Keywords: eye movements, testing, schwannoma, vestibular, vestibulo-ocular reflex
Abstract
Imbalance and dizziness are disabling symptoms for many patients with vestibular schwannomas (VS) but symptom severity typically does not correlate with the vestibulo-ocular reflex (VOR) amplitude-based metrics used to assess peripheral vestibular damage. In this study, we tested the hypothesis that imbalance and dizziness in patients with VS relate to VOR metrics that are not based on response amplitude. Twenty-four patients with unilateral, sporadic VS tumors were studied, and objective (balance) and subjective (dizziness) vestibular dysfunction was quantified. The VOR was tested using two yaw-axis motion stimuli, low-frequency en-bloc sinusoidal, and high-frequency head-on-body impulsive rotations. Imbalance correlated with VOR precision (the inverse of the trial-to-trial variability) and with low-frequency VOR dynamics (quantified with the time constant), and these two metrics were also strongly correlated. Dizziness correlated with the VOR bias caused by an imbalance in static central vestibular tone, but not with dynamic VOR metrics. VOR accuracy (mean response amplitude relative to the ideal response) was not correlated with the severity of imbalance or dizziness or with measures of VOR precision or time constant. Imbalance in patients with VS, therefore, scales with VOR precision and time constant, both of which appear to reflect the central vestibular signal-to-noise ratio, but not with VOR slow-phase accuracy, which is based on the magnitude of the central vestibular signals. Dizziness was related to the presence of a static central tone imbalance but not to any VOR metrics, suggesting that abnormal perception in VS may be affected by factors that are not captured by yaw-axis VOR measurements.
NEW & NOTEWORTHY The severity of symptoms associated with unilateral vestibular schwannomas (VS) is poorly correlated with standard yaw-axis vestibulo-ocular reflex (VOR) metrics that are based on response amplitude. In this study, we show that the balance and perceptual dysfunction experienced by patients with VS scales with VOR metrics that capture information about the central signal-to-noise ratio (balance) and central static tone (dizziness), but are not correlated with the VOR gain, which reflects central signal amplitude.
INTRODUCTION
This paper is part of the Journal of Neurophysiology issue honoring the scientific contributions of W. Mike King, entitled “Vestibular and oculomotor function in health and disease.” As family (S.K.) and friends (F.K. and R.F.L.) of Mike, we are grateful for the opportunity to help honor him, as he has been an exemplary scientist and a mentor to us and many others. Our topic is particularly relevant to Mike’s career because it combines aspects of his clinical and scientific interests, as we examined the relationship between clinical vestibular tests that employ yaw-axis vestibulo-ocular reflex (VOR) measurements and the severity of vestibular symptoms in a cohort of patients with unilateral vestibular dysfunction. The study population consisted of patients with sporadic, unilateral vestibular schwannomas (VS), which are benign, slowly growing tumors that originate from Schwann cells on the vestibular portion of the 8th cranial nerve (1). These tumors damage the vestibular nerve and the labyrinth, resulting in imbalance, dizziness, and other vestibular symptoms (2). Although VOR parameters based on response amplitude that are derived from standard low-frequency (en-bloc) and high-frequency (head-on-body) yaw-axis motion stimuli typically provide evidence of peripheral vestibular damage in patients with VS, these abnormalities are not well correlated with symptom severity (3–5).
Although objective (balance) and subjective (dizziness) vestibular dysfunction in patients with VS could diverge from yaw-axis angular VOR metrics for many reasons, we focused on the possibility that yaw-axis VOR features that are based on slow-phase variability rather than slow-phase amplitude could potentially provide information that is relevant to the postural and perceptual symptoms experienced by patients with VS. Specifically, the yaw VOR is typically characterized by its gain which is measure of slow-phase response accuracy (defined as the mean response amplitude relative to the ideal response amplitude), but can also be characterized by its precision (defined as the inverse of the trial-by-trial variability). Accuracy and precision reflect different aspects of vestibular processing, since accuracy is determined by the central signal magnitude, while precision is determined by the central signal-to-noise ratio (SNR) (6, 7). In a vestibular context, signal is defined as neuronal activity that encodes head motion and orientation whereas noise is random neuronal activity which does not provide the brain with motion or orientation information (8).
We hypothesized that changes in the central SNR may be more closely related to clinical vestibular symptoms in patients with VS than changes in signal magnitude, and therefore that vestibular symptoms could scale with VOR precision but not with VOR accuracy. Furthermore, the low-frequency dynamics of the VOR, typically quantified as the time constant of the eye movement response, are determined by the “velocity storage” integrator in the brain (9), and are affected by the integrity of the peripheral vestibular inputs for reasons that remain uncertain (10). Since the efficacy of velocity storage may be determined by the central SNR (11), we also examined the relationship between vestibular symptoms and the VOR time constant and between the time constant and VOR precision.
We found that the severity of imbalance in VS correlated closely with the yaw-axis VOR precision and time constant, but not with the VOR gain. In contrast, dizziness scaled with the bias of the VOR, which was produced by a static imbalance in vestibular tone, but did not scale with any of the dynamic VOR metrics we examined. Although VOR precision and time constant measures were also closely correlated, the VOR gain did not correlate with either VOR precision or time constant. These results suggest that imbalance in patients with VS is related to the central SNR and can be identified by analyzing the yaw-axis angular VOR variability and velocity storage. Dizziness was only influenced by the presence of an aberrant vestibular tone imbalance, which implies that vestibular features we did not assess or extra-vestibular factors such as anxiety or autonomic dysfunction may also contribute to the severity of perceptual dysfunction in patients with VS.
MATERIALS AND METHODS
The study was approved by the Massachusetts Eye and Ear Institutional Review Board, and all patients signed written informed consents before participation.
Patients and Control Subjects
Twenty-eight patients with unilateral, untreated, sporadic VS tumors were recruited from the large Otology and Neuro-otology clinics at Massachusetts Eye and Ear. These patients had received their VS diagnosis at least 6 mo before testing, and therefore had chronic damage to one vestibular periphery caused by the tumor but normal function in the contralateral ear/nerve and in the brain. Exclusionary criteria for patients with VS (and normal controls) were: other otologic or neurologic disorders (except presbycusis or migraine); psychiatric disorders other than anxiety or depression; chronic use of vestibular suppressants (benzodiazepines and antihistamines); corrected visual acuity worse than 20/20; and orthopedic problems that affected balance or gait. Patients with neurofibromatosis type 2 were also excluded from the study because they typically have bilateral vestibular nerve abnormalities (12).
Four of the patients with VS were subsequently excluded because their VOR characteristics differed markedly from the other patients (see below), so twenty-four patients with VS (16 females; age range 26–67 yr, mean age of 52 yr) and twenty-three normal controls subjects (8 females; age range 31–72 yr, mean age of 53 yr) served as test subjects. All patients with VS completed the vestibular symptom assessment and at least one of the two VOR test batteries. They were also examined by one author (R.F.L.), who performed a vestibular, oculomotor, and neurologic exam—the presence of spontaneous or inducible nystagmus was evaluated by observing eye movements behind video “Frenzel” glasses with the eyes straight ahead and the head upright (spontaneous nystagmus), and during eccentric gaze, Valsalva, compression of the tragus, hyperventilation, neck vibration, changes in head position using standard Hallpike–Dix head positions, and following horizontal and vertical head shaking. If nystagmus was observed, the direction of slow phases in the orbit (horizontal, vertical, and torsional) and relative to the abnormal vestibular nerve were noted.
Assessment of Vestibular Symptoms
The severity of balance and perceptual dysfunction in the VS and control populations was measured using standard, well-validated metrics. Balance was quantified with the Functional Gait Assessment (FGA), a measure of gait performance based on 10 tasks that are scored by a trained observer using a 30 (normal) to 0 (worst possible) scale (13). Dizziness was quantified using the Dizziness Handicap Inventory (DHI) which provides a subjective assessment of the severity of vestibular symptoms over a 0 (normal) to 100 (worst possible) scale (14).
Assessment of the Yaw-Axis Angular VOR
The VOR was assessed using two approaches that are frequently used in clinical vestibular testing laboratories (15, 16). Both methods used video eye tracking systems, and since these can make small errors due to pupillary tracking, we measured errors with an artificial eye during motion and found that tracking errors were very small (e.g., 0.2°/s) relative to the size of the VOR responses.
Sinusoidal en-bloc yaw-axis rotations employed low-frequency (0.01–1.0 Hz) and low-velocity (40°/s peak velocity) motion stimuli provided by a Neurokinetics (NKI) rotational chair, and eye movements were measured with a commercial (NKI) video system in complete darkness. Eye movements were desaccaded with a semiautomated system, reviewed by an experienced operator, and then slow phases were fit to a sine function with minimum mean-squared error. For each subject, the gain, phase, and bias values across frequencies were fit to functions and these data were used to calculate the gain, time, and bias constants (17) that characterized the sinusoidal VOR response (Fig. 1A). The gain is a measure of VOR slow phase accuracy (e.g., eye velocity/head velocity); the time constant, which was calculated as 1/[2πfc], where fc is the corner frequency (the stimulus frequency associated with an eye movement phase lead of 45°), quantifies velocity storage; and the bias measures the symmetry of the dynamic VOR response (slow phases toward the tumor ear—slow phases toward the normal ear for patients with VS, right-left slow phases for control subjects) combined with any superimposed spontaneous nystagmus (slow phases toward the tumor ear were designated as positive) caused by an imbalance in static central vestibular tone (18).
Figure 1.
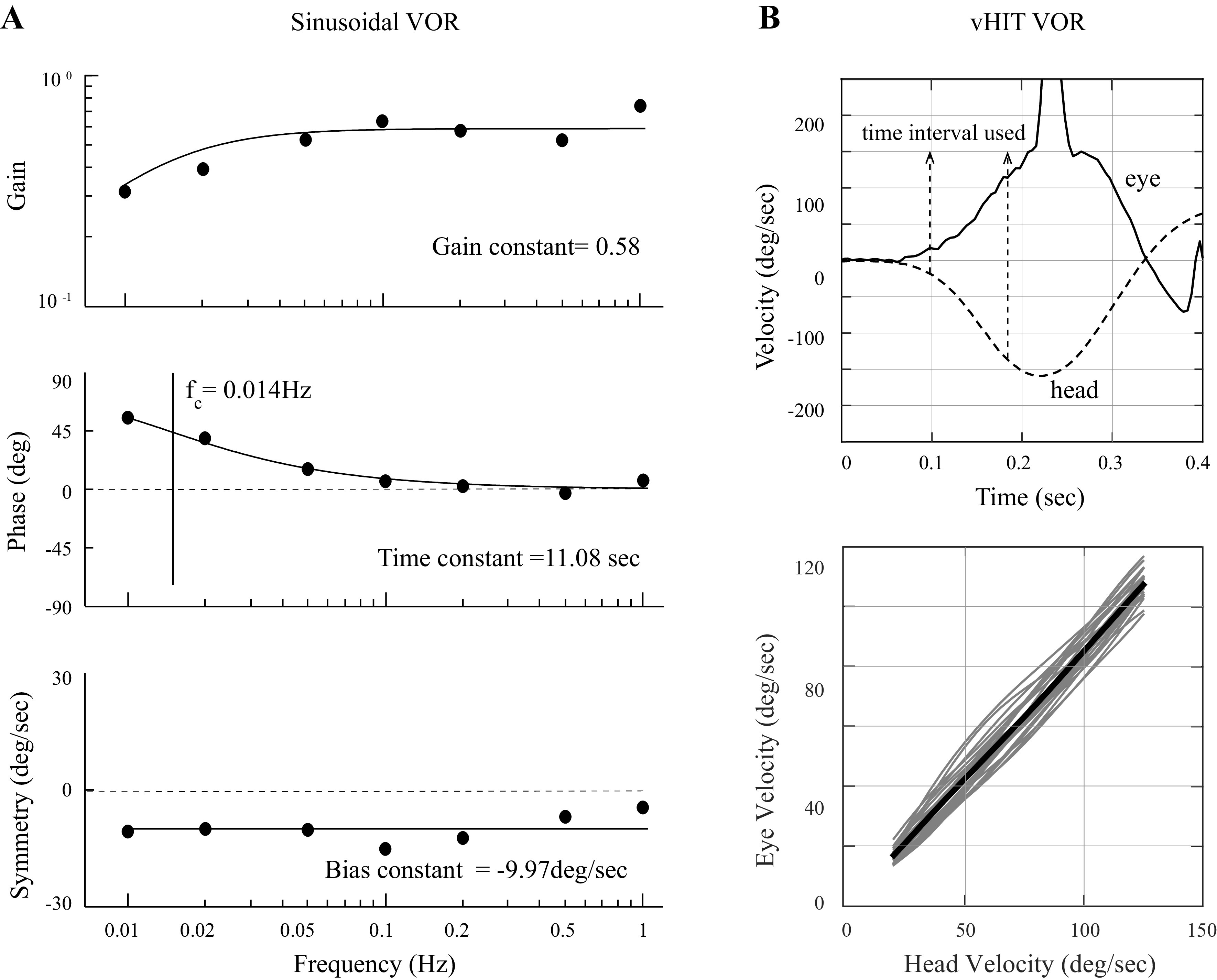
A: sinusoidal VOR data for a single patient with VS showing the gain, phase, and symmetry values for each of the seven tested frequencies. Each of the three sets of seven data points were fit to functions as illustrated using previously published methods (17), and these functions yielded the gain, time, and bias constant values that were used to characterize the patient’s sinusoidal VOR response. The time constant was calculated from the corner frequency (fc) of the phase data (the frequency where the phase lead is 45°) as 1/[2πfc]. B: data for the head-on-body VOR (vHIT) test for a single patient with VS, rotation toward the ear with the tumor. The top panel shows a single trial and illustrates the characteristic head and eye motion dynamics and the data section used to calculate the VOR gain and variability; the bottom panel shows the individual trials for this patient as thin gray lines and the mean response (thick black line). The VOR gain was calculated as the slope of the mean response and the VOR variability was calculated as the average of the root-mean-squared error for each individual trial relative to the mean response. VOR, vestibulo-ocular reflex; VS, vestibular schwannoma.
Passive head-on-body impulsive yaw-axis rotations (abbreviated as vHIT for vestibular head-impulse tests; 19, 20) were used to measure VOR gain (accuracy) and variability (precision). Head movements had bell-shaped velocity profiles that were relatively high-frequency (period of ∼0.2 s, consistent with a single acceleration sinusoid of ∼5 Hz) with high peak velocity (circa 150°/s) characteristics (Fig. 1B, top). Tests were performed in dim light and eye movements were recorded with a commercial (Eyeseecam) video system. Subjects fixated a target straight ahead, 96.5 cm from the head’s rotational axis, then the head was rotated to the left or right (direction and timing of onset randomized). VOR gain and variability were calculated separately for rotations toward the tumor and normal ears and a minimum of 10 trials were performed for each subject and direction of head rotation. vHIT analysis focused on the first 150 ms of the slow-phase VOR response since the VOR is open-loop with regard to visual feedback over that timeframe. The data segment that was analyzed for each trial began when the head velocity first exceeded 20°/s and ended before the first saccade, quick phase, or blink (Fig. 1B, top), and these data segments ranged from 75 to 120 ms in length (average duration of 100 ms). To calculate the VOR gain for each subject and head turn direction, the average eye versus head velocity trajectory was calculated for all trials (thick black line, Fig. 1B, bottom) and the gain was calculated as the slope of a linear regression of this line. To calculate VOR variability, the difference between each trial (thin gray lines, Fig. 1B, bottom) and the average trajectory was quantified as the root-mean-squared error between the trial and the mean response, and then these variability measures were averaged (21). Since we focused on the characteristics of the VOR slow phases in this study, quick phases that occurred during or after head rotations (22) were not tabulated.
Statistical Analysis
The parameters measured in the VS and normal subjects were normally distributed based on the Shapiro–Wilk test (P > 0.05 for each parameter), and statistical comparisons therefore used standard parametric methods. Comparisons between mean values were performed using t tests (corrected for repeated measures), and correlations were evaluated for statistical significance with the Pearson R test. P values < 0.05 were considered statistically significant. As noted, four of the 28 patients with VS were major outliers because they had VOR variability values that were much larger than the rest of the patients with VS (> 5 standard deviations from the mean), and these four patients were therefore excluded from our analyses. The data for this small subset of patients and the potential reason(s) why they different so markedly from the large majority of patients with VS are considered in the results and discussion, respectively.
RESULTS
VOR Metrics, Balance, and Dizziness Were Abnormal in the Population with VS
As summarized in Table 1, all measured parameters were abnormal in the VS population compared to the normal control subjects (t test: P < 0.05), with the exception of the sinusoidal VOR gain which was borderline abnormal (P = 0.06). In particular, for sinusoidal rotations the VOR gain and time constant were reduced in VS and there was a bias toward the side with the tumor. For vHIT testing, the VOR gain for rotations toward the tumor ear were reduced in the patients with VS and the VOR variability was increased. The perceptual (DHI scores) and balance (FGA scores) were also abnormal in the patients with VS compared with the control subjects (t test: P < 0.05 for DHI and FGA). The relevant physical exam parameters are described in the appropriate sections of the results.
Table 1.
VOR parameters and measures of vestibular disability
| Test/Subject | Normal | VS |
|---|---|---|
| VOR Sinusoids | ||
| Gain | 0.67 ± 0.11 | 0.59 ± 0.14 |
| Time constant, s | 19.8 ± 5.2 | 10.5 ± 4.9 |
| Bias, deg/s | −0.59 ± 5.1 | 1.14 ± 6.6 |
| VOR head impulses | ||
| Gain | 0.88 ± 0.1 | 0.71 ± 0.2 |
| Variability, deg/s | 11.3 ± 6.5 | 17.7 ± 6.6 |
| Vestibular disability | ||
| DHI | 1.7 ± 3.0 | 22.1 ± 17.8 |
| FGA | 29 ± 0.7 | 24.0 ± 4.2 |
Means ± 1 standard deviation for the normal and vestibular schwannoma (VS) subjects for the vestibulo-ocular reflex (VOR) parameters derived from sinusoidal rotations and head-on-body impulsive rotations, and for the measures of vestibular disability [DHI, subjective dizziness scaled from 0 (normal) to 100; FGA, gait and balance dysfunction scaled from 0 to 30 (normal)]. for sinusoidal VOR bias, positive values were toward the tumor ear in patients with VS and toward the right in normal subjects. Normal distributions were confirmed for all measurements using the Shapiro–Wilk test (P > 0.05 for all variables), and all comparisons between normal and VS subjects were significant (P < 0.05) except for the sinusoidal gain (P = 0.06). DHI, Dizziness Handicap Inventory; FGA, Functional Gait Assessment.
As noted, four of the patients with VS we tested had VOR variability values that were markedly different than the other 24 patients. In particular, the mean VOR variability for these four patients with VS was 54.4°/s, which is more than 5 standard deviations from the mean of the of the other 24 patients (Table 1). The other vestibular parameters for these four patients were less dissimilar from the majority of patients with VS and included mean vHIT gain of 0.87, sinusoidal gain of 0.56, sinusoidal time constant of 8.8 s, sinusoidal bias of 4.2 s, DHI of 6, and FGA of 27. Since their VOR variability values were such extreme outliers, we elected to remove these four patients from the data set (see discussion).
Association between Balance (FGA Score) and VOR Metrics
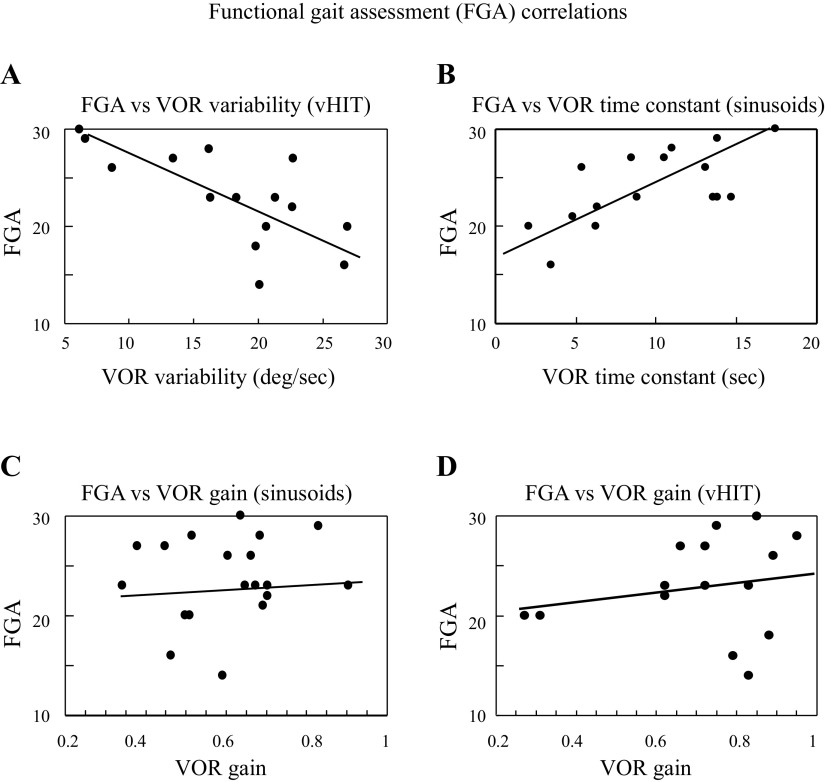
Figure 2A shows the correlation between the FGA and VOR variability—there was a strong negative correlation between these variables, as higher VOR variability values were associated with lower (worse) FGA scores (Pearson R: r = −0.7, P = 0.004). Figure 2B shows that the efficacy of velocity storage, as measured with the sinusoidal VOR time constant, was positively correlated with the FGA score (Pearson R: r = 0.58, P = 0.009)—shortening of the VOR time constant was correlated with worse balance, as evidenced by lower FGA scores. In contrast to the VOR variability and time constant, there were no significant correlations between the FGA score and the VOR gain, measured with either low-frequency sinusoids (Fig. 2C; r = 0.13, P = 0.55) or with impulsive (vHIT) stimuli (Fig. 2D; r = 0.25, P = 0.29), and the FGA did not correlate with the bias of the sinusoidal VOR (Pearson R: r = 0.23, P = 0.29).
Figure 2.
Scatter plots and linear regressions that demonstrate FGA correlations with vHIT VOR variability (regression: FGA = 32.3 − 0.5 × VOR variability; Pearson R: r = 0.71, P = 0.003; A) and sinusoidal VOR time constant (regression: FGA = 18.7 + 0.6 × VOR time constant; Pearson R: r = 0.66, P = 0.005; B); VOR gains calculated with sinusoidal (regression: FGA = 21.0 + 4.3 × sinusoidal gain; Pearson R: r = 0.14, P = 0.55; C) or vHIT (regression: FGA = 21.2 + 2.1 × vHIT gain; Pearson R: r = 0.12, P = 0.67; D) motion trajectories were not correlated with FGA scores. FGA is scaled from 30 (normal) to 0 (worst). FGA, Functional Gait Assessment; vHIT, vestibular head-impulse tests; VOR, vestibulo-ocular reflex; VS, vestibular schwannoma.
Association between Subjective Dizziness (DHI Score) and VOR Metrics
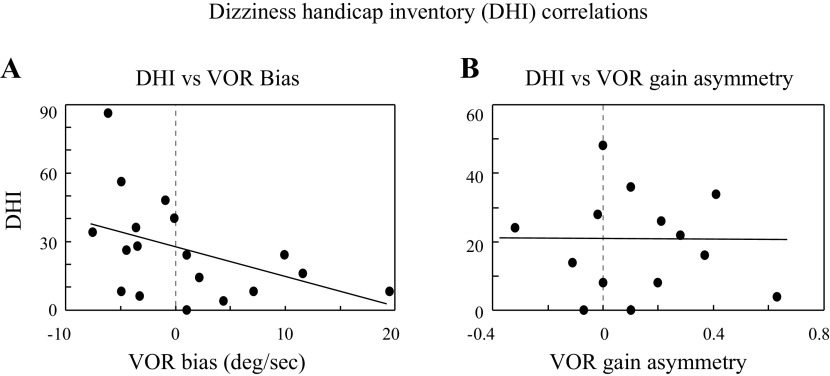
Unlike the FGA, the DHI was not correlated with VOR variability (r = 0.3, P = 0.22) or time constant (r = 0.32, P = 0.19), nor was it correlated with the VOR gain (sinusoids: r = 0.23, P = 0.31; vHIT: r = 0.06, P = 0.8). In contrast, the VOR bias calculated from sinusoidal rotations was significantly correlated with the DHI score (r = −0.47, P = 0.03), with higher DHI scores (more dizziness) associated with larger VOR bias toward the normal ear (negative, Fig. 3A). The VOR bias has two potential contributing factors—asymmetry of the dynamic VOR response can generate a slow phase bias, and an imbalance in static vestibular tone can produce spontaneous slow phases which also bias the VOR response (cf., 18, which is the most definitive study demonstrating how dynamic VOR asymmetry and spontaneous nystagmus interact to bias the net VOR response). These two potential contributors to the VOR bias cannot be separated from the sinusoidal VOR data.
Figure 3.
Scatter plots and regressions showing the relationship between the DHI score [scaled from 0 (normal) to 100 (worst)] and the VOR bias. A: bias measured with sinusoidal motion stimuli. On the x-axis, positive values indicate slow phase bias toward the tumor ear and negative values indicate a bias toward the normal ear. These bias measures include both dynamic VOR asymmetry (difference in VOR gains) and any spontaneous slow phases (reflecting tone asymmetry; 18) and were significantly correlated with the DHI score (regression: DHI = 5.0 − 0.1 × VOR bias; Pearson R: r = 0.47, P = 0.04). B: DHI vs. the symmetry of the dynamic VOR slow phases (gains) elicited with vHIT stimuli. x-Axis is the gain asymmetry (slow phases toward tumor ear − slow phases toward normal ear) so like A, positive values represent a bias toward the tumor ear. vHIT gain asymmetry was not correlated with the DHI score (regression: DHI = 0.2 − 0.01 × gain asymmetry; Pearson R: r = 0.16, P = 0.6). DHI, Dizziness Handicap Inventory; vHIT, vestibular head-impulse tests; VOR, vestibulo-ocular reflex.
Although we did not record spontaneous nystagmus (e.g., slow phase eye velocity with the head upright and stationary), we do have an independent measure of the dynamic VOR symmetry based on the response amplitudes generated by the vHIT rotations toward the normal and tumor ears (albeit at a higher frequency than the sinusoidal testing), and the vHIT VOR gain asymmetry (Fig. 3B) was not correlated with the DHI (Pearson R: r = −0.07, P = 0.79). The presence and direction of spontaneous nystagmus was identified for each patient with VS during the physical exam, however, and the DHI score correlated with these physical findings. The DHI score (means ± 1 SD) was 31 ± 10.3 for patients with VS with spontaneous slow phases toward the normal ear, 20.3 ± 8.2 for patients with VS with no spontaneous slow phases, and 12.4 ± 6.4 for patients with VS with slow phases directed toward the tumor ear (ANOVA: F = 4.9, P = 0.02). Furthermore, the eight patients with VS who had a VOR bias toward the normal ear that was >1°/s in magnitude, all had spontaneous nystagmus with slow phases directed toward the normal ear. In contrast, the patients with VS with a bias magnitude <1°/s or a bias >1°/s toward the tumor ear had either no spontaneous nystagmus or had nystagmus with slow phases toward the tumor ear.
Hyperventilation-induced nystagmus has been described in patients with VS (23), and 5 of the 24 patients with VS we examined did have this finding. Although subject numbers are very small, there was no suggestion in our results that hyperventilation-induced nystagmus was associated with any specific pattern of spontaneous nystagmus (1/5 had spontaneous nystagmus with slow phases toward the normal ear, 2/5 had no nystagmus, and 2/5 had nystagmus with slow phases toward the tumor ear). Taken together, our results are highly suggestive that the correlation between the DHI and the VOR bias is due to the association of higher DHI values with spontaneous nystagmus with slow phases toward the normal ear, rather than with an asymmetry of the dynamic VOR slow phases.
Association between Different VOR Metrics
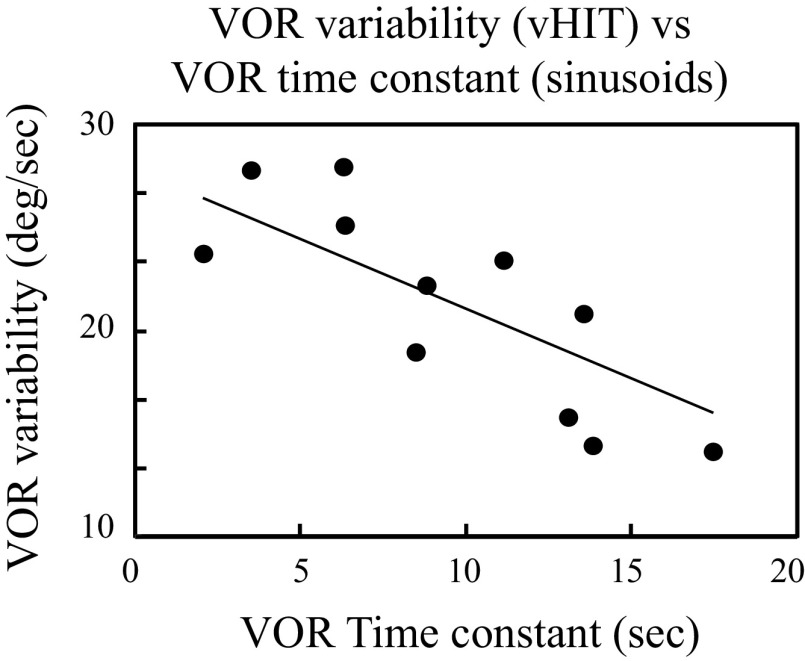
If velocity storage (quantified as the VOR time constant) and VOR variability depend on a shared parameter, then they should be correlated, and we found a strong negative correlation between these two VOR metrics (Fig. 4; Pearson R: r = −0.68, P = 0.01). In contrast, the VOR gain and VOR time constant calculated from the sinusoidal data were not correlated (Pearson R: r = 0.23, P = 0.33) nor were the VOR gain and VOR variability calculated from the vHIT data (Pearson R: r = −0.38, P = 0.16).
Figure 4.
Scatter plot and linear regression showing the relationship between the VOR variability calculated from vHIT testing and the VOR time constant calculated from sinusoidal testing. These two parameters were significantly correlated (regression: VOR variability = 18.4 − 0.5 × VOR time constant; Pearson R: r = 0.82, P = 0.002). vHIT, vestibular head-impulse tests; VOR, vestibulo-ocular reflex.
Aberrant velocity storage can generate central positional nystagmus (e.g., Ref. 24) and we did observe a tendency for VS patients with positional nystagmus to have shorter VOR time constants (7.7 ± 2.4 s) than patients without positional nystagmus (12.1 ± 4.5 s). This difference was not significant (t test: P = 0.14), however, possibly because the number of subjects where this comparison was possible was very small (n = 15).
DISCUSSION
Our principal finding is that patients with sporadic, unilateral VS tumors have postural and perceptual abnormalities that correlate with eye movement metrics derived from yaw-axis clinical VOR tests. Below, we consider the implications of these findings and the advantages and limitations of our approach.
Pathologic Effects of VS Tumors on the Vestibular Periphery
These tumors arise from the Schwann cells that form the myelin sheath of the vestibular portion of the 8th cranial nerve, and pathologic studies indicate that the tumor can damage the nerve in several ways (1). Specifically, the VS can cause axonal death, which results in the degeneration of the affected nerve fibers, and sublethal axonal damage and myelin damage, which degrade nerve function but do not cause axonal degeneration (25). The vestibular labyrinth can also be affected by VS tumors as evidenced by damage and death of hair cells (26). The mechanism(s) responsible for nerve and labyrinthine damage in VS appear to be multifactorial and most likely include direct compression of the nerve by the tumor, ischemia caused by occlusion of the vascular supply, and toxic effects of agents excreted by the tumor. Anatomic (27) and molecular biologic (28) features that are responsible for these different modes of nerve and labyrinthine damage have not been adequately clarified to date, but probably relate to tumor size, location, invasive characteristics, mobility (1, 29), and the specific profile of excreted toxins (12).
Pathophysiologic Effects of VS Tumors on Peripheral and Central Vestibular Function
Vestibular pathophysiology can be understood in terms of the signal and noise characteristics of the damaged periphery and the resulting changes in the vestibular signal magnitude and the signal-to-noise ratio (SNR) in the brain (8, 30). Each vestibular nerve carries both signal and noise to the brain, and the magnitude of the central vestibular signal and the central SNR are normally improved by the convergence of the two vestibular inputs. When two normal vestibular nerves provide the input to the brain, for example, the central signal magnitude is two times larger and the central SNR is √2 times larger than the situation when a single vestibular nerve provides the input to the brain (assuming the noise in the two vestibular nerves is not correlated, 31). Axonal death silences the affected neurons and therefore eliminates their signal and noise, reducing the central signal magnitude and SNR in tandem. In contrast, damage to the axons or myelin that does not silence the axons but produces random neural discharges would lower the central SNR (due to added neural noise) and increase the static tone provided by the damaged nerve (due to an increase in the mean discharge rate), but would not alter the central signal magnitude. The effects of hair cell damage or death caused by VS on afferent nerve function are not known, but in a gentamicin model of hair cell death (32), the afferent signal was reduced but the noise was not affected. In patients with VS, hair cell death may also reduce signal while sparing noise, and in this situation both the central signal magnitude and the central SNR would be reduced.
Taken together, the net effect of these pathologic features appears to be a reduction in the central signal magnitude, a reduction in the central SNR (which could be more pronounced than the change in signal magnitude if the damaged nerve generates random, noisy discharges), and a change in static vestibular tone. The tone imbalance reflects a change in the mean firing rate in the nerve with the VS, and this change could be either a reduction in tonic activity (due to axonal death; 18) or an increase in tonic activity (due to the addition of random, noisy discharges caused by axonal or myelin damage; 23).
Four of the twenty-eight patients with VS studied had extremely high VOR variability values (>5 SD from the mean) but their other vestibular parameters were not markedly different than the majority of the patients with VS. We do not know why these four patients had such enormous VOR variability values—they could have suffered from a neural or labyrinthine mechanism related to the VS tumor that degraded their central vestibular SNR (thereby raising their VOR variability) which did not affect the other 24 patients with VS, but it is also possible that the VOR variability data in these subjects were simply degraded by artifact introduced during the testing procedure.
Since these four patients with VS were such extreme outliers in terms of their VOR variability and because we could not exclude the possibility that these findings were artifactual in nature, we did not include them in the remainder of the data analysis applied to the other 24 subjects.
Balance and the Yaw-Axis VOR—Accuracy versus Precision
The key difference between the accuracy and precision of the VOR slow phases is that accuracy depends on the magnitude of the vestibular signal in the brain whereas precision depends on the central vestibular signal-to-noise ratio (8, 30). Based on the pathophysiologic considerations reviewed above, we propose several possible explanations for the correlation we observed in patients with VS between balance impairment and VOR precision (variability, SNR) but the lack of correlation between balance and VOR accuracy (gain, signal amplitude): 1) the central SNR may be more behaviorally relevant than the signal magnitude because the SNR directly affects the brain’s ability to extract sensory information from its inherently noisy neural environment (33). It is notable that the brain expends considerable computing power to improve precision, often in accordance with Bayesian optimization rules (34), so high precision must be inherently important; 2) changes in VOR precision caused by the tumor may be less amenable to adaptation than changes in VOR accuracy, so precision may provide a more accurate estimate of the severity of vestibular nerve damage after compensation has occurred. The accuracy of the VOR has been shown to be highly adaptable after peripheral damage, and this improvement may primarily result from amplification of the residual afferent vestibular signal by the brain (e.g., Ref. 35). In contrast, amplifying the residual input does not improve the SNR because both the signal and noise increase in concert, and presumably for this reason, the variability (or precision) of vestibular behaviors appears to be less amenable to adaptation (e.g., Refs. 36 and 37, which examined motion perception); or 3) given the complex pathologic effects of the VS on the vestibular nerve and labyrinth, the tumor may induce larger changes in the central SNR than in the signal magnitude due to noise generated by axonal and/or myelin damage, a situation in which VOR precision would be a more sensitive indicator of peripheral vestibular dysfunction than VOR accuracy. In this regard, it is notable that the brain appears to adapt poorly to time-variant changes in vestibular inputs (cf., Refs. 38–40), so the extent of central adaptation to increases in afferent noise caused by tumor-induced nerve damage is presumably small.
Interestingly, we did not observe a significant correlation between the sinusoidal VOR gain and time constant or between the vHIT VOR gain and variability, relationships that would be predicted if axonal death was the sole feature of VS, as tumors that kill more axons would result in lower VOR gain, reduced time constant, and increased variability. The lack of correlation between the gain-time constant and gain-variability, however, is consistent with two of the three mechanisms we propose—central adaptation that affects the gain more than the time constant or variability, or afferent noise caused by nerve fiber damage that alters the time constant and variability but not the gain, as would both serve to decorrelate the gain from the variability and time constant measurements. Beyond these observations, our results do not distinguish between the potential explanations for the correlation between VOR precision (but not accuracy) and balance, but highlight the functional relevance of precision measurements when assessing peripheral vestibular dysfunction in patients with VS.
Dizziness and the Yaw-Axis VOR—Role of Static Vestibular Tone
Similar to studies of other peripheral vestibular disorders (e.g., Ref. 41), we found in patients with VS that the severity of perceptual dysfunction (subjective dizziness) did not correlate with any of the dynamic yaw-axis VOR parameters we quantified (3, 4). The only correlate with dizziness severity was the presence of spontaneous nystagmus with slow phases directed toward the normal ear, indicative of a central static tone imbalance with higher tone on the side of the tumor. This finding is counter-intuitive because unilateral vestibular damage typically reduces static tone on the side of the lesion, resulting in slow phases directed toward the abnormal ear (18). Furthermore, during compensation this abnormality gradually resolves (42) as the central static tone rebalances (43), and persistence of spontaneous nystagmus with slow phases toward the abnormal ear is considered evidence of impaired compensation.
The presence of spontaneous slow phases toward the normal ear could be due to either increased tonic activity in the damaged nerve (e.g., resulting from aberrant spontaneous discharges caused by axonal or myelin damage) or by central overcompensation to the unilateral vestibular deficit. Regarding the former explanation, it is notable that hyperventilation-induced slow phases toward the normal ear have been reported in patients with VS (23), and these slow phases were primarily horizontal with smaller vertical and torsional components. This observation suggests that hyperactivity in the damaged nerve can be elicited by the ion shifts induced by the hypocapnic state.
Dizziness in patients with VS, therefore, appears to relate in part to abnormal activation of the damaged vestibular nerve or abnormal overcompensation to the unilateral deficit, but the absence of correlates between dizziness severity and dynamic VOR metrics suggests that perceptual dysfunction is most likely not related to the severity of peripheral vestibular damage. The subjective nature of dizziness scales such as the DHI complicates this analysis since these scores depend on a patient’s perceived symptom severity and this could vary widely between two patients with the exact same symptoms. More generally, dizziness severity in patients with VS may depend on peripheral or central vestibular characteristics that are not captured by the yaw-axis angular VOR or on non-vestibular characteristics such as psychogenic (44, 45) or autonomic dysfunction (46).
Relationship between VOR Precision and Velocity Storage
Peripheral vestibular damage increases the low-frequency VOR phase lead during sinusoidal rotation, which can be quantified as a reduction in the VOR time constant. Despite the relevance of this observation for clinical diagnosis (47, 48), the mechanism(s) that relate the VOR time constant to the status of the vestibular periphery is unknown. The time constant is determined by velocity storage, a central process that temporally integrates the semicircular canal inputs and thereby improves the accuracy of the central estimate of angular head velocity (11). Velocity storage presumably integrates both signal and noise, however, and the latter would result in drift that degrades the veracity of the central motion estimate (49). Velocity storage has been modeled, therefore, using Bayesian methods that assume that the brain optimizes velocity storage to balance accuracy and precision given the statistics of vestibular noise (11).
These approaches predict that VOR variability, which is determined by the SNR ratio in the brain, should correlate negatively with the VOR time constant (50)—higher noise levels (which lower the SNR and increase VOR variability) should also shorten the VOR time constant. Our results (Fig. 4A) demonstrate this negative correlation in the VS population and therefore support the contention that the brain adjusts VOR dynamics depending on noise levels in the central VOR pathways.
This conclusion is tempered by the fact that we did not measure vertical canal and otolith function in this study, nor did we quantify positional nystagmus which can be a manifestation of dysfunction in canal-otolith synthesis mediated by velocity storage. We did perform positional testing during the physical exam, however, and VS patients with positional nystagmus tended to have shorter VOR time constants than patients without positional nystagmus, although this relationship was not significant in our relatively small sample of subjects. Furthermore, although repeated vestibular stimulation (e.g., due to episodic vertigo attacks) could engender habituation of the VOR and shorten the VOR time constant (51), none of the patients with VS we studied experienced vertigo episodes but rather noted gradually progressive imbalance (2) as their sole vestibular symptom, so habituation should not have affected VOR responses in these patients with VS.
Study Limitations
We focused on a specific peripheral disorder (vestibular schwannomas) and employed testing protocols that only assessed the function of lateral canals, their afferent innervation, and the central processing related to eye movement responses. Here we consider the implications of these constraints in terms of the potential applicability of our results to other peripheral vestibular disorders and the potential utility of implementing a wider array of tests to assess other vestibular end-organs and nonoculomotor vestibular-mediated behaviors.
Are VOR results in VS applicable to other unilateral peripheral vestibular disorders.
The effects of VS tumors on the vestibular periphery are complex and include nerve and labyrinthine damage mediated by both direct physical and indirect toxic mechanisms (12). The functional consequences of combined nerve and labyrinthine damage in VS, however, can be reduced to two essential features—damage that reduces the central vestibular signal and SNR (due to hair cell or axonal death), and damage that activates the vestibular nerve by adding random discharges or noise, thereby reducing the central SNR (but not the signal) and potentially increasing static vestibular tone in the damaged nerve. In contrast, other monophasic peripheral vestibular disorders (e.g., vestibular neuritis; 52) cause hair cell or axonal death which results in static, rather than time-variant, changes in vestibular nerve function. These non-VS disorders, therefore, should reduce the central vestibular signal and SNR by damaging the vestibular periphery (similar to VS) but not activate the labyrinth or nerve by introducing random neuronal activity.
Of the three explanations considered earlier for the correlation between VOR precision (but not accuracy) and imbalance, it is notable that two are applicable to non-VS disorders—if the magnitude of the central SNR is more relevant to postural control than the magnitude of the central signal, or if precision scales with the extent of peripheral vestibular damage better than accuracy because the former is less susceptible to central compensation than the latter, then the precision-balance correlate should hold in non-VS disorders as well as in VS. Only the third potential mechanism, the effects of time-variant neural noise introduced by the VS on the SNR, is not relevant to most non-VS peripheral disorders, although similar noisy discharges could occur with non-VS lesions that compress the vestibular nerve and thereby damage axons and their myelin sheaths, for example, meningiomas, arachnoid cysts, or vascular loops (53). In contrast, the severity of perceptual dysfunction (dizziness) correlated with increased tonic activity on the side of the tumor (evidenced by spontaneous nystagmus with slow phases toward the normal ear), but not with any dynamic VOR metric. This observation suggests that unlike balance, dizziness in VS may be related to aberrant neural discharges from the damaged nerve and hence the dizziness-nystagmus correlation is unlikely to generalize to the majority of peripheral disorders as most of these are not expected to engender aberrant, spontaneous neural activity.
In summary, vestibular schwannomas are a complex form of peripheral vestibular damage, and it is unlikely that other peripheral vestibular disorders recapitulate all of the pathophysiologic features of these tumors. The aforementioned considerations suggest, however, that the precision-balance correlate we observed may generalize to non-VS disorders whereas the dizziness-nystagmus correlate is more likely to be relevant only to VS and the small number of other disorders that activate as well as damage the vestibular nerve. Given the absence of definitive information about the effects of different peripheral vestibular lesions on afferent nerve activity, however, the most direct way to determine if our findings in VS are generalizable to non-VS disorders would be to apply an analytic approach similar to the one we used in patients with VS in future studies to patients with unilateral vestibular damage caused by other (non-VS) etiologies.
Relevance of other vestibular inputs, other vestibular-mediated behaviors, and non-vestibular information.
This study focused on the yaw-axis angular VOR because these tests are commonly performed in many clinical vestibular laboratories—yaw-axis stimuli are readily elicited with en-bloc or head-on-body rotations and eye movements are straightforward to measure and quantify. We did not employ other vestibular tests in this study such as calorics or vestibular evoked myogenic potentials because our goal was to evaluate the information that could be extracted from the yaw VOR slow phase responses; our study was not designed to examine the utility of combining the results of the different available vestibular tests. Even with these constraints, there are a number of limitations to the approach we employed in this study.
Only lateral canal pathways were assessed with yaw-axis rotation.
Studies that relate imbalance and fall risk to the function of specific vestibular sensors suggest that the brain’s sensitivity to interaural (y-axis) translation and roll tilt about an earth-horizontal naso-occipital (x-axis) are best correlated with postural control (54, 55). Furthermore, during gait head movements in patients with VS are primarily translational along the earth-vertical z-axis and tilt about the pitch earth-horizontal y-axis, although smaller head movements in other rotational and translational axes also occur (56). Translational and tilt movements of the head are sensed by the otolith organs and the vertical canals but do not activate the lateral canals. Furthermore, velocity storage modulates the interaction between canal and otolith inputs (57) so the absence of information about otolith and vertical canal function may have relevant and unexplored implications regarding canal-otolith synthesis in the brain in patients with VS.
Despite the limitations associated with exploring only lateral canal function in the VS and normal subjects, we found that the severity of imbalance scales with the precision of yaw-axis VOR slow phases. The exact reason for the relationship between yaw-axis VOR precision and imbalance is not certain, but the most logical explanation is that the VS tumor damages all (or most) vestibular afferent inputs in a roughly correlated manner, so the severity of lateral canal damage can be considered a proxy for the severity of damage to the other vestibular end-organs and their innervation. This correlation would not hold if the tumor only affected selected end-organ afferents, for example, if it only damaged the inferior vestibular nerve (58), but our results did not suggest this type of selective damage in any patients with VS we studied.
Eye movements were the only vestibular-mediated behavior assessed.
While vestibular information contributes to balance and perception, we characterized vestibular dysfunction using oculomotor (VOR) measurements. Could the VS affect the peripheral vestibular afferents that generate the VOR differently than the afferents responsible for posture and perception? Anatomic and physiologic information do not definitively answer this question, but the available data suggest that these vestibular behaviors use overlapping, rather than segregated, afferent inputs (60, 61). More definitively, studies in normal subjects have shown clear correlations between VOR and perceptual measures (62), and between perceptual and postural parameters (55), indicating that some peripheral (and early central processing) is shared between the VOR, perception, and balance. Anatomic or physiologic divergence between the VOR and other vestibular-mediated behaviors, therefore, does not appear to explain the correlation patterns we observed, but future studies could examine these issues more carefully by comparing oculomotor (VOR), perceptual (e.g., motion and orientation thresholds; 63), and postural [e.g., gait kinematics (56), response to perturbations (64)] in patients with VS and other peripheral vestibular disorders.
Potential role of nonvestibular contributions to imbalance and dizziness.
Although VOR metrics scaled with the severity of imbalance, this does not preclude the possibility that other, nonvestibular information contributes to postural dysfunction in patients with VS. The role of nonvestibular contributions is more apparent for perceptual dysfunction since static vestibular tone but no dynamic VOR features correlated with the severity of dizziness. These perceptual results are consistent with the view that dizziness severity is not primarily related to the extent of peripheral vestibular damage in most patients (65), but rather is determined by nonvestibular factors which likely include psychologic (44), autonomic (46), and perhaps other unknown modalities.
Conclusions
Our primary conclusions are that yaw-axis VOR slow phase precision (but not gain) provides information about postural control in patients with unilateral vestibular deficits caused by sporadic VS, possibly by more accurately marking the extent of peripheral damage, and that the VOR time constant provides similar information about SNR as VOR precision. These conclusions are aligned with the methods used to evaluate other sensory systems, since they generally rely on precision rather than accuracy measures. For example, threshold measurements (which are directly related to precision by signal detection theory; 66) are the basis of the audiogram used to test hearing, spatial acuity used to test vision, and tactile sensitivity used to test somatosensation.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant DC018287 (to R.F.L. and F.K.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.K. and R.F.L. conceived and designed research; S.K. and K.D. performed experiments; S.K., K.D., and R.F.L. analyzed data; S.K., F.K., K.M.S., D.B.W., and R.F.L. interpreted results of experiments; S.K. and K.D. prepared figures; R.F.L. drafted manuscript; S.K., F.K., K.M.S., D.B.W., and R.F.L. edited and revised manuscript; S.K., K.D., F.K., K.M.S., D.B.W., and R.F.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Balkwill for technical assistance and Dr. Kathleen Cullen for helpful discussions.
REFERENCES
- 1.Jacob A, Robinson LL Jr, Bortman JS, Yu L, Dodson EE, Welling DB. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope 117: 2087–2092, 2007. doi: 10.1097/MLG.0b013e3181453a07. [DOI] [PubMed] [Google Scholar]
- 2.Nam GS, Jung CM, Kim JH, Son EJ. Relationship of vertigo and postural instability in patients with vestibular schwannoma. Clin Exp Otorhinolaryngol 11: 102–108, 2018. doi: 10.21053/ceo.2017.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CS, Peskoe SB, Risoli T Jr, Garrison DB, Kaylie DM. Associations of video head impulse test and caloric testing among patients with vestibular schwannoma. Otolaryngol Head Neck Surg 161: 324–329, 2019. doi: 10.1177/0194599819837244. [DOI] [PubMed] [Google Scholar]
- 4.Tranter-Entwistle I, Dawes P, Darlington CL, Smith PF, Cutfield N. Video head impulse in comparison to caloric testing in unilateral vestibular schwannoma. Acta Otolaryngol 136: 1110–1114, 2016. doi: 10.1080/00016489.2016.1185540. [DOI] [PubMed] [Google Scholar]
- 5.Brown CS, Cooper MW, Peskoe SB, Risoli T Jr, Kaylie DM. Associations of vestibular tests with penn acoustic neuroma quality of life scores after resection of vestibular schwannoma. Otol Neurotol 41: e241–e249, 2020. doi: 10.1097/MAO.0000000000002462. [DOI] [PubMed] [Google Scholar]
- 6.Karmali F. The velocity storage time constant: balancing between accuracy and precision. Prog Brain Res 248: 269–276, 2019. doi: 10.1016/bs.pbr.2019.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bays PM, Wolpert DM. Computational principles of sensorimotor control that minimize uncertainty and variability. J Physiol 578: 387–396, 2007. doi: 10.1113/jphysiol.2006.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Artiles A, Karmali F. Vestibular precision at the level of perception, eye movements, posture, and neurons. Neuroscience 468: 282–320, 2021. doi: 10.1016/j.neuroscience.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakushin SB, Raphan T, Cohen B. Coding of velocity storage in the vestibular nuclei. Front Neurol 8: 386, 2017. doi: 10.3389/fneur.2017.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hain TC, Zee DS. Velocity storage in labyrinthine disorders. Ann N Y Acad Sci 656: 297–304, 1992. doi: 10.1111/j.1749-6632.1992.tb25216.x. [DOI] [PubMed] [Google Scholar]
- 11.Karmali F, Merfeld DM. A distributed, dynamic, parallel computational model: the role of noise in velocity storage. J Neurophysiol 108: 390–405, 2012. doi: 10.1152/jn.00883.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Y, Chari DA, Vasilijic S, Welling DB, Stankovic KM. New developments in neurofibromatosis type 2 and vestibular schwannomas. Neurooncol Adv 3: vdaa153, 2021. doi: 10.1093/noajnl/vdaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phy Ther 84: 906–918, 2004. doi: 10.1093/ptj/84.10.906. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg 116: 424–427, 1990. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 15.Fife TD, Tusa RJ, Furman JM, Zee DS, Frohman E, Baloh RW, Hain T, Goebel J, Demer J, Eviatar L. Assessment: vestibular testing techniques in adults and children: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 55: 1431–1441, 2000. doi: 10.1212/wnl.55.10.1431. [DOI] [PubMed] [Google Scholar]
- 16.van de Berg R, Rosengren S, Kingma H. Laboratory examinations for the vestibular system. Curr Opin Neurol 31: 111–116, 2018. doi: 10.1097/WCO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 17.Dimitri PS, Wall C 3rd, Oas JG. Classification of human rotation test results using parametric modeling and multivariate statistics. Acta Otolaryngol 116: 497–506, 1996. doi: 10.3109/00016489609137880. [DOI] [PubMed] [Google Scholar]
- 18.Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol 59: 370–393, 1988. doi: 10.1152/jn.1988.59.2.370. [DOI] [PubMed] [Google Scholar]
- 19.Stevens MN, Garrison DB, Kaylie DM. What is the potential clinical utility of vHIT when assessing adult patients with dizziness? Laryngoscope 127: 2689–2690, 2017. doi: 10.1002/lary.26774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Halmagyi GM. Video head impulse testing: from bench to bedside. Semin Neurol 40: 5–17, 2020. doi: 10.1055/s-0039-3402063. [DOI] [PubMed] [Google Scholar]
- 21.Cleworth TW, Carpenter MG, Honegger F, Allum JHJ. Differences in head impulse test results due to analysis techniques. J Vestib Res 27: 163–172, 2017. doi: 10.3233/VES-170614. [DOI] [PubMed] [Google Scholar]
- 22.Janky KL, Patterson J, Shepard N, Thomas M, Barin K, Creutz T, Schmid K, Honaker JA. Video head impulse test (vHIT): the role of corrective saccades in identifying patients with vestibular loss. Otol Neurotol 39: 467–473, 2018. doi: 10.1097/MAO.0000000000001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minor LB, Haslwanter T, Straumann D, Zee DS. Hyperventilation-induced nystagmus in patients with vestibular schwannoma. Neurology 53: 2158–2168, 1999. doi: 10.1212/wnl.53.9.2158. [DOI] [PubMed] [Google Scholar]
- 24.Lewis RF, Priesol AJ, Nicoucar K, Lim K, Merfeld DM. Dynamic tilt thresholds are reduced in vestibular migraine. J Vestib Res 21: 323–330, 2011. doi: 10.3233/VES-2011-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunaga T, Kanzaki J, Hosoda Y. Gliosis of the eighth nerve transitional region in patients with cerebellopontine angle schwannoma. Acta Otolaryngol 114: 393–398, 1994. doi: 10.3109/00016489409126076. [DOI] [PubMed] [Google Scholar]
- 26.Dilwali S, Landegger LD, Soares VY, Deschler DG, Stankovic KM. Secreted factors from human vestibular schwannomas can cause cochlear damage. Sci Rep 5: 18599, 2015. doi: 10.1038/srep18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walz PC, Bush ML, Robinett Z, Kirsch CF, Welling DB. Three-dimensional segmented volumetric analysis of sporadic vestibular schwannomas: comparison of segmented and linear measurements. Otolaryngol Head Neck Surg 147: 737–743, 2012. doi: 10.1177/0194599812447766. [DOI] [PubMed] [Google Scholar]
- 28.Ren Y, Hyakusoku H, Sagers JE, Landegger LD, Welling DB, Stankovic KM. MMP-14 (MT1-MMP) is a biomarker of surgical outcome and a potential mediator of hearing loss in patients with vestibular schwannomas. Front Cell Neurosci 14: 191, 2020. doi: 10.3389/fncel.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunniway HM, Welling DB. Intracranial tumors mimicking benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 118: 429–436, 1998. doi: 10.1177/019459989811800401. [DOI] [PubMed] [Google Scholar]
- 30.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci 9: 292–303, 2008. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Körding KP, Wolpert DM. Bayesian integration in sensorimotor learning. Nature 427: 244–247, 2004. doi: 10.1038/nature02169. [DOI] [PubMed] [Google Scholar]
- 32.Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol 93: 643–655, 2005. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- 33.Knill DC, Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci 27: 712–719, 2004. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- 35.Paige GD. Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. II. Response characteristics and plasticity following unilateral inactivation of horizontal canal. J Neurophysiol 49: 152–168, 1983. doi: 10.1152/jn.1983.49.1.152. [DOI] [PubMed] [Google Scholar]
- 36.Klaus MP, Schöne CG, Hartmann M, Merfeld DM, Schubert MC, Mast FW. Roll tilt self-motion direction discrimination training: First evidence for perceptual learning. Atten Percept Psychophys 82: 1987–1999, 2020. doi: 10.3758/s13414-019-01967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann M, Furrer S, Herzog MH, Merfeld DM, Mast FW. Self-motion perception training: thresholds improve in the light but not in the dark. Exp Brain Res 226: 231–240, 2013. doi: 10.1007/s00221-013-3428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins JJ. The effects of stochastic galvanic vestibular stimulation on human postural sway. Exp Brain Res 124: 273–280, 1999. doi: 10.1007/s002210050623. [DOI] [PubMed] [Google Scholar]
- 39.Clark TK, Galvan-Garza RC., Bermudez Rey MC, Yi Y, Merfeld DM. . Perceptual noise and sensorimotor adaptation. In: NASA Human Research Program Investigator’s Workshop, Galveston TX, 2015. [Google Scholar]
- 40.Lee TL, Shayman CS, Oh Y, Peterka RJ, Hullar TE. Reliability of vestibular perceptual threshold testing about the yaw axis. Ear Hear 41: 1772–1774, 2020. doi: 10.1097/AUD.0000000000000859. [DOI] [PubMed] [Google Scholar]
- 41.Yip CW, Strupp M. The dizziness handicap inventory does not correlate with vestibular function tests: a prospective study. J Neurol 265: 1210–1218, 2018. doi: 10.1007/s00415-018-8834-7. [DOI] [PubMed] [Google Scholar]
- 42.Lewis RF, Carey JP. Images in clinical medicine. Abnormal eye movements associated with unilateral loss of vestibular function. N Engl J Med 355: e26, 2006. doi: 10.1056/NEJMicm031134. [DOI] [PubMed] [Google Scholar]
- 43.Curthoys IS, Halmagyi GM. Vestibular compensation. Adv Otorhinolaryngol 55: 82–110, 1999. doi: 10.1159/000059059. [DOI] [PubMed] [Google Scholar]
- 44.Staab JP, Eckhardt-Henn A, Horii A, Jacob R, Strupp M, Brandt T, Bronstein A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestib Res 27: 191–208, 2017. doi: 10.3233/VES-170622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herdman D, Norton S, Pavlou M, Murdin L, Moss-Morris R. Vestibular deficits and psychological factors correlating to dizziness handicap and symptom severity. J Psychosom Res 132: 109969, 2020. doi: 10.1016/j.jpsychores.2020.109969. [DOI] [PubMed] [Google Scholar]
- 46.Yates BJ, Bolton PS, Macefield VG. Vestibulo-sympathetic responses. Compr Physiol 4: 851–887, 2014. doi: 10.1002/cphy.c130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimitri PS, Wall C 3rd, Oas JG, Rauch SD. Application of multivariate statistics to vestibular testing: discriminating between Menière’s disease and migraine associated dizziness. J Vestib Res 11: 53–65, 2001. [PubMed] [Google Scholar]
- 48.Maire R, van Melle G. Vestibulo-ocular reflex characteristics in patients with unilateral Ménière's disease. Otol Neurotol 29: 693–698, 2008. doi: 10.1097/MAO.0b013e3181776703. [DOI] [PubMed] [Google Scholar]
- 49.Laurens J, Droulez J. Bayesian processing of vestibular information. Biol Cybern 96: 389–404, 2007. [Erratum in Biol Cybern 96: 405, 2007]. doi: 10.1007/s00422-006-0133-1. [DOI] [PubMed] [Google Scholar]
- 50.Cousins S, Kaski D, Cutfield N, Seemungal B, Golding JF, Gresty M, Glasauer S, Bronstein AM. Vestibular perception following acute unilateral vestibular lesions. PLoS One 8: e61862, 2013. doi: 10.1371/journal.pone.0061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res 90: 526–538, 1992. doi: 10.1007/BF00230935. [DOI] [PubMed] [Google Scholar]
- 52.Davis LE. Viruses and vestibular neuritis: review of human and animal studies. Acta Otolaryngol Suppl 503: 70–73, 1993. doi: 10.3109/00016489309128077. [DOI] [PubMed] [Google Scholar]
- 53.Schwaber MK, Whetsell WO. Cochleovestibular nerve compression syndrome. II. Vestibular nerve histopathology and theory of pathophysiology. Laryngoscope 102: 1030–1036, 1992. doi: 10.1288/00005537-199209000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Beylergil SB, Karmali F, Wang W, Bermúdez Rey MC, Merfeld DM. Vestibular roll tilt thresholds partially mediate age-related effects on balance. Prog Brain Res 248: 249–267, 2019. doi: 10.1016/bs.pbr.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Karmali F, Bermúdez Rey MC, Clark TK, Wang W, Merfeld DM. Multivariate analyses of balance test performance, vestibular thresholds, and age. Front Neurol 8: 578, 2017. [Erratum in Front Neurol 11: 556797, 2020]. doi: 10.3389/fneur.2017.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zobeiri OA, Mischler GM, King SA, Lewis RF, Cullen KE. Effects of vestibular neurectomy and neural compensation on head movements in patients undergoing vestibular schwannoma resection. Sci Rep 11: 517, 2021. doi: 10.1038/s41598-020-79756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res 210: 407–422, 2011. doi: 10.1007/s00221-011-2568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baguley DM, Jones SEM, Moffat DA. A small vestibular schwannoma arising from the inferior vestibular nerve. J Laryngol Otol 117: 498–500, 2003. doi: 10.1258/002221503321892398. [DOI] [PubMed] [Google Scholar]
- 59.Priesol AJ, Cao M, Brodley CE, Lewis RF. Clinical vestibular testing assessed with machine-learning algorithms. JAMA Otolaryngol Head Neck Surg 141: 364–372, 2015. doi: 10.1001/jamaoto.2014.3519. [DOI] [PubMed] [Google Scholar]
- 60.Mackrous I, Carriot J, Cullen KE, Chacron MJ. Neural variability determines coding strategies for natural self-motion in macaque monkeys. eLife 9: e57484, 2020. doi: 10.7554/eLife.57484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamali M, Chacron MJ, Cullen KE. Self-motion evokes precise spike timing in the primate vestibular system. Nat Commun 7: 13229, 2016. doi: 10.1038/ncomms13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nouri S, Karmali F. Variability in the vestibulo-ocular reflex and vestibular perception. Neuroscience 393: 350–365, 2018. doi: 10.1016/j.neuroscience.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King S, Priesol AJ, Davidi SE, Merfeld DM, Ehtemam F, Lewis RF. Self-motion perception is sensitized in vestibular migraine: pathophysiologic and clinical implications. Sci Rep 9: 14323, 2019. doi: 10.1038/s41598-019-50803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterka RJ. Sensory integration for human balance control. Handb Clin Neurol 159: 27–42, 2018. doi: 10.1016/B978-0-444-63916-5.00002-1. [DOI] [PubMed] [Google Scholar]
- 65.Brandt T. Phobic postural vertigo. Neurology 46: 1515–1519, 1996. doi: 10.1212/wnl.46.6.1515. [DOI] [PubMed] [Google Scholar]
- 66.Karmali F, Chaudhuri SE, Yi Y, Merfeld DM. Determining thresholds using adaptive procedures and psychometric fits: evaluating efficiency using theory, simulations, and human experiments. Exp Brain Res 234: 773–789, 2016. doi: 10.1007/s00221-015-4501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]