Keywords: dendritic cell, macrophage, menopause, sex differences, T cell
Abstract
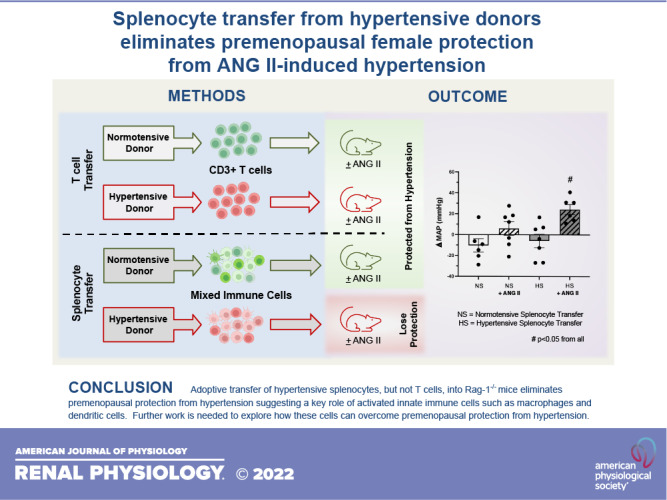
Premenopausal females are protected from angiotensin II (ANG II)-induced hypertension following the adoptive transfer of T cells from normotensive donors. For the present study, we hypothesized that the transfer of hypertensive T cells (HT) or splenocytes (HS) from hypertensive donors would eliminate premenopausal protection from hypertension. Premenopausal recombination-activating gene-1 (Rag-1)−/− females received either normotensive (NT) or hypertensive cells 3 wk before ANG II infusion (14 days, 490 ng/kg/min). Contrary to our hypothesis, no increase in ANG II-induced blood pressure was observed in the NT/ANG or HT/ANG groups. Flow cytometry demonstrated that renal FoxP3+ T regulatory cells were significantly decreased, and immunohistochemistry showed an increase in renal F4/80+ macrophages in the HT/ANG group, suggesting a shift in the renal inflammatory environment despite no change in blood pressure. Renal mRNA expression of macrophage chemoattractant protein-1 (MCP-1), endothelin-1 (ET-1), and G protein-coupled estrogen receptor-1 (GPER-1) was significantly decreased in the HT/ANG group. The adoptive transfer of hypertensive splenocytes before ANG II infusion (HS/ANG) eliminated premenopausal protection from hypertension and significantly decreased splenic FoxP3+ T regulatory cells compared with females that received normotensive splenocytes (NS/ANG). Expression of macrophage inflammatory protein 1α/chemokine (C-C motif) ligand 3 (MCP-1/CCL3), a potent macrophage chemokine, was elevated in the HS/ANG group; however, no increase in renal macrophage infiltration occurred. Together, these data show that in premenopausal females, T cells from hypertensive donors are not sufficient to induce robust ANG II-mediated hypertension; in contrast, transfer of hypertensive splenocytes (consisting of T/B lymphocytes, dendritic cells, and macrophages) is sufficient. Further work is needed to understand how innate and adaptive immune cells and estrogen signaling coordinate to cause differential hypertensive outcomes in premenopausal females.
NEW & NOTEWORTHY Our study is the first to explore the role of hypertensive T cells versus hypertensive splenocytes in premenopausal protection from ANG II-induced hypertension. We show that the hypertensive status of T cell donors does not impact blood pressure in the recipient female. However, splenocytes, when transferred from hypertensive donors, significantly increased premenopausal recipient blood pressure following ANG II infusion, highlighting the importance of further investigation into estrogen signaling and immune cell activation in females.
INTRODUCTION
Hypertension remains one of the most prevalent diseases in the world despite significant advancements in clinical screening and therapeutics. It is estimated that nearly 50% of all United States adults are currently living with hypertension, a large proportion of which are undiagnosed (1, 2). Patients with hypertension have a significantly higher risk of myocardial infarction, stroke, heart failure, kidney disease, and ultimately death compared with normotensive individuals, leading to a significant financial burden on our healthcare system (3, 4).
Up until the onset of menopause, women are relatively protected from the development of hypertension and the associated end-organ damage compared with men of similar age (5, 6). After menopause, this trend reverses, and postmenopausal women have higher rates of hypertension than their male counterparts. Sex differences are also seen in the effectiveness of commonly used antihypertensive therapeutics across the life span: women have decreased responsiveness and increased side effects to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers compared with men of similar age (7). Despite these known sex differences in clinical hypertension and responsiveness to antihypertensive therapeutics, little is known about the sex-specific physiological mechanisms at play that protect women from disease progression before menopause.
Sex differences have been reported in multiple animal models of hypertension, including the angiotensin II (ANG II) mouse model (8), the spontaneously hypertensive rat model (9, 10), and the Dahl salt-sensitive rat model (11). Much of this work has focused on traditional hypertensive mediators such as the vasculature, brain, and kidneys to elucidate the underlying sex-specific mechanisms. Recently, a growing body of work has moved beyond these traditional organ systems and demonstrated the importance of a novel hypertensive mediator: the immune system.
It was first shown that male recombination-activating gene-1 (Rag-1)−/− mice, which lack an adaptive immune system, have a blunted hypertensive response to ANG II (12). However, when Rag-1−/− males received a T cell adoptive transfer before ANG II infusion, a robust hypertensive response was restored, validating that T cells are required for ANG II-induced hypertension in male mice (12).
Our group furthered this work by demonstrating a sex difference in this immune-mediated hypertension. We found, using the Rag-1−/− model, that a sex difference is present in T cell-mediated hypertension, as premenopausal females were protected from ANG II before and after adoptive transfer of T cells (13). Using our mouse model of menopause [4-vinylcyclohexene diepoxide (VCD) model], we demonstrated that menopausal Rag-1−/− females lose protection against T cell-mediated hypertension and that ANG II is able to elicit a robust increase in blood pressure (14, 15). While this previous work identified a sex difference in immune-mediated hypertension, it did not clarify the underlying mechanisms that drive this premenopausal protection.
Hypertensive stimuli are known to cause T cell activation and infiltration into organs such as the kidney (16, 17), leading to elevations in blood pressure in male mice (12, 18, 19). Data from our Rag-1−/− studies suggested that premenopausal females (estrogen replete) suppress this T cell activation in response to a hypertensive stimulus and inhibit the infiltration of T cells into the kidney, preventing renal inflammation. Thus, in the present study, we hypothesized that transfer of hypertensive immune cells, either T cells or a mixed immune cell population (total splenocytes), from male hypertensive mice would eliminate premenopausal protection from ANG II-induced hypertension via the activation of inflammatory pathways in the kidney.
METHODS
Animals
Donor mice (male C57BL6/J mice, strain 000664, Jackson Laboratory, 10 wk old) were used as donors in both the T cell and splenocyte experiments.
Recipient mice (female and male Rag-1−/− mice, 8−10 wk old) were obtained in house at the University of Arizona. Original breeders were purchased from the Jackson Laboratory in 2014 (strain 002216, B6.129S7-Rag1tm1Mom/J) (13, 15) and used as recipients for both T cell and splenocyte transfer experiments.
All mice were housed in standard polypropylene cages in a temperature- and humidity-controlled facility and were maintained on a 14:10-h light-dark cycle with normal mouse chow (0.25% NaCl, No. 7013, Harlan Tecklad) and had water available ad libitum. All methods were approved by the Institutional Animal Care and Use Committee of the University of Arizona.
Experimental Protocol
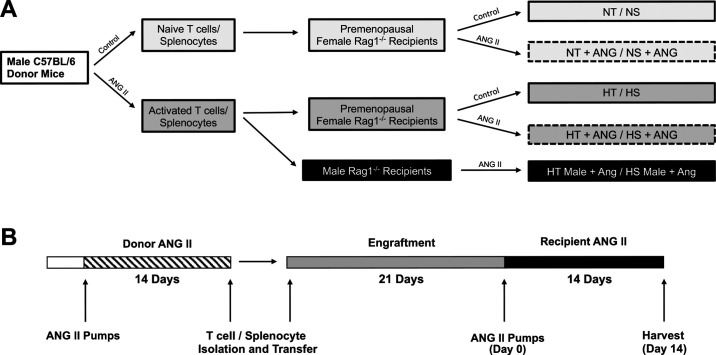
A shared timeline for all experimental protocols is shown in Fig. 1B.
Figure 1.
Experimental design and timeline. A: to determine the impact of transferring hypertensive T cells/splenocytes into premenopausal Rag-1−/− females, wild-type male donors (C57BL6/J) were split into control and angiotensin II (ANG II) donor groups. Normotensive/hypertensive T cells (NT/HT, respectively) and normotensive/hypertensive splenocytes (NS/HS, respectively) were then isolated and transferred into Rag-1−/− premenopausal female recipients as shown. Following immune cell engraftment, female recipients were further split into control and ANG II subgroups based on the schematic shown (NT/NS ± ANG and HT/HS ± ANG). One group of male Rag-1−/− recipients was included as a positive control group (HT/HS male + ANG). B: experimental timeline of donor ANG II infusion, T cell isolation, adoptive transfer into Rag-1−/− recipients, T cell engraftment period, recipient ANG II infusion, and final harvest/tissue collection. Rag-1, recombination-activating gene-1.
T Cell Adoptive Transfer Donors
For hypertensive T cell donors, male C57BL/6J mice received 14 days of ANG II infusion (800 ng/kg/min, A9525, Sigma) via an osmotic minipump (model 1004, Alzet), which induced a significant increase in mean arterial blood pressure (ΔMAP: 30.5 ± 8.4 mmHg). Normotensive T cell donor mice received sham surgery but no ANG II infusion 14 days before T cell isolation (△MAP: −1.7 ± 3.3 mmHg). CD3+ T cells were isolated from donor spleens using a Miltenyi T cell enrichment kit (Pan T cell Isolation Kit, No. 130-095-130, Miltenyi) according to the manufacturer’s protocol and pooled within the respective groups before adoptive transfer via tail vein injection (∼7.0 × 106 cells per recipient).
T Cell Adoptive Transfer Recipients
All donors used in this study were male. Rag-1−/− premenopausal female recipient mice received adoptive transfer of NT or HT male CD3+ T cells (∼7.0 × 106 cells per recipient) 3 wk before ANG II infusion (14 days, 490 ng/kg/min, A9525, Sigma) as previously described (13, 15).
As shown in Fig. 1A, the premenopausal experimental groups were as follows: normotensive donor T cells into Rag-1−/− premenopausal females (NT group), normotensive donor T cells into Rag-1−/− premenopausal females with ANG II infusion (NT/ANG group), hypertensive donor T cells into Rag-1−/− premenopausal females (HT group), and hypertensive donor T cells into Rag-1−/− premenopausal females with ANG II infusion (HT/ANG group).
A control male recipient group was used: hypertensive donor T cells into Rag-1−/− males with ANG II infusion (HT male/ANG group) to assess maximum blood pressure responses (results are shown in Supplemental Fig. S2; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.16587431.v1).
Splenocyte Adoptive Transfer Donors
For splenocyte donors, male C57BL/6J mice received 14 days of ANG II infusion (800 ng/kg/min, A9525, Sigma) via a osmotic minipump (model 1004, Alzet), which induced a significant increase in MAP (ΔMAP: 33.1 ± 7.8 mmHg). Normotensive splenocyte donor mice received sham surgery but no ANG II infusion 14 days before splenocyte isolation (ΔMAP: 6.4 ± 6.9 mmHg). Splenocyte samples were obtained by manual disruption of spleens through a 40-µm cell filter and pooled within the respective groups before adoptive transfer via tail vein injection (∼1.0 × 107 cells per recipient).
Splenocyte Adoptive Transfer Recipients
Rag-1−/− premenopausal female recipient mice received adoptive transfer of male normotensive (NS) or hypertensive splenocytes (HS) (∼1.0 × 107 cells per recipient) 3 wk before ANG II infusion (14 days, 490 ng/kg/min, A9525, Sigma). As shown in Fig. 1A, premenopausal splenocyte experimental groups were as follows: normotensive donor splenocytes into Rag-1−/− premenopausal females (NS group), normotensive donor splenocytes into Rag-1−/− premenopausal females with ANG II infusion (NS/ANG group), hypertensive donor splenocytes into Rag-1−/− premenopausal females (HS group), and hypertensive donor splenocytes into Rag-1−/− premenopausal females with ANG II infusion (HS/ANG group).
A control male recipient group was used: hypertensive donor splenocytes into males with ANG II infusion (HS male/ANG) to assess maximum blood pressure responses (results are shown in Supplemental Fig. S2).
ANG II Infusion and Blood Pressure Measurements
Mice were anesthetized using isoflurane for subcutaneous insertion of osmotic minipumps (model 1004, Alzet) containing ANG II (A9525, Sigma, donors: 800 ng/kg/min and recipients: 490 ng/kg/min).
A noninvasive tail cuff measurement system (MC4000, Hatteras Instruments) was used to monitor blood pressure in all experiments. Animals were not sedated, and all blood pressure readings were made in the morning following completion of the dark cycle. Mice were acclimated to the tail cuff measurement system for 3 consecutive days for 1 and 2 wk before the collection of baseline blood pressure measurements. Baseline blood pressures were read for 3 consecutive days before the insertion of the ANG II pumps, and the final day of measurement was used for analysis (baseline day 3). Changes in blood pressure (Δ) were calculated for each animal using the difference between ANG II day 14 and baseline day 3.
Flow Cytometry
Splenic and renal T cell expression patterns were analyzed by flow cytometry as previously described (13, 15). Briefly, the spleen and kidney were digested in Accutase for 30 min at 37°C and then manually disrupted through a 40-µm cell filter. The kidneys were further processed with a 30%/70% Percoll gradient to isolate immune cells. Cell suspensions were then stained with a saturated concentration of surface antibodies (1:100) and a live/dead discriminator dye (1:1,000). Fixation and permeabilization were performed with a Foxp3 Staining Buffer Set (Cat. No. 00-5521, eBioscience) followed by intracellular antibody staining (1:100). A BD Fortessa instrument (BD FACS Diva software, Becton Dickinson) was used to acquire data, and analysis was performed using FlowJo software (Tree Star).
T cell FCM antibodies and reagents were as follows: Live/Dead (Aqua, Cat. No. L34957, Invitrogen), CD45.2 (A700, Cat. No. 109822, BioLegend), CD3 (APCe780, Cat. No. 47-0032-82, Invitrogen), CD4 (FITC, Cat. No. 100406, BioLegend), CD8 (BV650, Cat. No. 100742, BioLegend), and FoxP3 (PEe610, Cat. No. 61-5773-82, Invitrogen).
Immunohistochemistry
Immunohistochemistry was performed as previously described and in accordance with the guidelines for authors and reviewers on the use of antibodies in physiology studies (15, 20). Briefly, the kidneys were removed, decapsulated, and fixed in 4% paraformaldehyde for 24 h before paraffin embedding and sectioning (5 µm).
Total macrophage infiltration was determined by F4/80 staining as previously described (15). Briefly, sections were deparaffinized, endogenous horseradish peroxidase (HRP) was blocked in 0.3% H2O2 in methanol, and antigen retrieval was performed in citrate buffer at 37°C. Nonspecific binding was blocked by 10% goat serum in 2% BSA before overnight incubation at 4°C with rat anti-mouse F4/80 (1:20, MCA497GA, Bio-Rad) or rabbit anti-mouse osteopontin (OPN; 1:100, MAB808, R&D Systems) followed by HRP-conjugated goat anti-rat IgG-HRP (1:200, Cat. No. 305005, Bio-Rad) or HRP-conjugated goat anti-rabbit (1:200, Cat. No. 7074S, Cell Signaling), respectively, for 1 h at room temperature. Positive staining was visualized with a Diaminobenzidine Substrate Kit (Cat. No. 002020, Life Technologies) and counterstained with hematoxylin (Cat. No. 7211, ThermoFisher). Images were acquired at ×20 magnification on a Nikon Eclipse 50i using NIS Elements software. A total of 10 cortical images from each section were analyzed with a multistep binary algorithm using ImageJ software. Data are presented as a percentage of the total tissue area (21).
RNA Isolation and Quantitative Real-Time PCR
RNA isolation and quantitative real-time PCR experiments were performed as previously described (13, 15). Briefly, one kidney per mouse was excised and used for RNA isolation. The kidneys were mechanically disrupted in RLT buffer + β-mercaptoethanol using RNAse-free beads (Cat. No. RDE5-RNA, Next Advance) in a bullet blender bead homogenizer (Next Advance). RNA was then isolated according to the manufacturer’s instructions (RNeasy Mini Kit, Cat. No. 74101, Qiagen). A DNase (Cat. No. 79254, Qiagen) incubation was performed to remove potential DNA contamination. A Nanodrop ND 1000 spectrophotometer (Wilmington, DE) was used to quantify RNA before reverse transcription. The resulting cDNA was used for real-time PCR experiments on a RotorGene RG3000 (Qiagen). Expression levels of the genes of interest were normalized to dynactin mRNA after samples were run in parallel (13).
Primer sequences were as follows: macrophage chemoattractant protein-1 (MCP-1), forward 5′-TTAAAAACCTGGATCGGAACCAA-3′ and reverse 5′-GCATTAGCTTCAGATTTACGGGT-3′; TNF-α, forward 5′-GATTCTTCCCTGAGGTGCAA-3′ and reverse 5′-AAGACAGCTTCCCACACTGG-3′; OPN, forward 5′-AGAGGCAAAAACACAGTTCCTT-3′ and reverse 5′-TTGGTTACAACGGTGTTTGC-3′; endothelin-1 (ET-1), forward 5′-CAGCATCCTTGATCCAAACA-3′ and reverse 5′-TGTGGAATCTCCTGGCTCTC-3′; G protein-coupled estrogen receptor-1 (GPER-1), forward 5′-TCCTGAGTGAACAGCGTGTC-3′ and reverse 5′-ACCCAGTCTCCTTCCACCTT-3′; IL-2, forward 5′-AAAGGGCTCTGACAACACATTT-3′ and reverse 5′-AGGGCTTGTTGAGATGATGC-3′; IL-6, forward 5′-GTGGCTAAGGACCAAGACCA-3′ and reverse 5′-AGGCATAACGCACTAGGTTTG-3′; IL-10, forward 5′-CCCTTTGCTATGGTGTCCTT-3′ and reverse 5′-AGTAGGGGAACCCTCTGAGC-3′; and macrophage inflammatory protein-1α (MIP-1α)/chemokine (C-C motif) ligand 3 (CCL3), forward 5′-CATATGGAGCTGACACCCCG-3′ and reverse 5′-GAGCAAAGGCTGCTGGTTTC-3′.
Statistics
Data were analyzed with Graph Pad Prism software (version 8) by ordinary two-way ANOVA with Tukey’s multiple comparison post hoc tests or an unpaired Student’s t test where appropriate. Data are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
Adoptive Transfer of T Cells From Hypertensive Donors Did Not Eliminate Premenopausal Protection From ANG II-Induced Hypertension
Our previous study demonstrated that premenopausal Rag-1−/− females are protected from ANG II-induced hypertension following the transfer of T cells from normotensive male donor mice (normotensive T cells) (13). In contrast, Rag-1−/− males became hypertensive when ANG II was infused following transfer the transfer of T cells from normotensive male donor mice. In a subsequent study, we showed that menopausal Rag-1−/− female mice became hypertensive when ANG II was infused following the transfer of T cells from normotensive male donor mice, highlighting that after menopause, females lose protection from T cell-mediated ANG II-induced hypertension.
Here, we examined if the transfer of T cells from hypertensive donors would eliminate premenopausal protection from ANG II-induced hypertension. Premenopausal Rag-1−/− females were infused with ANG II following the transfer of T cells from normotensive male donors or from hypertensive male donors (∼7.0 × 106 cells per recipient).
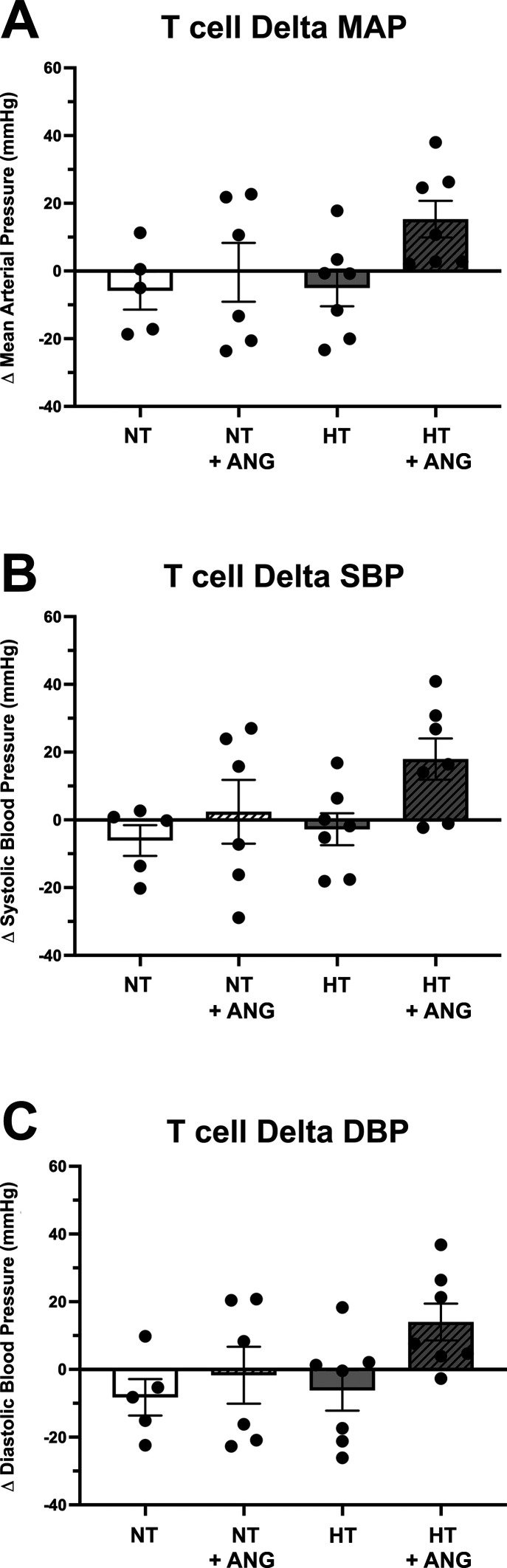
Three weeks following the adoptive transfer of T cells, but before ANG II infusion, baseline blood pressure measurements [MAP, systolic blood pressure (SBP), or diastolic blood pressure (DBP)] were not significantly different between the four groups of premenopausal females (Supplemental Fig. S1, A−C). Baseline MAP values were as follows: 96.2 ± 2.5 mmHg in the NT group, 95.7 ± 7.0 mmHg in the NT/ANG group, 97.4 ± 5.2 in the HT group, and 96.4 ± 3.7 mmHg in the HT/ANG group. Following ANG II infusion, the HT/ANG group demonstrated a small increase in ΔMAP, ΔSBP, and ΔDBP compared with all other groups; however, this was not statistically significant between any groups (Fig. 2, A–C, and Supplemental Table S1). Final MAP values were as follows: 90.4 ± 5.0 mmHg in the NT group, 95.3 ± 3.3 mmHg in the NT/ANG group, 92.3 ± 3.4 mmHg in the HT group, and 111.7 ± 5.5 mmHg (P < 0.05) in the HT/ANG group. Two-way ANOVA demonstrated a small but significant increase in MAP for the HT/ANG group compared with the NT and HT controls, similar to our previous studies where we transferred T cells from normotensive male mice into premenopausal female mice.
Figure 2.
Blood pressure response of Rag-1−/− females to 14 days of angiotensin II (ANG II) infusion (490 ng/kg/min) following adoptive transfer of normotensive T cells (NT) or hypertensive T cells (HT). No significant difference was seen in mean arterial blood pressure (ΔMAP; A), systolic blood pressure (ΔSBP; B), or diastolic blood pressure (ΔDBP; C) in premenopausal females following normotensive T cell transfer (NT + ANG) or hypertensive T cell transfer (HT + ANG) compared with the respective controls (NT and HT). The change (Δ) in blood pressure was calculated using the difference between the final ANG II day 14 and baseline blood pressure recording (baseline = following T cell adoptive transfer, before ANG II infusion); n = 5 in the control group and n = 6/7 in the experimental groups. No significant difference via two-way ANOVA was found. Rag-1, recombination-activating gene-1.
Also included in this experiment were male Rag-1−/− mice that received hypertensive T cells before ANG II infusion (HT male/ANG group) as a positive control group. ANG II induced a significant increase in ΔMAP of 52.2 ± 16.3 mmHg in the males (Supplemental Fig. S2, A−C).
Frequency of Renal T Regulatory Cells Decreased in Premenopausal Females That Received Hypertensive T Cells
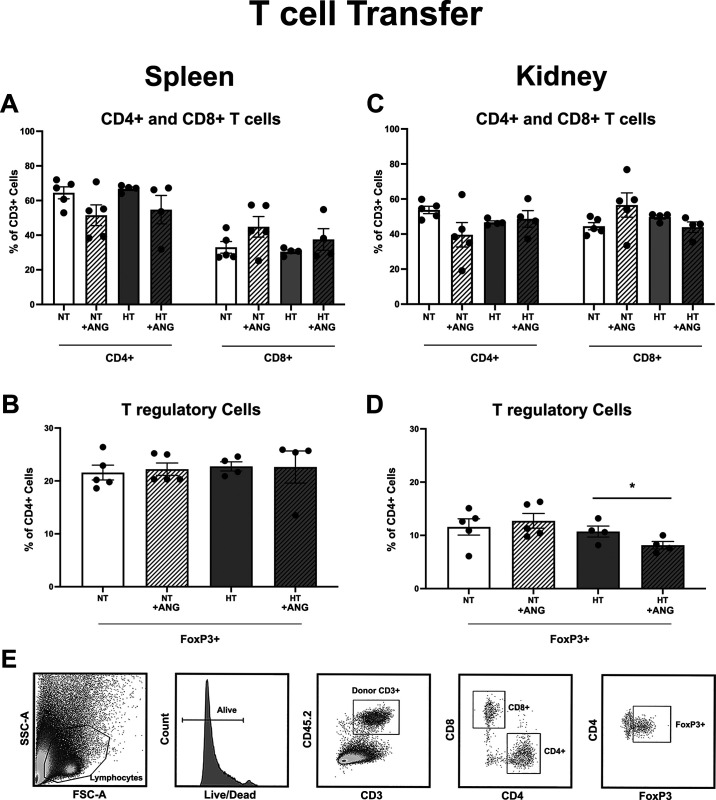
Flow cytometric analysis of the spleens from premenopausal females demonstrated no significant differences in splenic CD4+ or CD8+ T cell frequencies irrespective of donor T cell population (NT or HT; Fig. 3A). Similarly, no significant difference was seen in splenic FoxP3+ T regulatory cell frequencies across the premenopausal groups (Fig. 3B).
Figure 3.
Flow cytometric analysis of splenic and renal T cells in Rag-1−/− females following normotensive T cell (NT) or hypertensive T cell (HT) transfer and angiotensin II (ANG II) infusion. No significant difference was found in the frequency of CD4+ or CD8+ splenic T cells (A) or in the frequency of FoxP3+ splenic T regulatory cells (B) regardless of donor T cell activation (NT vs. HT) or ANG II infusion (control vs. control + ANG). Similarly, no significant difference was found in the frequency of CD4+ or CD8+ renal T cells (C) regardless of donor T cell activation (NT vs. HT) or ANG II infusion (control vs. ANG). However, females that received hypertensive T cells (HT + ANG) had a significantly lower frequency of renal FoxP3+ T regulatory cells compared with the respective control (HT control) and compared with females that received NT before ANG II infusion (NT + ANG) (D). E: representative flow cytometric gating. n = 5 in the NT groups and n = 4 in the HT groups. *P ≤ 0.05 via two-way ANOVA with a Tukey multiple comparison test. Rag-1, recombination-activating gene-1; FSC-A, forward scatter area; SSC-A, side scatter area.
Flow cytometric analysis of the kidneys demonstrated no significant difference in renal CD4+ or CD8+ T cell frequencies across the premenopausal groups (Fig. 3C). In contrast, there was a significant decrease following ANG II infusion in the frequency of FoxP3+ T cells in the kidneys of premenopausal females that received hypertensive T cells (HT/ANG group; Fig. 3D) compared with premenopausal females that received normotensive T cells (NT/ANG group). Gating strategies for all T cells are shown in Fig. 3E
Adoptive Transfer of Hypertensive T Cells Did Not Increase the Expression of Renal Inflammatory Markers
We have previously reported that the expression of renal inflammatory markers in premenopausal Rag-1−/− females following the transfer of male normotensive T cells was not impacted by ANG II infusion. In contrast, transfer of male normotensive T cells into Rag-1−/− postmenopausal females significantly increased renal inflammatory markers, indicating in our previous studies a protective role of estrogen signaling in T cell-mediated hypertension and renal inflammation (13, 15).
To determine if the expression of renal inflammatory markers was impacted in premenopausal females following the transfer of hypertensive T cells by ANG II infusion, we measured RNA expression profiles via RT-PCR. As shown in Table 1, no significant increase in inflammatory markers was identified; in contrast, ANG II significantly decreased mRNA expression of MCP-1 and ET-1 in the kidneys of premenopausal females that received hypertensive T cells. We also saw a significant decrease in GPER-1 expression following ANG II infusion. A recent study reported that GPER-1 plays a protective role in females against renal injury (22). However, in the present study, mRNA expression of the proinflammatory cytokine TNF-α and OPN, a marker for renal damage, was not changed (Table 1).
Table 1.
Renal gene expression following adoptive transfer of hypertensive T cells
| Gene | HT | HT + ANG |
|---|---|---|
| TNF-α | 1.0 ± 0.25 | 0.40 ± 0.14 |
| MCP-1 | 1.0 ± 0.13 | 0.45 ± 0.11* |
| Endothelin-1 | 1.0 ± 0.15 | 0.56 ± 0.04* |
| GPER-1 | 1.0 ± 0.03 | 0.54 ± 0.11* |
| OPN | 1.0 ± 0.14 | 0.85 ± 0.05 |
Results are expressed as means ± SE; n = 3 mice/group. RT-PCR data are shown as mean relative fold changes in the whole kidney. Assays for dynactin were run in parallel and used for normalization of each sample. ANG, angiotensin II; HT, hypertensive T cells; TNF-α, tumor necrosis factor-α; MCP-1, macrophage chemoattractant protein-1; GPER-1, G protein-coupled estrogen receptor-1; OPN, osteopontin. *P < 0.05 vs. control via an unpaired Student’s t test.
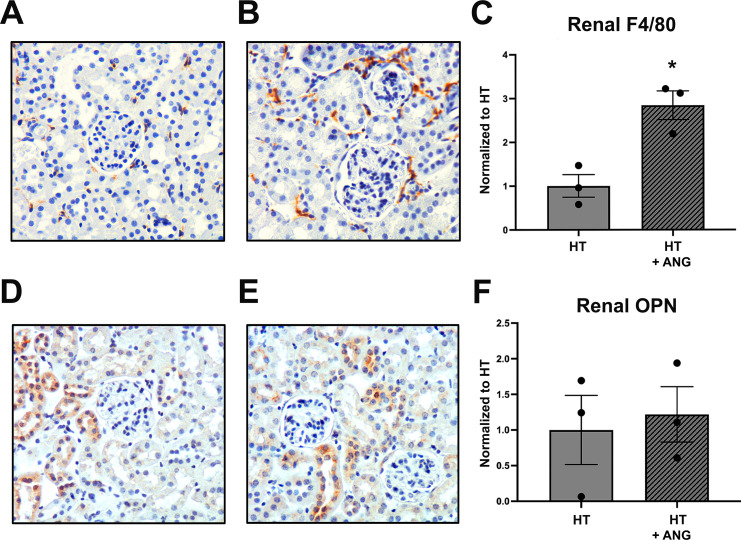
Using immunohistochemistry, we demonstrated a significant increase in macrophage staining (F4/80) in the kidneys of premenopausal females that received hypertensive T cells following ANG II infusion (Fig. 4, A–C), with no change in OPN-1 protein expression between the groups (Fig. 4, D–F), suggesting no ANG II-induced renal damage in the female kidneys.
Figure 4.
Renal immunohistochemistry in premenopausal female Rag-1−/− mice following hypertensive T cell (HT) transfer and angiotensin II (ANG II) infusion. A and B: representative images of F4/80-stained renal macrophages for premenopausal HT (A) and HT + ANG (B) groups. Renal F4/80 macrophages were significantly increased following ANG II infusion in premenopausal females that received HT (C). D and E: representative osteopontin (OPN) images for HT (D) and HT + ANG (E) groups. F: no significant difference was seen in renal OPN staining in premenopausal females that received HT. Results were normalized to HT. Magnification: ×200; n = 3 mice/group. *P < 0.05 via an unpaired Student’s t test. Rag-1, recombination-activating gene-1.
Adoptive Transfer of Hypertensive Splenocytes Eliminates Premenopausal Protection From ANG II-Induced Hypertension
Previous work in male rats has shown that the transfer of splenocytes from donors fed a high- or low-salt diet increased salt-sensitive hypertension in the recipient (23). The source of the splenocytes (from a hypertensive or normotensive donor) did not significantly affect the hypertensive response in the recipient.
In the present study, using premenopausal Rag-1−/− females, we examined whether the transfer of total splenocytes could induce a hypertensive response to ANG II and if the source of the splenocytes, from normotensive or hypertensive donors (∼1.0 × 107 cells per recipient), impacted the blood pressure response.
Three weeks following the adoptive transfer of splenocytes, baseline blood pressures (MAP, SBP, or DBP) were not significantly different in any of the four groups of premenopausal females (Supplemental Fig. S1, D−F). Baseline MAP values were as follows: 107.0 ± 7.2 mmHg in the NS group, 98.9 ± 5.7 mmHg in the NS/ANG group, 101.7 ± 5.6 mmHg in the HS group, and 97.6 ± 4.7 mmHg in the HS/ANG group. ANG II infusion induced a significant increase in ΔMAP, ΔSBP, and ΔDBP (Fig. 5, A–C) in premenopausal Rag-1−/− mice, thus eliminating premenopausal female protection from hypertension. This robust blood pressure response to ANG II infusion was not observed in premenopausal females that received splenocytes from normotensive donors (Fig. 5, A–C). Final MAP values were as follows: 96.6 ± 1.7 mmHg in the NS group, 104.9 ± 3.5 mmHg in the NS/ANG group, 95.8 ± 4.5 in the HS group, and 121.9 ± 7.1 mmHg (P < 0.05) in the HS/ANG group. Two-way ANOVA demonstrated a significant increase in MAP for the HS/ANG group compared with all other groups (Supplemental Table S1).
Figure 5.
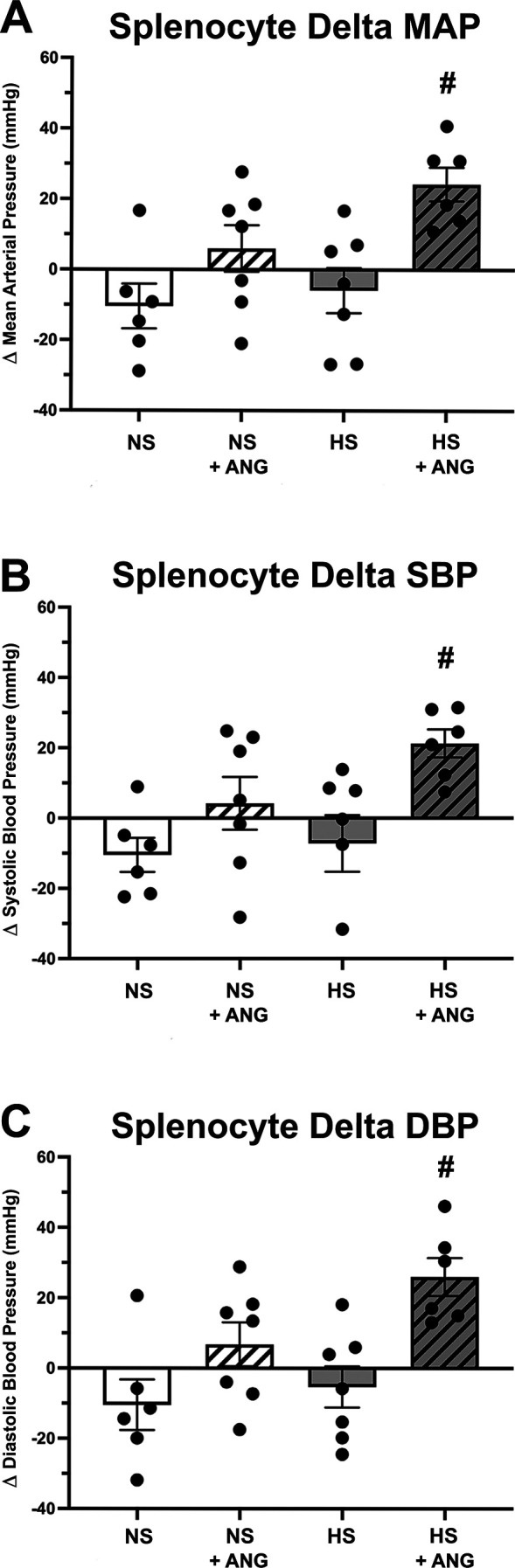
Blood pressure response of premenopausal Rag-1−/− females to 14 days of angiotensin II (ANG II) infusion (490 ng/kg/min) following adoptive transfer of normotensive splenocytes (NS) or hypertensive splenocytes (HS). A significant increase in mean arterial blood pressure (ΔMAP; A), systolic blood pressure (ΔSBP; B), and diastolic blood pressure (ΔDBP; C) was seen in premenopausal females following HS transfer and ANG II infusion (HS + ANG) compared with control (HS). No difference was seen in blood pressure following transfer of NS in premenopausal Rag-1−/− females (NS vs. NS + ANG; A−C). The change (Δ) in blood pressure was calculated using difference between the final ANG II day 14 and baseline blood pressure recording (baseline = following splenocyte adoptive transfer, before ANG II infusion); n = 6−7 mice/group. #P < 0.05 via two-way ANOVA with a Tukey’s multiple comparison test. Rag-1, recombination-activating gene-1.
Frequency of Splenic T Regulatory Cells Decreased in Premenopausal Females That Received Hypertensive Splenocytes
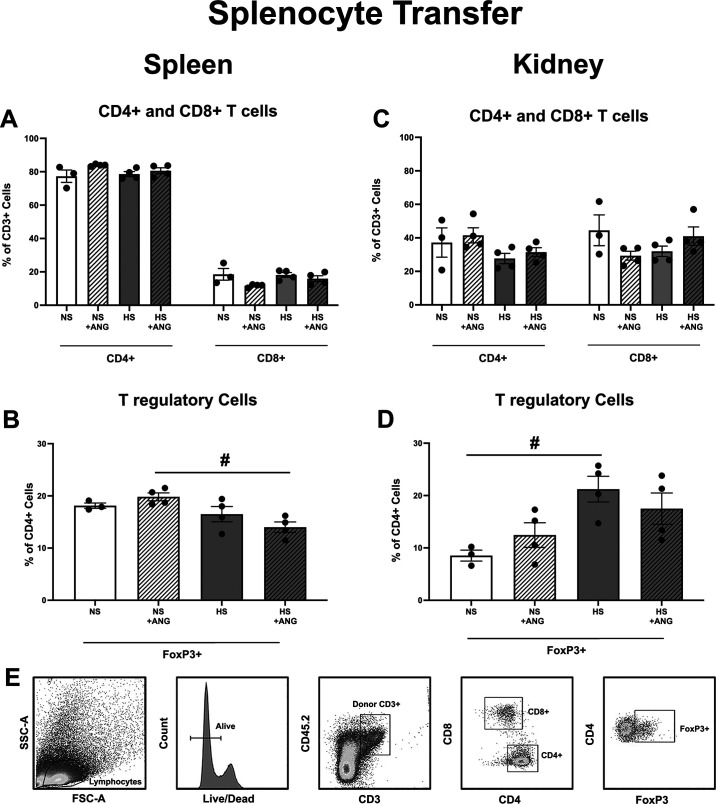
Flow cytometric analysis of the spleen from premenopausal females demonstrated no significant differences in splenic CD4+ or CD8+ T cell frequencies irrespective of donor splenocyte populations (NS or HS; Fig. 6A). Similarly, no significant difference was seen in renal CD4+ or CD8+ T cell frequencies across the premenopausal groups (Fig. 6C).
Figure 6.
Flow cytometric analysis of splenic and renal T cells in Rag-1−/− females following normotensive splenocyte (NS) or hypertensive splenocyte (HS) transfer and angiotensin II (ANG II) infusion. No significant difference was found in the frequency of CD4+ or CD8+ splenic (A) or renal (C) T cells regardless of donor splenocyte (NS vs. HS) or ANG II infusion (control vs. control + ANG). However, females that received HS + ANG had a significantly lower frequency of splenic FoxP3+ T regulatory cells compared with those that received NS + ANG (B). Interestingly, transfer of HS alone increased renal FoxP3+ T regulatory cells compared with NS transfer, but no impact of ANG II was seen (D). E: representative flow cytometric gating; n = 3−4 mice/group. #P < 0.05 via two-way ANOVA with a Tukey’s multiple comparison test. Rag-1, recombination-activating gene-1; FSC-A, forward scatter area; SSC-A, side scatter area.
In contrast, there was a significant decrease in the frequency of splenic FoxP3+ T cells in premenopausal females that received splenocytes from hypertensive donors following ANG II infusion (Fig. 6B) compared with females that received splenocytes from normotensive donors. The decrease in the frequency of FoxP3+ T cells did not occur in the kidneys; rather, the transfer of splenocytes from hypertensive donors significantly increased the frequency of FoxP3+ T cells in the kidneys of premenopausal females in the absence of ANG II infusion (Fig. 6D). The gating strategies for all T cells are shown in Fig. 6E.
Increase in Blood Pressure in Premenopausal Females Following the Adoptive Transfer of Splenocytes From Hypertensive Donors Is Associated With an Increase mRNA Expression of MIP-1α/CCL3 in the Kidneys
As the transfer of splenocytes from hypertensive donors into premenopausal females significantly increased MAP, we examined if this increase in blood pressure was accompanied by an increase in renal inflammatory markers.
As shown in Table 2, ANG II infusion had no impact on the renal expression of IL-2 or IL-6 in premenopausal females following the transfer of splenocytes from normotensive or hypertensive donors. ANG II decreased the renal expression of IL-10 in premenopausal females that received normotensive splenocytes. ANG II significantly increased the renal expression of MCP-1 in premenopausal females following the transfer of splenocytes from normotensive donors; no other comparisons within the groups demonstrated a significant difference in MCP-1 expression (Supplemental Table S4). ANG II infusion significantly increased the renal expression of MIP-1α (also known as CCL3) in premenopausal females that received splenocytes from hypertensive donors; no other comparisons within the groups demonstrated a significant difference in MIP-1α/CCL3 expression (Supplemental Table S4). There was no significant difference in GPER-1 or OPN expression in any of the splenocyte groups before or after ANG II infusion (Table 2).
Table 2.
Renal gene expression following adoptive transfer of normotensive or hypertensive splenocytes
| Gene | NS | NS + ANG | HS | HS + ANG |
|---|---|---|---|---|
| IL-2 | 1.0 ± 0.15 | 0.91 ± 0.06 | 1.0 ± 0.12 | 0.72 ± 0.10 |
| IL-6 | 1.0 ± 0.06 | 0.84 ± 0.06 | 1.0 ± 0.10 | 1.25 ± 0.12 |
| IL-10 | 1.0 ± 0.04 | 0.69 ± 0.02* | 1.0 ± 0.12 | 0.84 ± 0.08 |
| MCP-1 | 1.0 ± 0.15 | 1.85 ± 0.40* | 1.0 ± 0.10 | 1.37 ± 0.22 |
| MIP-1α | 1.0 ± 0.22 | 1.30 ± 0.41 | 1.0 ± 0.12 | 1.81 ± 0.11* |
| GPER-1 | 1.0 ± 0.04 | 1.34 ± 0.13 | 1.0 ± 0.09 | 1.19 ± 0.13 |
| OPN | 1.0 ± 0.02 | 1.18 ± 0.06 | 1.0 ± 0.06 | 0.95 ± 0.03 |
Results are expressed as means ± SE; n = 3 mice/group. RT-PCR data are shown as mean relative fold changes in the whole kidney. Assays for dynactin were run in parallel and used for normalization of each sample. ANG, angiotensin II; NS, normotensive splenocytes; HS, hypertensive splenocytes; IL, interleukin; MCP-1, macrophage chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; GPER-1, G protein-coupled estrogen receptor-1; OPN, osteopontin. *P < 0.05 vs control via an unpaired Student’s t test.
Using immunohistochemistry, we saw no change in renal macrophage infiltration (F4/80) in the kidneys of any premenopausal females following splenocyte transfers (Supplemental Fig. S5, A and B) and no change in OPN expression (Supplemental Fig. S5, C and D), suggesting no renal damage despite the increase in blood pressure.
DISCUSSION
The goal of the present study was to explore if premenopausal protection from ANG II-induced hypertension was eliminated following the transfer of hypertensive CD3+ T cells or hypertensive splenocytes (consisting of T/B lymphocytes, dendritic cells, and macrophages) and to determine if these donor immune cells impacted renal T cell infiltration and the renal inflammatory environment. Our major findings were 1) premenopausal females remain protected against ANG II-induced hypertension following the transfer of hypertensive CD3+ T cells despite a decrease in renal FoxP3+ T regulatory cell expression and increased renal infiltration of F4/80 macrophages and 2) the transfer of hypertensive splenocytes significantly increased the blood pressure response to ANG II in premenopausal females, thus eliminating premenopausal female protection, and was associated with an increase in renal expression of MIP-1α/CCL3 expression.
Hypertensive stimuli are known to cause T cell activation and infiltration into organs such as the kidney (16, 17), leading to elevations in blood pressure in male mice (12, 18, 19). Early studies in male Rag-1−/− mice demonstrated that without T cells, ANG II does not induce a hypertensive response (12). However, transfer of normotensive CD3+ T cells (from normotensive mice) restored ANG II-induced hypertension.
Our studies in female Rag-1−/− mice have highlighted a sex difference in T cell-mediated hypertension. Transfer of normotensive CD3+ T cells (male donors) into Rag-1−/− males produced robust ANG II-induced hypertension (13). The same T cells did not elicit an increase in blood pressure in Rag-1−/− premenopausal females; thus, T cell-mediated ANG II-induced hypertension is blunted in premenopausal females. In a followup study, we showed that transfer of normotensive CD3+ T cells (male donors) into Rag-1−/− menopausal females caused a significant increase in ANG II-mediated hypertension and renal inflammation (15). These studies highlighted that T cell-mediated ANG II-induced hypertension was independent of the sex of the T cell and suggested that estrogen was protective against T cell-mediated hypertension (13, 15). The mechanism by which premenopausal females suppress T cell-mediated ANG II-induced hypertension is unknown; however, loss of estrogen and a change in the estrogen-to-testosterone ratio are key physiological transitions in menopause.
Given that T cell activation is a key driver of hypertension development in male animals, in the present study we hypothesized that the transfer of hypertensive T cells into a premenopausal female would bypass the ability of estrogen to suppress an ANG II-induced blood pressure response. Results from the present study contradict our hypothesis: following the transfer of hypertensive T cells, premenopausal females remained protected from ANG II-induced hypertension, suggesting that activation of T cells alone is not sufficient to overcome the protective premenopausal hormonal environment.
Despite no significant impact on blood pressure, we found that renal FoxP3+ T regulatory cells were decreased in premenopausal females following the transfer of hypertensive T cells, suggesting a possible shift in the renal inflammatory environment. T regulatory cells are known to be an anti-inflammatory T cell subtype that act by decreasing the activity of other proinflammatory T cells during an immune response (24–26). Premenopausal females have increased T regulatory populations compared with males in several models of hypertension, including the spontaneously hypertensive rat model (27, 28) and the Dahl salt-sensitive rat model (29). We have previously demonstrated that selective depletion of splenic and renal T regulatory cells, using repeated injections of PC-61, eliminates premenopausal protection from ANG II-induced hypertension (15), and others have shown that T regulatory cell depletion is associated with increased renal proinflammatory cytokine secretion and elevated blood pressure (30).
Females that received T cells from hypertensive donors had a significant decrease in renal FoxP3+ T regulatory cells; thus, it is possible that extending the timeframe of ANG II infusion beyond 2 wk may identify a progressive loss in premenopausal protection when donor T cells are from a hypertensive environment. However, further studies are needed to confirm this speculation.
We also demonstrated in this study that MCP-1 and TNF-α were not increased by ANG II in the premenopausal female kidney; indeed, the expression levels were decreased in females following the transfer of T cells from hypertensive donors. It has recently been shown that enhanced TNF-α signaling may be protective in the kidney by decreasing the activity of proinflammatory T helper 17 cells (31). Despite the decrease in renal MCP-1 mRNA expression, we saw an increase of F4/80+ macrophages in the kidneys, demonstrating that MCP-1 gene expression is not a solid predictor of F4/80+ macrophage infiltration.
While our previous work has focused on the role of T cells in estrogen-mediated premenopausal protection from hypertension, it is important to note that many other immune cell subsets contribute to hypertensive outcomes. Dendritic cells and macrophages, cells of the innate immune system, have come to the forefront as playing a vital role in hypertension (32).
In male mice, it has been shown that ANG II infusion increases dendritic cell activation (16) and that adoptive transfer of dendritic cells from ANG II-infused donors increases the hypertensive response in male recipients (16, 33, 34). Both macrophages and dendritic cells can be directly activated by high salt media in vitro (35), and Dahl salt-sensitive rats have an enhanced population of proinflammatory M1 macrophages in the kidney (36). Furthermore, the adoptive transfer of salt-activated dendritic cells increases hypertension in male mice (33), and high salt-induced high blood pressure has been shown to be dependent on serum and glucocorticoid-regulated kinase-1 signaling directly on CD11c+ dendritic cells (37). Despite these advances in understanding the innate immune system in male hypertension, similar studies had yet to be performed in females.
Here, we demonstrated that adoptive transfer of splenocytes from hypertensive donors eliminated premenopausal female protection from hypertension and was associated with an increase in the renal expression of MIP-1α (also known as CCL3). MIP-1α/CCL3 is a potent chemokine known to aid in macrophage recruitment via chemokine (C-C motif) receptor 5 receptor (38), and CCL3 knockout mice have been shown to have decreased renal inflammation and macrophage recruitment (39). The presence of hypertensive dendritic cells in the donor splenocyte population likely contributes to the robust blood pressure response to ANG II, as has been shown in males; however, it is possible that additional immune cells are involved as MIP-1α/CCL3 is also known to aid in the attraction of neutrophils to target organs (40). The role of neutrophils in the female kidney is unknown and warrants further study.
We did not observe an increase in F4/80+ macrophage infiltration in the kidneys of premenopausal females who received splenocytes, either from normotensive or hypertensive donors. This contrasts to our previous work in menopausal females where T cell-mediated ANG II-induced hypertension induced a significant increase in total renal macrophage infiltration and a decrease in M2 macrophage subtypes (15).
Using our VCD model of menopause, we demonstrated that estrogen replacement prevents ANG II-induced postmenopausal hypertension (14). Using aged menopausal females, a recent study demonstrated that ANG II induced a significant increase in blood pressure that was reversed by estrogen replacement in a mechanism that is dependent on ANG II type 2 receptor (AT2R) signaling (41). Agonism of the AT2R signaling pathway in the kidney has also been shown to increase renal anti-inflammatory T regulatory cells and IL-10 secretion in Sprague-Dawley rats (42). Immune cells from both sexes express estrogen receptors, including T cells (43, 44), dendritic cells (45), and macrophages (46); thus, further studies are needed to examine the role of estrogen signaling on immune cell infiltration into the kidney during a hypertensive stimulus.
Perspectives and Significance
In conclusion, the present work highlights the fact that activation of T cells alone is not sufficient to overcome premenopausal protection from hypertension, likely due to the estrogen-rich environment in suppressing renal inflammation. In contrast, transfer of splenocytes (consisting of T/B lymphocytes, dendritic cells, and macrophages) from hypertensive donors overcomes premenopausal protection from hypertension. Together the studies presented here highlight the need for specificity when examining sex differences in renal function, inflammation, and hypertension as a whole.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S5 and Supplemental Tables S1–S3:https://doi.org/10.6084/m9.figshare.16587431.v1.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL131834 (to H.L.B.) and T32HL007249 (to M.A.S.).
DISCLOSURES
H.L.B. is the Editor-in-Chief of the American Journal of Physiology-Renal Physiology and was not involved and did not have access to information regarding the peer review process or final disposition of this article. An alternate editor oversaw the peer review and decision-making process for this article.
AUTHOR CONTRIBUTIONS
M.A.S., D.P.P., and H.L.B. conceived and designed research; M.A.S., D.P.P., C.M. and W.N. performed experiments; M.A.S., D.P.P., C.M., and W.N. analyzed data; M.A.S., D.P.P., J.L.U., and H.L.B. interpreted results of experiments; M.A.S. prepared figures; M.A.S. drafted manuscript; M.A.S., J.L.U., and H.L.B. edited and revised manuscript; M.A.S., D.P.P., C.M., W.N., J.L.U., J.N.-Z., and H.L.B. approved final version of manuscript.
REFERENCES
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global burden of hypertension: analysis of population-based studies from 89 countries. J Hypertens 33: e2, 2015. doi: 10.1097/01.hjh.0000469726.59998.cc. [DOI] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension 71: e13–e115, 2018. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 3.Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med 30: 160–164, 2020. doi: 10.1016/j.tcm.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Waeber B, de la Sierra A, Ruilope LM. Target organ damage: how to detect it and how to treat it? J Hypertens Suppl 27: S13–18, 2009. doi: 10.1097/01.hjh.0000356767.24507.8d. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 31: 1247–1254, 2018. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 141, e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 288: 321–333, 2002. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- induced hypertension. Braz J Med Biol Res 40: 727–734, 2007. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension 45: 406–411, 2005. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- 10.Gillis EE, Musall JB, Baban B, Sullivan JC. IL-10 treatment decreases blood pressure in male, but not female, spontaneously hypertensive rats. Am J Physiol Renal Physiol 319: F359–F365, 2020. doi: 10.1152/ajprenal.00206.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 35: 484–489, 2000. doi: 10.1161/01.HYP.35.1.484. [DOI] [PubMed] [Google Scholar]
- 12.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollow DP, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol 309: R1546–R1552, 2015. doi: 10.1152/ajpregu.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollow DP, Uhlorn J, Sylvester MA, Romero-Aleshire MJ, Uhrlaub JU, Lindsey M, Nikolich-Zugich J, Brooks HL. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated angiotensin II hypertension. Am J Physiol Heart Circ Physiol 317: H415–H423, 2019. doi: 10.1152/ajpheart.00792.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen S, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsheikh AJ, Dasinger JH, Abais-Battad JM, Fehrenbach DJ, Yang C, Cowley AW, Mattson DL. CCL2 mediates early renal leukocyte infiltration during salt-sensitive hypertension. Am J Physiol Renal Physiol 318: F982–F993, 2020. doi: 10.1152/ajprenal.00521.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 42: 31–38, 2003. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 20.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irsik DL, Romero-Aleshire MJ, Chavez EM, Fallet RW, Brooks HL, Carmines PK, Lane PH. Renoprotective impact of estrogen receptor-α and its splice variants in female mice with type 1 diabetes. Am J Physiol Renal Physiol 315: F512–F520, 2018. doi: 10.1152/ajprenal.00231.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gohar EY, Almutlaq RN, Daugherty EM, Butt MK, Jin C, Pollock JS, Pollock DM, De Miguel C. Activation of G protein-coupled estrogen receptor 1 ameliorates proximal tubular injury and proteinuria in Dahl salt-sensitive female rats. Am J Physiol Regul Integr Comp Physiol 320: R297–R306, 2021. doi: 10.1152/ajpregu.00267.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehrenbach DJ, Dasinger JH, Lund H, Zemaj J, Mattson DL. Splenocyte transfer exacerbates salt-sensitive hypertension in rats. Exp Physiol 105: 864–875, 2020. doi: 10.1113/EP088340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep 18: 21, 2016. doi: 10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 25.Ozdemir C, Akdis M, Akdis CA. T regulatory cells and their counterparts: masters of immune regulation. Clin Exp Allergy 39: 626–639, 2009. doi: 10.1111/j.1365-2222.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 26.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol 29: 543–548, 2013. doi: 10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tipton AJ, Musall JB, Crislip GR, Sullivan JC. Greater transforming growth factor-β in adult female SHR is dependent on blood pressure, but does not account for sex differences in renal T-regulatory cells. Am J Physiol Renal Physiol 313: F847–F853, 2017. doi: 10.1152/ajprenal.00175.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor LE, Gillis EE, Musall JB, Baban B, Sullivan JC. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 315: H1713–H1723, 2018. doi: 10.1152/ajpheart.00389.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogulamudi VR, Mani I, Subramanian U, Pandey KN. Genetic disruption of Npr1 depletes regulatory T cells and provokes high levels of proinflammatory cytokines and fibrosis in the kidneys of female mutant mice. Am J Physiol Renal Physiol 316: F1254–F1272, 2019. doi: 10.1152/ajprenal.00621.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen Y, Rudemiller NP, Zhang J, Robinette T, Lu X, Ren J, Privratsky JR, Nedospasov SA, Crowley SD. TNF-α in T lymphocytes attenuates renal injury and fibrosis during nephrotoxic nephritis. Am J Physiol Renal Physiol 318: F107–F116, 2020. doi: 10.1152/ajprenal.00347.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira NS, Tostes RC, Paradis P, Schiffrin EL. Aldosterone, inflammation, immune system, and hypertension. Am J Hypertens 34: 15–27, 2021. doi: 10.1093/ajh/hpaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 21: 1009–1020, 2017. doi: 10.1016/j.celrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res 118: 1233–1243, 2016. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol 317: F361–F374, 2019. doi: 10.1152/ajprenal.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Beusecum JP, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, Itani HA, Himmel LE, Harrison DG, Kirabo A. High salt activates CD11c+ antigen presenting cells via serum glucocorticoid kinase 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 74: 555–563, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keepers TR, Gross LK, Obrig TG. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect Immun 75: 1229–1236, 2007. doi: 10.1128/IAI.01663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correa-Costa M, Braga TT, Felizardo RJF, Andrade-Oliveira V, Perez KR, Cuccovia IM, Hiyane MI, da Silva JS, Câmara NO. Macrophage trafficking as key mediator of adenine-induced kidney injury. Mediators Inflamm 2014: 291024, 2014. doi: 10.1155/2014/291024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner JE, Paust HJ, Bennstein SB, Bramke P, Krebs C, Steinmetz OM, Velden J, Haag F, Stahl RA, Panzer U. Protective role for CCR5 in murine lupus nephritis. Am J Physiol Renal Physiol 302: F1503–F1515, 2012. doi: 10.1152/ajprenal.00382.2011. [DOI] [PubMed] [Google Scholar]
- 41.Barsha G, Mirabito Colafella KM, Walton SL, Gaspari TA, Spizzo I, Pinar AA, Hilliard Krause LM, Widdop RE, Samuel CS, Denton KM. In aged females, the enhanced pressor response to angiotensin II is attenuated by estrogen replacement via an angiotensin type 2 receptor-mediated mechanism. Hypertension 78: 128–137, 2021. doi: 10.1161/HYPERTENSIONAHA.121.17164. [DOI] [PubMed] [Google Scholar]
- 42.Ali R, Patel S, Hussain T. Angiotensin type 2 receptor activation limits kidney injury during the early phase and induces Treg cells during the late phase of renal ischemia. Am J Physiol Renal Physiol 320: F814–F825, 2021. doi: 10.1152/ajprenal.00507.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernardi AI, Andersson A, Stubelius A, Grahnemo L, Carlsten H, Islander U. Selective estrogen receptor modulators in T cell development and T cell dependent inflammation. Immunobiology 220: 1122–1128, 2015. doi: 10.1016/j.imbio.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Lélu K, Laffont S, Delpy L, Paulet P-E, Périnat T, Tschanz SA, Pelletier L, Engelhardt B, Guéry J-C. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol 187: 2386–2393, 2011. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 45.Laffont S, Seillet C, Guéry J-C. Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol 8: 108, 2017. doi: 10.3389/fimmu.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294: 63–69, 2015. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]