Abstract
Women who have had preeclampsia demonstrate microvascular endothelial-dysfunction, mediated in part by reduced nitric oxide (NO)-dependent dilation. Preeclamptic pregnancies are associated with elevated inflammation, and inhibition of inflammation attenuates endothelial damage in animal models of preeclampsia. However, it is unclear if inhibition of vascular inflammation improves endothelial function in women after a preeclamptic pregnancy. Using the cutaneous microcirculation as a model, we hypothesized that acute systemic inhibition of vascular inflammation (oral salsalate; 1500mg/twice daily, 4 days) would improve endothelium- and NO-dependent vasodilation in women with a history of preeclampsia (PE) but not in women with a history of uncomplicated pregnancy (HC). Twelve HC (30±1yrs, 10±2 months postpartum) and 10 PE (30±2yrs, 8±2 months postpartum) participated in a double-blind placebo-controlled study. Following each treatment, 2 intradermal microdialysis fibers were placed in the skin of the ventral forearm for graded infusion of acetylcholine (Ach, 10−7–102mM) or Ach+15mM L-NAME (NO synthase antagonist). Red blood cell flux was measured over each site by laser-Doppler flowmetry (LDF). Cutaneous vascular conductance was calculated (CVC=LDF/mean arterial pressure) and normalized to maximum (%CVCmax; 28mM SNP + local heat 43°C). ACh-induced (77±3 vs. 92±3%CVCmax; p=0.01) and NO-dependent (20±6 vs. 33±4%; p=0.02) vasodilation were attenuated in PE compared to HC. Salsalate augmented ACh-induced (95±2%CVCmax; p=0.002) and NO-dependent (39±3%; p=0.009) dilation in PE compared to placebo but had no effect in HC (all p>0.05). Salsalate treatment augmented endothelium-dependent vasodilation via NO-mediated pathways in women who have had preeclampsia, suggesting that inflammatory signaling mediates persistent endothelial dysfunction following preeclampsia.
Keywords: microvascular, endothelial dysfunction, preeclampsia, inflammation
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death among women worldwide and women who develop preeclampsia during pregnancy are at a significantly greater risk for the development of CVD across their lifespan (1, 2). These women suffer from CVD at a younger age and with greater frequency than women who have healthy pregnancies (1, 3–5), and are significantly more likely to die of CVD (2, 6, 7). As such, women who have had preeclampsia represent a high-risk pre-clinical cohort that require early intervention strategies to prevent or mitigate this accelerated progression of CVD. However, while the association between preeclampsia during pregnancy and elevated lifetime CVD risk is apparent, the vascular mechanism(s) responsible for this association remain unclear.
Numerous investigations have demonstrated that women with a history of preeclampsia have attenuated conduit artery vascular endothelial function compared to women with a history of uncomplicated pregnancy (8–13). We have recently demonstrated that otherwise healthy women with a history of preeclampsia have similarly attenuated microvascular endothelium-dependent dilation, mediated in part by reductions in nitric oxide (NO)-dependent dilation (14–16). This evidence suggests that vascular dysfunction observed during preeclamptic pregnancy remains aberrant following the pregnancy, and this residual dysfunction likely contributes to the increased lifetime risk of CVD in these women (17, 18).
Preeclampsia is a pregnancy disorder effecting ~5–7% of pregnancies in the United States, and ~8 million pregnancies worldwide (19) and is characterized by new onset hypertension (systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mmHg) accompanied by proteinuria (0.3g per 24 hrs) and/or thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms after 20 weeks gestation (20). Although the etiology of preeclampsia is currently unclear, pregnancies complicated by preeclampsia are associated with an exaggerated immune response leading to chronically elevated inflammation throughout pregnancy (21–25). This immune imbalance creates a chronic and uncontrolled state of inflammation which likely initiates and perpetuates the wide-spread endothelial dysfunction in the peripheral, glomerular, and cerebral vessels of preeclamptic women (26, 27). In support of this hypothesis, immunosuppression with cyclosporin A improves clinical characteristics of preeclampsia in a lipopolysaccharide induced preeclampsia rat model (28). Specifically, in the reduced uterine perfusion pressure rat model of preeclampsia, tumor necrosis factor alpha (TNFα) blockade lowers blood pressure and improves organ function (29). Similarly, in the pregnant stroke-prone spontaneously hypertensive rat TNFα blockade improves pregnancy outcomes, blood pressure, and uterine artery function (30). In women, prophylactic daily aspirin treatment during pregnancy delays the onset and attenuates the severity of preeclampsia in patients with elevated preeclampsia risk (31, 32), an effect that may be mediated by the anti-inflammatory actions of aspirin. These data suggest that exaggerated inflammation plays a role in vessel dysfunction during preeclampsia. However, whether vascular inflammation contributes to residual endothelial dysfunction and elevated lifetime CVD risk in postpartum women with a history of preeclampsia remains unexplored.
Given the mechanistic role of inflammation in the vascular sequelae of preeclampsia during pregnancy, and the evidence that endothelial dysfunction that manifests during preeclampsia remains aberrant in the year(s) following the affected pregnancy, the purpose of this study was to systematically examine the role of inflammation in attenuated endothelial function in the cutaneous microvasculature of women with a history of preeclampsia compared to women with a history of healthy pregnancy. We hypothesized that short-term, high dose (1500mg twice daily for 4 days) systemic treatment with the nonsteroidal anti-inflammatory drug salsalate would increase endothelium-dependent dilation in women with a history of preeclampsia, and that this would be mediated by an increase in NO-dependent dilation.
METHODS
Study Population
22 healthy normotensive postpartum women who had been pregnant and delivered within the past 18 months (range 2–18 months) participated in the study. Ten women had a history of preeclampsia diagnosed by their obstetrician during pregnancy (preeclamptic; PE) and 12 women had a history of healthy pregnancy and served as controls (healthy control; HC). Experimental protocols were approved by the Institutional Review Boards of The Pennsylvania State University and the University of Iowa, and the United States Food and Drug Administration (IND#124,294). Written and verbal consent were obtained voluntarily from all subjects prior to participation according to the Declaration of Helsinki and the U.S. Code of Federal Regulations, Part 46 (NCT03482440). Subjects were screened for neurological, cardiovascular, and metabolic diseases and underwent a complete medical screening including physical examination, lipid profile, and blood chemistry (Quest Diagnostics, Pittsburgh, PA; University of Iowa Diagnostic Lab, Iowa City, IA). All subjects were non-hypertensive at the time of testing (seated blood pressure <140/<90mmHg), non-diabetic, healthy non-smokers who were not taking prescription medications with primary or secondary vascular effects (e.g. statins, antihypertensives, anticoagulants, etc). All women met the inclusion criteria of no history of gestational diabetes, and no history of hypertension (SBP ≥140mmHg and/or DBP ≥90mmHg(33)) before pregnancy. Four women (3 HC/1 PE) were using hormonal contraceptives at the time of testing. Not all women were normally cycling, and subjects were tested without consideration for phase of the menstrual cycle. Subjects were matched for parity (number of times that a woman had given birth to a fetus with a gestational age of 24 weeks or more), time postpartum (months), body mass index (BMI), and blood chemistry. Subject characteristics are presented in Table 1.
Table 1.
Mean ± SE (range) HC, women who had an uncomplicated pregnancy; PE, women who had preeclampsia; BMI, body mass index; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; BUN, blood urea nitrogen.
| Table 1. Subject Characteristics | HC (n=12) | PE (n=10) | p-value |
|---|---|---|---|
| Age (yrs) | 30±1 (25–37) | 30±2 (20–41) | 0.82 |
| Parity (number) | 2±0 (1–3) | 2±0 (1–3) | 1.00 |
| Time post partum (mos) | 10±2 (2–17) | 8±2 (2–18) | 0.61 |
| BMI (kg·m−2) | 30±2 (20–36) | 28±1 (23–33) | 0.48 |
| MAP (mmHg) | 90±2 (82–98) | 91±2 (83–98) | 0.52 |
| SBP (mmHg) | 120±2 (107–132) | 123±2 (110–131) | 0.35 |
| DBP(mmHg) | 75±1 (69–84) | 75±2 (70–80) | 0.76 |
| Total Cholesterol (mg·dl−1) | 175±9 (132–250) | 189±14 (152–263) | 0.41 |
| HDL (mg·dl−1) | 58±4 (40–70) | 47±3 (34–67) | 0.07 |
| LDL (mg·dl−1) | 99±9 (81–153) | 117±11 (86–182) | 0.20 |
| HbAlc (%) | 5.2±0.1 (4.7–5.6) | 5.0±0.1 (4.7–5.4) | 0.18 |
| BUN (mg·dl−1) | 12±1 (7–16) | 11±1 (10–14) | 0.78 |
| Creatinine (mg·dl−1) | 0.72±0.02 (0.62–0.84) | 0.77±0.03 (0.56–0.90) | 0.18 |
| BUN/Creatinine | 16.1±0.2 (10.0–25.8) | 14.8±0.8 (11.7–18.6) | 0.41 |
Study Design and Salsalate Administration
Subjects were randomly assigned oral salsalate (1500mg, twice daily) or placebo for 4 consecutive 24-hour periods, in a randomized, double blind, crossover design. Subjects began the treatment with one dose on the evening of day 1 and took the last dose the morning of the experimental visit (day 5). Treatments were separated by at least 14 days. This dosing regimen was chosen based on pilot data conducted in our laboratory in this subject population, to produce steady state plasma salicylate concentrations in the therapeutic range of 10 to 30mg/dL (unpublished data). The treatment duration was chosen because maximal plasma concentrations of salicylate are achieved ~72 hours after the initiation of treatment, and because previous work has demonstrated that this duration acutely inhibits vascular nuclear factor kappa-B (NFκB) signaling (34, 35). Plasma salicylate concentrations were measured on day 3 of treatment and at the study visit. Subjects were monitored for side effects and none of the subjects dropped out during the intervention. All subjects completed both treatment arms of the study.
Microvascular Endothelium-Dependent Vasodilation
Following each treatment, each subject participated in one laboratory visit that lasted approximately 3.5 hours. Two intradermal microdialysis fibers (CMA 31 Linear Microdialysis Probe, 55kDa MWCO; CMA, Holliston, MA) were placed into the dermal layer of the ventral forearm and randomly assigned for the local delivery of lactated Ringer’s (control) or 15mmol/L NG-nitro-L-arginine methyl ester (L-NAME, Calbiochem, EMD Millipore, Billerica, MA) for the inhibition of nitric oxide synthase (NOS) (14). Pharmacological agents were mixed just prior to use, dissolved in lactated Ringer’s solution, sterilized using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), wrapped in foil to prevent degradation due to light exposure, and perfused through the microdialysis fibers at a rate of 2μL/min (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems, IN, USA). The concentrations of the pharmacological agents utilized were determined based on previous work utilizing intradermal microdialysis to assess endothelial function in our model (14, 36). Following an initial hyperemia-resolution period (~60–90 mins), cutaneous red blood cell flux was continually measured directly over each microdialysis site with an integrated laser-Doppler flowmetry probe placed in a local heating unit (MoorVMS-HEAT; Moor Instruments, DE, USA) which was set to thermoneutral (33°C). Automated brachial blood pressure (Cardiocap; GE Healthcare, WI, USA) was measured every 5 minutes throughout each protocol. Following baseline measurements (~10 minutes), ascending concentrations of ACh (10−7 – 102 mmol/L; USP, Rockville, MD), mixed with the site-specific treatment, were perfused sequentially for 5 minutes each. Following ACh doses, 28 mmol/L sodium nitroprusside (USP, Rockville, MD) was perfused and local temperature increased to 43°C to elicit maximal dilation (CVCmax) (37).
Plasma Cytokines
Whole blood samples were collected (12 HC/7 PE) via venipuncture into EDTA treated collection tubes, centrifuged, and plasma was isolated and stored at −80°C prior to analysis. Inflammatory cytokines IL-6 and TNFα were assessed in duplicate via ELISA (Inter-assay %CV= 2.3 and 0.4, Intra-assay %CV=1.5 and 1.1, for IL-6 and TNFα respectively; V-plex, Meso Scale Discovery, Rockville, MD).
Data and Statistical Analysis
All data collection and analysis procedures were standardized prior to testing. Blood flow data were digitized at 40Hz, recorded, and stored for offline analysis using Windaq software and Dataq data acquisition system (Dataq Instruments, Akron, OH). Cutaneous vascular conductance was calculated (CVC = laser Doppler flux/mean arterial pressure) and normalized to a percentage of site-specific maximum (%max). NO-dependent dilation was calculated for each subject as the difference between peak ACh-mediated dilation in the control (Ringers) and NOS-inhibited (L-NAME) sites.
Sample size was determined a priori by power analysis (power=0.80, α=0.05) using previously published data with similar primary outcomes in subjects with microvascular dysfunction (38, 39) and significance was set a priori at α=0.05. CVC data were analyzed using a 3-way repeated measures ANOVA (group*treatment*pharmacological site; SAS 9.4, Cary, IN) with post hoc Tukey corrections applied for specific planned comparisons, when appropriate. Linear regression analysis was used to examine the effect of time postpartum on our measures of microvascular endothelial function (Prism 8.1.2; Graphpad, San Diego, CA). Values are presented as mean ± SEM.
RESULTS
Oral salsalate increased plasma salicylate to therapeutic concentrations in both HC and PE. Plasma salicylate was in the therapeutic range on day 3 (HC: 13.8±1.5, PE: 18.2±0.9mg/dL, p=0.09 between groups) and the day of the experimental visit (HC: 19.1±2.3, PE: 21.2±2.1mg/dL; p=0.51 between groups) during the salsalate treatment. Plasma salicylate was undetectable during placebo treatment in both groups.
Table 2 presents plasma concentrations of inflammatory cytokines IL-6 and TNFα in HC and PE following placebo and salsalate treatments. There were no group or treatment differences in plasma IL-6 or TNFα (all p>0.05).
Table 2.
Plasma concentrations of inflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor α (TNFα). HC, women with a history of healthy pregnancy. PE, women with a history of preeclampsia. No differences (t-tests, all p>0.05).
| Table 2. | HC (n=12) | PE (n=7) |
|---|---|---|
| IL-6 (pg/ml) | ||
| placebo | 0.59±0.08 | 0.48±0.07 |
| salsalate | 0.51±0.07 | 0.61±0.15 |
| TNFα (pg/ml) | ||
| placebo | 1.5±0.2 | 1.5±0.3 |
| salsalate | 1.6±0.1 | 1.5±0.3 |
Table 3 presents baseline and maximal CVC values at control (Ringers) and NOS-inhibited (L-NAME) sites in HC and PE following placebo and salsalate treatments. There were no differences in baseline or maximal CVC between groups or across sites or treatments (all p>0.05).
Cutaneous vascular conductance at baseline and maximum (28mM SNP + 43°C local heat) in control (Ringers) and NOS-inhibited (L-NAME) microdialysis sites following placebo and salsalate treatments in women with a history of healthy pregnancy (HC) and women with a history of preeclampsia (PE). No differences (all p>0.05).
| Table 3. Cutaneous Vascular Conductance (flux·mmHg−1) | HC (n=12) | PE (n=10) |
|---|---|---|
| Baseline | ||
| Ringers | ||
| placebo | 0.2±0.1 | 0.2±0.0 |
| salsalate | 0.1±0.0 | 0.1±0.0 |
| L-NAME | ||
| placebo | 0.1±0.0 | 0.2±0.0 |
| salsalate | 0.1±0.0 | 0.1±0.0 |
| Maximal | ||
| Ringers | ||
| placebo | 1.8±0.2 | 1.8±0.2 |
| salsalate | 1.5±0.2 | 1.8±0.3 |
| L-NAME | ||
| placebo | 1.9±0.2 | 1.7±0.2 |
| salsalate | 1.6±0.1 | 1.9±0.3 |
Figure 1 presents cutaneous vasodilation (%CVCmax) responses to acetylcholine in control and L-NAME sites, and NO-dependent dilation (%) following placebo (panels A, B) or salsalate (panels C, D) treatment in women who had a healthy pregnancy (HC) and women who had preeclampsia (PE). PE had an attenuated vasodilation response to acetylcholine (p=0.01 main effect of group) and attenuated NO-dependent dilation (p=0.02) compared to HC following placebo. Oral salsalate increased cutaneous vasodilation responses (p<0.001 main effect of treatment within PE) and NO-dependent dilation (p=0.009) compared to placebo in PE but had no effect in HC (p>0.05 for both). There were no differences between groups or across treatments at the NOS-inhibited sites (all p>0.05). There was no relation between time postpartum and acetylcholine-mediated dilation or NO-dependent dilation in either HC or PE (all p>0.05).
Figure 1.
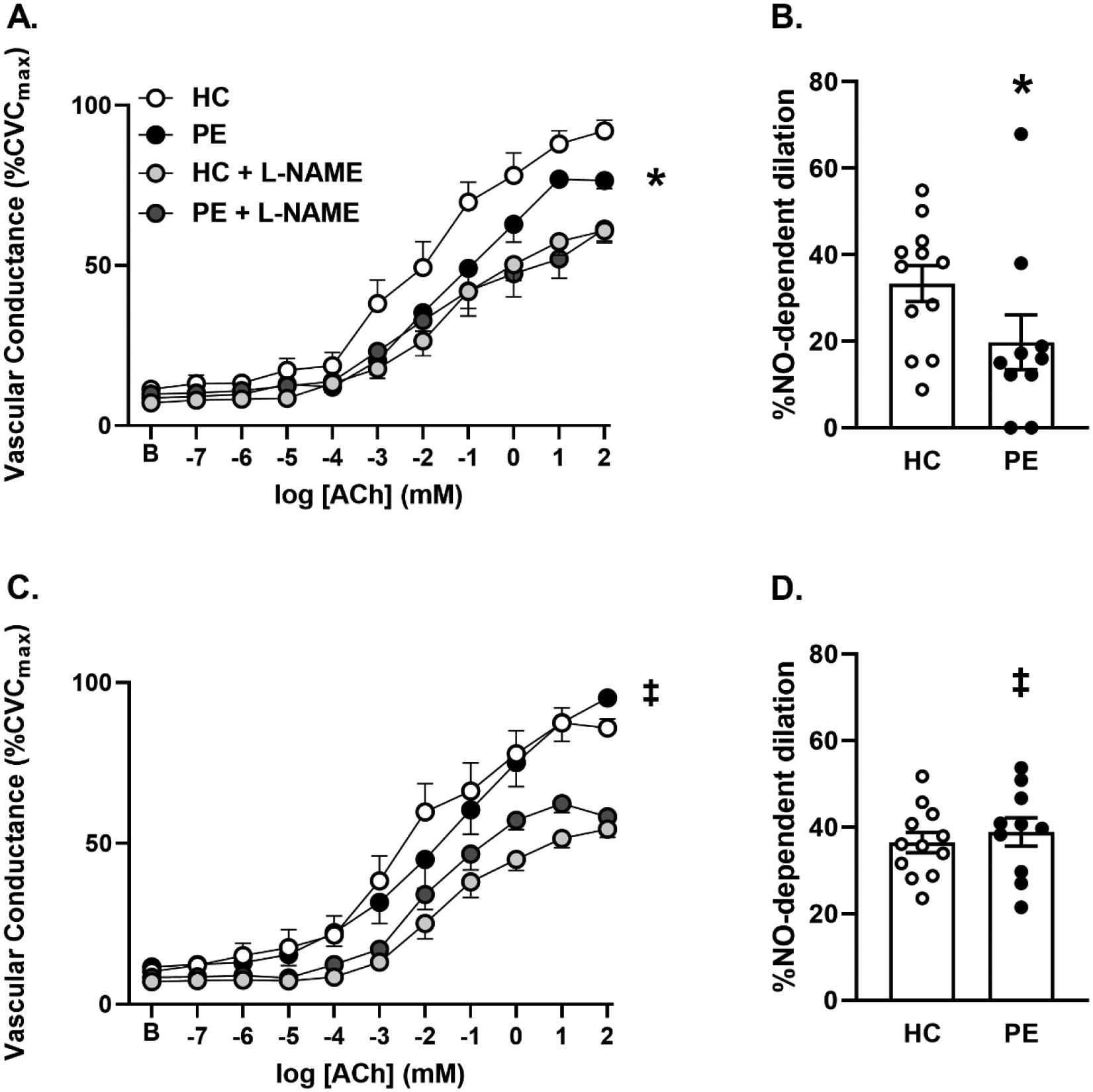
Mean ± SEM vasodilation (cutaneous vascular conductance, %CVCmax) and NO-dependent dilation (%) responses to acetylcholine (ACh) in control and NOS-inhibited (L-NAME) sites following placebo (panels A and B) and salsalate (1500mg twice daily for 4 days; panels C and D) treatments in postpartum women who have had a healthy pregnancy (HC) and women who have had preeclampsia during pregnancy (PE). *P < 0.05 PE vs HC. ‡P<0.05 vs control within PE.
DISCUSSION
The primary finding of this study was that acute oral salsalate treatment augmented endothelium-dependent dilation via NO-dependent mechanisms in the microvasculature of women with a history of preeclamptic pregnancy. These findings suggest that tonic vascular inflammation contributes to residual microvascular endothelial dysfunction in women who have had preeclampsia, and that interventional approaches that target this inflammation may be viable strategies for the treatment and/or prevention of microvascular dysfunction in these women.
Despite the remission of the clinical symptoms of preeclampsia following delivery, women with a history of preeclamptic pregnancy are 2–4 times more likely to develop CVD across their lifespan compared to women with a healthy pregnancy(18, 40). These women develop primary hypertension at an earlier age and with greater frequency than women who have healthy pregnancies (1, 4, 5, 41), and are more likely to die of stroke, myocardial infarction, and end-stage renal disease (2, 6, 7, 18). This increased risk may be explained by the residual vascular dysfunction that persists postpartum (17, 42). Several studies demonstrate that subclinical indices of vascular dysfunction -such as reduced brachial artery flow-mediated dilation and attenuated cutaneous microvascular vasodilator responses - persist for weeks, months, and even years after the affected pregnancy, even in the absence of traditional CVD risk factors (3, 8, 14–16, 43–46). As such, interventional approaches that repair or restore vascular endothelial function in women with a history of preeclampsia may prevent or slow the progression of overt CVD in this at-risk group. Our data suggest that tonic vascular inflammation contributes to residual microvascular endothelial dysfunction following preeclampsia, and that interventions that reduce this inflammation may represent mechanism-specific strategies to improve endothelial function in these women.
Preeclampsia is associated with chronically elevated inflammation throughout pregnancy (21–25), which likely contributes to the wide-spread endothelial dysfunction in the peripheral, glomerular, and cerebral microvessels of preeclamptic women (26, 27). Specifically, inhibiting this inflammation prevents the vascular sequalae of preeclampsia in animal models (29, 30, 47). The anti-inflammatory action of aspirin has been proposed as one of the mechanisms by which its prophylactic use exerts positive therapeutic effects during pregnancy in women with high preeclampsia risk (32). To our knowledge, we are the first to explore the role of inflammation in attenuated endothelium- and NO-dependent vasodilation in vivo in women who have had preeclampsia. Our data demonstrate that acute, potent anti-inflammatory treatment with salsalate improves microvascular endothelial function in postpartum women with a history of preeclampsia, suggesting that similar to vessel dysfunction observed during the affected pregnancy, inflammation plays a mechanistic role in the vascular dysfunction observed after preeclampsia.
Salsalate is a non-steroidal anti-inflammatory drug that exerts its therapeutic effects by inhibiting activation of the transcription factor NFκB and thereby preventing its downstream effects on the production of pro-inflammatory cytokines. Pierce et al. have shown that increasing plasma salicylate concentrations to a similar therapeutic range and for the same duration as the current protocol reduces total and nuclear vascular endothelial cell NFκB expression in endothelial biopsies from adults with elevated baseline NFκB expression but has no effect on cyclooxygenase expression (34). Similarly, Jablonski et al report that although this same protocol did not reduce vascular endothelial NFκB in participants who did not have elevated baseline expression, vascular endothelial cell IL-6 was attenuated following salsalate treatment (48). These data underscore the mechanistic specificity of salicylate as an inhibitor of NFκB. In this context, our data suggest that the NFκB signaling pathway and its downstream pro-inflammatory cytokine release contributes to endothelial dysfunction following preeclampsia. However, these results may not be generalizable to all anti-inflammatory treatments or targets for reducing inflammation. Future work with other anti-inflammatory targets and approaches are necessary to further delineate the mechanisms by which vascular inflammation contributes to persistent vessel dysfunction following preeclampsia.
Circulating biomarkers of inflammation such as IL-6 and TNFα are associated with increased risk of cardiovascular events (49), and are elevated during preeclampsia (24, 50) and up to 12–14 weeks after preeclampsia (21). We did not see differences in circulating IL-6 or TNFα between groups (history of preeclampsia vs. controls) or within groups across treatments (salsalate vs. placebo). Our data agree in part with prior studies that have shown increasing plasma salicylate to therapeutic concentrations reduces NFκB expression and/or downstream pro-inflammatory cytokine production in biopsied vascular endothelial cells and improves endothelium-dependent dilation, independent of changes in circulating factors or clinical characteristics in participants with subclinical vascular dysfunction (34, 35, 48, 51). We hypothesize that salsalate worked similarly in our study, inhibiting intracellular NFκB and attenuating endothelial cell expression of proinflammatory cytokines, thereby reducing vascular inflammation. However, because we did not directly assess biopsied vascular endothelial cells from our participants, this is speculative. Further work to define the inflammatory milieu of the vascular endothelium in otherwise healthy women with a history of preeclampsia is required to confirm this hypothesis, and to strengthen the evidence for the efficacy of anti-inflammatory intervention strategies for improved vascular endothelial function in these women.
We acknowledge that traditional CVD risk factors (e.g. hypertension, diabetes, elevated BMI, dyslipidemia) increase the risk of preeclampsia as well as negatively affect microvascular responses. In an attempt to limit these confounding variables, we excluded potential participants who had been diagnosed with overt cardiovascular and/or metabolic disease(s) prior to pregnancy. However, we cannot rule out subclinical alterations in cardiovascular or metabolic health that may have preceded the pregnancy and contributed to our findings in the months and years postpartum. Longitudinal studies that examine vascular function prior to, during, and after pregnancy are needed to fully elucidate whether subclinical vascular dysfunction precedes or predicts preeclampsia and what role, if any, this may play in mediating the development and severity of preeclampsia symptoms and subsequent lifetime CVD risk.
The clinical symptoms of preeclampsia (high blood pressure, proteinuria, edema, etc.) typically resolve within 12 weeks postpartum. However, otherwise healthy women who have had preeclampsia demonstrate attenuated endothelium-dependent dilation in the months and years following the effected pregnancy, indicating the need for early intervention. In this study, we employed a short (4 day), high-dose oral salsalate regimen that had been previously demonstrated to directly inhibit vascular NFkB and downstream vascular endothelial cell cytokine production, to assess the contribution of inflammation to endothelial dysfunction following preeclampsia. To the best of our knowledge, these are the first human data to suggest that suppression of inflammation augments endothelium-dependent vasodilation via NO-mediated mechanisms in women with a history of preeclampsia. As such, pharmacological treatments or lifestyle changes that reduce inflammation may be effective intervention strategies to improve microvascular function and slow or prevent the development of clinical CVD in this at-risk population.
HIGHLIGHTS.
Women who have had preeclampsia demonstrate microvascular endothelial dysfunction.
Preeclampsia is associated with elevated inflammation, but it is unknown if inflammation alters endothelial function after preeclampsia.
We show that acute systemic inhibition of inflammation improves endothelium-dependent vasodilation in women with a history of preeclampsia.
These data suggest that inflammatory signaling may be a target for improving cardiovascular health outcomes in women with a history of preeclampsia.
ACKNOWLEDGEMENTS
We would like to acknowledge the participants for contributing their time and effort. We would also like to acknowledge Kaila Brustkern, Jane Pierzga, MS and Susan Slimak, RN for their assistance throughout the project.
FUNDING
This project was supported by NIH HL129677-03 (AES), NIH HL138133-04 (AES), NIH HL093238-09 (LMA), NIH CTSA UL1TR002537 (UI ICTS), and NIH T-32-5T32AG049676 (GAD, CS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST/DISCLOSURES: NONE
Clinicaltrials.gov Registration #: NCT03482440
REFERENCES
- 1.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Alvarez B, Martell-Claros N, Abad-Cardiel M, Garcia-Donaire JA. [Hypertensive disorders during pregnancy: Cardiovascular long-term outcomes]. Hipertension y riesgo vascular. 2016. [DOI] [PubMed] [Google Scholar]
- 3.Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM). European journal of preventive cardiology. 2012;19(5):1138–44. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. Journal of the American College of Cardiology. 2014;63(18):1815–22. [DOI] [PubMed] [Google Scholar]
- 5.Haukkamaa L, Salminen M, Laivuori H, Leinonen H, Hiilesmaa V, Kaaja R. Risk for subsequent coronary artery disease after preeclampsia. The American journal of cardiology. 2004;93(6):805–8. [DOI] [PubMed] [Google Scholar]
- 6.Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta obstetricia et gynecologica Scandinavica. 1995;74(10):772–6. [DOI] [PubMed] [Google Scholar]
- 7.Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG : an international journal of obstetrics and gynaecology. 2005;112(3):286–92. [DOI] [PubMed] [Google Scholar]
- 8.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. American journal of physiology Heart and circulatory physiology. 2004;286(4):H1389–93. [DOI] [PubMed] [Google Scholar]
- 9.Aykas F, Solak Y, Erden A, Bulut K, Dogan S, Sarli B, et al. Persistence of cardiovascular risk factors in women with previous preeclampsia: a long-term follow-up study. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2015;63(4):641–5. [DOI] [PubMed] [Google Scholar]
- 10.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA : the journal of the American Medical Association. 2001;285(12):1607–12. [DOI] [PubMed] [Google Scholar]
- 11.Hamad RR, Eriksson MJ, Silveira A, Hamsten A, Bremme K. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. Journal of hypertension. 2007;25(11):2301–7. [DOI] [PubMed] [Google Scholar]
- 12.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478–87. [DOI] [PubMed] [Google Scholar]
- 13.Scholten RR, Thijssen DJ, Lotgering FK, Hopman MT, Spaanderman ME. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. American journal of obstetrics and gynecology. 2014;211(5):516 e1–e11. [DOI] [PubMed] [Google Scholar]
- 14.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased Angiotensin II Sensitivity Contributes to Microvascular Dysfunction in Women Who Have Had Preeclampsia. Hypertension. 2017;70(2):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin Sci (Lond). 2017;131(23):2777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanhewicz AE, Alexander LM. Local angiotensin-(1–7) administration improves microvascular endothelial function in women who have had preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology. 2020;318(1):R148–R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanhewicz AE. Residual vascular dysfunction in women with a history of preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology. 2018;315(6):R1062–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosla K, Heimberger S, Nieman KM, Tung A, Shahul S, Staff AC, et al. Long-Term Cardiovascular Disease Risk in Women After Hypertensive Disorders of Pregnancy: Recent Advances in Hypertension. Hypertension. 2021:HYPERTENSIONAHA12116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vascular health and risk management. 2011;7:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of O, Gynecologists’ Committee on Practice B-O. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–e60. [DOI] [PubMed] [Google Scholar]
- 21.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–14. [DOI] [PubMed] [Google Scholar]
- 22.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–43. [DOI] [PubMed] [Google Scholar]
- 23.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183(11):7023–30. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Chen S, Zhao C, Xia F. Maternal Immune System and State of Inflammation Dictate the Fate and Severity of Disease in Preeclampsia. Journal of immunology research. 2021;2021:9947884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumbreras-Marquez MI, Lumbreras-Marquez J, Barraza-Salas M, Castillo-Reyther RA, De la Maza-Labastida S, Hernandez-Rayon YI, et al. Maternal and umbilical cord procalcitonin, high-sensitivity C-reactive protein, and interleukin-6 levels in preeclamptic and normotensive patients: A cross-sectional study. Pregnancy hypertension. 2020;21:218–23. [DOI] [PubMed] [Google Scholar]
- 26.Shaw J, Tang Z, Schneider H, Salje K, Hansson SR, Guller S. Inflammatory processes are specifically enhanced in endothelial cells by placental-derived TNF-alpha: Implications in preeclampsia (PE). Placenta. 2016;43:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Lamarca B The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva ginecologica. 2010;62(2):105–20. [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B, Yang J, Huang Q, Bao J, Brennecke SP, Liu H. Cyclosporin A significantly improves preeclampsia signs and suppresses inflammation in a rat model. Cytokine. 2016;81:77–81. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham MW, Jayaram A, Deer E, Amaral LM, Vaka VR, Ibrahim T, et al. Tumor necrosis factor alpha (TNF-alpha) blockade improves natural killer cell (NK) activation, hypertension, and mitochondrial oxidative stress in a preclinical rat model of preeclampsia. Hypertension in pregnancy. 2020;39(4):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small HY, Nosalski R, Morgan H, Beattie E, Guzik TJ, Graham D, et al. Role of Tumor Necrosis Factor-alpha and Natural Killer Cells in Uterine Artery Function and Pregnancy Outcome in the Stroke-Prone Spontaneously Hypertensive Rat. Hypertension. 2016;68(5):1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huai J, Lin L, Juan J, Chen J, Li B, Zhu Y, et al. Preventive effect of aspirin on preeclampsia in high-risk pregnant women with stage 1 hypertension. Journal of clinical hypertension. 2021;23(5):1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. The New England journal of medicine. 2017;377(7):613–22. [DOI] [PubMed] [Google Scholar]
- 33.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA : the journal of the American Medical Association. 2014;311(5):507–20. [DOI] [PubMed] [Google Scholar]
- 34.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119(9):1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin Sci (Lond). 2014;127(11):645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanhewicz AE, Alexander LM. Local Angiotensin 1–7 Administration Improves Microvascular Endothelial Function in Women Who Have Had Preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6(4):337–46. [DOI] [PubMed] [Google Scholar]
- 38.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension. 2011;58(5):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. American journal of physiology Heart and circulatory physiology. 2008;294(1):H466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, et al. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2017;49(1):143–9. [DOI] [PubMed] [Google Scholar]
- 42.Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of Vascular Dysfunction After Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Hypertension. 2016;68(6):1447–58. [DOI] [PubMed] [Google Scholar]
- 43.Timokhina E, Kuzmina T, Strizhakov A, Pitskhelauri E, Ignatko I, Belousova V. Maternal Cardiac Function after Normal Delivery, Preeclampsia, and Eclampsia: A Prospective Study. J Pregnancy. 2019;2019:9795765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blaauw J, Souwer ET, Coffeng SM, Smit AJ, van Doormaal JJ, Faas MM, et al. Follow up of intima-media thickness after severe early-onset preeclampsia. Acta obstetricia et gynecologica Scandinavica. 2014;93(12):1309–16. [DOI] [PubMed] [Google Scholar]
- 45.Evans CS, Gooch L, Flotta D, Lykins D, Powers RW, Landsittel D, et al. Cardiovascular system during the postpartum state in women with a history of preeclampsia. Hypertension. 2011;58(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald SD, Ray J, Teo K, Jung H, Salehian O, Yusuf S, et al. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis. 2013;229(1):234–9. [DOI] [PubMed] [Google Scholar]
- 47.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52(6):1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriya J Critical roles of inflammation in atherosclerosis. J Cardiol. 2019;73(1):22–7. [DOI] [PubMed] [Google Scholar]
- 50.Szarka A, Rigo J Jr., Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC immunology. 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, et al. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor kappa B signalling. Journal of hypertension. 2015;33(12):2477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


