Abstract
Cannabis usage has steadily increased as acceptance is growing for both medical and recreational reasons. Medical cannabis is administered for treatment of chronic pain based on the premise that the endocannabinoid system signals desensitize pain sensor neurons and produce anti-inflammatory effects. The major psychoactive ingredient of cannabis is Δ9-tetrahydrocannabinol (THC) that signals mainly through cannabinoid receptor-1 (CBr), which is also present on nonneuron cells including blood platelets of the circulatory system. In vitro, CBr-mediated signaling has been shown to acutely inhibit platelet activation downstream of the platelet collagen receptor glycoprotein (GP)VI. The systemic effects of chronic THC administration on platelet activity and function remain unclear. This study investigates the effects of chronic THC administration on platelet function using a nonhuman primate (NHP) model. Our results show that female and male NHPs consuming a daily THC edible had reduced platelet adhesion, aggregation, and granule secretion in response to select platelet agonists. Furthermore, a change in bioactive lipids (oxylipins) was observed in the female cohort after THC administration. These results indicate that chronic THC edible administration desensitized platelet activity and function in response to GPVI- and G-protein coupled receptor-based activation by interfering with primary and secondary feedback signaling pathways. These observations may have important clinical implications for patients who use medical marijuana and for providers caring for these patients.
Keywords: blood cells, cannabis, nonhuman primates, platelets, THC
INTRODUCTION
Marijuana is the most commonly used federally illegal drug in the United States and worldwide with increasing popularity as both a recreational and medicinal drug (1, 2). The prevalence of use is on the rise with ∼192 million people (3.9%) worldwide using marijuana in 2016, a 16% increase from 2006 (2). In the United States, there are ∼22.2 million marijuana users each month (3). This high prevalence is due in part to the recent legalization and decriminalization at the state level, which promotes the availability of marijuana and its perceived safety. Marijuana use can lead to the development of problem use, and in severe cases, it takes the form of addiction. Approximately a third of marijuana users have some degree of marijuana use disorder (4) and 4 million people in the United States met diagnostic criteria in 2015 (5). Of equal concern is that the content of Δ9-tetrahydrocannabinol (THC), marijuana’s main psychoactive component, has increased from less than 4% in the early 1990s to more than 15% in 2018 in confiscated marijuana samples (2).
THC acts through the endocannabinoid system that is composed of endogenously produced cannabinoids, cannabinoid receptors, and regulating enzymes. Both cannabinoid 1 (CB1) and CB2 receptors are G protein-coupled receptors (GPCRs) (6, 7) and facilitate specific downstream signaling resulting in regulation in neurological processes and immune modulation (8). Although marijuana has been used for the treatment of chronic pain, insomnia, nausea, and vomiting, studies have also suggested that marijuana use is associated with cardiovascular system dysfunction including both thrombosis and comprised vascular integrity in select cases (9). Blood platelets, which occupy essential physiological and pathological cardiovascular roles, have been shown to express CB1 and CB2 receptors on their cell membrane, suggesting a possible direct effect of THC on platelet function (10, 11).
A potential mechanistic role of cannabinoids (CBs) in regulating platelet function is still being explored. Earlier studies found that in vitro addition of the endocannabinoids 2-arachidonoylglycerol (2-AG) promoted platelet aggregation, demonstrating a prohemostatic or prothrombotic role for CB receptor signaling in platelet function and concluded its role in prothrombotic events (12). Yet, later studies showed a reduced platelet response to platelet glycoprotein (GP)VI immunoreceptor tyrosine-based activation motif (ITAM) stimulation under static and flow conditions, suggesting that CB receptor-dependent signaling would dampen essential platelet function (13). Thus, it still remains to be seen how chronic exposure of marijuana components affect systemic platelet function in vivo. Therefore, our aim was to study the effects of chronic controlled THC edible exposure on platelet function in nonhuman primates (NHPs). This study builds on previous work by De Angelis et al. (13), where acute endocannabinoid exposure as self-reported by people induced a reduction in platelet surface adhesion. In the current study, we demonstrate that the administration of chronic THC edibles in NHPs had desensitizing effects on platelet adhesion, aggregation, and granule secretion functions. Changes in oxylipin metabolite profile were observed with THC administration and potentially play a role in the desensitization of platelet functionality. Together, our observations may have important implications for clinical health policies regarding the use of medical marijuana.
METHODS
Reagents
Cross-linked collagen-related peptide (CRP-XL) was from R. Farndale (CambCol Laboratories, Cambridge University, UK). Fibrillar collagen was from Chrono-Log Corporation (Havertown, PA). Adenosine diphosphate (ADP) and bovine serum albumin (BSA) were obtained from Sigma Aldrich (St. Louis, MO). Thrombin receptor activator peptide 6 (TRAP6; SFLLRN) was obtained from Tocris (Bristol, UK). Protease-activated receptor 4 agonist (GFPKF-NH2) was obtained from Genescript (Piscataway, NJ). Pam2CSK4 was from Invivogen (San Diego, CA). Prothrombin time (PT) Dade Innovin obtained from Siemens Healthcare Diagnostics (Germany) and activated partial thromboplastin time (aPTT) reagent from Thermo Fisher Scientific (Middletown, VA). 18-HEPE, 13(S)-HODE, 12(S)-HETE, 9(R)-HETE, and 9,10-DiHOME were obtained from Cayman Chemical (Ann Arbor, MI).
Antibodies
Flow cytometry antibodies human CD62-FITC (Cat. No. 550866) and human PAC1-FITC (Cat. No. 340507) were from BD biosciences (San Diego, CA). Antibodies specificity was tested with untreated controls and inhibitors to same target as can be seen in Supplemental Fig. S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.16785766).
Ethical Statement
All animal procedures and experimental THC administration has been approved by the Oregon Health & Science University, Oregon National Primate Research Center Institutional Animal Care and Use Committee (IACUC) and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for humane animal care were followed (IP0001389). The THC used in our study was supplied directly from the National Institute of Drug Abuse (NIDA) Drug Supply Program. Permission was obtained for all experimental protocols involving THC administration before the initiation of the study, and the study was conducted in accordance with institutional and national guidelines.
Experimental Design
A cohort of 10 (n = 7 female, n = 3 male) young, sexually mature Rhesus macaques (Macaca mulatta) were indoor-housed under controlled conditions. Animal ages ranged from 6.7 to 12.7 yr, with a mean age of ∼10 yr. Animals were maintained on a standard chow diet (TestDiet, St. Louis, MO). To minimize potential confounders and interindividual variability, each individual animal served as its own control during the evaluation of platelet function obtained at the different time points of THC induction.
THC Induction and Measurements
In addition to a standard chow diet, animals were given one cookie containing THC (THC edible) per day that was made using research-grade THC obtained directly from the National Institute of Drug Administration (NIDA). All cookies were administered in the morning before the animal’s daily chow so that they were consumed on an empty stomach and to confirm they were completely ingested. Animals were slowly titrated up to 2.5 mg/7 kg/day of THC over approximately a 3-mo time period to model Colorado state’s medical marijuana acclimation recommendations for the female cohort. Male animals were titrated up to 2.5 mg/7 kg/day of THC over approximately a 7-mo time period. Animals were maintained on a dose for 21 days for females and 70 days for males before THC dosage increase. Dosing regimen of the animal cohorts was based on the menstrual cycle for female animals and the renewal rate of semen production for the male animals. The THC dosage was calculated from the recommended THC starting dose of 5 mg (standard research unit of THC per NIDA) for a 68 kg adult (the average rhesus macaque weighs ∼6–7.5 kg), followed by titration to 10 mg for moderate users (standardized serving size for edible retail marijuana products in Colorado), and 20–30 mg for heavy users, as described earlier (7). Serum was drawn at each dose adjustment time point during THC induction, 3 h following edible consumption, to determine THC concentrations with each increase in dosage and analyzed as described before (7).
Blood Collection
Blood (∼12 mL) was collected from female and male Rhesus macaques by venipuncture and anticoagulated with 3.2% sodium citrate. To obtain platelet-rich plasma (PRP), whole blood was centrifuged for 8 min at 1,000 rpm. Platelet poor plasma (PPP) was obtained by spinning down remaining red blood cells at 10,000 rpm for 3 min.
Platelet Aggregation under Flow
Channels of Ibidi µ-slide VI0.1 chamber were coated with 100 µg/mL of fibrillar type I collagen (Chrono-Log Corp, Havertown, PA) for 1 h at room temperature (RT). Channels were washed with Hepes Tyrodes buffer and then blocked with 5 mg/mL denatured bovine serum albumin for 1 h at RT before connecting the outflow ports of the chamber to a syringe pump. PRP from nonhuman primates was perfused into the chambers at a shear rate of 300 s−1 for 5 min. Channels were fixed with 4% paraformaldehyde (PFA) and washed with Hepes Tyrodes buffer postperfusion. Three random fields were imaged for each channel at ×40 magnification using a Zeiss Axiovert 200 M microscope and SlideBook 6 software (RRID:SCR_014300). Surface area and number of platelet aggregates on each image were measured using ImageJ software (RRID:SCR_003070) as previously described (14).
Platelet Aggregation and Thromboxane Generation
Platelet rich plasma (PRP; 300 µL per sample) were preincubated in glass cuvettes and warmed to 37°C. Platelet aggregation under stirring conditions was initiated by CRP-XL (1 µg/mL) or ADP (3 µM), and changes in light transmission were monitored for 5 min using a PAP-4 aggregometer (Chrono-Log Corporation). After 5 min, solutions were removed from cuvettes and cleared by centrifugation before analysis for thromboxane B2 (TXB2) content by ELISA assay (Enzo life sciences). Fold-change of platelet aggregation relative to baseline control and measured TXB2 concentrations were analyzed for statistical significance by one-way ANOVA testing for multiple comparisons using GraphPad PRISM 8 software (RRID:SCR_002798).
Human Platelet Aggregation with Oxylipins
Isolated washed platelets were obtained from healthy volunteers and platelets were prepared as described before (15). 2 × 108 platelet/mL were pretreated with select oxylipins (50 µM) or vehicle control (0.1% DMSO) for 10 min. Platelet aggregation under stirring conditions was initiated by CRP-XL (1 µg/mL), and changes in light transmission were monitored for 5 min using a PAP-4 aggregometer (Chrono-Log Corporation).
Platelet Flow Cytometry
PRP was diluted 1:4 in Hepes-Tyrode’s buffer before stimulation with the agonists CRP-XL (1 µg/mL), TRAP-6 (30 µM) and GFPKF-NH2 (200 µM), ADP (30 µM), and Pam-2CSK4 (10 µg/mL) in the presence of either anti-CD62P-FITC antibody (1:25 dilution) or human PAC-1-FITC antibody (1:25 dilution) for 20 min at 37°C. Reactions were stopped by adding 2% PFA to samples, and platelet activation was determined by flow cytometry analysis using Canto II machine. Platelet activation on agonist stimulation was analyzed relative to unstimulated platelets and compared with baseline control for statistical significance.
Clotting Times
Clotting times of PPP (33% final volume) were measured with a KC4 Coagulation Analyzer at 37°C for 3 min with activated partial thromboplastin time (aPTT) reagent. Clotting was initiated with addition of 25 mM CaCl2, and clotting time was recorded. For prothrombin time (PT), clotting was initiated with the addition of Dade Innovin reagent to 50% PPP.
Sample Preparation for Oxylipin Analysis
Oxylipins were extracted from PPP using the approach described by Pedersen et al. (16) with minor modifications by García-Jaramillo et al. (17). In short, 100-µL PPP was transferred to 2-mL polypropylene tubes. Cold LC–MS-grade methanol (35 μL) and an antioxidant solution [0.2 mg/mL solution BHT (butylated hydroxytoluene) in 1:1 methanol:water; 5 μL] was added to each sample. Each sample also received 10 μL of a deuterated oxylipin recovery standard solution; the standards included 22 deuterated oxylipins in methanol at a concentration of 5 ng/µL. PPP was transferred to a 96-well Ostro Pass Through Sample Preparation Plate (Waters Corp, Milford, MA) and eluted into glass inserts containing10-μL 20% glycerol in methanol by applying a vacuum for 10 min. Eluents were dried by vacuum centrifugation in a Labconco centrivap vacuum concentrator for 2 h at RT. Once dry, samples were reconstituted with 100 μL of methanol:acetonitrile (50:50), containing the internal standard [1-cyclohexyl-dodecanoic acid (CUDA) at 50 ng/mL]. Samples were transferred to a spin filter (0.22-μm PVDF membrane, Millipore-Sigma, Burlington, MA) and centrifuged (3 min at 6°C at 9,000 rpm) before transferred to 2-mL amber LC–MS vials. Extracts were analyzed by ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). The internal oxylipin standards added to the samples were used to correct the recovery of the quantified oxylipins.
LC-MS/MS Analysis
High-performance liquid chromatography (HPLC) was performed using a Shimadzu system (Shimadzu, Columbia, MD) coupled to a QTRAP 4000 (AB SCIEX, Framingham, MA). Chromatographic separation of lipids was achieved on a Waters Acquity UPLC CSH C18 column [100 mm length × 2.1 mm inner diameter (ID); 1.7-μm particle size] with an additional Waters Acquity VanGuard CSH C18 precolumn (5 mm × 2.1 mm ID; 1.7-μm particle size) held at 60°C. The mobile phase consisted of water containing 0.1% acetic acid (A) and acetonitrile/isopropanol (B; ACN/IPA; 90/10, vol/vol) containing 0.1% acetic acid. Gradient elution conditions were: 0–1.0 min, 25%–40% B; 1.0–2.5 min, 40%–42% B; 2.5–4.5 min, 42%–50% B; 4.5–10.5 min, 50%–65% B; 10.5–12.5 min, 65%–75% B; 12.5–14 min, 75%–85% B; 14–14.5 min, 85%–95% B; 14.5–21 min, 95%–95% B; 21–22.5 min, 95%–25% B; and 22.5–27 min, 25%–25% B. A 5-μl aliquot of each sample was injected onto the column. The column effluent was introduced via an electrospray ion source. The flow rate was 0.15 mL/min. All samples were kept 10°C throughout the analysis. The MS/MS was performed on an Applied Biosystems 4000 QTRAP hybrid linear ion trap-triple quadrupole instrument (AB Sciex, Concord, ON, Canada) operated at a source temperature of 525°C with a needle voltage of −4,500 kV. The operation parameters of the MS/MS detector was collisionally activated dissociation −2 psi, curtain gas 30 psi, source gas 1 40 psi, source gas 2 40 psi, collision cell exit potential −15 V, and entrance potential −10 V. Nitrogen was used as the source gas, curtain gas, and collision gas. Dynamic multiple reaction monitoring (dMRM) experiments were conducted at collision energies ranging from −15 to −50 eV (Supplemental Table S2). Concentrations were calculated using the internal calibration method and MultiQuant (v. 3.0.2, Sciex) software.
Statistical Analysis
Data are presented as means ± standard error of mean (SEM). One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used to compare between baseline and dosage groups. Kruskal–Wallis with Dunn post hoc test was used to compare between groups when the data did not qualify for parametric statistics. P < 0.05 was considered significant. All statistical analyses were conducted using GraphPad Prism 9 (RRID:SCR_002798).
RESULTS
Chronic Edible Administration of THC to Nonhuman Primates Reduces Platelet Adhesion and Aggregate Formation under Flow
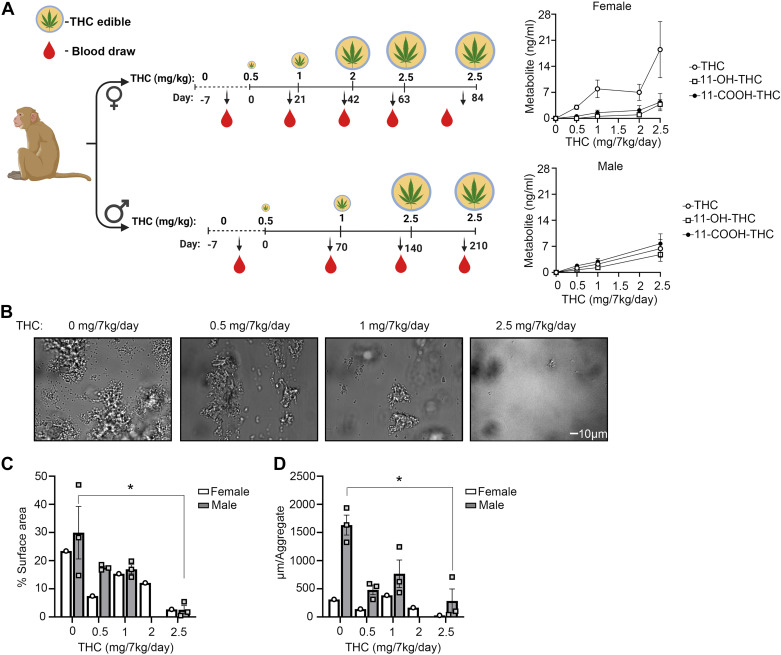
Endocannabinoid receptor agonists have been shown to reduce ex vivo platelet activation and aggregate formation on collagen under flow (13). We therefore first sought to examine whether chronic THC edible administration in nonhuman primates (NHPs) affected ex vivo platelet adhesion on collagen under flow. As shown in Fig. 1A, our model consisted of two cohorts: 7 female NHPs fed a daily THC edible for a total of 84 days and 3 male NHPs fed a daily THC edible for a total of 210 days. In the female cohort, the THC dose was increased every 21 days until a final concentration of 2.5 mg/7 kg/day was achieved, whereas in the male cohort, the THC dose was increased every 70 days until a final concentration of 2.5 mg/7 kg/day was achieved. These distinct THC administration timelines for the female and male cohorts were chosen due to sex differences in reproductive health, with THC administration for females being timed in accord with their menstrual cycle, whereas THC administration for males being timed in accord with the kinetics of sperm production. An increasing concentration of plasma THC levels and corresponding metabolites were observed in both cohorts as a function of dose (Fig. 1A). Complete blood counts including platelets remained largely constant for both cohorts (Tables 1 and 2), although a slight increase was observed for neutrophils in the female cohort, perhaps consistent with prior observations related to the presence of CB2 on neutrophils.
Figure 1.
Assessment of platelet adhesion on a collagen surface under flow after chronic THC edible administration. A: timeline of THC edible administration in females (n = 7) and males (n = 3) and measured plasma THC levels. B: platelet-rich plasma (PRP) from n = 3 male animals were perfused at 300 s−1 over collagen coated surface for 5 min prior to fixating. Representative images were taken and quantified for platelet surface coverage per time point (C) and aggregate size on collagen surface for female (n = 1) and male (n = 3; D). Data are means ± SE. Data were analyzed using ANOVA with repeated measures and Kruskal–Wallis with Dunn post hoc test. *P < 0.05 vs. baseline. Figure created with BioRender.com. THC, Δ9-tetrahydrocannabinol.
Table 1.
Female NHP complete blood count
| THC, mg/7 kg/day | 0 | 0.5 | 1 | 2 | 2.5 |
|---|---|---|---|---|---|
| WBC, k/μL | 4.35 (±1.0) | 4.76 (±1.79) | 5.33 (±1.22) | 4.81 (±1.05) | 5.49 (±1.14) |
| Neutrophils, k/μL | 0.38 (±0.52) | 1.43 (±0.53) | 1.84 (±0.86) | 1.82 (±0.81) | 2.37 (±0.91) |
| Hb, g/dL | 8.90 (±0.60) | 9.53 (±0.66) | 9.97 (±0.73) | 10.43(±1.17) | 10.64 (±0.76) |
| HCT, % | 28.21 (±3.03) | 31.20 (±2.73) | 32.09 (±1.32) | 31.50 (±2.80) | 30.74 (±1.41) |
| RBC, M/μL | 4.21 (±0.44) | 4.56 (±0.41) | 4.71 (± 0.27) | 4.66 (±0.48) | 4.54 (±0.30) |
| Lymphocyte, k/μL | 10.46 (±1.41) | 11.73 (±1.05) | 12.31 (±0.51) | 12.14 (±0.60) | 12.31 (±0.32) |
| Monocyte, k/μL | 0.17 (±0.13) | 0.28 (±0.18) | 0.26 (±0.13) | 0.25 (±0.11) | 0.38 (±0.26) |
| Eosinophils, k/μL | 0.12 (±0.11) | 0.33 (±0.16) | 0.31 (±0.18) | 0.32 (±0.13) | 0.40 (±0.22) |
| PLT, k/μL | 374.3 (±38.2) | 304.9 (±55.2) | 308.3 (±50.4) | 344.6 (±36.6) | 318.4 (±76.2) |
| MCV, fL | 67.11 (±2.56) | 68.19 (±2.00) | 69.74 (±3.03) | 67.81 (±3.34) | 66.31 (±3.04) |
| MCH, pg | 21.36 (±2.18) | 21.67 (±1.95) | 21.40 (±1.26) | 22.40 (±1.32) | 23.17 (±1.97) |
| MCHC, g/dL | 31.79 (±2.92) | 31.81 (±2.94) | 30.73 (±1.63) | 33.10 (±2.26) | 34.86 (±1.72) |
| RDW, % | 13.74 (±0.43) | 13.44 (±0.53) | 13.31 (±0.63) | 13.31 (±0.74) | 13.30 (±0.74) |
| MPV, fL | 8.87 (±1.80) | 8.97 (±1.29) | 9.43 (±2.42) | 8.40 (±1.79) | 8.87 (±2.70) |
Means ± SE. HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume; NHP, nonhuman primates; PLT, platelets; RBC, red blood cells; THC, Δ9-tetrahydrocannabinol; WBC, white blood cells.
Table 2.
Male NHP complete blood count
| THC, mg/7 kg/day | 0 | 0.5 | 1 | 2.5 |
|---|---|---|---|---|
| WBC, k/μL | 7.66 (±3.10) | 5.58 (±2.51) | 6.51 (±2.25) | 7.14 (±1.82) |
| Neutrophils, k/μL | 4.37 (±2.63) | 2.67 (±1.70) | 2.54 (±1.37) | 3.16 (±1.39) |
| Hb, g/dL | 11.70 (±0.64) | 10.37 (±0.34) | 11.63 (±0.42) | 9.83 (±0.26) |
| HCT, % | 37.43 (±1.04) | 34.53 (±1.70) | 40.63 (±1.22) | 41.77 (±0.21) |
| RBC, M/μL | 5.16 (±0.20) | 4.78 (±0.22) | 5.19 (±0.14) | 5.61 (±0.11) |
| Lymphocyte, k/μL | 2.54 (±0.30) | 2.33 (±0.56) | 3.17 (±0.56) | 2.51 (±0.27) |
| Monocyte, k/μL | 0.31 (±0.14) | 0.25 (±0.13) | 0.35 (±0.18) | 0.68 (±0.30) |
| Eosinophils, k/μL | 0.42 (±0.24) | 0.33 (±0.15) | 0.44 (±0.16) | 0.76 (±0.21) |
| PLT, k/μL | 404.33 (±31) | 339.33 (±45) | 381.33 (±24) | 330.67 (±43) |
| MCV, fL | 72.63 (±3.07) | 72.23 (±1.15) | 78.27 (±0.31) | 74.40 (±1.19) |
| MCH, pg | 22.73 (±1.99) | 21.73 (±0.41) | 22.40 (±0.22) | 17.53 (±0.65) |
| MCHC, g/dL | 31.27 (±1.55) | 30.03 (±0.48) | 28.60 (±0.16) | 23.57 (±0.74) |
| RDW, % | 13.07 (±0.50) | 13.27 (±0.39) | 13.23 (±0.33) | 13.77 (±0.56) |
| MPV, fL | 6.87 (±0.95) | 7.20 (±1.36) | 7.40 (±0.62) | 9.03 (±2.37) |
Means ± SE. HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume; NHP, nonhuman primates; PLT, platelets; RBC, red blood cells; RDW, red cell distribution width; THC, Δ9-tetrahydrocannabinol; WBC, white blood cells.
Blood drawn at select time points was perfused over a collagen-coated surface and assessed for platelet adhesion and aggregate formation for both males (Fig. 1B) and one female (Supplemental Fig. S2). Consistent with the studies using endocannabinoid receptor agonists (13), we show that chronic edible administration of THC significantly reduced platelet surface area coverage and aggregate size on collagen under physiologically relevant levels of shear flow (Fig. 1, C and D). The data suggest that chronic THC edibles may impair select platelet functions including thrombus formation under flow.
Chronic Edible Administration of THC to Nonhuman Primates Reduces Agonist-Induced Platelet Aggregation and Thromboxane Production
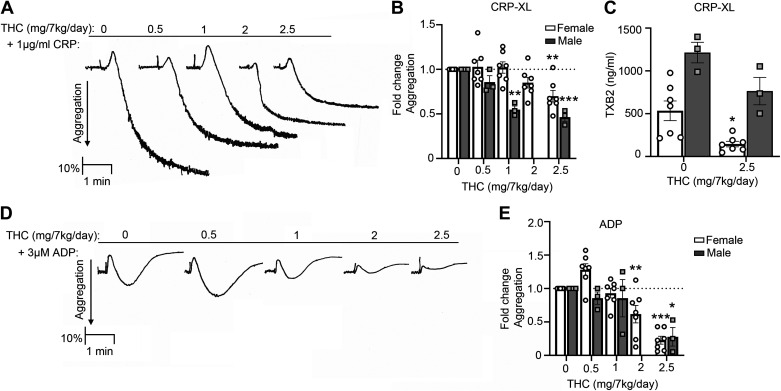
Next, we performed studies to determine the effects of chronic THC administration on platelet functions including aggregation and granule secretion in solution. Our data demonstrate robust aggregation of NHP platelets in response to the GPVI-agonist CRP-XL before chronic THC administration (Fig. 2A, baseline). A dose-dependent inhibition of aggregation to CRP-XL was observed following chronic THC administration, as quantified by fold change in aggregation per animal for both female (at 2.5 mg/kg/day dose) and male (at both 1 and 2.5 mg/7 kg/day doses) cohorts (Fig. 2B). Aligned with the fact that GPVI-mediated platelet activation is largely dependent on granule secretion of the secondary mediators including thromboxane and ADP, we found that chronic THC administration reduced GPVI-mediated thromboxane secretion in the female cohort (Fig. 2C). Moreover, a direct effect on ADP-mediated platelet activation was observed as a dose-dependent inhibition of ADP-induced platelet aggregation for both female (at both 2 and 2.5 mg/7 kg/day doses) and male (at 2.5 mg/7 kg/day dose) cohorts following THC administration (Fig. 2, D and E).
Figure 2.
THC edibles effect on platelet aggregation and thromboxane production after CRP-XL and ADP stimulation. A: representative PRP aggregation traces over time from one animal after CRP-XL stimulation. B: quantification of aggregation fold change compared with baseline for 1 µg/mL CRP-XL for 7 females and 3 males. C: thromboxane production measured by ELISA for 1 µg/mL CRP-XL. D: representative aggregation traces from 1 animal after ADP stimulation. E: quantification of fold-change aggregation in response to 3 µM ADP for 7 females and 3 males. Data are means ± SE with every animal representing 1 data point. Data were analyzed using ANOVA with repeated measures and Kruskal–Wallis with Dunn post hoc test. *P < 0.05; ** P < 0.001; ***P < 0.0001 vs. baseline. CRP-XL, collagen-related peptide-XL; PRP, platelet rich plasma; THC, Δ9-tetrahydrocannabinol.
Chronic Edible Administration of THC to Nonhuman Primates Reduces Agonist-Induced Platelet α-Granule Secretion
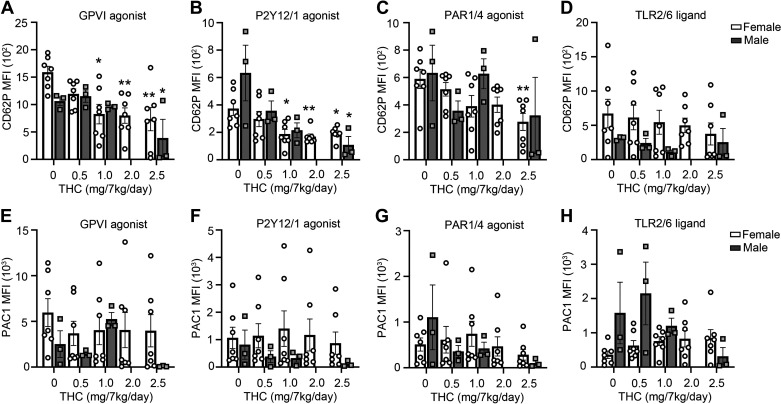
Platelet granule secretion and integrin activation are hallmarks of platelet activation and are essential hemostatic functions. In light of the inhibitory effects observed for THC on platelet aggregation, we next sought to determine if THC edibles were able to inhibit platelet α-granule secretion as measured by P-selectin expression in response to select platelet agonists. As shown in Fig. 3, A–D, we found that platelet P-selectin expression in response to the GPVI-agonist CRP-XL was significantly reduced at the highest THC dose for both the female and male cohorts (Fig. 3A). A similar trend in reduced P-selectin expression in the platelets from THC-treated animals was observed for the P2Y12/P2Y1 agonist ADP (Fig. 3B), whereas a reduced P-selectin expression in response to the PAR-agonists TRAP6 and GFPKF-NH2 was only observed for the female cohort (Fig. 3C). Platelet P-selectin expression in response to the TLR2/6 ligand Pam2CSK4 was insensitive to chronic administration of THC (Fig. 3D). Similarly, chronic edible administration of THC did not affect agonist-induced activation of the platelet integrin αIIbβIII receptor under the conditions tested herein as measured by PAC-1 binding (Fig. 3, E–H). Taken together, these results suggest that chronic edible administration of THC inhibits platelet α-granule secretion to select agonists that signal via either the ITAM- or GPCR-signaling pathways.
Figure 3.
THC edibles effect on platelet α-granule secretion and integrin receptor activation measured by flow cytometry. PRP was analyzed by flow cytometry for P-selectin (CD62P) expression after stimulation for 20 min with the GPVI receptor agonists CRP-XL (A), P2Y12/Y1 agonist ADP (B), PAR1 agonist TRAP-6 and PAR4 agonist GFPKF-NH2 (C), and TLR2/6 ligand Pam-2 (D). PRP was analyzed by flow cytometry for integrin activation (PAC1) after stimulation for 20 min with the agonists CRP-XL (E), P2Y12/Y1 agonist ADP (F), PAR1 agonist TRAP-6 and PAR4 agonist GFPKF-NH2 (G), and TLR2/6 ligand Pam-2CSK4 (H). Data are means ± SE for female (n = 7) and male (n = 3) representing each data point per animal per time point. Data were analyzed using ANOVA with repeated measures and Kruskal–Wallis with Dunn post hoc test. *P < 0.05; **P < 0.001 vs. baseline. CRP-XL, collagen-related peptide-XL; PRP, platelet rich plasma; THC, Δ9-tetrahydrocannabinol.
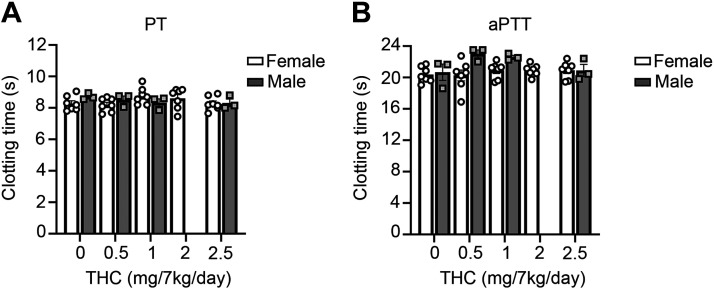
Effect of Chronic THC Edibles on Plasma Clotting Times
As platelet activity and activation of the coagulation cascade are both requisite for normal hemostasis, we next assessed whether THC edibles affected plasma clotting times. We therefore measured the clotting times of platelet poor plasma (PPP) from the female or male cohorts initiated by either lipidated tissue factor (Innovin) or aPTT reagent. We did not observe any differences in either clotting times for animals on chronic edible THC (Fig. 4).
Figure 4.
THC edibles effect on plasma clotting times. A: platelet poor plasma (PPP) was stimulated by TF Innovin to measure prothrombin time (PT). B: clotting times were measured by the addition of aPTT reagent for 3 min at 37°C and clotting was initiated with CaCl2 and clotting time was recorded. Data are means ± SE for female (n = 7) and male (n = 3) representing each data point per animal per time point. aPTT, activated partial thrombospondin time; THC, Δ9-tetrahydrocannabinol.
Effect of Chronic THC Edibles on Oxylipin Levels in Plasma
Endocannabinoids are derived from polyunsaturated fatty acids (PUFAs), including arachidonic acid (ARA), and these lipid mediators can signal through CB receptors (18).
Another class related to endocannabinoids are oxylipins that are derived also from PUFAs, including ARA, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). These unique lipid mediators are generated by the three major oxygenases: cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome P450 (CYP450) (19). Platelet activation is associated with significant changes of membrane lipids and formation of oxylipins, which are potent bioactive lipid mediators amplifying platelet activation in a paracrine and autocrine manner. To study more broadly the potential effects of chronic THC edible dosing on the blood environment, we analyzed the plasma from the female cohort for metabolic changes by mass spectrometry. Employing dynamic multiple reaction monitoring (dMRM), we evaluated 66 oxylipins, 22 deuterated oxylipins, CUDA, and the deuterated surrogates eicosapentaenoic acid-d5 (EPA-d5), docosahexaenoic acid-d5 (DHA-d5), linolenic acid-d5 (ALA-d5), linoleic acid-d4 (LA-d4), and arachidonic acid-d8 (ARA-d8) in a 27-min LC-run in a targeted approach and 29 oxylipins were detected (Supplemental Table S1). The detailed list of MRM transitions can be found in Supplemental Table S2. Our study focused on quantifying 29 detectable oxylipin metabolites found in plasma (Fig. 5A) and Z scores were determined as shown in the heatmap. The major precursors for oxylipin generation, DHA, ARA, and EPA, showed reduced levels with increased THC concentrations (Fig. 5B). In particular, the administration of THC edibles showed an increased trend of 12(S)-HETE, 9(R)-HETE, 18-HEPE, and 8-HODE (Fig. 5C). These results give an insight in plasma changes of oxylipin levels in response to chronic THC concentrations in vivo.
Figure 5.
THC edibles effect on oxylipin metabolome in plasma from female animals. A: platelet poor plasma (PPP) was obtained from n = 7 females, and lipid mediators were extracted, identified, and quantified using LC-MS/MS-based lipid profiling. B: oxylipin concentrations from the precursors arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). C: fold-change of the oxylipins 18-HEPE, 12S-HETE, 13S-HODE, and 9 R-HETE. Data are means ± SE. THC, Δ9-tetrahydrocannabinol.
DISCUSSION
Medical cannabis is administered for chronic pain treatment based on the premise that the endocannabinoid system both signals and desensitizes pain sensor neurons and has anti-inflammatory effects (7). The chronic effects of marijuana have not been extensively studied, and the effects on physiology remains unclear. Here, we used controlled administration of chronic THC edibles in both a female and male NHP cohort to study the effects of chronic THC edibles on platelet function (Fig. 6). Our results show a reduction of platelet adhesion on collagen under flow. Also, we observed significant reduction in platelet aggregation in response to the GPVI-agonist CRP-XL in combination with a reduction in platelet thromboxane production. Platelet aggregation in response to the P2Y1/Y12 receptor, ADP, was completely abolished in both cohorts with the highest dose of THC edible administration. Similar effects were observed in a reduction in platelet P-selectin expression. Additionally, chronic THC administration resulted in changes in oxylipin production in plasma. The underlying mechanism of desensitized platelet activation states associated with chronic THC edible administration is potentially by interference of the secondary feedback loop of platelet activation.
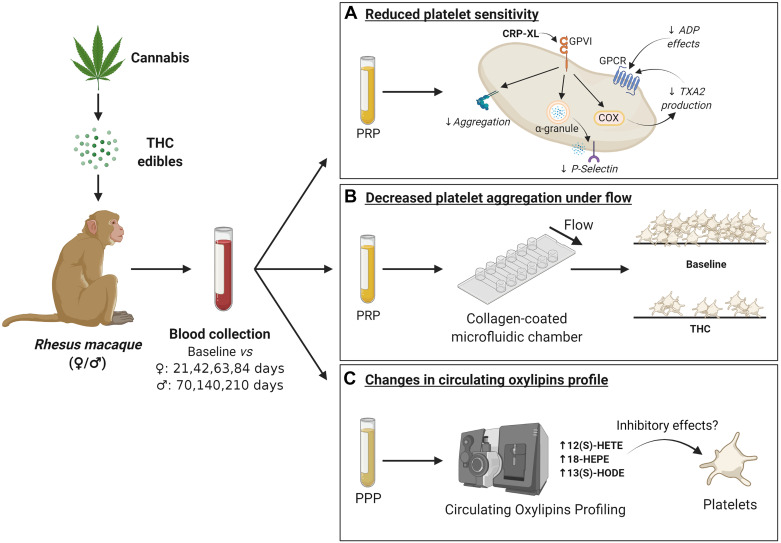
Figure 6.
Schematic representation of THC edibles effects on platelet function. Administration of THC edible dosing in a female and male cohort was studied by collecting blood samples and analyzed for platelet function. In A, the systemic THC exposure resulted in a reduction of platelet sensitivity, which can be seen as a reduction in CRP-XL- and ADP-induced platelet aggregation, a reduction in integrin receptor activation and thromboxane production, as well as a reduction in P-selectin exposure from the α granules. In B, we observed a reduction in platelet aggregate formation under shear flow on a collagen coated surface with THC edible administration. In C, we observed changes in plasma oxylipin levels between baseline and THC edible administration that may have inhibitory effects on platelets. Figure created with BioRender.com. CRP-XL, collagen-related peptide-XL; THC, Δ9-tetrahydrocannabinol.
The policy changes within individual states and provinces in the United States and Canada have led to significant increases in the use of recreational and medical marijuana (20). Associated with this overall increase is a trend of substance abuse greater in males and an increase in medical marijuana use largely in females (21). Several animal model studies suggest that females may be more sensitive than males to the reinforcing and discriminative effects of cannabinoids (21). Differences between sexes have also been observed in the metabolic processing of THC or the levels of endocannabinoids in the brain and periphery (22). Consideration of the metabolism of THC is also important in this context and is often not measured in most analyses despite the fact that the metabolites of THC are highly bioactive, influenced by type of administration and impacted by sex, as females metabolize THC at a faster rate than males (23). Differences in cannabinoid sensitivity could occur from the direct influence of sex chromosomes that control cannabinoid receptor expression, as receptor density and efficacy for GPCR activation differences were observed in the brains of both male and female rats (24). Female rodents were also more sensitive to the effects of THC (25) with a potential role for the estrous cycle on THC sensitivity as fluctuations across the estrous cycle change THC sensitivity (7, 26). In our study, we studied the effects of chronic THC edibles in both a female and a male NHP cohort. Both cohorts showed a significant reduction in platelet activation effect. However, we observed differences in THC metabolites. THC is metabolized mainly by the liver, resulting in the two main metabolites 11-OH-THC and 11-COOH-THC. In our female cohort, the plasma THC levels were higher compared with the males. However, the males had increased levels of THC metabolites. Our study utilizes sex different cohorts based on select timelines, 3 mo for females versus 7 mo for males. This therefore limited our ability to directly compare the results from the female and male cohorts; this was further compounded by a low number of males available for this study. Still, interestingly, although the males were on a longer timeline of THC administration, the impairment of their platelet function was somewhat diminished as compared with the female cohort, perhaps as a result from this differing THC metabolism between males and females. These differences could also be reflective of inherent sex differences in platelet function. Future studies are required to elucidate the chronic effects of cannabis use and sex differences on platelet and immune cell biological functions.
The endocannabinoid system is known to be expressed by the central nervous system, wherein THC signals through CB1 in neuronal and nonneuronal cells alike (6, 27). THC can act as an agonist and antagonist at CB receptors depending on receptor density (7). Studies have shown that CB2 signaling in cells drives the secretion of interleukin (IL)6 and IL10 (28). However, in our study, we did not see any detectable changes in IL6 or IL10 expression in serum in either of our female and male cohort at baseline or at highest dose of THC administered (data not shown). Platelets have been reported to express cannabinoid receptors (11); however, some studies contradict these findings and failed to detect either CB1 or CB2 proteins or their mRNAs in platelets (29, 30). An additional hypothesis could be that platelet function is effected by the uptake and metabolization of the endocannabinoid components and that this can lead to changes in platelet functional effects.
Platelet function is mainly driven by the receptor-mediated signaling leading to secondary feedback activation via secretion of ADP and production of thromboxane by the platelet membrane. Oxylipins are known to be important bioactive lipids that regulate platelet activation and function (19). We observed THC-dependent changes in plasma lipid metabolites in our female cohort. An important change was observed in 12(S)-HETE, 9(R)-HETE, 18-HEPE, 13(S)-HODE, 9(S)-HOTrE, and 13(S)-HOTrE. Studies have demonstrated a role for THC in affecting arachidonic acid metabolism and changes in oxylipin secretion by cells in vitro (31, 32). Oxylipin metabolites are involved in modulating immune responses such as 15(S)-HETE inhibiting polymorphonuclear leukocytes, and increasing levels of 18-HEPE and 13(S)-HOTrE have been shown to exhibit anti-inflammatory effects in modulating macrophage function (33–35). Moreover, 11β-prostaglandin F2α (11β-PGF2α) is the primary metabolite of PGD2 and has pro- and anticoagulant effects (36). Further, THC has also been proposed to be a potential COX-2 inhibitor (37); this might have a resemblance to aspirin treatment, where a reduction in oxylipin profile in platelets was observed in a patient cohort on aspirin (38). Levels of 13(S)-HODE limit platelet aggregation and endothelial cell interaction (39). The production of 12S-HETE by platelets can both exhibit platelet activation and platelet aggregate inhibition depending on agonists used and concentrations in vitro (19, 40). Patients who have a low-platelet 12-HETE production are at an increased risk to present with bleeding (41); this may be a predictive outcome of chronic THC use based on our study in which we observed increased levels of 12(S)-HETE in plasma in addition to diminished agonist-induced platelet activation (42). We demonstrate for the first time changes in oxylipin metabolite profile in the presence of chronic THC administration. Moreover, we tested a selection of oxylipins on its effects on platelet aggregation in human platelets, where we observed some had a reducing effect on platelet aggregation (Supplemental Fig. S3). The observed changes in oxylipin profile might be related to our observed reduced platelet functions. However, further studies need to be conducted to locate the source of oxylipin production in the presence of chronic THC administration.
The clinical implications of chronic cannabis use in the population not only have effects on behavioral sciences but also impact the cardiovascular system (9). Studies have shown that endocannabinoids such as anandamide and 2-AG were able to promote platelet aggregation (43, 44). Yet, anandamide was also shown to inhibit platelet activation by the collagen receptor GPVI under static and flow conditions (13). The early studies were limited by reliance on the sole use of synthetic agonists, whereas the later work included a pilot study of self-reported cannabis users without control of dosage. Platelets also have additional functions beyond maintaining vessel integrity, such as in wound healing and angiogenesis. As cannabis is the most commonly used illicit drug in pregnancy (45), this is concerning because during pregnancy and placental development, platelets play an important role in providing necessary growth factors and support for placental angiogenesis to occur (46). The underlying mechanism for this is not well understood, but these findings suggest the importance of taking a thorough patient drug history for those undergoing surgery or are pregnant. As the effect of THC use on platelets may impair its role in normal placental development, it is important that health care providers appropriately counsel women who use marijuana and are planning to conceive or pregnant. In conclusion, our study expands our current knowledge regarding the effects of cannabinoids on platelet reactivity by studying the direct effects of chronic THC use in both female and male NHPs. Our observations may have important implications for clinical health policies regarding the use of medical marijuana in the future.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.16785766
GRANTS
All Oregon National Primate Research Center (ONPRC) cores and units were supported by the National Institutes of Health (NIH) Grant P51 OD011092. Research reported in this publication was supported by the Reproductive Scientist Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Drug Abuse (NIDA) Drug Supply Program, and Silver Family Innovation Award under Award Numbers K12 HD000849, R03 HD097116 (to J.O.L.), American Heart Association (828839; to S.E.R.), and the National Heart, Lung, and Blood Institute (HL101972 and HL144113) and National Institute of Allergy and Infectious Diseases (AI157037; to O.J.T.M and M.T.H.).
DISCLAIMERS
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of the AHA or NIH NICHD and NIDA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.R., M.T.H., J.E.A., O.J.T.M., and J.O.L. conceived and designed research; S.E.R., H.H.S.L., J.J., J.P., I.P-I., A.R.M., and J.C. performed experiments; S.E.R., H.H.S.L., and J.C. analyzed data; S.E.R., I.P.-I., J.C., D.E.J.A., J.F.S., O.J.T.M., and J.O.L. interpreted results of experiments; S.E.R. prepared figures; S.E.R., O.J.T.M., and J.O.L. drafted manuscript; S.E.R., M.T.H., J.F.S., J.E.A., O.J.T.M., and J.O.L. edited and revised manuscript; S.E.R., H.H.S.L., J.J., J.P., I.P.-I., A.R.M., J.C., D.E.J.A., M.T.H., J.F.S., J.E.A., O.J.T.M., and J.O.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the veterinary and husbandry staff for providing excellent care for the animals used in this study, in particular Dr. Lauren Drew Martin and Travis Hodge. Additionally, we thank the Bioanalytical Shared Resource/Pharmacokinetics Core and Oregon Clinical and Translational Research Institute (OCTRI) Laboratories at OHSU.
REFERENCES
- 1.NIDA. What is the scope of marijuana use in the United States? https://www.drugabuse.gov/publications/research-reports/marijuana/what-scope-marijuana-use-in-united-states [17 Aug 2021].
- 2.United Nations Office on Drugs and Crime. World Drug Report 2018. Vienna: 2018. https://www.unodc.org/wdr2018/. [Google Scholar]
- 3.SAMHSA, CBHSQ. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. [17 Aug 2021].
- 4.Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72: 1235–1242, 2015. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quality SACfBHSa. Results from the 2018 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables. [17 Aug 2021].
- 6.Lu HC, Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging 6: 607–615, 2021. doi: 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan KS, Mahalingaiah S, Campbell LR, Roberts VHJ, Terrobias JJD, Naito CS, Boniface ER, Borgelt LM, Hedges JC, Hanna CB, Hennebold JD, Lo JO. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F S Sci 2: 287–294, 2021. doi: 10.1016/j.xfss.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffens S, Pacher P. The activated endocannabinoid system in atherosclerosis: driving force or protective mechanism? Curr Drug Targets 16: 334–341, 2015. doi: 10.2174/1389450115666141202113225. [DOI] [PubMed] [Google Scholar]
- 9.Latif Z, Garg N. The impact of marijuana on the cardiovascular system: a review of the most common cardiovascular events associated with marijuana use. J Clin Med 9: 1925, 2020. doi: 10.3390/jcm9061925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg 99: 1127–1130, 2004. doi: 10.1213/01.ANE.0000131505.03006.74. [DOI] [PubMed] [Google Scholar]
- 11.Catani MV, Gasperi V, Catanzaro G, Baldassarri S, Bertoni A, Sinigaglia F, Avigliano L, Maccarrone M. Human platelets express authentic CB(1) and CB(2) receptors. Curr Neurovasc Res 7: 311–318, 2010. doi: 10.2174/156720210793180774. [DOI] [PubMed] [Google Scholar]
- 12.Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg 99: 1127–1130, 2004. doi: 10.1213/01.ANE.0000131505.03006.74. [DOI] [PubMed] [Google Scholar]
- 13.De Angelis V, Koekman AC, Weeterings C, Roest M, de Groot PG, Herczenik E, Maas C. Endocannabinoids control platelet activation and limit aggregate formation under flow. PLoS One 9: e108282, 2014. doi: 10.1371/journal.pone.0108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garland KS, Reitsma SE, Shirai T, Zilberman-Rudenko J, Tucker EI, Gailani D, Gruber A, McCarty OJT, Puy C. Removal of the C-terminal domains of ADAMTS13 by activated coagulation factor XI induces platelet adhesion on endothelial cells under flow conditions. Front Med (Lausanne) 4: 232, 2017. doi: 10.3389/fmed.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reitsma SE, Pang J, Raghunathan V, Shatzel JJ, Lorentz CU, Tucker EI, Gruber A, Gailani D, McCarty OJT, Puy C. Role of platelets in regulating activated coagulation factor XI activity. Am J Physiol Cell Physiol 320: C365–C374, 2021. doi: 10.1152/ajpcell.00056.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen TL, Newman JW. Establishing and performing targeted multi-residue analysis for lipid mediators and fatty acids in small clinical plasma samples. Methods Mol Biol 1730: 175–212, 2018. doi: 10.1007/978-1-4939-7592-1_13. [DOI] [PubMed] [Google Scholar]
- 17.García-Jaramillo M, Lytle KA, Spooner MH, Jump DB. A lipidomic analysis of docosahexaenoic acid (22:6, ω3) mediated attenuation of western diet induced nonalcoholic steatohepatitis in male Ldlr−/− mice. Metabolites 9: 252, 2019. doi: 10.3390/metabo9110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cascio MG. PUFA-derived endocannabinoids: an overview. Proc Nutr Soc 72: 451–459, 2013. doi: 10.1017/S0029665113003418. [DOI] [PubMed] [Google Scholar]
- 19.Yeung J, Hawley M, Holinstat M. The expansive role of oxylipins on platelet biology. J Mol Med (Berl) 95: 575–588, 2017. doi: 10.1007/s00109-017-1542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greaves L, Hemsing N. Sex and gender interactions on the use and impact of recreational cannabis. Int J Environ Res Public Health 17: 509, 2020. doi: 10.3390/ijerph17020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 43: 34–51, 2018. doi: 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci 5: 64, 2011. doi: 10.3389/fnbeh.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene NZ, Wiley JL, Yu Z, Clowers BH, Craft RM. Cannabidiol modulation of antinociceptive tolerance to Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 235: 3289–3302, 2018. doi: 10.1007/s00213-018-5036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, Wiley JL. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend 194: 20–27, 2019. doi: 10.1016/j.drugalcdep.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanton HL, Barnes RC, McHann MC, Bilbrey JA, Wilkerson JL, Guindon J. Sex differences and the endocannabinoid system in pain. Pharmacol Biochem Behav 202: 173107, 2021. doi: 10.1016/j.pbb.2021.173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiers CE, Shokri-Kojori E, Wong CT, Abi-Dargham A, Demiral ŞB, Tomasi D, Wang G-J, Volkow ND. Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology 41: 2596–2605, 2016. doi: 10.1038/npp.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 43: 155–172, 2018. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL. Cannabinoid receptor 2 (CB2) signals via G-alpha-s and induces IL-6 and IL-10 cytokine secretion in human primary leukocytes. ACS Pharmacol Transl Sci 2: 414–428, 2019. doi: 10.1021/acsptsci.9b00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldassarri S, Bertoni A, Bagarotti A, Sarasso C, Zanfa M, Catani MV, Avigliano L, Maccarrone M, Torti M, Sinigaglia F. The endocannabinoid 2-arachidonoylglycerol activates human platelets through non-CB1/CB2 receptors. J Thromb Haemost 6: 1772–1779, 2008. doi: 10.1111/j.1538-7836.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- 30.Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 97: 1049–1070, 2015. doi: 10.1189/jlb.3RU0115-021R. [DOI] [PubMed] [Google Scholar]
- 31.Currais A, Quehenberger O, M Armando A, Daugherty D, Maher P, Schubert D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging Mech Dis 2: 16012, 2016. doi: 10.1038/npjamd.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz S, Specter S, Vanderhoek JY, Coffey RG. The effect of delta-9-tetrahydrocannabinol on arachidonic acid metabolism in human peripheral blood mononuclear cells. J Pharmacol Exp Ther 268:1289–1296, 1994. [PubMed] [Google Scholar]
- 33.Kumar N, Gupta G, Anilkumar K, Fatima N, Karnati R, Reddy GV, Giri PV, Reddanna P. 15-Lipoxygenase metabolites of α-linolenic acid [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci Rep 6: 31649, 2016. doi: 10.1038/srep31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Chen CY, Arita M, Kim K, Li X, Zhang H, Kang JX. An omega-3 polyunsaturated fatty acid derivative, 18-HEPE, protects against CXCR4-associated melanoma metastasis. Carcinogenesis 39: 1380–1388, 2018. doi: 10.1093/carcin/bgy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr 6: 513–540, 2015. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braune S, Kupper JH, Jung F. Effect of prostanoids on human platelet function: an overview. Int J Mol Sci 21: 9020, 2020. doi: 10.3390/ijms21239020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPartland JM. Cannabis and eicosanoids. J Cannabis Ther 1: 71–83, 2001. doi: 10.1300/J175v01n01_06. [DOI] [Google Scholar]
- 38.Ellero-Simatos S, Beitelshees AL, Lewis JP, Yerges. -Armstrong LM, Georgiades A, Dane A, Harms AC, Strassburg K, Guled F, Hendriks MMWB, Horenstein RB, Shuldiner AR, Hankemeier T, Kaddurah. -Daouk R, Null N; Pharmacometabolomics Research Network. Oxylipid profile of low-dose aspirin exposure: a pharmacometabolomics study. J Am Heart Assoc 4: e002203, 2015. doi: 10.1161/JAHA.115.002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas TA, Bastida E, Nakamura K, Hullin F, Admirall L, Buchanan MR. Binding of 13-HODE and 5-, 12- and 15-HETE to endothelial cells and subsequent platelet, neutrophil and tumor cell adhesion. Biochimica et Biophysica Acta 961: 153–159, 1988. doi: 10.1016/0005-2760(88)90108-7. [DOI] [PubMed] [Google Scholar]
- 40.Coffey MJ, Jarvis GE, Gibbins JM, Coles B, Barrett NE, Wylie ORE, O’Donnell VB. Platelet 12-lipoxygenase activation via glycoprotein VI. Circ Res 94: 1598–1605, 2004. doi: 10.1161/01.RES.0000132281.78948.65. [DOI] [PubMed] [Google Scholar]
- 41.Walenga RW, Sunderji S, Stuart MJ. Formation of hydroxyeicosatetraenoic acids (HETE) in blood from adults versus neonates: reduced production of 12-HETE in cord blood. Pediatr Res 24: 563–567, 1988. doi: 10.1203/00006450-198811000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Turnbull RE, Sander KN, Turnbull J, Barrett DA, Goodall AH. Profiling oxylipins released from human platelets activated through the GPVI collagen receptor. Prostaglandins Other Lipid Mediat 158: 106607, 2022. doi: 10.1016/j.prostaglandins.2021.106607. [DOI] [PubMed] [Google Scholar]
- 43.Baldassarri S, Bertoni A, Bagarotti A, Sarasso C, Zanfa M, Catani MV, Avigliano L, Maccarrone M, Torti M, Sinigaglia F. The endocannabinoid 2-arachidonoylglycerol activates human platelets through non-CB1/CB2 receptors. J Thromb Haemost 6: 1772–1779, 2008. doi: 10.1111/j.1538-7836.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- 44.Maccarrone M, Bari M, Menichelli A, Del Principe D, Agrò AF. Anandamide activates human platelets through a pathway independent of the arachidonate cascade. FEBS Lett 447: 277–282, 1999. doi: 10.1016/S0014-5793(99)00308-7. [DOI] [PubMed] [Google Scholar]
- 45.Martin CE, Longinaker N, Mark K, Chisolm MS, Terplan M. Recent trends in treatment admissions for marijuana use during pregnancy. J Addict Med 9: 99–104, 2015. doi: 10.1097/ADM.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 46.Kohli S, Isermann B. The role of platelets during development and reproduction. In: Platelets in Thrombotic and Non-Thrombotic Disorders, edited by Gresele P, Kleiman N, Lopez J, Page C.. Cham: Springer, p. 531–539. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.16785766