ABSTRACT
Objectives:
This study retrospectively investigated the prevalence and clinical features of trephine syndrome, which is a late complication of craniectom, in patients who underwent craniectomy decompression.
Methods:
Trephine syndrome was defined as an increase of ≥2 points in the functional independent measure (FIM) score at 7 days after cranioplasty compared with that 3 days before cranioplasty. Patients who underwent craniectomy at Kawasaki Medical School Hospital between January 1, 2010, and March 15, 2020, were included in the study.
Results:
During the observation period, 102 patients underwent craniectomy decompression; 71 of them later underwent cranioplasty. In total, 12 and 59 patients were assigned to the trephine and non-trephine syndrome groups, respectively. The patients in the trephine syndrome group were significantly younger than those in the non-trephine syndrome group (P<0.05). The mean durations±standard deviations (in days) from craniectomy decompression to cranioplasty were 57.1±38.9 and 83.6±69.3 for the trephine and non-trephine syndrome groups, respectively (P<0.05). Improvements in the FIM motor scores were greater than the improvements in the cognitive scores for all but one case (P<0.05). The frequency with which patients experienced exacerbation (worsened consciousness and sudden anisocoria) after hospitalization was significantly higher in the trephine syndrome group than in the non-trephine syndrome group (P<0.05).
Conclusions:
Performing cranioplasty as early as possible in young patients may lead to functional improvement. In the trephine syndrome group, the improvement in motor FIM score was greater than that of the cognitive score. Moreover, post-hospitalization exacerbation was more frequent in the trephine syndrome group.
Keywords: function, cranioplasty, craniectomy decompression, retrospective study
INTRODUCTION
Complications following craniectomy may occur early (i.e., bleeding, cerebral herniation toward the craniectomy, cerebrospinal fluid leakage, and epilepsy) or late (i.e., subdural edema, hydrocephalus, and trephine syndrome, which is also known as sinking skin flap syndrome).1) Among these complications, trephine syndrome has gained increased attention in recent years. The syndrome is characterized by symptoms such as headache, dizziness, pain and discomfort at the site of the cranial defects, mood swings, and deterioration of abilities. Trephine syndrome may occur weeks to months after craniectomy decompression and markedly disappears after osteoplasty. It is believed to be caused by the effect of atmospheric pressure associated with the cranial defects, decreased cerebral blood flow, decreased cerebrospinal fluid flow, and impaired glucose metabolism.2) However, the definitions of the functional improvement seen in trephine syndrome are inconsistent and include a marked improvement in neuropsychological tests 2 weeks postoperatively compared with that immediately before surgery,3) an increase of ≥2 points in the functional independent measure (FIM) score within 7 days after cranioplasty compared with that 3 days before cranioplasty,4) and an improvement in consciousness disturbance within 5 days after cranioplasty compared with that immediately before surgery.5)
In Japan, it is also known that higher brain dysfunction may be improved after cranioplasty. Interestingly, there have been a few reports of patients with markedly improved function after cranioplasty.6,7) Furthermore, to our best knowledge, no study in Japan has compared and examined multiple cases of marked functional improvement after cranioplasty. It is hoped that useful information for predicting functional prognosis may be obtained by examining the characteristics of patients with recognizable functional improvement after cranioplasty. Consequently, we retrospectively investigated the prevalence and patient characteristics of trephine syndrome, which was diagnosed based on FIM score changes in patients who underwent craniectomy decompression in our hospital.
MATERIALS AND METHODS
This study was performed according to a protocol approved by the Research Ethics Committee of Kawasaki Medical School Hospital (No. 5312–00). Because of the retrospective nature of the study, written informed consent was not required, but refusal to participate was allowed through an opt-out method.
Participants
Patients who underwent craniectomy at Kawasaki Medical School Hospital between January 1, 2010, and March 15, 2020, were included in this study. The inclusion criterion was a modified Rankin Scale (mRS) score of 0 or 1 before illness. The exclusion criteria included changes in the drug regimen that could affect cognitive function, such as the main effects of psychotropic and nootropic drugs, a week before or after cranioplasty, and conditions, such as infection, that worsened the general condition of the patient 1 week before or after cranioplasty.
Survey Items
The medical records of the included patients were reviewed. The analyzed data included age, sex, causative disease, mRS score, date and time of onset of primary disease, Glasgow Coma Scale (GCS) score on acute admission, number of comorbidities, date and time of craniectomy decompression, date of cranioplasty, presence of complications before cranioplasty, materials used for cranioplasty, drugs used for the central nervous system, and the FIM score. In this study, trephine syndrome was defined as an increase of ≥2 points in the FIM score 7 days after cranioplasty compared to that 3 days before cranioplasty.
Analysis
The data of participants in the trephine syndrome group and the non-trephine syndrome group were compared. The t-test was used to analyze the ages of the patients and the times from craniectomy decompression to cranioplasty, whereas the Mann–Whitney U test was used to analyze the GCS scores during transport and the number of comorbidities. Fisher’s exact test was used for all other items. The statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). The level of significance was set at P<0.05.
RESULTS
During the observation period, 102 patients underwent craniectomy decompression. Of these, 71 subsequently underwent cranioplasty. All patients who underwent cranioplasty had a premorbid mRS score of ≤1, and none presented with comorbidities (such as new infections) that worsened their general condition at 1 week before or after surgery.
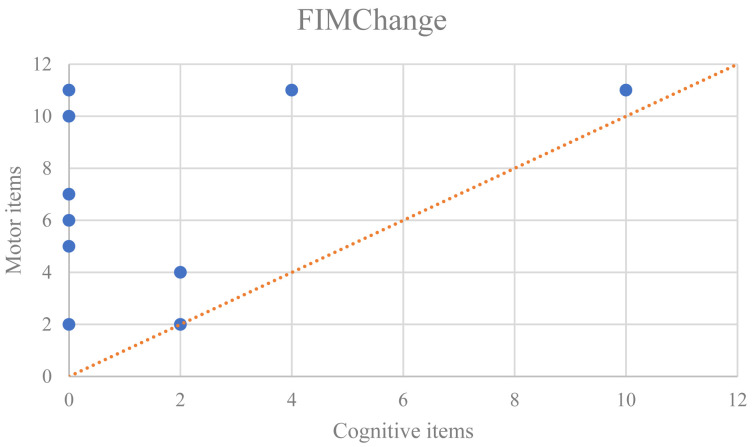
Twelve patients showed an improvement in activities of daily living (an increase of ≥2 points in the FIM score) 7 days after cranioplasty compared with that 3 days before cranioplasty. These 12 patients were assigned to the trephine syndrome group; the other 59 patients were assigned to the non-trephine syndrome group. The mean ages±standard deviations (SDs) of the participants in the trephine syndrome and non-trephine syndrome groups were 43.8±23.0 and 56.9±16.0 years, respectively (P<0.05). Moreover, trephine syndrome was significantly more common in patients who could wait >48 h between primary disease onset and craniectomy decompression, i.e., patients requiring surgery due to post-hospitalization exacerbation (worsened consciousness and sudden anisocoria), compared with those who underwent surgery within 48 h. Furthermore, the mean durations±SDs between craniectomy decompression and cranioplasty were 57.1±38.9 and 83.6±69.3 days, respectively (P<0.05). No significant differences in sex, causative disease, GCS score during transport, number of comorbidities, lesion site, presence of complications before cranioplasty, materials used for cranioplasty, or the use of preoperative central nervous system drugs were observed between the groups (Table 1). The FIM score changes before and after cranioplasty in the trephine syndrome group were divided into motor and cognitive score changes. Interestingly, improvements in the motor score were greater than those in the cognitive score for all but one case, in which the improvements were equal (Fig. 1).
Table 1. Clinical characteristics of patients with trephine syndrome.
| Trephine syndrome present |
Trephine syndrome absent |
P-value | |
| Sex (male/female) | 9/3 | 35/24 | 0.181 |
| Age (mean) | 43.8±23.0 | 56.9±16.0 | 0.019 |
| Causative disease (trauma/illness) | 7/5 | 23/36 | 0.337 |
| Median GCS score during transfer | 5.5 (3,10) | 10 (5,12) | 0.127 |
| Number of comorbidities (median) | 1 (0,2) | 1 (1,2) | 0.427 |
| Unilateral/bilateral (site of injury) (unilateral/bilateral) |
11 (right, 7; left, 4)/1 |
57 unilateral (right, 36; left, 21)/2 |
0.431 |
| Pre-cranioplasty complications (yes/no) | 6/6 | 20/39 | 0.335 |
| Materials (autologous/artificial) | 7/5 | 21/38 | 0.197 |
| Central nervous system drugs before surgery (yes/no) | 7/5 | 24/35 | 0.343 |
| Exacerbation (worsened consciousness and sudden anisocoria) was observed within 48 h after onset (yes/no) |
3/9 | 5/54 | 0.039 |
| Duration from craniectomy decompression to cranioplasty (days) | 57.1±38.9 | 83.6±69.3 | 0.008 |
GCS, Glasgow coma scale.
Fig. 1.
Motor and cognitive FIM score improvements after cranioplasty in patients with trephine syndrome. In the graph, most plots are above the X=Y line. This means that the cases judged as having trephine syndrome had more improvement in the motor than in the cognitive FIM items.
DISCUSSION
In 1939, Grant et al. reported on cases of trephine syndrome, as characterized by severe headaches, dizziness, pain, adverse effects of cranial defects, and depressive symptoms that improved after cranioplasty.8) In 1977, Yamaura et al. described similar symptoms that improved with cranioplasty as the sinking skin flap syndrome.9) Following these reports, several studies were conducted on the impact of cranioplasty on functional impairment. As a result, physiological changes, such as functional improvement10) and the normalization of intracranial pressure after cranioplasty,11) have become apparent; however, a universal definition of trephine syndrome has not yet been established. A systematic review of trephine syndrome by Ashayeri et al. summarized three features: symptoms appearing from weeks to months after craniectomy decompression, symptoms appearing regardless of the site of injury, and clinical functional improvement after cranioplasty.2) In the current study, we reviewed the medical records retrospectively and therefore could not investigate the time of onset of symptoms; the shortest time between craniectomy and cranioplasty was 18 days, and, on average, several weeks had elapsed between the two surgical procedures. There were more cases of right-sided injuries than of bilateral or left-sided injuries; however, the differences were not significant. For assessing clinical improvement after cranioplasty, Di Stefano et al. reported using the modified Wisconsin card sorting test, the letter fluency test, the simplified London Tower test, and the Rivermead behavioral memory test.3) Honeybul et al. reported an improvement of ≥2 points in the FIM score, whereas Jeyaraj et al. reported improvement in consciousness. However, it was not possible for Jeyaraj et al. to determine the significance of changes in neurological test results or to examine whether the change in consciousness disturbance was circadian or changed with the descriptor.5) Pain from trauma could not be distinguished from headaches caused by trephine syndrome. Consequently, in the current study, patients with improved activities of daily living (an increase of ≥2 points in the FIM score) 7 days after craniectomy compared with 3 days before craniectomy were diagnosed as having trephine syndrome. We could not investigate the time of onset of symptoms because our information was based on a survey of medical records. However, we discovered that the shortest duration from craniectomy decompression to cranioplasty was 18 days, indicating that at least several weeks had passed by the time cranioplasty was performed; no significant relationship with the injury site, which could be bilateral, left-sided, or right-sided, was observed.
Kurland et al. reported that 10% of patients had trephine syndrome after craniectomy decompression.12) In the current study, 11.7% of all patients who underwent craniectomy (19% of those who underwent cranioplasty) were diagnosed with trephine syndrome, which was a slightly greater percentage than that reported by Kurland et al.
The patients in the trephine syndrome group were significantly younger than those in the non-trephine syndrome group. Trephine syndrome is caused by decreased cerebral blood flow, decreased cerebrospinal fluid flow, and impaired glucose metabolism resulting from impaired intracranial pressure regulation associated with cranial defects.1) In this study, the changes in the FIM motor score in the trephine syndrome group were greater than those in the cognitive score, and we observed a short-term improvement in the FIM score. Therefore, we believe that trephine syndrome is more likely to occur in young people with relatively intact muscle strength.
The trephine syndrome group had a significantly shorter time between craniectomy and cranioplasty compared with the non-trephine syndrome group. Alexander et al. reported a marked reduction in intracranial pulse-wave amplitude when changing from the supine to the sitting position in patients before cranioplasty.11) These effects suggest that the longer duration of cranial defects had irreversible adverse effects on brain function, and cranioplasty may not have resulted in a sharp functional improvement despite the improvement of the intracranial pressure impairment. Cola et al. reported that early cranioplasty may lead to functionally favorable outcomes; however, it may also result in a high incidence of complications associated with cranioplasty (i.e., abscesses) or the inability to adequately decompress it.13) However, we believe that cranioplasty should be performed early for rapid functional improvement after cranioplasty.
The incidence of trephine syndrome was significantly higher in the group of patients who could wait for 48 h between primary disease onset and craniectomy, i.e., in patients who required surgery for post-hospitalization exacerbation (worsened consciousness and sudden anisocoria). Previous studies have examined the ideal timing of cranioplasty for achieving functional recovery. Nevertheless, no previous study has reported an association between the timing of craniectomy and the occurrence of trephine syndrome. Such findings may help to predict patient prognosis.
CONCLUSIONS
This study investigated the clinical features and prevalence of trephine syndrome at our hospital. After comparing patient age and the elapsed time to cranioplasty for the trephine syndrome and non-trephine syndrome groups, we found that performing cranioplasty as early as possible in young patients may lead to functional improvement. Furthermore, in the trephine syndrome group, improvements in motor FIM scores were greater than the improvements in cognitive scores. Moreover, in the trephine syndrome group, there was a higher proportion of patients with post-hospitalization exacerbation first observed >48 h from primary disease onset than there were patients who underwent craniectomy within 48 h.
ACKNOWLEDGMENTS
The authors would like to thank Kensuke Tochio for his assistance in data collection.
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Gopalakrishnan MS,Shanbhag NC,Shukla DP,Konar SK,Bhat DI,Devi BI: Complications of decompressive craniectomy. Front Neurol 2018;9:977. 10.3389/fneur.2018.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashayeri K,Jackson EM,Huang J,Brem H,Gordon CR: Syndrome of the trephined: a systematic review. Neurosurgery 2016;79:525–534. 10.1227/NEU.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 3.Di Stefano C,Sturiale C,Trentini P,Bonora R,Rossi D,Cervigni G,Piperno R: Unexpected neuropsychological improvement after cranioplasty: a case series study. Br J Neurosurg 2012;26:827–831. 10.3109/02688697.2012.692838 [DOI] [PubMed] [Google Scholar]
- 4.Honeybul S,Janzen C,Kruger K,Ho KM: The impact of cranioplasty on neurological function. Br J Neurosurg 2013;27:636–641. 10.3109/02688697.2013.817532 [DOI] [PubMed] [Google Scholar]
- 5.Jeyaraj P: Importance of early cranioplasty in reversing the “syndrome of the trephine/motor trephine syndrome/sinking skin flap syndrome”. J Maxillofac Oral Surg 2015;14:666–673. 10.1007/s12663-014-0673-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okuma Y,Kegoya Y,Sotome Y,Matsuda Y,Sato Y,Tanabe T,Muraoka K,Hirotsune N,Nishino S: A case of neurological recovery after cranioplasty following removal of acute subdural hematoma [in Japanese]. Neurotraumatology 2018;41:81–85. [Google Scholar]
- 7.Sugiyama K,Kondo T,Hirayama K,Tobimatsu Y,Urushiyama Y,Izumi S: A case of neurological improvement and facilitation of rehabilitation after cranioplasty [in Japanese]. J Rehabil Med 2004;41:104–109. [Google Scholar]
- 8.Grant FC,Norcross NC: Repair of cranial defects by cranioplasty. Ann Surg 1939;110:488–512. 10.1097/00000658-193910000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaura A,Sato M,Meguro K,Nakamura T,Uemura K: Cranioplasty following decompressive craniectomy – analysis of 300 cases (author’s transl) [in Japanese]. No Shinkei Geka 1977;5:345–353. [PubMed] [Google Scholar]
- 10.Stefano CD,Rinaldesi ML,Quinquinio C,Ridolfi C,Vallasciani M,Sturiale C,Piperno R: Neuropsychological changes and cranioplasty: a group analysis. Brain Inj 2016;30:164–171. 10.3109/02699052.2015.1090013 [DOI] [PubMed] [Google Scholar]
- 11.Lilja-Cyron A,Andresen M,Kelsen J,Andreasen TH,Fugleholm K,Juhler M: Long-term effect of decompressive craniectomy on intracranial pressure and possible implications for intracranial fluid movements. Neurosurgery 2020;86:231–240. 10.1093/neuros/nyz049 [DOI] [PubMed] [Google Scholar]
- 12.Kurland DB,Khaladj-Ghom A,Stokum JA,Carusillo B,Karimy JK,Gerzanich V,Sahuquillo J,Simard JM: Complications associated with decompressive craniectomy: a systematic review. Neurocrit Care 2015;23:292–304. 10.1007/s12028-015-0144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corallo F,De Cola MC,Lo Buono V,Marra A,De Luca R,Trinchera A,Bramanti P,Calabrò RS: Early vs late cranioplasty: what is better? Int J Neurosci 2017;127:688–693. 10.1080/00207454.2016.1235045 [DOI] [PubMed] [Google Scholar]