Abstract
A systematic literature review and meta-analysis was undertaken of the lateral flow-based FebriDx immunoassay for triaging patients with suspected coronavirus disease 2019 (COVID-19) upon admission to healthcare facilities. An electronic search was conducted in Scopus and Medline using the keywords ‘FebriDx’ AND ‘COVID-19’ OR ‘SARS-CoV-2’, with no language or date (i.e. up to 4th February 2022) limits, selecting studies where FebriDx was used for triaging patients with suspected COVID-19 in acute care settings, and reporting sufficient data to construct a 2×2 table. Five studies were included in the final analysis, totalling 2309 patients. The pooled diagnostic sensitivity and specificity were 0.91 [95% confidence interval (CI) 0.88–0.93] and 0.92 (95% CI 0.90–0.93), whilst the area under the curve, accuracy and kappa statistics were 0.971 (95% CI 0.962–0.980), 91.4% (95% CI 90.2–92.5%) and 0.762 (95% CI 0.731–0.793), respectively, thus reflecting substantial agreement with reference molecular testing techniques. Negative and positive predictive values were 0.974 (95% CI 0.966–0.981) and 0.742 (95% CI 0.711–0.770), respectively. This pooled analysis demonstrated that FebriDx has clinical value for rapid screening of patients with suspected COVID-19 in acute care settings, especially in regions with high viral circulation in which the pre-test probability is high, and enables prioritization for confirmatory laboratory testing.
Keywords: SARS-CoV-2, COVID-19, Immunoassay, Diagnosis, Antigen
Introduction
The use of rapid and accurate coronavirus disease 2019 (COVID-19) screening tools is essential in healthcare settings, especially upon hospital or emergency department (ED) admission, where rapid diagnosis or exclusion of severe acute respiratory syndrome coronavirus disease-2 (SARS-CoV-2) infection can enable more appropriate and timely treatment of symptomatic patients, as well as the dedicated triage of subjects with asymptomatic infection seeking care for other pathologies, who may be responsible for nosocomial transmission, potentially leading to large hospital clusters [1,2]. Although the use of traditional, laboratory-based nucleic acid amplification assays (NAATs) remains the gold standard for detecting viral RNA and hence diagnosing SARS-CoV-2 infection accurately, the systematic use of these techniques for screening patients is inherently unfeasible, mainly due to low throughput and relatively long turnaround times [3]. Neither the surrogate nor subsidiary usage of rapid molecular assays as point-of-care (POC) at the site of patient admission seems a pervasive solution, as the accuracy of some of these methods is considerably lower compared with laboratory-based NAATs (as low as 0.60), and these devices are not constitutively suited to manage large volumes of tests [4]. The latter aspect has garnered magnified relevance with the dramatic surge of COVID-19 cases worldwide since the emergence of the highly contagious Omicron variant of SARS-CoV-2 [5]. Several approaches have been proposed for rapid screening of patients for COVID-19 during the pandemic in acute care settings, including the use of rapid diagnostic tests to detect SARS-CoV-2 antigens (Ag-RDTs), body temperature screening and calculation of diagnostic predictive scores. For different reasons, however, all of these approaches have important drawbacks.
The diagnostic accuracy of Ag-RDTs has been reviewed by many recent meta-analyses, which revealed that their sensitivity compared with reference molecular techniques is low, typically in the range of 0.59–0.76 [6], 0.66–0.88 [7] or 0.57–0.71 [8], depending on the different populations and according to the distinct strategy used for pooling data. Therefore, although they can be performed rapidly outside of conventional clinical laboratories, the low accuracy does not enable efficient triage of patients with COVID-19, leaving up to one-third of subjects with SARS-CoV-2 infection under-diagnosed [9].
With respect to the assessment of body temperature, several lines of evidence now attest that this approach is highly unreliable for screening due to a number of factors, including: elevated body temperature is not always present in patients with viral and/or SARS-CoV-2 infection; body temperature thresholds that are used for this purpose vary widely in the current scientific literature; body temperature differs according to the part of the body and may be altered dramatically by environmental conditions (especially hot or cool temperatures), and may be falsely low in patients taking antipyretic drugs; and variability between devices used to measure body temperature [10]. Due to these caveats, it is not surprising that a recent meta-analysis revealed that the overall predictive value of fever is approximately 80% and 50% in adult and paediatric populations, respectively [11].
The use of so-called ‘predictive scores’ has been proposed for COVID-19 screening, although recent evidence underpins that their clinical efficiency is rather modest (i.e. 50–75%) [12,13].
Taken together, the above data suggest that none of these three potential approaches are foolproof. As such, there is a need to identify and validate additional solutions to screen patients with suspected COVID-19 on admission to healthcare facilities, such as FebriDx which is designed to distinguish rapidly between bacterial and viral infections.
Methods
Device description
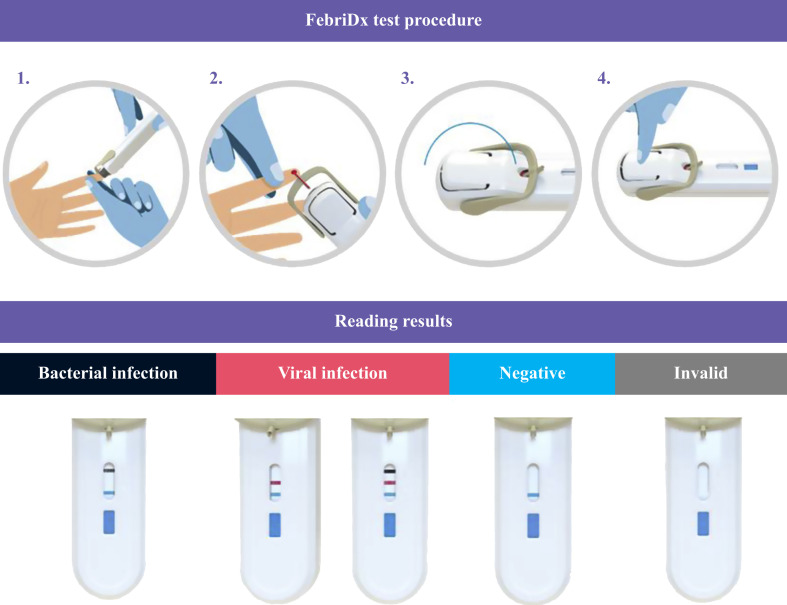
FebriDx (Lumos Diagnostics, Sarasota, FL, USA) is a CE-approved, fast, self-contained lateral flow-based POC immunoassay, developed specifically to differentiate between viral and bacterial acute respiratory infections, as described comprehensively elsewhere [14]. Briefly, this device functions by simultaneous and qualitative detection of C-reactive protein (CRP) and myxovirus resistance protein A (MxA) by means of specific monoclonal anti-MxA and anti-CRP antibodies present within the single lateral-flow test strip. The test requires a small amount of peripheral whole blood (i.e. 5 μL), which can be collected by fingertip puncture using a dedicated integral lancet (Figure 1 ). Blood is conveyed to the lateral flow section of the device, which is activated by pressing a start button which releases the buffer release button. After 10 min, test results are interpreted by visual inspection as presence or absence of three lines in the diagnostic window, corresponding to CRP value >20 mg/L (grey line at the top), MxA value >40 ng/mL (red line in the middle), and positive control (blue line at the bottom) (Figure 1) [14]. According to the manufacturer's guidance, a positive MxA test result with or without a positive CRP test result may reflect viral infection (thus including COVID-19), a positive CRP test result with a negative MxA test result is suggestive of bacterial infection, and negative results for both tests is indicative of no infection [14].
Figure 1.
Structure and function of FebriDx.
Search strategy
An electronic search in Scopus and Medline (using the PubMed interface) was undertaken using the keywords ‘FebriDX’ AND ‘COVID-19’ OR ‘SARS-CoV-2’ within all search fields, without language or date (i.e. up to 4th February 2022) limits. An initial screening of all documents was carried out by two authors (G.L. and C.M.) to select those studies where FebriDX was used to triage patients with suspected COVID-19, and which specifically reported the number of true-positive, true-negative, false-positive and false-negative cases thus enabling construction of a 2×2 table. The references of selected documents were analysed to identify other trials for potential inclusion. A pooled analysis using the Mantel–Haenszel approach and random effects model was then performed to estimate diagnostic sensitivity, specificity and accuracy [estimated as summary receiver operating characteristic curve (SROC), agreement and Kappa statistics] of FebriDx in COVID-19 triage. The heterogeneity of studies was estimated using χ2 test and I 2 statistic. Statistical analysis was performed using Meta-DiSc 1.4 (Unit of Clinical Biostatistics Team, Ramón y Cajal Hospital, Madrid, Spain) [15]. The analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Appendix 1, see online supplementary material), in accordance with the Declaration of Helsinki and within the terms of local legislation. Ethical approval was not required as this was a systematic literature review.
Results
The electronic search conducted according to the aforementioned criteria enabled the identification of seven articles following elimination of between-database duplicates. One of the identified items was not included in this analysis as it was a review article [16], and one other article only reported data on MxA [17]. Thus, five studies, totalling 2309 patients, were included in the final analysis [[18], [19], [20], [21], [22]], as summarized in Table I . Two included studies were published by the same team of authors on different cohorts [18,22]. Four studies were conducted in the UK and one was conducted in Italy. In four studies, FebriDx was used to screen patients with suspected COVID-19 at ED admission, whilst in the remaining study, the test was used more generally to screen patients with suspected COVID-19 upon hospital admission. The sample sizes ranged between 47 and 958 patients, with a generally old median age (four studies reported a median age >60 years) and greater prevalence of males (i.e. 51–68%). The reference techniques used for diagnosing SARS-CoV-2 were laboratory-based real-time polymerase chain reaction (RT-PCR) assay on nasopharyngeal swab (NPS) (two studies), rapid POC PCR test on NPS (two studies), and either laboratory-based RT-PCR assay on NPS and/or positive SARS-CoV-2 serology (one study).
Table I.
Summary of studies that investigated the diagnostic performance of FebriDx in patients with suspected coronavirus disease 2019 (COVID-19)
| Study | Country | Setting | Date | Study population | Sample size | Disease prevalence | COVID-19 diagnosis |
|---|---|---|---|---|---|---|---|
| Clark et al., 2021 [18] | UK | Screening of suspected COVID-19 at ED admission | 20th March and 12th April 2020 | Median age 70 (IQR 52–81) years; 46% females | 248 | 47.6% (95% CI 41.2–54.0%) | Laboratory-based RT-PCR assay on NPS |
| Houston et al., 2021 [19] | UK | Screening of suspected COVID-19 at ED admission | 10th August and 4th November 2020 | IQR 49–84 years; 48% females | 958 | 4.7% (95% CI 3.4–6.2%) | Rapid RT-PCR assay on NPS |
| Karim et al., 2021 [20] | UK | Screening of suspected COVID-19 at ED admission | 16th March and 3rd April 2020 | Median age 67 (IQR 53–77) years; 32% females | 47 | 72.3% (95% CI 57.4–84.4%) | Laboratory-based RT-PCR assay on NPS and/or anti-SARS-CoV-2 antibodies |
| Lagi et al., 2021 [21] | Italy | Screening of suspected COVID-19 at hospital admission | 1st August 2020 and 31st January 2021 | Median age 66 (IQR 52–80) years; 38% females | 200 | 68.0% (95% CI 61.0–74.4%) | Laboratory-based RT-PCR assay on NPS |
| Mansbridge et al., 2022 [22] | UK | Screening of suspected COVID-19 at ED admission | 22nd September 2020 and 7th January 2021 | Median age 62 (IQR 40–78) years; 49% females | 856 | 17.9% (95% CI 15.4–20.6%) | Rapid multiplex PCR testing on NPS |
CI, confidence interval; ED, emergency department; IQR, interquartile range; NPS, nasopharyngeal swab; PCR, polymerase chain reaction; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
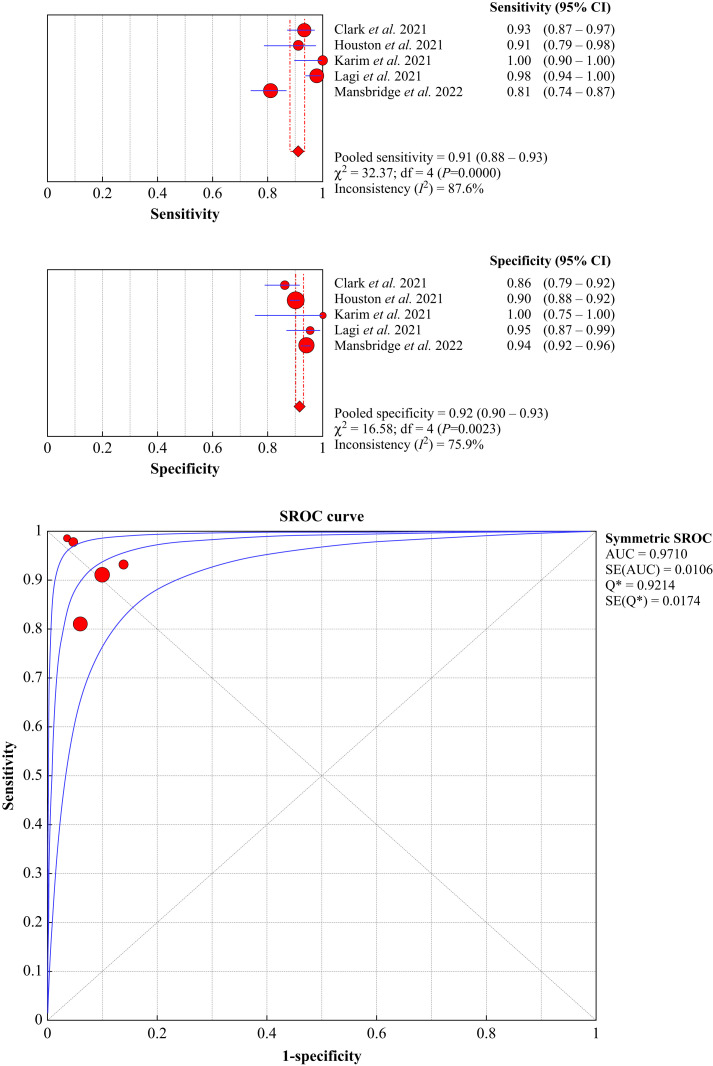
The cumulative diagnostic accuracy of FebriDx for screening patients with suspected COVID-19 is shown in Figure 2 . Overall, the diagnostic sensitivity and specificity were 0.91 (95% CI 0.88–0.93, I 2=87.6%) and 0.92 (95% CI 0.90–0.93, I 2=75.9%), respectively, whilst the area under the SROC, accuracy and kappa statistics were 0.971 (95% CI 0.962–0.980), 91.4% (95% CI 90.2–92.5%) and 0.762 (95% CI 0.731–0.793), respectively, thus reflecting substantial agreement with molecular testing [23]. The negative and positive predictive values were 0.974 (95% CI 0.966–0.981) and 0.742 (95% CI 0.711–0.770), respectively.
Figure 2.
Pooled diagnostic performance of FebriDx in patients with suspected coronavirus disease 2019 (COVID-19) upon hospital (or emergency department) admission. CI, confidence interval; AUC, area under the curve; SROC, summary receiver operating characteristic.
Discussion
Rapid triage of patients with suspected COVID-19 is a key aspect for preventing ED overcrowding and delivering the best possible care to patients [24]. Over 2 years since COVID-19 was declared as a pandemic, a huge number of clinical laboratories are still facing enormous challenges to provide timely test results of SARS-CoV-2 testing and, even worse, cannot provide reliable solutions to facilitate the fast and accurate diagnosis that is required in acute care and short-stay hospital units [25]. Several strategies have been proposed to overcome the main drawbacks (i.e. turnaround time) of molecular detection of SARS-CoV-2 RNA in these clinical settings, but most have failed to enter routine clinical practice for a variety of inherent reasons reviewed comprehensively elsewhere [26]. FebriDx is a single-use, portable test that allows simultaneous qualitative assessment of two infective biomarkers (i.e. CRP and MxA), the combined detection of which enables differentiation between acute viral infections, bacterial infections requiring antibiotics, and other non-infectious conditions. Therefore, its use for triaging patients with suspected symptomatic SARS-CoV-2 infection deserves scrutiny.
To the authors' knowledge, this is the first systematic literature review and meta-analysis of FebriDx for rapid screening of patients with suspected COVID-19 upon hospital admission. The results of this pooled analysis are extremely favourable, in that FebriDx displayed 91% sensitivity, 92% specificity and up to 91% accuracy in identifying symptomatic patients with COVID-19. This translates into several favourable outcomes, encompassing faster diagnosis or exclusion of COVID-19 in acute care settings, thus primarily allowing more appropriate and timely treatment of infected patients and/or enabling patient prioritization for molecular testing. The negative predictive value of FebriDx was as high as 0.974, thus reflecting excellent efficiency for ruling out symptomatic SARS-CoV-2 infection. This would realistically translate into the capability to exclude COVID-19 in the vast majority of symptomatic patients presenting at the ED or in other acute care wards, who could hence be triaged rapidly for other respiratory and non-respiratory pathologies. Interestingly, the diagnostic accuracy of FebriDx seems to be even better than reported previously for other forms of respiratory infections, where the test displayed cumulative sensitivity and specificity of 0.84 (95% CI 0.75–0.90) and 0.93 (95% CI 0.90–0.95) for bacterial infections, and 0.87 (95% CI 0.72–0.95) and 0.82 (95% CI 0.66–0.86) for viral infections, respectively, resulting in the avoidance of inappropriate antibiotic therapy in approximately 5% of patients [27]. The study by Mansbridge et al. was the largest (n=856) of the studies in this review, and employed rapid multiplex PCR testing to diagnose SARS-CoV-2 on NPS samples [22]. In fact, this real-world assessment of the device within an ED not only found good diagnostic accuracy (0.81 sensitivity and 0.94 specificity, respectively), leading to improvement in the number of patients moved from high-risk to lower-risk areas, but also enabled the length of ED stay to be reduced (-15 min; 95% CI -28 to -3 min), thus improving the efficiency of triage.
It is noteworthy that the excellent diagnostic performance of FebriDx found in this systematic analysis of current scientific literature was estimated in cohorts of symptomatic patients with high baseline risk and/or clinical suspicion of COVID-19. Therefore, translating such high diagnostic accuracy into comparable efficiency to diagnose any type of SARS-CoV-2 infection (thus including asymptomatic or mildly symptomatic cases) is unfeasible and misleading. This is particularly true as the concentrations of the two analytes measured by FebriDx (i.e. CRP and MxA) are illness-dependent, especially the concentration of CRP which is directly correlated with the severity of SARS-CoV-2 infection [28], and is not significantly elevated in the vast majority of patients with mild or asymptomatic infection [29]. Likewise, Mataki et al. showed that the concentration of MxA is over three-fold lower in patients with mild SARS-CoV-2 infection compared with those with severe illness, with the range of values (2.6–9.6 ng/mL) lying below the diagnostic cut-off for FebriDx (i.e. 40 ng/mL) [17].
At this point in time, it seems inadvisable to envisage FebriDx as a surrogate for NAATs or laboratory-based antigenic immunoassays, and its use should be limited to screening patients with highly suggestive signs and symptoms of COVID-19 in regions with high community circulation of SARS-CoV-2 and thus higher pre-test probability. Positive patients could then be prioritized for further confirmatory laboratory testing. Further studies are needed to determine whether the excellent test accuracies reported in studies during the early stages of the pandemic will be replicated across different prevalence rates of SARS-CoV-2 infection, especially in periods when SARS-CoV-2 may be circulating concomitantly with other respiratory viruses.
In conclusion, the pooled evidence from the relatively limited number of studies published to date suggests that FebriDx has clinical value for rapid triage of patients with suspected COVID-19 upon hospital admission, especially in regions with high viral circulation in which the pre-test probability is high, as it allows rapid screening and can help to overcome the current shortage of reagents and personnel for performing NAATs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2022.02.009.
Author contributions
Methodology: G.L. and C.M. Investigation: G.L., R.N. and B.M.H. Formal analysis: G.L. and B.M.H. Writing – original draft: G.L. Writing – review and editing: B.M.H.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pantazopoulos I., Tsikrika S., Kolokytha S., Manos E., Porpodis K. Management of COVID-19 patients in the emergency department. J Pers Med. 2021;11:961. doi: 10.3390/jpm11100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alqahtani F., Alanazi M., Alassaf W., Aleanizy F.S., Aljahany M., Joseph M., et al. Preventing SARS-CoV-2 transmission in the emergency department by implementing a separate pathway for patients with respiratory conditions. J Complement Integr Med. 2021 doi: 10.1515/jcim-2020-0422. [DOI] [PubMed] [Google Scholar]
- 3.Bohn M.K., Mancini N., Loh T.P., Wang C.B., Grimmler M., Gramegna M., et al. IFCC interim guidelines on molecular testing of SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1993–2000. doi: 10.1515/cclm-2020-1412. [DOI] [PubMed] [Google Scholar]
- 4.Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G., Mattiuzzi C., Henry B.M. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis. 2021;9:11–17. doi: 10.1515/dx-2021-0149. [DOI] [PubMed] [Google Scholar]
- 6.Lee J., Song J.U., Shim S.R. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: a systematic review and meta-analysis. J Clin Virol. 2021;144:104985. doi: 10.1016/j.jcv.2021.104985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y.H., Wu C.C., Bai C.H., Lu S.C., Yang Y.P., Lin Y.Y., et al. Evaluation of the diagnostic accuracy of COVID-19 antigen tests: a systematic review and meta-analysis. J Chin Med Assoc. 2021;84:1028–1037. doi: 10.1097/JCMA.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 8.Fujita-Rohwerder N., Beckmann L., Zens Y., Verma A. Diagnostic accuracy of rapid point-of-care tests for diagnosis of current SARS-CoV-2 infections in children: a systematic review and meta-analysis. BMJ Evid Based Med. 2022 doi: 10.1136/bmjebm-2021-111828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn M.K., Lippi G., Horvath A.R., Erasmus R., Grimmler M., Gramegna M., et al. IFCC interim guidelines on rapid point-of-care antigen testing for SARS-CoV-2 detection in asymptomatic and symptomatic individuals. Clin Chem Lab Med. 2021;59:1507–1515. doi: 10.1515/cclm-2021-0455. [DOI] [PubMed] [Google Scholar]
- 10.Lippi G., Nocini R., Mattiuzzi C., Henry B.M. Is body temperature mass screening a reliable and safe option for preventing COVID-19 spread? Diagnosis. 2021 doi: 10.1515/dx-2021-0091. [DOI] [PubMed] [Google Scholar]
- 11.Islam M.A., Kundu S., Alam S.S., Hossan T., Kamal M.A., Hassan R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 17515 patients. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elimian K.O., Aderinola O., Gibson J., Myles P., Ochu C.L., King C., et al. Assessing the capacity of symptom scores to predict COVID-19 positivity in Nigeria: a national derivation and validation cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G., Henry B.M., Hoehn J., Benoit S., Benoit J. Validation of the Corona score for rapid identification of SARS-CoV-2 infections in patients seeking emergency department care in the United States. Clin Chem Lab Med. 2020;58:e311–e313. doi: 10.1515/cclm-2020-1121. [DOI] [PubMed] [Google Scholar]
- 14.Shirley M. FebriDx®: a rapid diagnostic test for differentiating bacterial and viral aetiologies in acute respiratory infections. Mol Diagn Ther. 2019;23:803–809. doi: 10.1007/s40291-019-00433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamora J., Abraira V., Muriel A., Khan K.S., Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail S.A., Huntley C., Post N., Rigby S., Shrotri M., Williams S.V., et al. Horses for courses? Assessing the potential value of a surrogate, point-of-care test for SARS-CoV-2 epidemic control. Influenza Other Respir Viruses. 2021;15:3–6. doi: 10.1111/irv.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mataki N., Ohmura H., Kodama T., Nakamura S., Kichikawa Y., Nishimura K., et al. Myxovirus resistance protein A in peripheral blood predicts supplemental oxygen need in COVID-19. J Infect. 2021;82:186–230. doi: 10.1016/j.jinf.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark T.W., Brendish N.J., Poole S., Naidu V.V., Mansbridge C., Norton N., et al. Diagnostic accuracy of the FebriDx host response point-of-care test in patients hospitalised with suspected COVID-19. J Infect. 2020;81:607–613. doi: 10.1016/j.jinf.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houston H., Deas G., Naik S., Shah K., Patel S., Greca Dottori M., et al. Utility of the FebriDx point-of-care assay in supporting a triage algorithm for medical admissions with possible COVID-19: an observational cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim N., Ashraf M.Z., Naeem M., Anwar T., Aung H., Mallik S., et al. Utility of the FebriDx point-of-care test for rapid triage and identification of possible coronavirus disease 2019 (COVID-19) Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagi F., Trevisan S., Piccica M., Graziani L., Basile G., Mencarini J., et al. Use of the FebriDx point-of-care test for the exclusion of SARS-CoV-2 diagnosis in a population with acute respiratory infection during the second (COVID-19) wave in Italy. Int J Infect Dis. 2021;108:231–236. doi: 10.1016/j.ijid.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansbridge C.T., Tanner A.R., Beard K.R., Borca F., Phan H.T.T., Brendish N.J., et al. FebriDx host response point-of-care testing improves patient triage for COVID-19 in the emergency department. Infect Control Hosp Epidemiol. 2022 doi: 10.1017/ice.2021.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 24.May L., Tran N., Ledeboer N.A. Point-of-care COVID-19 testing in the emergency department: current status and future prospects. Expert Rev Mol Diagn. 2021;21:1333–1340. doi: 10.1080/14737159.2021.2005582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynard C., Allen J.A., Shinkins B., Prestwich G., Goves J., Davies K., et al. COVID-19 rapid diagnostics: practice review. Emerg Med J. 2022;39:70–76. doi: 10.1136/emermed-2021-211814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G., Horvath A.R., Adeli K. Editorial and executive summary: IFCC interim guidelines on clinical laboratory testing during the COVID-19 pandemic. Clin Chem Lab Med. 2020;58:1965–1969. doi: 10.1515/cclm-2020-1415. [DOI] [PubMed] [Google Scholar]
- 27.Carlton H.C., Savović J., Dawson S., Mitchelmore P.J., Elwenspoek M.M.C. Novel point-of-care biomarker combination tests to differentiate acute bacterial from viral respiratory tract infections to guide antibiotic prescribing: a systematic review. Clin Microbiol Infect. 2021;27:1096–1108. doi: 10.1016/j.cmi.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Yitbarek G.Y., Walle Ayehu G., Asnakew S., Ayele F.Y., Bariso Gare M., Mulu A.T., et al. The role of C-reactive protein in predicting the severity of COVID-19 disease: a systematic review. SAGE Open Med. 2021;9 doi: 10.1177/20503121211050755. 20503121211050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronbichler A., Kresse D., Yoon S., Lee K.H., Effenberger M., Shin J.I. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.