Keywords: contractility, histamine, histamine receptor, mouse, urinary bladder
Abstract
Histamine has been implicated in urinary bladder dysfunction as an inflammatory mediator driving sensory nerve hypersensitivity. However, the direct influence of histamine on smooth muscle has not been thoroughly investigated. We hypothesized that histamine directly contracts urinary bladder smooth muscle (UBSM) independent of effects on nerves. Single cell quantitative RT-PCR determined that only histamine H1 and H2 receptors were expressed on UBSM cells. In isolated tissue bath experiments, histamine (200 µM) caused a highly variable and rapidly desensitizing contraction that was completely abolished by the H1 receptor antagonist fexofenadine (5 µM) and the Gq/11 inhibitor YM254890 (1 µM). Neither the muscarinic receptor antagonist atropine (1 µM), the Na+ channel blocker tetrodotoxin (1 µM), nor the transient receptor potential vanilloid type 1 antagonist capsazepine (10 µM) altered responses to histamine, suggesting that nerve activation was not involved. UBSM desensitization to histamine was not due to receptor internalization, as neither the cholesterol-depleting agent methyl-β-cyclodextrin (10 mM), the dynamin-mediated endocytosis inhibitor dynasore (100 µM), nor the clathrin-mediated endocytosis inhibitor pitstop2 (15 µM) augmented or prolonged histamine contractions. Buffer from desensitized tissues still contracted histamine-naïve tissues, revealing that histamine was not metabolized. Prolonged exposure to histamine also had no effect on contractions due to electrical field stimulation, suggesting that both efferent nerve and UBSM excitability were unchanged. Together, these data suggest that histamine, although able to transiently contract UBSM, does not have a lasting effect on UBSM excitability or responses to efferent nerve input. Thus, any acute effects of histamine directly on UBSM contractility are unlikely to alter urinary bladder function.
NEW & NOTEWORTHY Histamine is commonly associated with inflammatory bladder pathologies. We sought to investigate the role of histamine on urinary bladder contractility. Histamine contracts the bladder, but this response is highly variable and desensitizes completely in minutes. This desensitization is not due to internalization of the receptor or metabolism of histamine. Because nerve-evoked contractions are also not increased in the presence of histamine, our findings suggest that histamine is not directly acting to change contractility.
INTRODUCTION
The urinary bladder is a distensible organ with two main functions: urine storage and urine elimination (1). Dysfunctional and/or diseased urinary bladders share common overlapping lower urinary tract symptoms (LUTS) of overactivity, hypersensitivity, and/or underactivity that are often associated with inflammatory responses that lead to histamine release (2). The most well-studied postulate regarding the role of histamine in bladder physiology involves the release of histamine from mast cells, which, in turn, drives central and peripheral nerve hyperexcitability in response to bladder distension (3–5). Thus, these findings suggest that histamine indirectly augments bladder contractility through neuronal hyperexcitability, as opposed to directly acting on histamine receptors in urinary bladder smooth muscle (UBSM) that disrupt micturition coordination (6–8). Histamine does directly contract porcine bladder urothelium/lamina propria, and histamine receptor expression is increased in detrusor muscle from patients with interstitial cystitis/painful bladder syndrome (9, 10). However, the direct effects of histamine on UBSM contractility and excitability are less clear. Furthermore, it is unclear if histamine alters the responsiveness of UBSM to other contractile stimuli.
As a vasoactive bioamine, histamine triggers both immediate immune signaling and subsequent pleiotropic effects in various organs depending on which of the four receptor subtypes is activated (11–13). These effects include increased neurotransmitter release (14) but also rapidly desensitizing smooth muscle contractions in other smooth muscle-rich organs such as the trachea and uterus (15, 16). Smooth muscle responses to histamine are typically mediated through histamine H1 receptors (Gq-coupled contraction) and H2 receptors (Gs-coupled relaxation) (17). H3 and H4 receptors appear to be expressed in human detrusor smooth muscle (18), although the significant phenotypic changes that smooth muscle cells undergo in culture make these data difficult to interpret. Although some of the effects of histamine on UBSM contractility have been investigated (10, 17, 19), it remains unclear if these responses are due to contractions of cells in the muscularis mucosa, release of contractile compounds after sensory nerve activation, or direct actions on histamine receptors in urinary bladder smooth muscle—or, for that matter, all three.
In this study, we found that histamine causes a rapidly desensitizing contraction in mouse urinary bladder strips that was mediated by H1 receptors. Histamine-induced contractions were independent of nerve activation and were also unaffected by removal of the urothelium. This contraction was repeatable only after prolonged washout (though reduced in amplitude), suggesting that receptors could recover. The rapid desensitization was not due to metabolism of histamine, as naïve tissues contracted when exposed to buffer taken from desensitized tissues. Desensitization was also not due to endocytosis; depletion of cholesterol or inhibition of dynamin-mediated endocytosis only further reduced the amplitude and area under the curve (AUC) of histamine contractions, whereas inhibition of clathrin-mediated endocytosis had no effect. Continued exposure to histamine also had no effect on electrical field-stimulated UBSM contractions or contractions in response to carbachol (CCh; 200 nM). Together, these data suggest that UBSM contractile responses to histamine rapidly desensitize through a mechanism other than receptor internalization or metabolism. Also, although capable of transiently contracting UBSM directly, histamine does not alter the ability of UBSM to respond to other physiologically relevant stimuli. Thus, any acute direct effects of histamine on urinary bladder smooth muscle appear negligible regarding normal contractile function.
MATERIALS AND METHODS
Animal Care and Use
All animal procedures followed institutional guidelines and were approved by the Institutional Animal Care and Use Committees of Michigan State University (National Institutes of Health Assurance D16-0054). Male C57BL/6 mice (9–17 wk old, Jackson Laboratory, Bar Harbor, ME) were group housed in a temperature- and humidity-controlled environment with a 12:12-h light-dark cycle. Mice were provided ad libitum access to standard chow and water. Before all experimental procedures, mice were euthanized by intraperitoneal injection of pentobarbital (>150 mg/kg) followed by decapitation.
Smooth Muscle Cell Dissociation
Urinary bladders were dissected and placed in ice-cold Ca2+-free HEPES dissection buffer containing (in mM) 134 NaCl, 6 KCl, 1.2 MgCl2, 10 HEPES, and 7 glucose (pH 7.4). Tissues were then cleaned of connective tissue, pinned flat, denuded of the urothelium by blunt dissection (when appropriate), and cut into ∼2-mm-wide strips. Bladder strips were incubated at 37°C for 18 min in dissociation buffer (consisting of Ca2+-free HEPES buffer with 2 mg/mL albumin) to which papain (1.0 mg/mL) and dithioerythritol (1.0 mg/mL) were added. Tissues were transferred into fresh dissociation buffer with collagenase (2.0 mg/mL) and CaCl2 (100 µM) and incubated at 37°C for 10 min. Following incubation, UBSM myocytes were placed on ice in fresh Ca2+-free dissection buffer and gently triturated with a glass Pasteur pipette. Cells were then placed in a custom chamber on an inverted microscope, and 10 UBSM cells (determined by fusiform morphology) were collected using a suction pipette for further experimentation.
Single Cell Quantitative RT-PCR
Isolated detrusor myocytes were used for single cell quantitative RT-PCR experiments using the Ambion Cells-to-CT qRT-PCR Kit (Thermo Fisher Scientific, Waltham, MA). Lysed samples were subjected to recommended thermal cycles for reverse transcription (25°C for 10 min, 42°C for 60 min, and 85°C for 5 min) using a Veriti Thermocycler (Applied Biosystems, Waltham, MA). Pooled TaqMan gene expression assays/samples were then preamplified via the following stages: enzyme activation at 95°C for 10 min, 14 amplification cycles of denaturing at 95°C for 15 s and annealing/extending at 60°C for 4 min, and finally enzyme deactivation at 99°C for 10 min. RT-PCR was performed using the QuantStudio 7 Flex Real-Time PCR System (Thermo Fisher Scientific). cDNA was amplified using recommended RT-PCR thermal cycles: uracil-DNA glycosylase incubation at 50°C for 2 min, enzyme activation at 95°C for 10 min, and 40 amplification cycles of denaturing at 95°C for 5 s and annealing/extending at 60°C for 1 min. Expression of the following mRNAs were measured: Hrh1 (Mm00434002_s1), Hrh2 (Mm00434009_s1), Hrh3 (Mm00446706_m1), and Hrh4 (Mm00467634_m1) for histamine H1—H4 receptors as well as Acta2 (Mm00725412_s1) for α-smooth muscle actin. All gene expression assays were obtained from Thermo Fisher Scientific, who also validated their specificity. Probe context sequences used are shown in Table 1. The results are reported as raw threshold cycle (CT) values per manufacturer recommendations.
Table 1.
Context sequences used for RT-PCR
| Gene | Sequence |
|---|---|
| Hrh1 | 5′-TGAGGGAGATGCCAGGGGCTCAAAG-3′ |
| Hrh2 | 5′-GGCTCCGCAGTCTGACCAATTGCTT-3′ |
| Hrh3 | 5′-CTTCCTCGTGGGTGCCTTCTGCATC-3′ |
| Hrh4 | 5′-AGTTTCAAATGCTGTGTCTTATAGG-3′ |
| Acta2 | 5′-TAGCCCTGGCCTAGCAACACTGATT-3′ |
Acta2, α-smooth muscle actin; Hrh1−Hrh4, histamine H1−H4 receptors, respectively.
Isometric Contractility
Whole mouse bladders were removed, placed in ice-cold Ca2+-free HEPES dissection buffer, and cut into four ∼2-mm-wide bladder strips with the urothelium denuded or intact for isometric contractility experiments. UBSM strips were hung in an 820MS Isolated Tissue Bath System (Danish Myo Technologies, Aarhus, Denmark) containing warm (37°C) bicarbonate-buffered physiological salt solution (PSS) consisting of (mM) 119 NaCl, 24 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgCl2, and 2 CaCl2. All chambers were aerated throughout the duration of the experiments with biological atmosphere gas (25% O2–5% CO2–70% N2) to maintain pH and tissue oxygenation. Passive tension (∼10 mN) was then applied, and strips were equilibrated for 1 h (exchanging fresh PSS every 15 min) before exposure to drugs. To ensure tissue strips were viable and to account for variability in strip volume, an initial contractile response was evoked by exposure to 60 mM KCl (20–22). Similarly, responses to 200 nM CCh were measured at the end of the experiments to verify tissue viability. Responses were recorded using a PowerLab ADC and LabChart 8 software (ADInstruments).
Histamine concentration-response experiments.
For histamine concentration-response experiments, increasing concentrations of histamine dihydrochloride (100 nM to 400 µM) were added directly to the bath. The next concentration was added immediately after the plateau of the previous response or after 2 min, if no response was noted.
Histamine contractility experiments.
For histamine contractility experiments, a bolus of histamine dihydrochloride (200 µM) was applied for 15–20 min. This concentration was chosen because it caused a near-maximal contraction in our isometric contractility assay and aligned closely with previously published works (10, 23). Following a 30-min washout/re-equilibration period, tissues were then incubated with vehicle (H2O, 1.0% DMSO) or drug for 15−60 min before a second exposure to histamine. The drugs used were the H1 receptor antagonist fexofenadine (5 µM), the H2 receptor antagonist cimetidine (5 µM), the transient receptor potential vanilloid type 1 (TRPV1) antagonist capsazepine (10 µM), the muscarinic receptor antagonist atropine (1 µM), the voltage-gated Na+ channel blocker tetrodotoxin (TTX; 1 µM), the clathrin-mediated endocytosis inhibitor pitstop2 (15 µM), the dynamin inhibitor dynasore (100 µM), or the Gq/Gs inhibitor YM254890 (1 µM). Tissue responses to CCh (1 µM) were measured after a 30-min incubation with antagonist. Maximum responses to histamine and the area under the curve (AUC) of the histamine contractions were measured using LabChart 8 software. AUC was calculated over equivalent time periods in all strips. Detrusor contractility data were normalized to the initial contraction to histamine (200 µM) or KCl (60 mM) as appropriate.
Buffer exchange experiments.
For buffer exchange experiments, histamine (200 µM) was added into a tissue bath for a minimum of 5 min or until UBSM strips contracted and desensitized back to baseline. Buffer from this tissue bath was then manually transferred to a separate bath containing a histamine-naïve UBSM strip for 10 min to measure the response. This protocol was repeated similarly using CCh and buffer alone.
Desensitization experiments.
For desensitization experiments, urothelium-intact UBSM strips were incubated with the cholesterol-depleting agent methyl β-cyclodextrin (MβCD; 10 mM) for 60 min before histamine (200 µM) exposure or cholesterol (5.1 mM) for 1.5 h following exposure to MβCD to measure histamine responses. Tissue responses were normalized to an initial contraction with 60 mM KCl.
Electrical field stimulation.
Electrical field stimulation (EFS) was conducted using a CS4/CS8 stimulator and MyoPULSE software (Danish Myo Technologies). Tissues received electrical field pulses (0.2-ms width, 2-s durations, 20 V) sequentially increasing from 0.5 to 50 Hz. Responses were recorded before and after a 1-h exposure to vehicle (distilled H2O) or histamine (200 µM).
Drugs and Chemicals
Fexofenadine and cimetidine were obtained from Fisher Scientific (Waltham, MA). Capsazepine was obtained from Cayman Chemical (Ann Arbor, MI). TTX was obtained from Hello Bio (Princeton, NJ). Unless otherwise noted, histamine, MβCD, and all other reagents/chemicals were obtained from Sigma-Aldrich (Cleveland, OH). Stock solutions of MβCD, histamine, and CCh were made in distilled H2O. Stock solutions for all other drugs were made with DMSO.
Statistical Analysis
Analyses were performed as previously described (24). The coefficient of variation (%) was calculated as (σ/mean) × 100. For comparisons of two unpaired samples of equal variance, statistical significance between groups was established using two-tailed, unpaired Student’s t tests (α = 0.05). In paired experiments, statistical significance between groups was established using two-tailed, paired Student’s t tests (α = 0.05). For samples of unequal variance, the Mann–Whitney U test was used (α = 0.05). For comparing multiple groups, Brown–Forsythe ANOVA (if unequal SD) was used followed by Dunnett’s T3 multiple comparisons test. For EFS experiments, two-way ANOVA was used followed by Tukey’s post hoc analysis to compare individual means. Calculations were performed using Microsoft Excel or GraphPad Prism (GraphPad Software, San Diego, CA). Values are expressed as means ± SE. Differences with P values of <0.05 were considered statistically significant. Where appropriate, N represents the number of animals and n represents the number of replicates from the same animal.
RESULTS
Histamine H1 and H2 Receptor mRNA Is Expressed in Isolated UBSM Cells
Single cell RT-PCR was used to determine which of the four subtypes of histamine receptors were expressed in freshly dissociated detrusor myocytes. UBSM cells expressed both Hrh1 and Hrh2 mRNA (Fig. 1). Hrh3 and Hrh4 mRNA were not detected after 40 amplification cycles. Acta2 mRNA was also measured to validate that samples were UBSM cells.
Figure 1.
Urinary bladder smooth muscle cells express histamine receptors. Single cell quantitative RT-PCR was used to measure histamine receptor expression in freshly isolated urinary bladder smooth muscle cells. Expression of histamine H1 and H2 receptor (Hrh1 and Hrh2) mRNA was detected, but no expression of H3 or H4 receptor (Hrh3 and Hrh4) mRNA was present after 40 cycles. α-Smooth muscle actin (Acta2) mRNA expression was used to verify that cells were smooth muscle. Data points represent each of N = 12 animals (n = 20 cells per animal). n.s. indicates no signal after 40 cycles. CT, threshold cycle.
UBSM Contracts and Rapidly Desensitizes to Histamine
Isometric contractility was performed to directly investigate the immediate effects of histamine on detrusor strips (Fig. 2). Histamine cumulative concentration-response curves (100 nM to 400 µM) were indicative rapid desensitization of the contractile response (Fig. 2A). Alternatively, a single bolus of 200 µM histamine rapidly contracted both urothelium-intact (Fig. 2B) and urothelium-denuded (Fig. 2C) bladder strips. As with cumulative concentration-response experiments, the histamine response was also transient and highly variable (Fig. 2D). Responses to histamine were reproducible after washout, albeit slightly reduced in both intact and denuded strips (68.87 ± 4.47% and 73.93 ± 4.07%, respectively; Fig. 2E).
Figure 2.
Histamine (Hist) causes urothelium-independent transient contractions of urinary bladder smooth muscle (UBSM) strips. A: representative traces of rapid desensitization of urothelium-intact urinary bladder strips in response to increasing concentrations of histamine (100 nM–400 µM). Representative traces of urothelium-intact (B) and urothelium-denuded (C) urinary bladder strips during bolus administrations of histamine (200 µM) before and after washout. Repeated washes between exposures to histamine were removed from the traces for clarity. Histamine-induced contractions with and without urothelium do not significantly differ, but the second contraction to histamine tended to be reduced compared with the first (D). Results are presented as percentages of the initial contraction to 60 mM KCl (C) or the control contraction to 200 µM histamine (E). P > 0.05 by Brown–Forsythe ANOVA. N = 5.
To assess the variability of histamine-induced contractions in UBSM strips, the coefficient of variation was calculated for responses to 60 mM KCl, 200 nM CCh, and 200 µM histamine (Table 2). Before normalization, both the first and second contractions to histamine showed a much larger degree of relative variability than contractions to either KCl or CCh. Normalization to the initial KCl response reduced the relative variability for CCh contractions, but the relative variability of each response to histamine was either marginally reduced or increased. This suggested that the relative variability in histamine contractions was not related to variations in strip volume or viability. When normalized to the initial histamine contraction, the relative variability of the second histamine response reduced nearly to that of the KCl contraction. Together, these data suggested that whereas UBSM contractions to KCl and CCh remained relatively consistent between mice, the magnitude to which histamine could contract UBSM varied between animals. Additionally, these data showed that repeated contractions to histamine in a UBSM strip from a single animal were relatively consistent. Since no differences were noted between intact and denuded tissues compared with the initial contraction to histamine (Fig. 2E), urothelium-intact UBSM strips were used in all remaining experiments.
Table 2.
Coefficient of variation of urinary bladder smooth muscle responses to agonists
| 60 mM KCl | 200 nM Carbachol | 1° Histamine (200 µM) | 2° Histamine (200 µM) | |
|---|---|---|---|---|
| Raw data, mN | 27.17% | 36.10% | 69.57% | 78.71% |
| %KCl response | 23.08% | 64.11% | 83.63% | |
| %Control histamine | 33.40% |
UBSM Contractions to Histamine Are Mediated by H1 Receptors
Previous research with porcine bladders determined that the H1 receptor mediates histamine contractions (10). Thus, we next determined which histamine receptor subtype and G protein pathway mediated histamine-induced contractions. After initial contraction with histamine, the H2 receptor antagonist cimetidine (5 µM) did not block the subsequent histamine-induced contraction (Fig. 3A). The H1 receptor antagonist fexofenadine (5 µM), however, abolished the response to histamine (Fig. 3B) compared with vehicle (Fig. 3C). The Gq/Gs inhibitor YM254890 also abolished the response to histamine compared with vehicle (Fig. 3C). The effect of fexofenadine was specific to histamine, as all tissues contracted similarly to 200 nM CCh in the presence of either histamine receptor antagonist (Fig. 3D). However, YM254890 also abolished contractions to CCh (1 µM), as muscarinic receptors are also coupled to Gq/11 (Fig. 3D).
Figure 3.
Histamine (Hist) H1 receptors mediate urinary bladder smooth muscle (UBSM) contractions to histamine. Representative traces of histamine-induced contractions from urothelium-intact urinary bladder strips, before and after exposure to the H2 receptor antagonist cimetidine (Cimet; 5 µM; A) or the H1 receptor antagonist fexofenadine (Fexo; 5 µM; B). Histamine-induced contractions were abolished by fexofenadine (P = 0.0003) and the Gq/Gs inhibitor YM254890 (P = 0.0003) but were unaffected by cimetidine (C). D: neither histamine antagonist significantly altered responses to carbachol (CCh), indicating that tissues remained viable and neither drug inhibited muscarinic receptors. Carbachol contractions were abolished by YM254890 (P = 0.0022). Results are shown as percentages of the control contraction to histamine (C) or to the initial contraction to 60 mM KCl (D). *P < 0.05 by Brown–Forsythe ANOVA. N = 5.
Contractions to Histamine Are Independent of Neurotransmitter Release
Histamine can drive the release of neurotransmitter from sensory nerves, which augments bladder sensory outflow and also contracts UBSM (4). Therefore, the effects of the voltage-gated Na2+ channel blocker TTX (1 µM) and the TRPV1 channel blocker capsazepine (10 µM) on histamine-induced UBSM contraction were tested (Fig. 4). Neither TTX (Fig. 4, A, D and E) nor capsazepine (Fig. 4, B, D and E) had a significant effect on contractions to histamine compared with the vehicle control. The muscarinic antagonist atropine also had no effect (Fig. 4, C–E).
Figure 4.
Histamine (Hist)-dependent contractions are not driven by nerves. Representative traces of histamine-induced contractions on urothelium-intact urinary bladder strips incubated with and without the Na+ channel blocker tetrodotoxin (TTX; A), the transient receptor potential vanilloid type 1 channel antagonist capsazepine (CPZ; B), or the muscarinic antagonist atropine (Atro; C). None of the antagonists/blockers significantly altered the amplitude (D) or integral (E) of histamine-induced contractions. P > 0.05 by Brown–Forsythe ANOVA. N = 4–6. AUC, area under the curve.
Removal of Cholesterol Diminished Histamine-Induced Contractions
Caveolae are small, cholesterol-rich membrane invaginations that play an important role in receptor internalization for some G protein-coupled receptors (25). In other tissues, disruption of caveolae with the cholesterol-removing agent MβCD prevented rapid desensitization to contractile agonists (26). Thus, to determine if the transient nature of histamine-induced UBSM contractions was due to caveolae-mediated receptor desensitization, contraction to a bolus of histamine (200 µM) was measured in the absence or presence of 10 mM MβCD (Fig. 5, A and B). In the presence of MβCD, histamine contractions were significantly diminished compared with vehicle controls (31.08 ± 5.18% versus 5.25 ± 0.29%, respectively; Fig. 5C). Cholesterol (5.1 mM) replenishment recovered histamine-induced contractions after exposure to MβCD (10 mM; Fig. 5D), suggesting that the effects of MβCD were specific to depletion of membrane cholesterol. These effects of MβCD were specific to the mechanism in which histamine induces contraction, as all tissues contracted similarly to 200 nM CCh (Fig. 5E).
Figure 5.
Methyl-β-cyclodextrin (MβCD) reduces histamine-induced contractions in a cholesterol-dependent manner. Representative traces of sequential histamine-induced contractions in the presence of the cholesterol-depleting agent MβCD (A) or MβCD followed by 5.1 mM cholesterol (B). C: MβCD significantly reduced urinary bladder contractions to histamine compared with vehicle (P = 0.0075 by Welch’s t test). D: cholesterol restores the reduced contractions to histamine induced by MβCD (P = 0.0072 by Welch’s t test). E: MβCD did not significantly alter the response to carbachol, indicating that tissues remained viable and MβCD had no effect on muscarinic receptor-dependent contractions. *P < 0.05. N = 5−7.
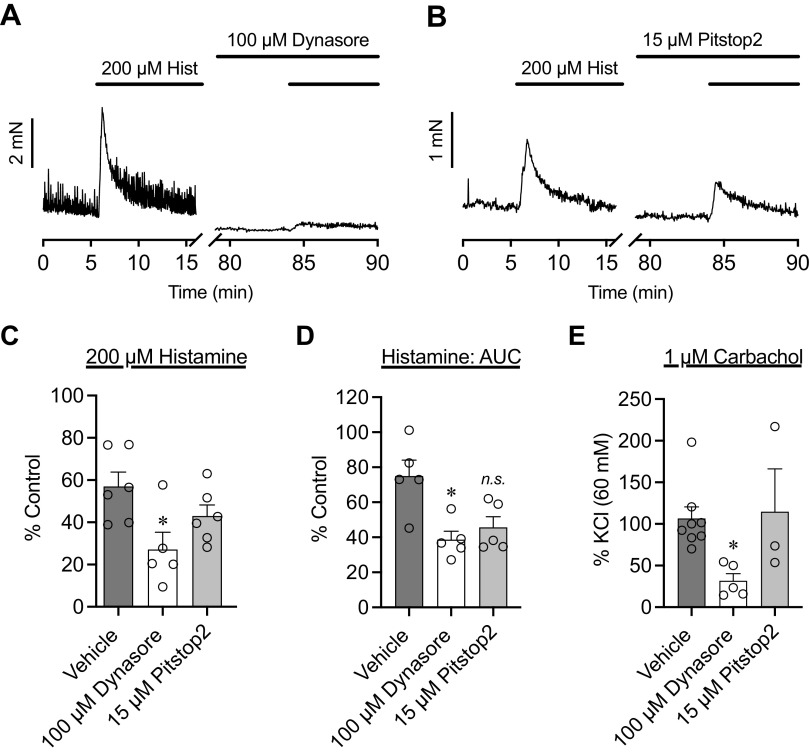
UBSM Desensitization to Histamine Is Independent of Dynamin-Mediated Endocytosis, Clathrin-Mediated Endocytosis, and Histamine Metabolism
Inhibitors of endocytosis were used to determine potential pathways by which UBSM desensitized to histamine (Fig. 6). Neither the dynamin-mediated endocytosis inhibitor dynasore (100 µM) nor the clathrin-mediated endocytosis inhibitor pitstop2 (15 µM) increased the amplitude (Fig. 6C) or integral (Fig. 6D) of histamine contractions. Instead, dynasore significantly reduced both the amplitude and integral of histamine-induced contractions. CCh-induced contractions were reduced in the presence of dynasore compared with vehicle control (Fig. 6E) but remained the same in the presence of pitstop2.
Figure 6.
Histamine (Hist)-induced desensitization is not dependent on dynamin- or clathrin-mediated endocytosis. Representative traces of histamine-induced contractions before and after exposure to the dynamin-mediated endocytosis inhibitor dynasore (100 µM; A) or the clathrin-mediated endocytosis inhibitor pitstop2 (15 µM; B). Dynasore significantly reduced both the amplitude (C) and integral (D) of contractions to histamine as opposed to augmenting them (P = 0.045 and P = 0.023, respectively). E: dynasore also reduced carbachol contractions, whereas pitstop2 did not. *P < 0.05 by Brown–Forsythe ANOVA. N = 5−6. AUC, area under the curve; ns, not significant.
A histamine buffer exchange bioassay was performed to determine if rapid metabolism was the cause of the short-lived contractions (Fig. 7). Buffer transferred from desensitized tissues still contracted naïve UBSM, whereas transferred buffer alone did not change the baseline.
Figure 7.
The transient nature of histamine-induced contractions is not due to metabolism. The summary bar graph indicates that, even after desensitizing one tissue, the same histamine-containing buffer contracted naïve urinary bladder smooth muscle strips. P > 0.05. Results are presented as percentages to 60 mM KCl. Dashed lines connect exposed tissue to naïve tissue from each experiment. N = 5.
Histamine Does Not Affect UBSM Contractions to EFS
EFS mimics normal physiological stimuli to the urinary bladder by causing the release of acetylcholine and ATP from cholinergic and purinergic efferent nerve terminals in the bladder wall (27–29). Contractions resulting from EFS are also completely blocked by TTX, suggesting that the responses are indeed nerve mediated and not due to depolarization of smooth muscle directly (30, 31). To determine if prolonged exposure to histamine altered UBSM contractile responses to other physiological stimuli, EFS frequency-response experiments were performed before and after a 1-h exposure to histamine (200 µM). Neither vehicle nor histamine altered contractions elicited by EFS (Fig. 8, A and B).
Figure 8.
Prolonged histamine exposure had no effect on nerve-mediated contractions of urinary bladder smooth muscle. Frequency-response curves from urothelium-intact urinary bladder strips prior to and during incubation with vehicle (A) or histamine (B). Histamine had no effect on contractions evoked by electrical field stimulation (EFS). P > 0.05 by two-way ANOVA. N = 4–6 for control and vehicle EFS; N = 6 for control and histamine EFS.
DISCUSSION
Using isometric contractility and pharmacological tools, our study thoroughly examined the role of histamine as a direct contractor of UBSM. We determined that UBSM contractions to histamine are due to direct activation of the histamine H1 receptor on smooth muscle and not due to release of neurotransmitter from afferent or efferent nerves. Furthermore, UBSM rapidly desensitizes to histamine, although not through receptor internalization or rapid histamine metabolism. Finally, although histamine does contract UBSM directly, it does not have any profound effect on nerve-evoked contractions. Together, our findings suggest that any direct role for histamine signaling in UBSM is independent of immediate changes to contractility or excitability.
Histamine in the Pathogenesis of Bladder Dysfunction
In terms of bladder disease, histamine contributes to pelvic pain and bladder hypersensitivity in interstitial cystitis (32). The expression of histamine receptors in the bladder wall is increased and responses to exogenous histamine are decreased in patients with interstitial cystitis, suggesting that the desensitization seen in our experiments also occurs in vivo (9, 33). Recently, Grundy et al. (4) also found that histamine was able to sensitize sensory nerves and increase afferent outflow in response to urinary bladder distention in the absence of a disease but without augmenting contractility. Increased sensory outflow due to histamine was related to the recruitment of TRPV1-positive C-fibers normally engaged only at supraphysiological intravesical pressures. Thusly, any apparent alteration in voiding was likely to be a result of improper efferent signals driving UBSM contractions at much lower pressures due to the increase in sensory outflow falsely signaling a full bladder. Although not necessarily linked to histamine, this aberrant recruitment of C-fibers also drives bladder overactivity in mouse models of social stress, which also strongly resembles an inflammatory response (34, 35). Taken together, it is likely that inflammatory insults alter both the amount and type of sensory outflow relayed to the central nervous system during filling to significantly impact voiding. Also, these findings support our conclusion that changes in urinary bladder function due to histamine release are not related to direct alteration of UBSM contractility but are instead due to increases in sensory outflow from the bladder during filling.
Although many other studies have focused on the additive role of histamine regarding urinary bladder pathophysiology that leads to LUTS, our findings suggest that, unto itself, histamine does not mediate UBSM contractility in the absence of disease and other procontractile mediators. Future experiments will assess if changes to UBSM contractility elicited by histamine differ in animal models of inflammatory bladder dysfunction (such as interstitial cystitis) or if the presence of other inflammatory cytokines in addition to histamine alters UBSM contractility directly.
Why Are Histamine Contractions Variable?
One of the more interesting findings of these experiments was the inherent variability of histamine-induced contractions between mice. Contractile responses in UBSM strips are often normalized to contractions to a depolarizing stimulus (e.g., 60 mM KCl), since this accounts for variability in strip length, width, and viability over multiple experiments (20–22). Central to this methodology is the idea that, in the absence of disease or other treatments, a contractile response to another agonist should be consistently proportional to the contraction to the depolarizing stimulus. This indeed holds true for responses driven by CCh in our experiments, which is expected given the role of muscarinic receptors in driving bladder contractility and their ability to initiate Ca2+ influx through voltage-gated Ca2+ channels (36). However, our finding that histamine responses are not proportional to either KCl or CCh contractions suggests that histamine receptor expression is extremely labile between mice and dependent on factors which are currently unclear. The mice used in these experiments were acquired from the same breeder, at the same age, and housed under the same conditions. Yet, the expression of Hrh1 and Hrh2 mRNA is also more variable than that of Acta2 (Fig. 1). Since the tissues used for single cell PCR and for tissue bath experiments came from different animals, we cannot correlate receptor expression with contractile responses directly; however, our overall findings suggest that this variability in histamine receptor expression may indeed match with changes in responses to histamine.
Additional experiments are needed determine if circulating histamine levels can affect histamine receptor expression in UBSM and if regional differences in histamine receptor expression exist throughout the bladder wall. Also, levels of circulating stress hormones and inflammatory cytokines should be assessed to determine if prior stresses or inflammatory insult drive can down histamine receptor expression through histamine release and receptor desensitization.
Why Are Histamine Contractions Transient?
Other studies measuring urinary bladder contractility to histamine have suggested that receptor desensitization plays an important role in mediating its effects (33). To test this, we used MβCD to determine if the desensitization of histamine-induced contractions could be prevented by inhibiting caveolae-dependent receptor internalization. In the rat aorta, MβCD prevents rapid tachyphylaxis and desensitization to subsequent contractions by angiotensin II (26). However, instead of prolonging contraction or augmenting the maximal response, MβCD significantly reduced UBSM contractions to histamine. This reduction could be prevented by reintroduction of cholesterol to the bath, suggesting that it was depletion of cholesterol mediating the effect of MβCD and not a nonspecific effect on other pathways. Our findings still suggest that caveolae (and perhaps membrane cholesterol in general) play an important role in histamine-induced UBSM contractions that is not necessary for responses to other agonists, namely, CCh. Also, UBSM contractions induced by serotonin and angiotensin II are also reduced in the presence of MβCD and recover upon reintroduction of cholesterol (37), suggesting that histamine is not alone in its requirement for membrane cholesterol to mediate a contractile response. In all of these cases, it may be the disruption of UBSM smooth cell Ca2+-induced Ca2+ release pathways by MβCD that attenuates the response (38), suggesting that histamine and other such amines may also be initiating different types of Ca2+ signals to mediate contraction compared with muscarinic agonists. So, although caveolae-mediated receptor internalization does not appear to be responsible for the desensitization of UBSM strips to histamine, caveolae do appear to play a pivotal role in the signaling cascade required to drive the contractile response.
We also investigated other possible internalization pathways, including dynamin- and clathrin-mediated endocytosis. To investigate this, the clathrin-mediated endocytosis inhibitor pitstop2 was used as a known pharmacological inhibitor that prevents receptor endocytosis via inhibition of the clathrin terminal domain (39). The dynamin-mediated endocytosis inhibitor was also used, as it blocks by disabling vesicle scission (40, 41). Neither of these prolonged histamine-induced contractions. Dynasore does appear to slightly reduce the histamine response; this could be due to the ability of the drug to reduce lipid levels as well (41). Nonetheless, if the desensitization of histamine receptors was due to dynamin-mediated endocytosis, the response to histamine should be increased or prolonged as opposed to reduced. Thus, our findings suggest that histamine contractile signaling does not depend on endocytosis via caveolae, dynamin, or clathrin.
What is causing this rapid desensitization, if not caveolae-mediated internalization? One alterative hypothesis we considered was that the effects of histamine are instead dependent on rapid metabolism of histamine. Based on our results exchanging the buffer from a UBSM strip desensitized with histamine to a naïve strip, we found that histamine was still present in the bath and thus did not undergo rapid metabolism. This finding shows that rapid desensitization to histamine is not dependent on metabolizing enzymes mentioned as a cause for the desensitization of the trachea, gut, and kidney (42–44).
Altogether, our findings suggest it is more likely that desensitization of the histamine response is caused by β-arrestin-dependent desensitization or G protein-coupled receptor kinase-mediated phosphorylation than receptor internalization (45). We attempted to test this with commercially available β-arrestin inhibitors, but problems with solubility prevented us from thoroughly testing this hypothesis. Future experiments, either with β-arrestin knockout mice or newer, more soluble β-arrestin inhibitors, will be needed to determine if this mechanism is involved. Nonetheless, UBSM appears keenly capable of mitigating any contractile response initiated by histamine without altering its ability to respond to other contractile signals. Thus, the nature of histamine-induced contractions suggests it does not directly contribute to UBSM dysfunction by altering contractility and further suggests that the smooth muscle itself has mechanisms in place protect against prolonged contractions driven directly by inflammatory mediators like histamine.
Species Differences in Urinary Bladder Responses to Histamine
Histamine alters detrusor contractility based on the histamine receptor subtypes in the urinary bladder of different species (or, in the case of the rat, not at all) (46). In pigs, H1 receptors drive contraction of UBSM strips, whereas H2 receptors in the urothelium oppose this response (10). In the guinea pig urinary bladder, histamine also potentiates purinergic nerve-evoked contractions in addition to causing transient contractions by itself (19). We tested both possibilities in the mouse urinary bladder and found neither to be the case. One possible explanation is the presence of a contractile muscularis mucosa in guinea pig bladders that, as of yet, is not found in mice (47). Another possible explanation for the lack of effect on EFS-induced contraction is that mice could lack a purinergic component to nerve-mediated contractions. This is likely not the case, as both purinergic and cholinergic components of nerve-evoked UBSM contraction have been differentiated using increasing frequencies of EFS (48). Had the purinergic component been altered, we would expect to see increased EFS contractions below 15 Hz (48); we indeed did not. None of these prior studies investigated if any of these species-specific differences involved indirect release of contractile compounds from sensory nerves or mast cells driving contractions to histamine as opposed to direct actions on UBSM, which also may be responsible for the perceived differences.
Other studies have also reported differences between histamine-induced contractions in porcine detrusor strips with and without urothelium, where responses to histamine are augmented once the urothelium was denuded (49). In our study, removal of the urothelium altered neither the maximum contractile response to histamine nor its desensitization (Fig. 2). However, the study in porcine bladder used strips selectively dissected from the bladder trigone; given the size of the mouse bladder, our strips encompassed the entire length from trigone to dome. Also, unlike the mouse bladder, these strips from porcine bladder trigone also were sympathetically innervated, suggesting that pig trigone smooth muscle contains a very different complement of receptors compared with other segments of the urinary bladder wall and makes any comparisons herein extremely difficult.
In summary, species-dependent differences that exist regarding the effects of histamine in the bladder may relate to muscularis mucosa contractility or the indirect effects of histamine on sensory nerves or purinergic neurotransmission. Investigation of regional differences in histaminergic contractility within the bladder may also be worthwhile, but this will require the use of larger animal models than mice and careful consideration of nerve innervation.
Limitations
Our study is not without its own limitations. We did not directly measure the histamine-specific effects on afferent or efferent nerves that could drive UBSM contractions in vivo. In addition, the exact mechanism by which histamine contractions desensitize remains unclear. Although we also attempted to interrogate this pharmacologically, the relative insolubility of pharmacological inhibitors of β-arrestin proved insurmountable; thus, this possibility requires further investigation using genetic knockout mouse models. Although this study is limited by only examining histamine effects on isolated UBSM strips instead of the whole intact bladder, these findings have revealed that the effects of histamine alone on UBSM are short lived and incapable of augmenting contractions induced by physiological stimuli, a finding that could otherwise be obscured in whole bladder in vivo experiments.
Conclusions
In closing, our findings suggest that histamine-induced UBSM contractions in mice are transient in nature, highly variable, dependent on histamine H1 receptors and membrane cholesterol, and independent of nerve activation. The transient nature of the contraction is not due to histamine metabolism or internalization of histamine receptors via clarthin- or dynamin-mediated endocytosis, suggesting a role for receptor desensitization by β-arrestins or G protein-couple receptor kinase-mediated receptor phosphorylation. Furthermore, since histamine does not alter nerve-evoked contractility or sensitivity to cholinergic agonists, UBSM contractility seems largely unaffected by prolonged exposure to histamine in the absence of disease. Although this study provides evidence for the non-neuronal role of histamine as a direct contractile agonist in UBSM, it also suggests that any role for histamine as a direct sensitizer or regulator of contraction in UBSM is unlikely.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K01DK103840 (to N.R.T.) and R01DK119615 (to N.R.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M.J. conceived and designed research; B.M.J. performed experiments; B.M.J. analyzed data; B.M.J. and N.R.T. interpreted results of experiments; B.M.J. and N.R.T. prepared figures; B.M.J. drafted manuscript; B.M.J. and N.R.T. edited and revised manuscript; B.M.J., G.C.M., and N.R.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. William F. Jackson and Dr. R. Clinton Webb for the assistance and suggestions regarding experimental design.
REFERENCES
- 1.Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154: 3–13, 2010. doi: 10.1016/j.autneu.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Jones BM, Tykocki NR. New direct evidence that histamine augments bladder sensory outflow during filling is nothing to sneeze at. Am J Physiol Renal Physiol 318: F455–F456, 2020. doi: 10.1152/ajprenal.00581.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover S, Srivastava A, Lee R, Tewari AK, Te AE. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 3: 19–33, 2011. doi: 10.1177/1756287211398255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy L, Caldwell A, Garcia Caraballo S, Erickson A, Schober G, Castro J, Harrington AM, Brierley SM. Histamine induces peripheral and central hypersensitivity to bladder distension via the histamine H1 receptor and TRPV1. Am J Physiol Renal Physiol 318: F298–F314, 2020. doi: 10.1152/ajprenal.00435.2019. [DOI] [PubMed] [Google Scholar]
- 5.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 69: 34–40, 2007. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 6.Granato C, Korstanje C, Guilloteau V, Rouget C, Palea S, Gillespie JI. Prostaglandin E2 excitatory effects on rat urinary bladder: a comparison between the β-adrenoceptor modulation of non-voiding activity in vivo and micro-contractile activity in vitro. Naunyn Schmiedebergs Arch Pharmacol 388: 727–735, 2015. doi: 10.1007/s00210-015-1139-9. [DOI] [PubMed] [Google Scholar]
- 7.Kazaryan KV, Danielyan MA, Chibukhchyan RG, Margaryan SG. Histamine-mediated regulation of electrical activity during the bladder–urethra interaction in rats. J Evol Biochem Phys 54: 50–58, 2018. doi: 10.1134/S0022093018010064. [DOI] [Google Scholar]
- 8.Wang M, Xing N, Wu L, Huang WC, Xu Z, Liu G. Regulation of spontaneous contractions in intact rat bladder strips and the effects of hydrogen peroxide. Biomed Res Int 2018: 2925985, 2018. doi: 10.1155/2018/2925985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan H, Zhang EW, Zhang P, Zhang XD, Zhang N, Du P, Yang Y. Differential expression of histamine receptors in the bladder wall tissues of patients with bladder pain syndrome/interstitial cystitis—significance in the responsiveness to antihistamine treatment and disease symptoms. BMC Urol 19: 115, 2019. doi: 10.1186/s12894-019-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stromberga Z, Chess-Williams R, Moro C. Histamine modulation of urinary bladder urothelium, lamina propria and detrusor contractile activity via H1 and H2 receptors. Sci Rep 9: 3899, 2019. doi: 10.1038/s41598-019-40384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks AC, Whelan CJ, Purcell WM. Reactive oxygen species generation and histamine release by activated mast cells: modulation by nitric oxide synthase inhibition. Br J Pharmacol 128: 585–590, 1999. doi: 10.1038/sj.bjp.0702838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa T, Hume JR, Keef KD. Modulation of K+ and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol 468: 379–400, 1993. doi: 10.1113/jphysiol.1993.sp019777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C, Diehl SA, Noubade R, Ledoux J, Nelson MT, Spach K, Zachary JF, Blankenhorn EP, Teuscher C. Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc Natl Acad Sci USA 107: 18967–18972, 2010. doi: 10.1073/pnas.1008816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchiya Y, Hosokawa T, Kasuya Y. Effects of histamine on neurally mediated contraction of canine tracheal smooth muscle. Jpn J Pharmacol 52: 647–651, 1990. doi: 10.1254/jjp.52.647. [DOI] [PubMed] [Google Scholar]
- 15.Anderson WH, Krzanowski JJ, Polson JB, Szentivanyi A. Characteristics of histamine tachyphylaxis in canine tracheal smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 308: 117–125, 1979. doi: 10.1007/BF00499053. [DOI] [PubMed] [Google Scholar]
- 16.Brighton PJ, Rana S, Challiss RJ, Konje JC, Willets JM. Arrestins differentially regulate histamine- and oxytocin-evoked phospholipase C and mitogen-activated protein kinase signalling in myometrial cells. Br J Pharmacol 162: 1603–1617, 2011. doi: 10.1111/j.1476-5381.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol 9: 1873, 2018. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuhaus J, Oberbach A, Schwalenberg T, Stolzenburg JU. Cultured smooth muscle cells of the human vesical sphincter are more sensitive to histamine than are detrusor smooth muscle cells. Urology 67: 1086–1092, 2006. doi: 10.1016/j.urology.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Patra PB, Westfall DP. Potentiation of purinergic neurotransmission in guinea pig urinary bladder by histamine. J Urol 151: 787–790, 1994. doi: 10.1016/S0022-5347(17)35088-7. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson AC, Sutton BW, Boone TB, Ford AP, Munoz A. Inhibition of urothelial P2X3 receptors prevents desensitization of purinergic detrusor contractions in the rat bladder. BJU Int 116: 293–301, 2015. doi: 10.1111/bju.13003. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes VS, Ribeiro AS, Martínez-Sáenz A, Blaha I, Serrano-Margüello D, Recio P, Martínez AC, Bustamante S, Vázquez-Alba D, Carballido J, García-Sacristán A, Hernández M. Underlying mechanisms involved in progesterone-induced relaxation to the pig bladder neck. Eur J Pharmacol 723: 246–252, 2014. doi: 10.1016/j.ejphar.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy L, Caldwell A, Garcia Caraballo S, Erickson A, Schober G, Castro J, Harrington AM, Brierley SM. Histamine induces peripheral and central hypersensitivity to bladder distension via the histamine H1 receptor and TRPV1. Am J Physiol Renal Physiol 318: F298–F314, 2019. doi: 10.1152/ajprenal.00435.2019. [DOI] [PubMed] [Google Scholar]
- 24.Tykocki NR, Heppner TJ, Dalsgaard T, Bonev AD, Nelson MT. The KV 7 channel activator retigabine suppresses mouse urinary bladder afferent nerve activity without affecting detrusor smooth muscle K(+) channel currents. J Physiol 597: 935–950, 2019. doi: 10.1113/JP277021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart EJ, Ying YS, Anderson RG. Hormonal regulation of caveolae internalization. J Cell Biol 131: 929–938, 1995. doi: 10.1083/jcb.131.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linder AE, Thakali KM, Thompson JM, Watts SW, Webb RC, Leite R. Methyl-β-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J Pharmacol Exp Ther 323: 78–84, 2007. doi: 10.1124/jpet.107.123463. [DOI] [PubMed] [Google Scholar]
- 27.Brading AF, Mostwin JL. Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br J Pharmacol 98: 1083–1090, 1989. doi: 10.1111/j.1476-5381.1989.tb12651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kura H, Obara K, Yabu H. Contractile responses to electrical field stimulation and ATP in guinea-pig urinary bladder. Comp Biochem Physiol C Comp Pharmacol Toxicol 102: 193–197, 1992. doi: 10.1016/0742-8413(92)90063-d. [DOI] [PubMed] [Google Scholar]
- 29.Nausch B, Heppner TJ, Nelson MT. Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. Am J Physiol Regul Integr Comp Physiol 299: R878–R888, 2010. doi: 10.1152/ajpregu.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillespie JI, Harvey IJ, Drake MJ. Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol 88: 343–357, 2003. doi: 10.1113/eph8802536. [DOI] [PubMed] [Google Scholar]
- 31.Ramos-Filho AC, Shah A, Augusto TM, Barbosa GO, Leiria LO, de Carvalho HF, Antunes E, Grant AD. Menthol inhibits detrusor contractility independently of TRPM8 activation. PLoS One 9: e111616, 2014. doi: 10.1371/journal.pone.0111616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One 3: e2096, 2008. doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol 150: 2007–2012, 1993. doi: 10.1016/S0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- 34.Mingin GC, Heppner TJ, Tykocki NR, Erickson CS, Vizzard MA, Nelson MT. Social stress in mice induces urinary bladder overactivity and increases TRPV1 channel-dependent afferent nerve activity. Am J Physiol Regul Integr Comp Physiol 309: R629–R638, 2015. doi: 10.1152/ajpregu.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingin GC, Peterson A, Erickson CS, Nelson MT, Vizzard MA. Social stress induces changes in urinary bladder function, bladder NGF content, and generalized bladder inflammation in mice. Am J Physiol Regul Integr Comp Physiol 307: R893–R900, 2014. doi: 10.1152/ajpregu.00500.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson KE. Muscarinic acetylcholine receptors in the urinary tract. Handb Exp Pharmacol : 319–344, 2011. doi: 10.1007/978-3-642-16499-6_16. [DOI] [PubMed] [Google Scholar]
- 37.Cristofaro V, Peters CA, Yalla SV, Sullivan MP. Smooth muscle caveolae differentially regulate specific agonist induced bladder contractions. Neurourol Urodyn 26: 71–80, 2007. doi: 10.1002/nau.20361. [DOI] [PubMed] [Google Scholar]
- 38.Hotta S, Yamamura H, Ohya S, Imaizumi Y. Methyl-beta-cyclodextrin prevents Ca2+-induced Ca2+ release in smooth muscle cells of mouse urinary bladder. J Pharmacol Sci 103: 121–126, 2007. doi: 10.1254/jphs.sc0060213. [DOI] [PubMed] [Google Scholar]
- 39.DiCello JJ, Rajasekhar P, Eriksson EM, Saito A, Gondin AB, Veldhuis NA, Canals M, Carbone SE, Poole DP. Clathrin and GRK2/3 inhibitors block δ-opioid receptor internalization in myenteric neurons and inhibit neuromuscular transmission in the mouse colon. Am J Physiol Gastrointest Liver Physiol 317: G79–G89, 2019. doi: 10.1152/ajpgi.00085.2019. [DOI] [PubMed] [Google Scholar]
- 40.Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol 438: 77–93, 2008. doi: 10.1016/S0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preta G, Cronin JG, Sheldon IM. Dynasore—not just a dynamin inhibitor. Cell Commun Signal 13: 24, 2015. doi: 10.1186/s12964-015-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohrui T, Yamauchi K, Sekizawa K, Ohkawara Y, Maeyama K, Sasaki M, Takemura M, Wada H, Watanabe T, Sasaki H. Histamine N-methyltransferase controls the contractile response of guinea pig trachea to histamine. J Pharmacol Exp Ther 261: 1268–1272, 1992. [PubMed] [Google Scholar]
- 43.Rangachari PK. Histamine: mercurial messenger in the gut. Am J Physiol 262: G1–G13, 1992. doi: 10.1152/ajpgi.1992.262.1.G1. [DOI] [PubMed] [Google Scholar]
- 44.Sudarikova AV, Fomin MV, Yankelevich IA, Ilatovskaya DV. The implications of histamine metabolism and signaling in renal function. Physiol Rep 9: e14845, 2021. doi: 10.14814/phy2.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Böhm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J 322: 1–18, 1997. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen ML, Drey K. Contractile responses in bladder body, bladder neck and prostate from rat, guinea pig and cat. J Pharmacol Exp Ther 248: 1063–1068, 1989. [PubMed] [Google Scholar]
- 47.Mitsui R, Lee K, Uchiyama A, Hayakawa S, Kinoshita F, Kajioka S, Eto M, Hashitani H. Contractile elements and their sympathetic regulations in the pig urinary bladder: a species and regional comparative study. Cell Tissue Res 379: 373–387, 2020. doi: 10.1007/s00441-019-03088-6. [DOI] [PubMed] [Google Scholar]
- 48.Werner ME, Knorn A-M, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- 49.Templeman L, Chapple CR, Chess-Williams R. Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J Urol 167: 742–745, 2002. doi: 10.1097/00005392-200202000-00076. [DOI] [PubMed] [Google Scholar]