Abstract
Mycobacterium bovis has the broadest host range of species in the Mycobacterium tuberculosis complex and is responsible for disease in humans and diverse animal species. We report on genotypic differences at multiple loci among 13 isolates derived from a range of human and animal infections. All isolates were classified as M. bovis by phenotypic analysis but could be subdivided into five distinct genotypes based on polymorphisms at the pncA and oxyR loci, the status of the RD5 deletion region, and the spoligotype pattern. These findings suggest the existence of a spectrum of strains with genotypic characteristics between those of M. tuberculosis and M. bovis.
Tuberculosis is caused by Mycobacterium tuberculosis, a slow-growing acid-fast bacterium which shares microbiological and biochemical characteristics with M. bovis. Together with other closely related organisms (M. microti, M. africanum, and M. canetti), these bacteria are referred to as the M. tuberculosis complex. M. bovis has the broadest host range of any member of the complex, causing disease in a wide range of mammals, including humans (21).
Members of the complex are traditionally characterized on the basis of a set of tests of microbiological and biochemical phenotypes, but these are time-consuming and consequently not always performed. Differentiation and drug susceptibility testing of M. tuberculosis complex members solely by conventional means may be complicated by the slow growth of the organisms in culture and their propensity to change growth characteristics (7). Species identification may sometimes be difficult with multiple-drug-resistant organisms; a nosocomial outbreak of multiple-drug-resistant M. tuberculosis was originally attributed incorrectly to M. bovis on the basis of phenotype (3, 14), which emphasises the benefits of genotyping methods, and indeed, a growing list of these is available.
Polymorphisms that have been used to discriminate between M. bovis and M. tuberculosis include single base changes in the pyrazinamidase gene (pncA) and in the oxyR pseudogene. M. bovis strains customarily contain guanine at position 169 in the pncA gene, and M. tuberculosis isolates contain cytosine at the same position. (23). This polymorphism renders the majority of M. bovis isolates resistant to treatment with pyrazinamide. The oxyR pseudogene in M. bovis is characterized by adenine at position 285, and M. tuberculosis is characterized at the same position by guanine (24).
In the classification scheme proposed for the division of the members of the tuberculosis complex into three major groups defined by single nucleotide polymorphisms in the catalase (katG463)- and gyrase A (gyrA95)-encoding genes (25), M. bovis (and M. microti, M. africanum, and some M. tuberculosis isolates) displays polymorphisms characteristic of group I, having the genotype katG463(Leu) gyrA95 (Thr). M. bovis and group I M. tuberculosis isolates also show greater differences in the oxyR pseudogene, which has been advanced as evidence for the ancestral nature of group I organisms (25).
M. bovis and M. bovis BCG vaccine strains have undergone a series of genomic deletion events compared to M. tuberculosis reference strains (9). M. microti and M. africanum appear to fall between the M. bovis and BCG strains and the reference strains in their deletion patterns (9). Associated with one of these deletions, namely, RD5, is the mtp40 element, a 3-kb fragment which encodes a section of a phospholipase C enzyme (9). This fragment is missing from the majority of M. bovis isolates and provides the basis for a PCR to distinguish M. tuberculosis from M. bovis (17).
Differences in the direct repeat (DR) locus, which are exploited by the DNA fingerprinting technique of spoligotyping, have also been used to discriminate between members of the M. tuberculosis complex as well as between host-specific strains of M. bovis (5, 15, 26). M. bovis characteristically lacks spacers 39 to 43 in the spoligotype system. Compared to other members of the M. tuberculosis complex, M. microti has a much smaller number of variable spacers in the DR region (K. Kremer, D. van Soolingen, J. van Embden, S. Hughes, J. Inwald, and G. Hewinson, Letter, J. Clin. Microbiol. 36:2793–2794, 1998).
Reports of studies applying biomolecular assays have occasionally described strains with unusual genotypic profiles between those of M. tuberculosis and M. bovis. One variant isolated from seals and other pinnipeds (22, 26) displays pncA and oxyR polymorphisms identical to those of M. tuberculosis but shares other biochemical and genetic properties with M. bovis. Caprine strains also appear to occupy an intermediate genotypic position, with pncA169 (C), typical of M. tuberculosis, and oxyR285 (A), characteristic of M. bovis (1, 6, 13). These strains also possess multiple copies of the repetitive element IS6110, a characteristic generally associated with M. tuberculosis (12, 27). These subtypes of M. bovis carry a zoonotic risk of disease for humans (13, 27), which adds clinical importance to their pyrazinamide-susceptible phenotype (20).
The present study, using a panel of diverse M. bovis isolates of human and animal origins, combines a number of these approaches and was undertaken to assess molecular assays as diagnostic tools as well as the extent of genotypic diversity in this taxon.
M. bovis BCG Pasteur and the H37Rv isolate of M. tuberculosis were used as the reference strains. A total of seven M. tuberculosis complex strains, including some from exotic species, were provided by the Veterinary Laboratory Agency (VLA), New Haw, Surrey United Kingdom. The strains were chosen to typify infections from geographic hot spot regions of bovine tuberculosis in the United Kingdom and from unrelated outbreaks outside these areas (Table 1). Thus, bovine and badger isolates were from cases in Cornwall, United Kingdom, the fur seal isolate was from an outbreak at a zoo in southern Britain, and the goat and llama isolates were from mid Wales, United Kingdom.
TABLE 1.
Available culture and biochemical data for veterinary isolates of M. bovis
| Animal isolatea | Growth onb:
|
Sensitivity toc:
|
Preferred media for growthd | |||
|---|---|---|---|---|---|---|
| Stonebrinks medium | 7H11 medium | Sodium salicylate | TCH | Pza | ||
| Cow | − | ++ | NDe | ND | ND | Pyr > Glu |
| Badger 1 | ND | ND | ND | ND | ND | ND |
| Badger 2 | ND | ND | S | S | R | Pyr > Gly |
| Goat | ++ | +++ | S | S | R | Pyr > Gly |
| Cat | ND | ND | ND | ND | ND | Pyr > Gly |
| Fur seal | ND | − | S | S | ND | Gly > Pyr |
| Llama | ++ | ++ | S | S | R | Pyr > Gly |
All isolates were microaerophilic.
−, no growth; ++, 10 to 20 colonies; +++, >20 colonies; ND, not determined.
Pza, pyrazinamide; R, resistant; S, sensitive.
Pyr, pyruvate; Gly, glycerol.
ND, not determined.
Six isolates of M. bovis strains that cause human disease were obtained from the Public Health Laboratory Service at Dulwich, London, United Kingdom (Table 2). Species identification by standard biochemical assays was performed at each source laboratory (4).
TABLE 2.
Clinical details of six patients from whom M. bovis was cultured and phenotypic strain data
| Patient | Sex | Age (yrs) | Site of infection | Strain oxygen requirementd | Strain sensitivity toa:
|
Preferred media for growthb | |
|---|---|---|---|---|---|---|---|
| TCH | Pza | ||||||
| 1 | Male | 56 | Lung | M | S | R | Pyr > Gly |
| 2 | Female | 63 | Lung | M | S | R | Pyr >> Gly |
| 3 | Female | —c | Endometrium | M | S | R | Pyr > Gly |
| 4 | Female | 88 | Vertebra | M | S | R | Pyr > Gly |
| 5 | Male | 63 | Urinary tract | M | S | R | Pyr > Gly |
| 6 | Female | 76 | Lung | M | S | R | Pyr > Gly |
Pza, pyrazinamide; R, resistant; S, sensitive.
Pyr, pyruvate; Gly, glycerol.
—, unknown.
M, Microaerophilic.
DNA was extracted by a standard lysozyme-proteinase K-phenol chloroform procedure, followed by ethanol precipitation (10). To maximize the integrity of the genomic DNA, cultures were heat killed by boiling for 5 min at 100°C (2).
Genotyping of the various M. bovis isolates was performed by using PCR assays directed towards a series of loci with defined specificity patterns. PCR was performed as described previously, with a number of measures taken to avoid contamination (18). All oligonucleotide primers were obtained from Sigma-Genosys Ltd., Cambridge, United Kingdom. Table 3 lists the sequences of these primers and the parameters of the various methods used. Gel electrophoretic analysis of PCR products and automated DNA sequencing were performed as previously reported (18).
TABLE 3.
Primer sequences and details of PCR methods used in genotyping of M. bovis isolates
| Locus (accession no.) | Primer sequencesa | PCR product size (bp) | [MgCl2] (mM) | PCR parameters (temps and times) | No. of cycles |
|---|---|---|---|---|---|
| rpoB1 | F1, 5′-CAGGACGTSGAGGCGATCAC-3′; R1, 5′-ACRTGGCGGTCCTCCTCGTC-3′ | 401 | 1.25 | 94°C, 10 s; 65°C, 30 s; 72°C, 15 s | 35 |
| rpoB2 (L27989) | F2, 5′-GACGAGGAGGACCGCCARGT-3′; R2, 5′-TTTCTTGCGACGACGACGTC-3′ | 337 | 1.25 | 94°C, 10 s; 65°C, 30 s; 72°C, 15 s | 35 |
| 16S rRNA | F, 5′-GRGRTACTCGAGTGGCGAAC-3′; R, 5′-GGCCGGCTACCCGTCGTC-3′ | 208 | 1.5 | 94°C, 10 s; 62°C, 30 s; 72°C, 15 s | 35 |
| mtp40 (M57952) | F, 5′-CTGGTCGAATTCGGTGGA-3′; R, 5′-ATGGTCTCCGACACGTTCGAC-3′ | 152 | 1.5 | 94°C, 10 s; 62°C, 30 s; 72°C, 15 s | 32 |
| pncA (U59967) | F, 5′-ATCAGCGACTACCTGGCCGA-3′; R, 5′-GATTGCCGACGTGTCCAGAC-3′ | 180 | 1.0 | 94°C, 20 s; 66°C, 30 s; 72°C, 30 s | 32 |
| oxyR (U16243) | F, 5′-CGCGCTGTCAGAGCTGACTTT-3′; R, 5′-TCTGCGGAATCAGTGTCACC-3′ | 150 | 2.0 | 94°C, 20 s; 66°C, 30 s; 72°C, 30 s | 32 |
| katG (X68081) | F, 5′-TCAGCCACGACCTCGTCGG-3′; R, 5′-AGGCGGATGCGACCACCGTT-3′ | 163 | 2.0 | 94°C, 40 s; 64°C, 30 s; 72°C, 20 s | 35 |
| gyrA (L27512) | F, 5′-CGAGACCATGGGCAACTACCA-3′; R, 5′-ATTGCCTGGCGAGCCGAA-3′ | 131 | 1.0 | 94°C, 40 s; 64°C, 30 s; 72°C, 20 s | 35 |
| RD7 | F, 5′-ACTTCAGTGCTGGTTCGTGG-3′; R, 5′-ATCTTGCGGCCCAATGAATC-3′ | 211 | 2.0 | 94°C, 20 s; 66°C, 30 s; 72°C, 20 s | 35 |
F, forward; R, reverse. Nucleotides: R, A+G; S, G+C.
The mycobacterial genomic regions amplified were the RNA polymerase beta gene (rpoB), a hypervariable region of the 16S rRNA gene (16), the pyrazinamidase gene (pncA), and the oxyR pseudogene (oxyR), which contains species-defining polymorphisms (6, 23, 24). Analysis of katG463 and gyrA95 for grouping was performed according to the Sreevatsan model (25). The isolates were examined for the presence or absence of the mtp40 element in the plcA gene. Additionally, primers spanning deletion region 7 (RD7) (9) were designed to amplify a PCR product from strains which have undergone this event. For consistency, we have adopted the numerical classification of deletion regions proposed by Gordon and colleagues (9). Spoligotyping (15) was used for further strain identification.
All clinical and veterinary isolates showed microbiological and biochemical characteristics typical of M. bovis, including resistance to pyrazinamide, susceptibility to thiophene-2-carboxylic acid hydrazide (TCH), and preferential growth on media containing pyruvate (Tables 1 and 2).
The results from screening a panel of M. bovis isolates of human and animal origin by using a range of genotypic criteria emphasize the complex phylogeny of such strains. In all, five different genotypes were distinguished among the 13 isolates (Table 4).
TABLE 4.
M. bovis isolates with intermediate genotypea
| Genotype identification | Result for genotypic criterionb
|
Isolate and/or source(s) | ||||
|---|---|---|---|---|---|---|
| pncA | oxyR | RD5 | RD7 | Spoligotype | ||
| M. bovis | B | B | − | − | B | M. bovis BCG, badger 1, cow, cat, Ilama, patients 2 to 6 |
| Strain with intermediate genotypes | B | B | + | − | B | Goat |
| T | B | − | − | B | Goatc | |
| T | T | + | − | B | Fur seal | |
| T | T | + | − | B | Fur seald | |
| T | T | + | − | M | Badger 2 | |
| T | T | − | − | T | Patient 1 | |
| M. tuberculosis | T | T | + | − | T | M. tuberculosis H37Rv |
Molecular characteristics of the H37Rv strain and previously reported genotypes of caprine and fur seal isolates are included for comparison.
T, M. tuberculosis genotype; B, M. bovis genotype; M, M. microti genotype; +, region present; −, region deleted.
Previously reported caprine isolate (1).
Previously reported fur seal isolates (27).
As expected, all of the isolates had the katG463 (CTG) gyrA95 (ACC) genotype characteristic of a group 1 organism (Table 5) (25). The M. tuberculosis H37Rv reference strain showed the predicted group 3 genotype. Furthermore, all the isolates as well as the reference strain (M. bovis BCG) generated the expected 211-bp product characteristic of the RD7 deletion event (9). As anticipated, no product was obtained from the M. tuberculosis laboratory strain H37Rv.
TABLE 5.
M. bovis genotyping: summary of main findings from veterinary, human, and reference strains
| Sample or straina | Genotypic resultb
|
Spoligotyping patternc | |||||
|---|---|---|---|---|---|---|---|
| katG463 | gyrA95 | pncA169 | oxyR285 | RD5 (mtp40) | RD7 | ||
| VLA isolates | |||||||
| Cow | CTG | ACC | G | A | − | − | M. bovis (9) |
| Badger 1 | CTG | ACC | G | A | − | − | M. bovis (9) |
| Badger 2 | CTG | ACC | C | G | + | − | M. microti (32) |
| Goat | CTG | ACC | G | A | + | − | M. bovis (22) |
| Cat | CTG | ACC | G | A | − | − | M. bovis (novel) |
| Fur seal | CTG | ACC | C | G | + | − | M. bovis (46) |
| Llama | CTG | ACC | G | A | − | − | M. bovis (22) |
| PHLS human isolates | |||||||
| 1 | CTG | ACC | C | G | − | − | M. tuberculosis |
| 2 | CTG | ACC | G | A | − | − | M. bovis |
| 3 | CTG | ACC | G | A | − | − | M. bovis |
| 4 | CTG | ACC | G | A | − | − | M. bovis |
| 5 | CTG | ACC | G | A | − | − | M. bovis |
| 6 | CTG | ACC | G | A | − | − | M. bovis |
| Reference strains | |||||||
| H37Rv | CGG | AGC | C | G | + | + | M. tuberculosis |
| M. bovis BCG | CTG | ACC | G | A | − | − | M. bovis |
PHLS, Public Health Laboratory Service.
+, region present; −, region deleted.
Numbers in parentheses refer to VLA pattern numbers.
Diverse patterns were seen in the pncA and oxyR loci. Five of the veterinary isolates had the pncA169 (G)-oxyR285 (A) genotype characteristic of M. bovis (Table 5). Typifying these, and consistent with their derivation from a single outbreak, the bovine and badger 1 isolates were identical according to all the criteria used. The strain is AF 2122/97, which was recently sequenced at the Sanger Centre and is now awaiting full description and commentary.
The typical M. bovis group included the feline isolate, which is a finding in contrast to those of previous reports of the acquisition of M. microti infections by cats through the hunting and consumption of small rodents (11).
Two isolates (from badger 2 and a fur seal) were found to have the M. tuberculosis pncA169 (C)-oxyR285 (G) genotype.
Three different patterns were observed among the animal isolates with genotypes between those of M. tuberculosis and M. bovis. Unexpectedly, three of the veterinary strains generated positive PCR products for mtp40, which is generally missing from M. bovis. These strains included the two isolates with the M. tuberculosis pncA169 (C)-oxyR285 (G) genotype as well as the caprine isolate which typifies M. bovis at these loci. Unlike the strains isolated from Spanish goats (1, 20), our goat isolate demonstrated the M. bovis-resistant allele pncA169 (G). This strain, originally isolated from a goat in mid Wales in 1996, shared a spoligotype identical with that recovered from a captive llama in Gwent, South Wales, United Kingdom, in 1999. The two strains differed in their mtp40 status (Table 5). This finding illustrates the fact that spoligotyping is limited in its ability to differentiate between epidemiologically related strains; identical spoligotypes do not exclude differences at other loci (19).
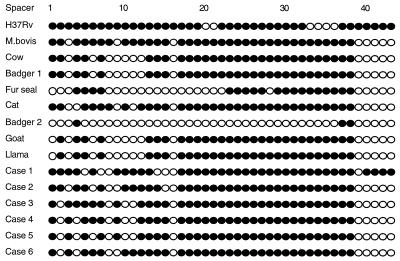
As found previously, the fur seal isolate retained the RD5-associated mtp40 element and showed polymorphisms at the pncA and oxyR loci characteristic of M. tuberculosis (22, 27). In contrast to the RD5 region, which was retained, the RD7 region had been deleted from all the isolates analyzed, including that from the fur seal, which suggests that this deletion event may have occurred early in the evolution of the M. tuberculosis complex. Undoubtedly, knowledge of deletion region subsets in M. bovis strains will play an increasingly important part in understanding the phenotype, pathogenesis, and evolution of individual isolates. The seal strain showed a typical M. bovis spoligotype in that it lacked spacers 39 to 43 in the DR region (Fig. 1). With minor differences, the pattern was very similar to the two examples previously recorded for seals (27).
FIG. 1.
Spoligotyping hybridization patterns obtained with animal and human isolates of M. bovis. The M. tuberculosis strain H37Rv and M. bovis BCG patterns are shown for reference. Filled and open circles indicate the presence and absence of spacers, respectively.
The spoligotype for badger 2 was characteristic of M. microti. The pattern is distinctive, with hybridization to only 3 of the possible 43 spacers of the DR region (spacers 4, 37, and 38) (Fig. 1). This pattern was previously found in a cat and a cow from the West Country, United Kingdom, is consistent with the transfer of disease between these species, and has been reported as part of a study of isolates from the United Kingdom and Holland (Kremer et al., letter). A second, similar pattern found in voles shows hybridization only to spacers 37 and 38 (Kremer et al., letter).
Five of the six human isolates and four of the animal isolates had classic M. bovis profiles in that they possessed the pyrazinamide-resistant pncA allele and lacked the RD5 region and the 3′ spoligotype spacers. The spoligotype patterns of these human isolates did not match any patterns of the animal isolates or any patterns in the M. bovis database kept at the VLA. Considering the ages of the patients (Table 2), it is likely that disease was the result of the reactivation of an infection acquired earlier in life.
In marked contrast, the isolate from patient 1 (Table 2) showed a pattern immediately suggestive of M. tuberculosis because of the retention of 3′-end spacers 40 to 43 (Fig. 1). This isolate was cultured from a patient of Indian origin who had been living in England for over 40 years and who presented with pulmonary tuberculosis. In spite of having the pncA169 (C) genotype, this strain displayed a pyrazinamidase-resistant phenotype in microbiological assays and deletion of the RD5 and RD7 regions, consistent with M. bovis. Spoligotyping data compiled on disease-producing strains of M. bovis in the United Kingdom have revealed very occasionally the presence of spacers in the 3′ spoligotype region; to date, a total of three other isolates with two or more spacers have been recorded (VLA, unpublished observations). Strains possessing spacers 4 and 5 of these 3′-terminal spacers have also been reported as appearing in isolates from patients born in Asia (5). Patient isolate 24 as described by this group (5) resembled patient isolate 1 in the present study in being negative for the mtp40 fragment.
Finally, the rpoB and 16S rRNA sequences were screened for differences as described in an earlier study of various mycobacterial species (8). Among the atypical mycobacterial species examined, intraspecies diversity in these genes was highest in M. smegmatis and M. gordonae (8). The study recorded a lack of genetic diversity among 63 M. tuberculosis isolates but did not include M. bovis or other members of the complex. Despite the diverse origins of the M. bovis isolates in the present study, no differences were found in either 738 bp of the rpoB gene or 208 bp of a hypervariable region of the 16S rRNA region, with all isolates displaying sequences identical to those of the reference strains. These findings therefore extend the observations made by Gingeras et al. (8) to members of the M. bovis lineage and are consistent with the lack of variation at these loci in the M. tuberculosis complex.
It was anticipated that the selection of strains from different animal sources might help uncover associations between genotype and host specificity in addition to exploring the scope of genotypic diversity. Our results are broadly consistent with those of previous reports which found that strains with a genotype between those of M. tuberculosis and M. bovis are often implicated in infections of fur seals and goats, but more studies at the molecular level are needed to confirm this. Investigations with multiple genetic markers can confound expectations: the cat in our study was not infected with M. microti, as might be expected, whereas one of the badgers was; the caprine subtype was not the cause of disease in the goat. Thus, while the current range of genotypic markers provides a useful resource for studying evolution within the M. tuberculosis complex, these basic observations suggest that they are probably not directly linked to the genetic determinants underlying host specificity.
Acknowledgments
This work was supported in part by a grant from MAFF (Ministry of Agriculture, Food and Fisheries), Whitehall, London, England.
REFERENCES
- 1.Aranaz A, Liebana E, Gomez-Mampaso E, Galán J C, Cousins D, Ortega A, Blázquez J, Baquero F, Mateos A, Suarez G, Dominguez L. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Bacteriol. 1999;49:1263–1273. doi: 10.1099/00207713-49-3-1263. [DOI] [PubMed] [Google Scholar]
- 2.Bemer-Melchior P, Drugeon H B. Inactivation of Mycobacterium tuberculosis for DNA typing analysis. J Clin Microbiol. 1999;37:2350–2351. doi: 10.1128/jcm.37.7.2350-2351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvet E, Casalino E, Mendoza-Sassi G, Lariven S, Vallée E, Pernet M, Gottot S, Vachon F. A nosocomial outbreak of multidrug-resistant Mycobacterium bovis among HIV-infected patients. A case-control study. AIDS. 1993;7:1453–1460. doi: 10.1097/00002030-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Collins C H, Grange J M, Yates M D. Tuberculosis bacteriology: organization and practice. 2nd ed. London, England: Butterworth-Heinemann; 1997. [Google Scholar]
- 5.Cousins D V, Williams S N, Dawson D J. Tuberculosis due to Mycobacterium bovis in the Australian population: DNA typing of isolates, 1970–1994. Int J Tuberc Lung Dis. 1999;3:722–731. [PubMed] [Google Scholar]
- 6.Espinosa de los Monteros L E, Galán J C, Gutiérrez M, Samper S, García Marín J F, Martín C, Domínguez L, de Rafael L, Baquero F, Gomez-Mampaso E, Blázquez J. Allele-specific PCR method based on pncA and oxyR sequences for distinguishing Mycobacterium bovis from Mycobacterium tuberculosis: intraspecific M. bovis pncA sequence polymorphism. J Clin Microbiol. 1998;36:239–242. doi: 10.1128/jcm.36.1.239-242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillespie J, Barton L L, Rypka E W. Phenotypic changes in mycobacteria grown in oxygen-limited conditions. J Med Microbiol. 1986;21:251–255. doi: 10.1099/00222615-21-3-251. [DOI] [PubMed] [Google Scholar]
- 8.Gingeras T R, Ghandour G, Wang E, Berno A, Small P M, Drobniewski F, Alland D, Desmond E, Holodniy M, Drenkow J. Simultaneous genotyping and species identification using hybridization pattern recognition analysis of genetic Mycobacterium DNA arrays. Genome Res. 1998;8:435–448. doi: 10.1101/gr.8.5.435. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S V, Brosch R, Billault A, Garnier T, Eiglemeier K, Cole S T. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol. 1999;32:643–655. doi: 10.1046/j.1365-2958.1999.01383.x. [DOI] [PubMed] [Google Scholar]
- 10.Goyal M, Shaw R J, Banerjee D K, Coker R J, Robertson B D, Young D B. Rapid detection of multidrug-resistant tuberculosis. Eur Respir J. 1997;10:1120–1124. doi: 10.1183/09031936.97.10051120. [DOI] [PubMed] [Google Scholar]
- 11.Gunn-Moore D A, Jenkins P A, Lucke V M. Feline tuberculosis: a literature review and discussion of 19 cases by an unusual mycobacterial variant. Vet Rec. 1996;138:53–58. doi: 10.1136/vr.138.3.53. [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez M, Samper S, Gavigan J-A, García Marín J F, Martin C. Differentiation by molecular typing of Mycobacterium bovis strains causing tuberculosis in cattle and goats. J Clin Microbiol. 1995;33:2953–2956. doi: 10.1128/jcm.33.11.2953-2956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez M, Samper S, Jiménez M S, van Embden J D A, García Marin J F, Martín C. Identification by spoligotyping of a caprine genotype in Mycobacterium bovis strains causing human tuberculosis. J Clin Microbiol. 1997;35:3328–3330. doi: 10.1128/jcm.35.12.3328-3330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez M C, Galán J C, Blázquez J, Bouvet E, Vincent V. Molecular markers demonstrate that the first described multidrug-resistant Mycobacterium bovis outbreak was due to Mycobacterium tuberculosis. J Clin Microbiol. 1999;37:971–975. doi: 10.1128/jcm.37.4.971-975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kox L F F, van Leeuwen J, Knijper S, Jansen H M, Kolk A H J. PCR assay based on DNA coding for 16S rRNA for detection and identification of mycobacteria in clinical samples. J Clin Microbiol. 1995;33:3225–3233. doi: 10.1128/jcm.33.12.3225-3233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liébana E, Aranaz A, Francis B, Cousins D. Assessment of genetic markers for species differentiation within the Mycobacteria tuberculosis complex. J Clin Microbiol. 1996;34:933–938. doi: 10.1128/jcm.34.4.933-938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mays S A, Taylor G M, Legge A J, Young D B, Turner-Walker G. A paleopathological and biomolecular study of tuberculosis in a Medieval skeletal collection from England. Am J Phys Anthropol. 2001;114:298–311. doi: 10.1002/ajpa.1042. [DOI] [PubMed] [Google Scholar]
- 19.Niemann S, Richter E, Rüsch-Gerdes S. Stability of Mycobacterium tuberculosis IS6110 restriction fragment length polymorphism patterns and spoligotypes determined by analyzing serial isolates from patients with drug-resistant tuberculosis. J Clin Microbiol. 1999;37:409–412. doi: 10.1128/jcm.37.2.409-412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemann S, Richter E, Rüsch-Gerdes S. Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J Clin Microbiol. 2000;38:152–157. doi: 10.1128/jcm.38.1.152-157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Reilly L M, Daborn C J. The epidemiology of tuberculosis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Suppl. 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 22.Romano M I, Alito A, Bigi F, Fisanotti J C, Cataldi A. Genetic characterisation of mycobacteria from South American wild seals. Vet Microbiol. 1995;47:89–98. doi: 10.1016/0378-1135(95)00103-h. [DOI] [PubMed] [Google Scholar]
- 23.Scorpio A, Collins D, Whipple D, Cave D, Bates J, Zhang Y. Rapid differentiation of bovine and human tubercle bacilli based on a characteristic mutation in the bovine pyrazinamidase gene. J Clin Microbiol. 1997;35:106–110. doi: 10.1128/jcm.35.1.106-110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sreevatsan S, Escalante P, Pan X, Gillies II D A, Siddiqui S, Khalaf C N, Kreiswirth B N, Bifani P, Adams L G, Ficht T, Perumaalla V S, Cave M D, van Embden J D A, Musser J M. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis. J Clin Microbiol. 1996;34:2007–2010. doi: 10.1128/jcm.34.8.2007-2010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreevatsan S, Pan X, Stockbauer K E, Connell N D, Kreiswirth B N, Whittam T S, Musser J M. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Soolingen D, de Haas P E W, Haagsma J, Eger T, Hermans P W M, Ritacco V, Alito A, van Embden J D A. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J Clin Microbiol. 1994;32:2425–2433. doi: 10.1128/jcm.32.10.2425-2433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zumárraga M, Bernadelli A, Bastida R, Quse V, Loureiro J, Cataldi A, Bigi F, Alito A, Ramos M C, Samper S, Otal I, Martin C, Romano M I. Molecular characterization of mycobacteria isolated from seals. Microbiology. 1999;145:22519–22526. doi: 10.1099/00221287-145-9-2519. [DOI] [PubMed] [Google Scholar]