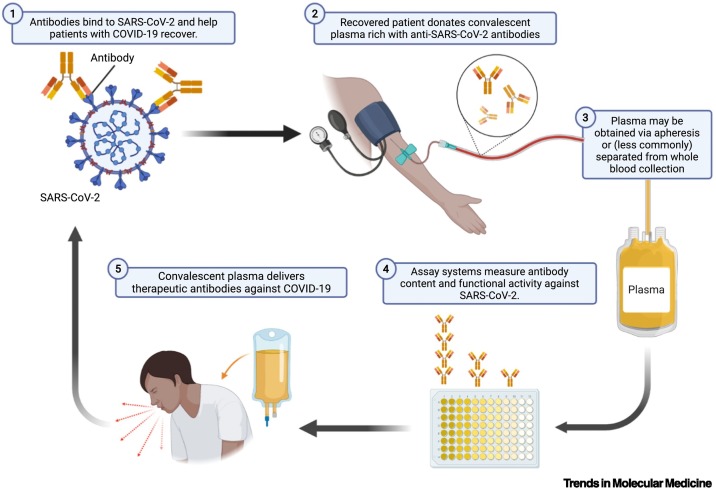
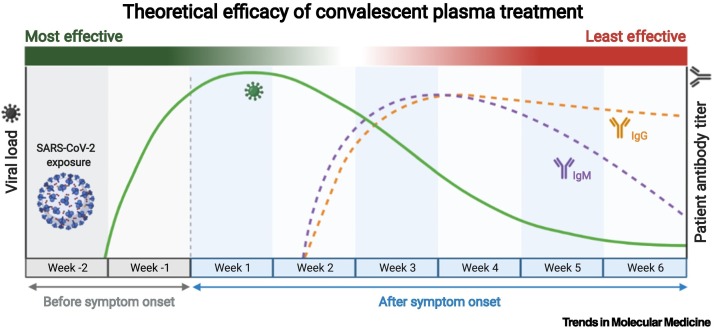
Coronavirus disease 2019 (COVID-19) revived interest in convalescent plasma (CP) as a therapeutic option against novel pathogens. CP therapy involves the administration of antibodies against a pathogen [e.g., severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] to prevent or treat an infectious disease, and has been used periodically for more than a century. Historical experiences suggest that CP therapy must be given early in disease course and contain sufficient, specific antibody content to obtain the best results. Antibodies in CP mediate their therapeutic effect through a variety of mechanisms, including: (i) viral neutralization, (ii) antibody-dependent cellular cytotoxicity, and (iii) phagocytosis.
CP leverages established blood collection and transfusion infrastructure globally, even in resource-limited settings. Apheresis allows for collection of plasma (containing proteins, coagulants, and immunoglobulins) and returns red blood cells back to the donor. Generally, CP donors must satisfy eligibility criteria for community blood donations and be recovered from COVID-19.
ADVANTAGES:
High titer, functional, polyclonal antibodies in CP can neutralize a broad range of SARS-CoV-2 variants and mutations.
CP therapy is safe with similar risks to plasma transfusion.
The potential benefits of CP are most apparent in patients with immunosuppression (from disease or treatment) and in patients treated early in disease course.
CHALLENGES:
The immunological profile of CP contains a wide distribution of antibody isotypes and subclasses raising concerns about standardization, optimal dosing, and potency.
Several aspects of the antibody profile are not fully understood, including assay systems to determine antibody profile and therapeutic target levels to confer protective immunity.
Recruitment and screening of potential CP donors pose logistical and regulatory challenges.
Potential adverse events of CP therapy include known risks associated with transfer of blood substances and theoretical risk of antibody-dependent enhancement of infection.
APPLICATIONS:
Transfusion of CP with high-titer, functional antibodies early in COVID-19 disease course may reduce hospitalization and risk of death.
Widespread access to CP for novel infectious diseases is feasible and was authorized by the US FDA during the COVID-19 pandemic, via an expanded access programi , ii and subsequent emergency use authorizationiii – v.
CP should be considered as a potential treatment in future infectious disease outbreaks.
Acknowledgments
Acknowledgments
The figures were generated with Biorender.com
Declaration of interests
No interests are declared.
Resources
ihttps://ccpp19.org/iiwww.uscovidplasma.orgiiiwww.fda.gov/media/141477/downloadivwww.fda.gov/media/141478/downloadvwww.fda.gov/media/141480/downloadLiterature
- 1.Bloch E.M., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A., et al. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner M.J., et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N. Engl. J. Med. 2021;384:1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury D.S., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 5.Klassen S.A., et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin. Proc. 2021;96:1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunze K.L., et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat. Commun. 2021;12:4864. doi: 10.1038/s41467-021-25113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ripoll J.G., et al. Convalescent plasma for infectious diseases: historical framework and use in COVID-19. Clin. Microbiol. Newsl. 2021;43:23–32. doi: 10.1016/j.clinmicnews.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senefeld J.W., et al. Access to and safety of COVID-19 convalescent plasma in the United States Expanded Access Program: a national registry study. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamatatos L., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021 doi: 10.1126/science.abg9175. Published online March 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson M.A. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. 2021;7:1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]