcting CFTR modulators, can further restore modulator-rescued CFTR function. Using functional electrophysiological assays, we investigated the efficacy and mechanism of action by which lubiprostone, as well as the endogenous prostaglandin E2, regulate CFTR activity in non-CF and CF airway epithelia.
METHODS
Cell Expansion and Maintenance
Human nasal epithelial cells were obtained under written informed consent by nasal brushings from non-CF individuals and individuals homozygous for F508del or heterozygous for G551D/R117H CFTR mutations. This protocol has been approved by the Institutional Review Board of National Jewish Health (HS-2832). Primary-derived human airway epithelial (HNE) cells were expanded on an irradiated NIH 3T3 feeder layer in the presence of the Y-27632 ρ-kinase inhibitor, then seeded at 2.5 × 105 cells·cm−2. All cells were plated on bovine collagen-coated 0.33-cm diameter cell culture inserts (Corning Costar Snapwell Inc., Kennebunk, ME) and kept at 37°C with culture media changes three times per week. For the first 2 days after seeding, cells were submerged in PneumaCult-EX Plus Medium (Stem Cell Technologies, Vancouver, BC, Canada). After 2 days, an air-liquid interface (ALI) was established by removing the apical solution and replacing the basolateral solution with PneumaCult-ALI Medium (Stem Cell Technologies). Cells were raised at ALI for 21–28 days before experimentation.
Fisher rat thyroid (FRT) cells expressing normal CFTR (CFTR-FRT) were a gift from Dr. Eric J. Sorscher (Emory University, Atlanta, GA). The media used to culture CFTR-FRT cells was a modified Ham’s F-12 media supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 0.2% hygromycin. CFTR-FRT cells were seeded on uncoated inserts at 1.5 × 104 cells·cm−2 and maintained submerged in media for 7–10 days before experimentation.
Electrophysiological Analyses and Drug Treatment
During pretreatment, cells were incubated with CFTR modulators for 24 h before electrophysiological analyses. The CFTR modulators used during 24 h drug treatment were tezacaftor (VX-661; 3 µM), ivacaftor (VX-770; 100 nM), elexacaftor (VX-445; 3 μM), lubiprostone (1 μM), and/or PGE2 (1 μM). The PGE2 receptor antagonists (20 μM) used during 24 h drug treatment were [−]EP1: SC-51089; [−]EP2: TG6-10-1; [−]EP3: L-798,106; [−]EP4: L-161,982 (Cayman Chemical Co., Ann Arbor, MI). These receptor antagonists are advertised as “selective” or “highly selective” for their target receptors by the manufacturer. Our preliminary validations indicated that a 20 µM concentration of these compounds achieved a saturating antagonism of a single receptor.
Electrophysiological analyses were carried out in an Ussing chamber apparatus (VCC MC8 and MC6; Physiologic Instruments, San Diego, CA). Before each experiment, the Ussing chamber was mounted with cell-free inserts and prewarmed to 37°C with identical Ringer’s solution in the apical and basolateral baths and the offset potential and fluid resistance between electrodes were corrected to zero. The Ringer’s solution was 120 mM NaCl, 10 mM d-glucose, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1.2 mM MgCl2, 1.2 mM CaCl2, saturated with 95% O2-5% CO2, pH 7.4. Cells on inserts were taken directly from culture and mounted onto the Ussing chamber. Transepithelial voltage was allowed to stabilize under an open circuit before beginning a run under voltage clamp mode (±5 mV pulses every 5 s).
Human nasal epithelial (HNE) cultures are inherently polarized epithelia, so all electrophysiological experiments with HNE cells were performed under symmetrical chloride conditions [see Bratcher et al. (20)]. In contrast, FRT cells do not form polarized epithelia, and the presence of a chloride gradient is required to establish directionality of chloride transport across the FRT epithelia. Therefore, experiments using FRT cells were performed under basolateral-to-apical chloride gradient conditions (chloride-free in apical bath). The chloride-free Ringer’s solution was 115 mM Na+ gluconate, 5 mM Ca2+ gluconate, 10 mM d-glucose, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1.2 mM MgSO4, saturated with 95% O2-5% CO2, pH 7.4.
During electrophysiological experiments on HN8E cells, the test compounds that were acutely applied were DMSO (volume-matched); amiloride (100 μM; apical); ivacaftor (VX-770; 1 μM; apical); forskolin alone (dose curve; apical and basolateral), forskolin (20 μM) and 3-isobutyl-1-methylxanthine (100 μM) (Fsk/IBMX or F/I; apical and basolateral); lubiprostone (dose curve, 100 nM, or 1 μM; apical and basolateral); PGE2 (dose curve or 1 μM; apical and basolateral); Cact-A1 (dose curve; apical and basolateral); CFTRinh-172 (10 μM; apical); adenosine triphosphate (ATP; 100 μM; apical). For electrophysiological experiments using FRT cells, all test compounds were applied to both surfaces using the concentrations listed above. Together, Fsk and IBMX increase intracellular cAMP by activating adenylate cyclase (Fsk) and inhibiting phosphodiesterases (IBMX). Cact-A1 is a cAMP-independent activator of CFTR (21).
Integrity of HNE cultures for electrophysiological analyses was assessed using the presence of mucosal secretions, beating cilia, and an adequate transepithelial resistance (>100 Ω cm−2) as indicators of fully differentiated, electrically tight epithelia. Electrophysiological recordings were obtained using Acquire and Analyze 2.3 software (Physiologic Instruments, Inc.).
Intracellular cAMP Determination
A commercial ELISA Kit (ADI-900-163, ENZO Life Sciences) was used to determine intracellular cAMP following the manufacturer’s protocol. HNE cultures were exposed for 30 min to Fsk, lubiprostone, PGE2, or Cact-A1 (all 10 μM), then removed from culture media and lysed in manufacturer-provided buffer. Cell lysates were spun down and the supernatant was analyzed for cAMP concentration. Intracellular cAMP levels were calculated based on a standard curve and expressed as pmol cAMP mg protein−1. Protein content was determined using the Pierce BCA Protein Assay (Thermo Fisher Scientific, Rockford, IL).
Gene Expression Analysis
Using the TRIzol method following manufacturer’s protocol (Molecular Research Center Inc.), total RNA was isolated from frozen cells. Total RNA was assessed for purity (A260/A280 > 1.9) and quantified using a NanoDrop spectrophotometer (ND-1000; NanoDrop Technologies). First-strand cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc.) following the manufacturer’s protocol. Quantitative polymerase chain reaction (RT-PCR) was performed using a StepOnePlus system (Applied Biosystems). Individual RT-PCR reactions were carried out in 10 μL volumes containing 20 ng of cDNA, 150 nM forward and reverse primers, and 1X SYBRselect master mix (Thermo Fisher Scientific), following the manufacturer’s protocol. The thermal profile of the reactions was 2 min at 50°C, 2 min at 95°C (holding and activation), 40 cycles of 15 s at 95°C, 30 min at 60°C, 30 s at 72°C (cycling). A melt curve analysis cycle from 60°C to 95°C was used to confirm a single product in each reaction. The comparative method (2−ΔΔCT) (22) was used to calculate relative mRNA expression using α-tubulin (ATUBA1A) as a reference gene. Nucleotide sequences of primer pairs for PGE2 receptors have been previously published (23). CFTR primer pairs were as follows: CFTR forward, 5′-GGA GAG CAT ACC AGC AGT GAC T-3′; CFTR reverse, 5′-TTC CAA GGA GCC ACA GCA CAA C-3′.
Western Blotting
Frozen monolayers were lysed with 1× radioimmunoprecipitation assay (RIPA) lysis and extraction buffer (Thermo Fisher Scientific). Equal amounts of protein (20 μg) were loaded for SDS-PAGE in an 8% gel prepared following manufacturer’s protocol (ProtoGel, National Diagnostics). After electrophoretic separation, protein was transferred on to nitrocellulose membrane (Bio-Rad). Blots were subsequently blocked with 5% nonfat dry milk (LabScientific) in PBS-T (phosphate-buffered saline with 0.05% Triton X-100) for 1 h at room temperature. Blots were probed with primary antibodies (1:1,000 dilution) at 4°C overnight. The antibodies used were as follows: α-tubulin (DSHB Hybridoma Product 12G10; mouse monoclonal; antibody registry ID: AB_1157911); EP2 (Alomone Labs Cat. No. APR-064; rabbit polyclonal; antibody registry ID: AB_2341058); EP4 (Alomone Labs Cat. No. APR-066; rabbit polyclonal; antibody registry ID: AB_ 2756762). After primary incubation, blots were washed with PBS-T then incubated in horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000 dilution) for 1 h at room temperature: goat-anti mouse (R&D Systems); goat anti-rabbit (Cell Signaling Technology). After secondary incubation, membranes were washed again in PBS-T and subjected to chemiluminescent detection (ECL Plus Western Blotting Substrate; Pierce). Antibodies for α-tubulin, EP2, and EP4 each detected bands at ∼50 kDa, which was the expected molecular mass for each target protein. EP2 and EP4 band densities were normalized to α-tubulin and quantified using ImageJ software (NIH).
Calculations and Statistical Analyses
As indicated in the figure captions, statistical analyses of experiments were made using one- or two-sample t tests or one-way ANOVA with Tukey’s post hoc analyses. An α-value of 0.05 was selected to denote statistical significance. All data are presented as means ± standard error. Figure assembly and all statistical analyses were completed using GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA).
RESULTS
Lubiprostone Activates CFTR in the Airway
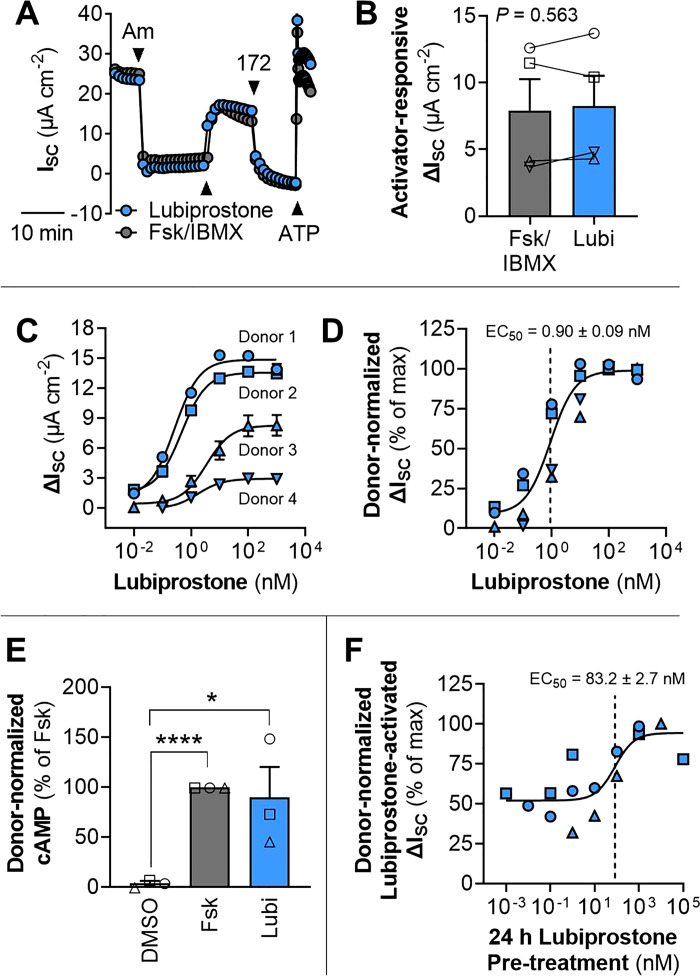
In our electrophysiological experiments, we observed rapid changes in ISC in response to acute drug application that were quick to stabilize, indicating that the epithelial cultures were differentiated and of adequate integrity. Predictably, amiloride and CFTRinh-172 resulted in rapid decreases in ISC, and forskolin and ATP resulted in rapid increases in ISC (Fig. 1A). Acute lubiprostone (100 nM) resulted in an equivalent CFTR-mediated ΔISC as Fsk/IBMX (Fig. 1, A and B). The concentration of lubiprostone necessary achieve a half-maximal ΔISC (EC50) in non-CF HNE was <1 nM (0.90 ± 0.09 nM) and saturating at >100 nM (Fig. 1, C and D).
Figure 1.
Lubiprostone activates CFTR in primary-derived human nasal epithelia. A: representative electrophysiological tracing for non-CF HNE after 21 days in culture demonstrating acute short-circuit current (ISC) responsiveness to pharmacological activation and inhibition. Compounds: amiloride (Am; 100 μM; apical), forskolin (Fsk; 20 μM), and 3-Isobutyl-1-methylxanthine (IBMX; 100 μM) (Fsk/IBMX; apical and basolateral), lubiprostone (Lubi; 100 nM; apical and basolateral), CFTRinh-172 (172; 10 μM; apical), ATP (100 μM; apical). B: comparison of acute CFTR activation by lubiprostone and Fsk/IBMX (n = 4 donors). C and D: dose-response curve for acute activation of CFTR by lubiprostone (n = 4 donors). E: stimulation of intracellular cAMP by forskolin and lubiprostone (1 μM) (n = 3 donors). F: dose-response curve for the effect of prolonged (24 h) treatment with lubiprostone on the acute lubiprostone responsiveness (n = 3 donors). For clarity, individual donors are presented as different symbols. Data are presented as means ± SE. *Significant differences (one- or two-sample t test; *P < 0.05, ****P < 0.0001). CFTR, cystic fibrosis transmembrane conductance regulator; CF, cystic fibrosis; HNE, human nasal epithelia.
In the single donor tested, it appeared that the ISC response to lubiprostone was similar whether administered to the apical or basolateral sides of the epithelia (Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.17315174). Acute CFTR activation by forskolin, lubiprostone, PGE2, and Cact-A1 were compared in HNE cells with a single non-CF donor (Supplemental Fig. S2). Lubiprostone, PGE2, and Cact-A1, all appeared to achieve a similar or greater maximal ΔISC as compared with forskolin, indicating that CFTR could be activated by each compound. Some apparent differences between compounds were observed in both maximum ΔISC and sensitivity (EC50) (Supplemental Fig. S2).
The cAMP stimulating effects of lubiprostone and forskolin in non-CF HNE cells were compared. Both compounds significantly increased intracellular cAMP above the DMSO control, but no difference was detected between lubiprostone and forskolin in the magnitude of cAMP stimulation achieved (Fig. 1E).
Non-CF HNE cultures were pretreated with prolonged (24 h) exposure to lubiprostone to examine whether prolonged CFTR activation by lubiprostone would limit acute responsiveness to the drug. The CFTR-mediated ΔISC from acute lubiprostone application increased with increasing concentrations of lubiprostone pretreatment; the EC50 of lubiprostone pretreatment was 83.2 ± 2.7 nM (Fig. 1F). Pretreatment with 1 µM lubiprostone was saturating and increased acute lubiprostone-responsiveness by ∼twofold (Fig. 1F). In the single donor tested, it appeared that prolonged pretreatment PGE2 also increased the acute responsiveness of CFTR activation, similar to the effect of pretreatment with lubiprostone (Supplemental Fig. S3).
Prostaglandin E2 Receptor EP2 Regulates Ion Transport across the Airway Epithelium
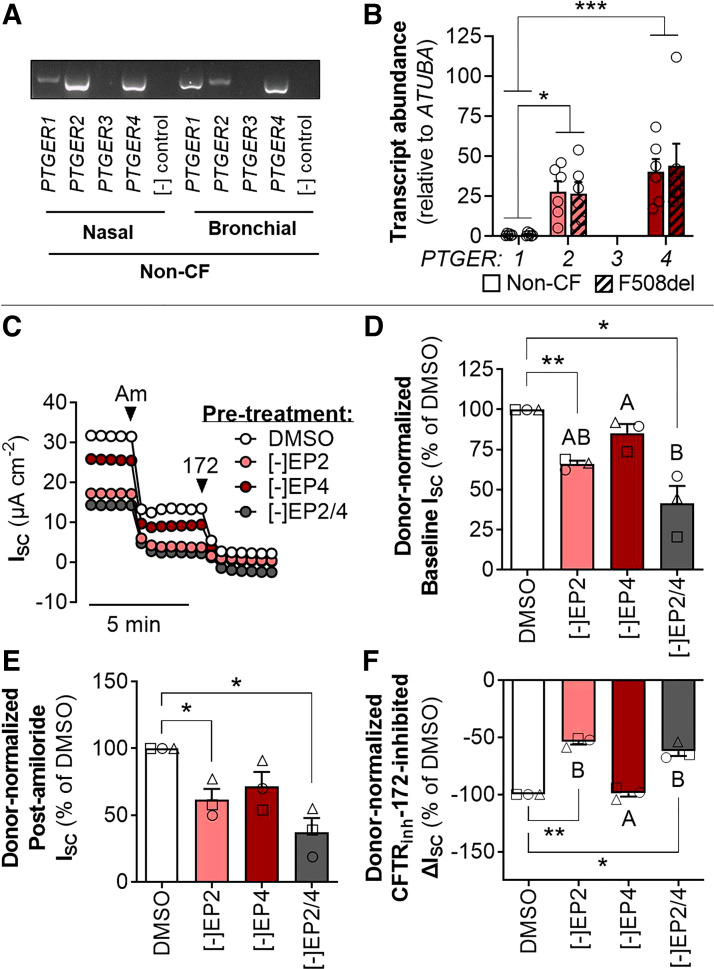
Using molecular probes for PGE2 receptors EP1–4 (gene names: PTGER1–4), we detected the presence of PTGER1, PTGER2, and PTGER4 in non-CF primary-derived nasal (HNE) and bronchial epithelial cultures (Fig. 2A). In non-CF HNE cells, mRNA of PTGER2 and PTGER4 were present in significantly higher abundance than PTGER1 and the mRNA expression of PTGER2 and PTGER4 did not differ between non-CF and F508del/F508del HNE epithelia (Fig. 2B).
Figure 2.
PGE2 receptor EP2 regulates ion transport across the airway epithelium. Representative banding (A) and relative gene expression (B) of PGE2 receptors EP1–4 (PTGER1–4) in non-CF and F508del/F508del HNE (n = 6 donors). Representative trace (C) of ISC across non-CF HNE cells after 24 h pretreatment with DMSO or antagonists to EP2 and/or EP4 then acute inhibition of ion transport by amiloride and CFTRinh-172. Donor-normalized baseline ISC (D), postamiloride ISC (E), and CFTRinh-172-inhibited ΔISC (F). For clarity, individual donors are presented as different symbols (n = 3 donors). Data are presented as means ± SE. Asterisks (one-sample t test) and letters (ANOVA with Tukey’s post hoc) indicate significant differences between groups (*P < 0.05, **P < 0.01, ***P < 0.001). ATUBA1A, α-tubulin; CFTR, cystic fibrosis transmembrane conductance regulator; CF, cystic fibrosis; HNE, human nasal epithelia; ISC, acute short-circuit current; PGE2, prostaglandin E2.
To examine whether EP2 and/or EP4 affect constitutive ion transport across HNE cells, we pretreated non-CF HNE cells for 24 h with selective antagonists to EP2 and/or EP4 and then analyzed the ISC at baseline and after ENaC inhibition by amiloride, and compared the ΔISC upon CFTR inhibition by CFTRinh-172 (Fig. 2, C–F). Antagonism of EP2, but not EP4, significantly reduced baseline and postamiloride ISC across HNE cells (Fig. 2, D and E). Likewise, the CFTRinh-172-inhibited ΔISC was significantly reduced by antagonism of EP2 but not EP4 (Fig. 2F).
CFTR Activation by Lubiprostone Is Mediated through Prostaglandin Receptors EP2 and EP4
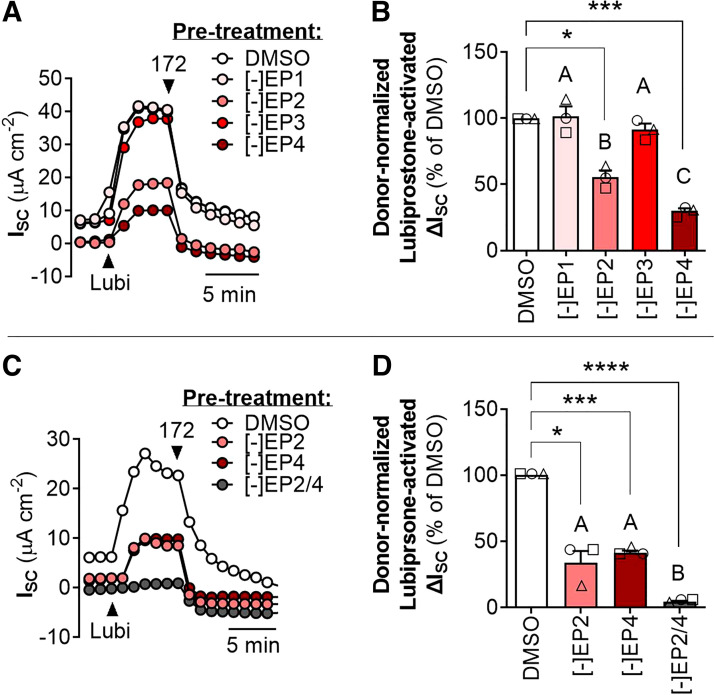
To identify whether and which PGE2 receptors were mediating CFTR activation by lubiprostone, we pretreated non-CF HNE cells for 24 h with selective antagonists to EP1, EP2, EP3, and/or EP4. Blockade of EP1 or EP3 had no effect on CFTR activation by lubiprostone compared with the DMSO control, whereas blockade of either EP2 or EP4 significantly reduced the CFTR-mediated ΔISC by lubiprostone (Fig. 3, A and B). Blockade EP2 or EP4 resulted in a ∼50% decrease in CFTR activation by lubiprostone, and simultaneous blockade of EP2 and EP4 abolished CFTR activation by lubiprostone altogether (Fig. 3, C and D).
Figure 3.
Lubiprostone activates CFTR on the airway epithelium via PGE2 receptors EP2 and EP4. Acute CFTR activation by lubiprostone in non-CF HNE after 24 h pretreatment with DMSO or antagonists to EP1, EP2, EP3, and/or EP4. Receptor antagonists were applied individually (A and B) or in combination (C and D), as indicated in panel legends and x-axes. For clarity, individual donors are presented as different symbols (n = 3 donors). Data are presented as means ± SE. Asterisks (one-sample t test) and letters (ANOVA with Tukey’s post hoc) indicate significant differences between groups (*P < 0.05, ***P < 0.001, ****P < 0.0001). CFTR, cystic fibrosis transmembrane conductance regulator; CF, cystic fibrosis; HNE, human nasal epithelia; ISC, acute short-circuit current; Lubi, lubiprostone.
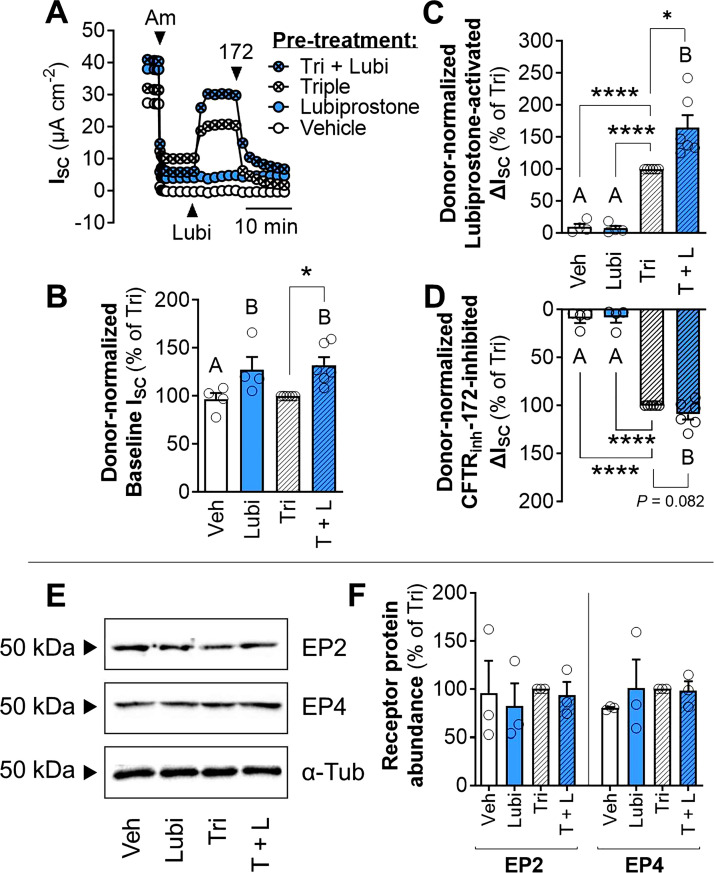
Cotreatment with Lubiprostone Increases Modulator-Rescued CFTR Function in CF Airway Epithelia
Lastly, we investigated the therapeutic potential of targeting the prostaglandin signaling pathways to improve mutant CFTR function. We tested the hypothesis that cotreatment with lubiprostone alongside established CFTR modulators would increase CFTR-mediated ion transport. In HNE cells derived from six individual donors homozygous for F508del-CFTR, 24 h cotreatment with lubiprostone and the triple combination of elexacaftor/tezacaftor/ivacaftor significantly increased baseline ISC and acute lubiprostone-activated ΔISC compared with the cells treated only with the triple combination (Fig. 4, A–C). Cotreatment with lubiprostone did not have a significant effect on CFTRinh-172-inhibited ΔISC (Fig. 4D). Additionally, in an experiment from a single donor heterozygous with G551D/R117H-CFTR, 24 h cotreatment with lubiprostone and ivacaftor significantly increased CFTR-mediated ISC compared with the ivacaftor-only control (Supplemental Fig. S4).
Figure 4.
Cotreatment with lubiprostone increases CFTR activity in modulator-rescued F508del/F508del nasal epithelia. A: Representative trace of acute CFTR activation by lubiprostone in F508del/F508del HNE after 24 h pretreatment (24 h) with vehicle (DMSO), lubiprostone (1 µM), the triple combination of elexacaftor (3 μM), tezacaftor (3 μM), and ivacaftor (100 nM) (Tri), or the triple combination and lubiprostone (T + L). Donor-normalized baseline ISC (B) and lubiprostone-activated (C) or CFTRinh-172-inhibited ΔISC (D; n = 6 donors). E and F: Western blot analysis of EP2, EP4, and α-tubulin (n = 3 donors). Data are presented as means ± SE (each circle indicates an individual donor). Asterisks (one-sample t test) and letters (ANOVA with Tukey’s post hoc) indicate significant differences between groups (*P < 0.05, ****P < 0.0001). Am, amiloride; α-Tub; α-tubulin; CFTR, cystic fibrosis transmembrane conductance regulator; HNE, human nasal epithelia; ISC, acute short-circuit current; Lubi, lubiprostone; Veh, vehicle.
We investigated whether 24 h pretreatment with lubiprostone and/or the triple combination affects EP2 or EP4 abundance. Western blotting detected the presence of EP2 and EP4, but the abundances of these receptors were unchanged by any treatment (Fig. 4, E and F). Likewise, no changes in CFTR, PTGER2, or PTGER4 transcript abundance compared with the vehicle control were observed (Supplemental Table S1).
DISCUSSION
The aim of the present work was to investigate whether the prostaglandin signaling pathway can be targeted to increase CFTR-mediated ion transport in non-CF and CF human airway epithelia. We demonstrated that lubiprostone and PGE2 can stimulate CFTR-mediated ion transport. Although only the PGE2 receptor EP2 appeared to be important in regulating constitutive CFTR activity on the airway epithelium, lubiprostone activation of CFTR occurred via the agonism of both EP2 and EP4. Importantly, the present study is the first investigation of GPCR activation of CFTR wherein prolonged treatment with GCPR stimulation did not affect the protein abundance of target receptors and actually enhanced modulator-rescued mutant CFTR function.
Our results in human airway epithelia showing that lubiprostone and PGE2 activate CFTR at nanomolar concentrations reflect similar findings in other model epithelia. Less than 1 µM lubiprostone is sufficient to increase ISC across T84 cell cultures (16, 17) as well as nonhuman mammalian native tracheal and intestinal tissue (17–19, 24). In the present study, all prolonged lubiprostone treatments of cells in culture were to the basolateral side and all acute exposures in the Ussing chamber were to both the basolateral and apical sides. In previous studies with lubiprostone, changes in ISC were slower to reach maximum values when lubiprostone was added to the basolateral surface compared with much more immediate responses in ISC when lubiprostone was added to the apical surface (16, 18, 19). The apical bias in the sidedness of lubiprostone action observed in these previous studies in gastrointestinal tissue could have two possible explanations: 1) that lubiprostone is directly interacting with ion channels that are predominantly expressed on the apical surface, such as CFTR or CLCN2; 2) that lubiprostone is activating membrane-bound receptors that are predominantly expressed on the apical surface. In an anecdotal experiment using a single donor, we observed that the side of the epithelium to which lubiprostone is acutely applied may not change the responsiveness or magnitude of the resulting ΔISC. We know CFTR has a prominent role in epithelial ion transport and that CFTR expression is confined to the apical surface. Therefore, given the apparent lack of sidedness of lubiprostone action in our HNE cells, it seems unlikely that lubiprostone is directly activating CFTR. Likewise, the classically proposed target of lubiprostone, apically located CLCN2, is unlikely to be a direct target of lubiprostone or simply does not have a prominent role in ion transport in our HNE cells, compared to in gastrointestinal epithelia. It is possible that prostaglandin receptors are expressed on both surfaces of the airway epithelial cultures and this is the reason for the lack of sidedness of lubiprostone action in our studies. It is also possible that absorption of lubiprostone occurs across airway epithelial cultures, unlike in gastrointestinal tissue where lubiprostone is not absorbed (2). Both the immunolocalization of target receptors of lubiprostone and the possible absorption of lubiprostone across native airway epithelia and airway epithelial cultures should be examined in future studies.
Prostaglandins are a large group of naturally occurring, bicyclic fatty-acid compounds that target an array of transmembrane, G protein-coupled prostaglandin receptors with varying specificity and cross-reactivity (25). Acute apical or basolateral exposure to PGE2 has been shown to stimulate CFTR-dependent and independent ISC in model airway epithelial cells 16-HBE14o-, CFBE41o-, and Calu-3 in the micromolar range (26, 27), a finding that we corroborate in the present work. There are four primary receptors to PGE2 (EP1-4) and these receptors can be distinguished by their G protein associations: EP1 is a contractile type receptor, which activates Gαq and stimulates intracellular Ca2+; EP2 and EP4 are relaxant type receptors, which activate Gαs and stimulate intracellular cAMP by adenylate cyclase; and EP3 is an inhibitory type receptor, which activates Gαi and inhibits cAMP production by adenylate cyclase. The relatively high expression of EP2 and EP4 that we observed in non-CF and CF HNE cells, and apparent lack of EP3 expression, corresponds well with our observations of cAMP- and ISC-stimulating effects of both lubiprostone and PGE2 in airway epithelial cells.
It is rather interesting that antagonism of EP2, but not EP4, appeared to affect constitutive (i.e., lubiprostone-independent) CFTR-mediated ion transport across the airway epithelium. One possibility for this is that EP2 is expressed in greater abundance on the membrane surface in CFTR-rich cells. Although our receptor transcription and protein abundance data show similar expression levels in crude homogenate preparations, it is possible that the receptor subtypes differ in their subcellular localization or colocalization with CFTR, which warrants future studies on this. Another, perhaps more likely, possibility is that EP2 and EP4 differ in their G protein associations in airway epithelial cells. For instance, it has been shown in other systems that, in addition to coupling to Gαs, EP4 can also associate with Gαi (28, 29). Where this is the case, cAMP stimulation by PGE2-activated EP2 is less transient than by PGE2-activated EP4. If it is the case in airway epithelial cells that EP2 and EP4 differ their G protein associations in this way, then it is conceivable that constitutive regulation of cAMP, and thus CFTR activity, is under greater control by EP2. Future analyses of potential differences in EP2 and EP4 G protein interactions and the transience or longevity of EP2 and EP4 stimulation in airway epithelial cells is warranted. Importantly, the cyclooxygenase-mediate synthesis and regulation of PGE2 and its role in mediating ion currents in airway epithelia should be investigated.
We sought to investigate the receptor-mediated mechanism of action of lubiprostone further. We used a widely used approach of pharmacologically blocking PGE2 receptors (17, 19, 26, 27, 30, 31) to identify the receptor targets of lubiprostone in HNE cells. From our studies, it was apparent that lubiprostone acts through both the EP2 and EP4 receptors to stimulate CFTR-dependent ISC. Here, it appeared that EP2 and EP4 each mediate ∼50% of the action of lubiprostone—when both EP2 and EP4 where blocked, lubiprostone action was abolished. Our findings here differ from work in intestinal epithelia wherein it has been shown that lubiprostone action was mediated primarily or solely by EP4 (9, 17, 31). The primary role of EP4 in mediating ISC stimulation by lubiprostone was also observed in the ovine airway (19). More work in primary-derived human airway epithelial is needed to further characterize the cell types in which EP2 and EP4 are expressed, and importantly, in which cells EP2 and/or EP4 are colocalized with CFTR.
Previous attempts at identifying receptor-mediated CFTR activators for use in vivo have been unsuccessful. Albuterol, a β2 adrenergic receptor (β2AR) agonist that increases intracellular cAMP, has been tested for its efficacy in activating CFTR. However, prolonged treatment with albuterol was found to downregulate cell-surface β2AR and reduce intrinsic cAMP generation in airway epithelia (6). Importantly, prolonged albuterol treatment was shown to decrease forskolin-responsive CFTR-mediated ISC across the airway epithelia. The results in the present manuscript demonstrating that prolonged exposure to lubiprostone increased CFTR-mediated ISC indicates that albuterol-induced CFTR-dysfunction is not an inherent property of all G protein-coupled receptor-mediated pathways to activate CFTR. That is, GPCR-mediated CFTR activation involving EP2/EP4 must be meaningfully different from other GPCR-mediated, CFTR-activating pathways.
In the present manuscript, we were unable to deliver a mechanistic explanation for how prolonged treatment with lubiprostone increases acute responsiveness to lubiprostone (Fig. 1F) or other CFTR activators such as Fsk/IBMX (Supplemental Fig. S3). A simple explanation for this would be that prolonged exposure to lubiprostone increases the expression of CFTR, EP2, and/or EP4. However, we observed that prolonged lubiprostone exposure did not affect gene expression of CFTR, PTGER2, or PTGER4, or protein expression of EP2 or EP4. Another possibility is that in addition to activating EP2 and EP4, prolonged exposure to lubiprostone and PGE2 is increasing the open probability of CFTR (32, 33), thereby resulting in the increased Fsk/IBMX-induced ΔISC observed. Future studies should investigate possible mechanistic explanations for the apparent benefit of prolonged EP2/EP4 stimulation on CFTR-mediated ion currents.
The clinical success of CFTR modulator therapy, notably the recent introduction of elexacaftor and the triple combination therapy, has been remarkable, particularly regarding improvements to lung function (as measured by forced expiratory volume; FEV1). However, FEV1 in patients with CF on the triple combination therapy is still well below age-respective predicted levels (3, 5) and pulmonary fungal and bacterial infections remain concerning (34–37). It seems likely that additional pharmacological intervention to further increase mutant CFTR function nearer to normal CFTR levels could translate to additional clinical improvement. Thus, we sought to investigate the use of lubiprostone as an additional CFTR modulator in restoring ion transport function to CF epithelia. Our results with cotreatment with lubiprostone alongside established CFTR modulators in F508del/F508del and G551D/R117H human nasal epithelia demonstrate the therapeutic potential of the prostaglandin signaling pathway in the airway as a means to treat CFTR dysfunction. It has been shown that lubiprostone and PGE2 stimulate mucin release in the small intestine (38). Therefore, future studies applying lubiprostone or other PGE2 derivatives to the airway epithelium should investigate physiological responses other than ion currents, such as airway surface liquid depth, ciliary beat frequency, and mucociliary clearance.
Prospective
An additional drug to improve CFTR function with a distinct mechanism-of-action compared with currently prescribed CFTR modulators, such as a receptor-mediated CFTR activator as presented in this study, is intriguing given the proven approach of combination drug therapy to treat cystic fibrosis. Theoretically, CFTR activation as a form of treatment could be applied to treat any class defects in CFTR. This would include class II, III, and IV mutations in CFTR, which have known structural deficiencies that cause CF. Importantly, this would also include class V and VI mutations of CFTR, which are characterized by low or less stable CFTR expression and cannot be treated with currently available structure-correcting CFTR modulators. Finally, although class I CFTR mutations (characterized by incomplete protein synthesis) are unable to be clinically targeted by CFTR modulators until a successful read-through agent emerges (39, 40), much work is already being conducted to screen currently available CFTR modulators for their ability to enhance function of CFTR read-through products (41–44). It would stand to reason that a CFTR activator could be yet another useful tool in increasing function of a CFTR read-through product. In conclusion, we have demonstrated that lubiprostone and PGE2 stimulate CFTR-mediated ion transport in primary-derived human airway epithelia. Importantly, we show for the first time, to our knowledge, that GPCR activation of CFTR can enhance modulator-rescued mutant CFTR function, even under prolonged treatment regimens. The work presented here may inspire future work using gene-specific approaches to more completely understand the importance of PGE2 signaling via EP2 and EP4 in regulating ion transport processes across the airway epithelium.
DATA AVAILABILITY
Data will be made available upon reasonable request. Please see the link to supplemental material in Supplemental Data.
SUPPLEMENTAL DATA
Supplemental Table S1 and Supplemental Figs. S1–S4: https://doi.org/10.6084/m9.figshare.17315174.
GRANTS
This research was supported by the Cystic Fibrosis Foundation Grant Nos. BRATCH16I0 (to P.E.B.) and ZEITLI2010 (to P.L.Z.), the Eugene F. and Easton M. Crawford Charitable Lead Unitrust (to C.A.S. and P.E.B.), the Gilead Sciences Research Scholars Program in Cystic Fibrosis (to P.E.B), the National Institutes of Health Grant Nos. F32HL158174 (to C.A.S.) and R01HL155325 (to P.E.B.), and the Department of Pediatrics at National Jewish Health.
DISCLOSURES
P.E.B and P.L.Z. are co-inventors on a patent application (17/130,580) concerning the use of prostaglandin analogues in the treatment of cystic fibrosis transmembrane conductance regulator dysfunction. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.A.S., P.E.B., and P.L.Z. conceived and designed research; C.A.S., S.Y., and P.E.B. performed experiments; C.A.S. analyzed data; C.A.S., P.E.B., and P.L.Z. interpreted results of experiments; C.A.S. prepared figures; C.A.S. drafted manuscript; C.A.S., P.E.B., and P.L.Z. edited and revised manuscript; C.A.S., P.E.B., and P.L.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the Cystic Fibrosis Foundation for the contribution of compounds to this work through the CFTR Chemical Compound Distribution Program.
REFERENCES
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Cousar JL, Munck A, McKone EF, Van Der Ent CK, Moeller A, Simard C, Wang LT, Ingenito EP, McKee C, Lu Y, Lekstrom-Himes J, Elborn JS. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 377: 2013–2023, 2017. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 3.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Rowe SM, Jain R; VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 381: 1809–1819, 2019. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasemann H. CFTR modulator therapy for cystic fibrosis. N Engl J Med 377: 2085–2088, 2017. doi: 10.1056/NEJMe1712335. [DOI] [PubMed] [Google Scholar]
- 5.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL; VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 379: 1612–1620, 2018. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewington JJ, Backstrom J, Feldman A, Kramer EL, Moncivaiz JD, Ostmann AJ, Zhu X, Lu LJ, Clancy JP. Chronic β2AR stimulation limits CFTR activation in human airway epithelia. JCI Insight 3: e93029, 2018. doi: 10.1172/jci.insight.93029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambizas EM, Ginzburg R. Lubiprostone: a chloride channel activator for treatment of chronic constipation. Ann Pharmacother 41: 957–964, 2007. doi: 10.1345/aph.1K047. [DOI] [PubMed] [Google Scholar]
- 8.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: A double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther 25: 1351–1361, 2007. doi: 10.1111/j.1365-2036.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 9.Akiba Y, Kaunitz JD. May the truth be with you: lubiprostone as EP receptor agonist/ClC-2 internalizing “inhibitor. Dig Dis Sci 57: 2740–2742, 2012. doi: 10.1007/s10620-012-2410-2. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald KD, McKenzie KR, Henderson MJ, Hawkins CE, Vij N, Zeitlin PL. Lubiprostone activates non-CFTR-dependent respiratory epithelial chloride secretion in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol 295: L933–L940, 2008. doi: 10.1152/ajplung.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalán MA, Julio-Kalajzić F, Niemeyer MI, Cid LP, Sepúlveda FV. Short chain fatty acids effect on chloride channel ClC-2 as a possible mechanism for lubiprostone intestinal activation. Cells 9: 1781, 2020. doi: 10.3390/cells9081781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiffhauer ES, Vij N, Kovbasnjuk O, Kang PW, Walker D, Lee S, Zeitlin PL. Dual activation of CFTR and CLCN2 by lubiprostone in murine nasal epithelia. Am J Physiol Lung Cell Mol Physiol 304: L324–L331, 2013. doi: 10.1152/ajplung.00277.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuppoletti J, Tewari KP, Chakrabarti J, Malinowska DH. Identification of the fatty acid activation site on human CLC-2. Am J Physiol Cell Physiol 312: C707–C723, 2017. doi: 10.1152/ajpcell.00267.2016. [DOI] [PubMed] [Google Scholar]
- 14.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 15.Norimatsu Y, Moran AR, MacDonald KD. Lubiprostone activates CFTR, but not ClC-2, via the prostaglandin receptor (EP4). Biochem Biophys Res Commun 426: 374–379, 2012. doi: 10.1016/j.bbrc.2012.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339–351, 2011. doi: 10.1007/s10620-010-1495-8. [DOI] [PubMed] [Google Scholar]
- 17.Bijvelds MJC, Bot AGM, Escher JC, de Jonge HR. Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology 137: 976–985, 2009. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Joo NS, Wine JJ, Cuthbert AW. Lubiprostone stimulates secretion from tracheal submucosal glands of sheep, pigs, and humans. Am J Physiol Lung Cell Mol Physiol 296: 811–824, 2009. doi: 10.1152/ajplung.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuthbert AW. Lubiprostone targets prostanoid EP4 receptors in ovine airways. Br J Pharmacol 162: 508–520, 2011. doi: 10.1111/j.1476-5381.2010.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bratcher PE, Yadav S, Shaughnessy CA, Thornell IM, Zeitlin PL. Effect of apical chloride concentration on the measurement of responses to CFTR modulation in airway epithelia cultured from nasal brushings. Physiol Rep 8: e14603, 2020. doi: 10.14814/phy2.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namkung W, Park J, Seo Y, Verkman AS. Novel amino-carbonitrile-pyrazole identified in a small molecule screen activates wild-type and Δf508 cystic fibrosis transmembrane conductance regulator in the absence of a cAMP agonist. Mol Pharmacol 84: 384–392, 2013. doi: 10.1124/mol.113.086348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E 2 that differentially modulate activation responses. Blood 107: 3243–3250, 2006. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fei G, Wang YZ, Liu S, Hu HZ, Wang GD, Qu MH, Wang XY, Xia Y, Sun X, Bohn LM, Cooke HJ, Wood JD. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol 296: G823–G832, 2009. doi: 10.1152/ajpgi.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 36: 1187–1205, 2004. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Sellers ZM, Illek B, Figueira MF, Hari G, Joo NS, Sibley E, Souza-Menezes J, Morales MM, Fischer H, Wine JJ. Impaired PGE2-stimulated Cl- and HCO3-secretion contributes to cystic fibrosis airway disease. PLoS One 12: e0189894–20, 2017. doi: 10.1371/journal.pone.0189894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joy AP, Cowley EA. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am J Respir Cell Mol Biol 38: 143–152, 2008. doi: 10.1165/rcmb.2006-0295OC. [DOI] [PubMed] [Google Scholar]
- 28.Vleeshouwers W, van den Dries K, de Keijzer S, Joosten B, Lidke DS, Cambi A. Characterization of the Signaling Modalities of Prostaglandin E2 Receptors EP2 and EP4 Reveals Crosstalk and a Role for Microtubules. Front Immunol 11: 1–13, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem 277: 2614–2619, 2002. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 30.Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, Belvisi MG. Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax 70: 740–747, 2015. doi: 10.1136/thoraxjnl-2014-206592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZH, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154: 126–135, 2008. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling BN, Kokko KE, Eaton DC. Prostaglandin E2 activates clusters of apical Cl- channels in principal cells via a cyclic adenosine monophosphate-dependent pathway. J Clin Invest 93: 829–837, 1994. doi: 10.1172/JCI117037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G234–G251, 2008. doi: 10.1152/ajpgi.00366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryant JM, Brown KP, Burbaud S, Everall I, Belardinelli JM, Rodriguez-Rincon D, Grogono DM, Peterson CM, Verma D, Evans IE, Ruis C, Weimann A, Arora D, Malhotra S, Bannerman B, Passemar C, Templeton K, MacGregor G, Jiwa K, Fisher AJ, Blundell TL, Ordway DJ, Jackson M, Parkhill J, Floto RA. Stepwise pathogenic evolution of Mycobacterium abscessus. Science 372: eabb8699, 2021. doi: 10.1126/science.abb8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, et al. Emergence and spread of a humantransmissible multidrug-resistant nontuberculous mycobacterium. Science 354: 751–757, 2016. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brugha R, Spencer H. Mycobacterium abscessus in cystic fibrosis. Science 372: 465–466, 2021. doi: 10.1126/science.abi5695. [DOI] [PubMed] [Google Scholar]
- 37.Bercusson A, Jarvis G, Shah A. CF fungal disease in the age of CFTR modulators. Mycopathologia 186: 655–664, 2021. doi: 10.1007/s11046-021-00541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Lisle RC. Lubiprostone stimulates small intestinal mucin release. BMC Gastroenterol 12: 156, 2012. doi: 10.1186/1471-230X-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutyam V, Du M, Xue X, Keeling KM, Lucile White E, Robert Bostwick J, Rasmussen L, Liu B, Mazur M, Hong JS, Falk Libby E, Liang F, Shang H, Mense M, Suto MJ, Bedwell DM, Rowe SM. Discovery of clinically approved agents that promote suppression of cystic fibrosis transmembrane conductance regulator nonsense mutations. Am J Respir Crit Care Med 194: 1092–1103, 2016. doi: 10.1164/rccm.201601-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopes-Pacheco M. CFTR modulators: the changing face of cystic fibrosis in the era of precision medicine. Front Pharmacol 10: 1–29, 2020. doi: 10.3389/fphar.2019.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutyam V, Sharma J, Li Y, Peng N, Chen J, Tang LP, Falk Libby E, Singh AK, Conrath K, Rowe SM. Novel correctors and potentiators enhance translational readthrough in CFTR nonsense mutations. Am J Respir Cell Mol Biol 64: 604–616, 2021. doi: 10.1165/rcmb.2019-0291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutyam V, Libby EF, Peng N, Hadjiliadis D, Bonk M, Solomon GM, Rowe SM. Therapeutic benefit observed with the CFTR potentiator, ivacaftor, in a CF patient homozygous for the W1282X CFTR nonsense mutation. J Cyst Fibros 16: 24–29, 2017. doi: 10.1016/j.jcf.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue X, Mutyam V, Tang L, Biswas S, Du M, Jackson LA, Dai Y, Belakhov V, Shalev M, Chen F, Schacht J, Bridges RJ, Baasov T, Hong J, Bedwell DM, Rowe SM. Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor. Am J Respir Cell Mol Biol 50: 805–816, 2014. doi: 10.1165/rcmb.2013-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe SM, Varga K, Rab A, Bebok Z, Byram K, Li Y, Sorscher EJ, Clancy JP. Restoration of W1282X CFTR activity by enhanced expression. Am J Respir Cell Mol Biol 37: 347–356, 2007. doi: 10.1165/rcmb.2006-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 and Supplemental Figs. S1–S4: https://doi.org/10.6084/m9.figshare.17315174.
Data Availability Statement
Data will be made available upon reasonable request. Please see the link to supplemental material in Supplemental Data.