Abstract
Neurobehavioral deficits emerge in nearly 50% of patients following a mild traumatic brain injury (TBI) and may persist for months. Ketamine is used frequently as an anesthetic, analgesic and for management of persistent psychiatric complications. Although ketamine may produce beneficial effects in patients with a history of TBI, differential sensitivity to its impairing effects could make the therapeutic use of ketamine in TBI patients unsafe. This series of studies examined male C57BL/6J mice exposed to a mild single blast overpressure (mbTBI) for indications of altered sensitivity to ketamine at varying times after injury. Dystaxia (altered gait), diminished sensorimotor gating (reduced prepulse inhibition) impaired working memory (step-down inhibitory avoidance) were examined in mbTBI and sham animals 15 minutes following intraperitoneal injections of saline or R,S-ketamine hydrochloride, from day 7–16 post injury and again from day 35–43 post injury. Behavioral performance in the forced swim test and sucrose preference test were evaluated on day 28 and day 74 post injury respectively, 24 hours following drug administration. Dynamic gait stability was compromised in mbTBI mice on day 7 and 35 post injury and further exacerbated following ketamine administration. On day 14 and 42 post injury, prepulse inhibition was robustly decreased by mbTBI, which ketamine further reduced. Ketamine-associated memory impairment was apparent selectively in mbTBI animals 1 h, 24 h and day 28 post shock (tested on day 15/16/43 post injury). Ketamine selectively reduced immobility scores in the FST in mbTBI animals (day 28) and reversed mbTBI induced decreases in sucrose consumption (Day 74). These results demonstrate increased sensitivity to ketamine in mice when tested for extended periods after TBI. The results suggest that ketamine may be effective for treating neuropsychiatric complications that emerge after TBI but urge caution when used in clinical practice for enhanced sensitivity to its side effects in this patient population.
Keywords: Mild traumatic brain injury, ketamine, antidepressant, side-effect, advanced blast simulator
INTRODUCTION
Ketamine is used frequently in medicine as an anesthetic, analgesic and more recently for treatment of depression and post-traumatic stress disorder. However, patients with a history of TBI may demonstrate different responses to the neurological and behavioral effects of ketamine. Administration of ketamine as an anesthetic during the peritrauma period has been shown to reduce intracranial pressure (Chang et al., 2013; Zeiler et al., 2014) and inhibit spreading depolarization (Carlson et al., 2018; Pacheco et al., 2019), two features that are associated with poor outcomes after traumatic brain injury (TBI). However, ketamine may be administered in the months to years following recovery from the initial injury to attenuate neurological deficits or aid in managing the psychiatric symptoms that emerge in 44–50% of patients diagnosed with a mild traumatic brain injury (Halbauer et al., 2009; Kalkstein et al., 2017; Scheenen et al., 2016; Vasterling et al., 2009; Zaninotto et al., 2016). Although a single infusion of ketamine (0.5 mg/kg over a 40 minute period) is known to cause rapid (within hours) remission of intractable symptoms of major depression including suicidal ideation (DiazGranados et al., 2010; Zarate et al., 2006), the usefulness of this treatment has not been formally tested in trials for depression in patients with a history of TBI. This indication may see greater use after intranasal administration of S-ketamine was approved for treating major depressive disorder by the US Food and Drug Administration in March 2019. Other indications in the literature support the potential of ketamine to alleviate post-traumatic stress disorder and chronic pain (Cohen et al., 2018; Hartberg et al., 2018). As these disorders are frequently comorbid with and can exacerbate the negative behavioral sequelae associated with TBI, there is an urgent need to assess the efficacy of ketamine for treating these disorders in TBI patients.
Successful treatment of long-term neurobehavioral deficits and functional impairment after mild TBI remains a significant unmet need for medicine. Although ketamine may be useful at treating many psychiatric symptoms, it also has a significant profile of negative behavioral and neurological side effects. It is not known whether TBI exposed subjects would be more sensitive to either the beneficial or negative behavioral effects of ketamine. Indeed, preclinical evidence indicates that anesthetic/sedative medications administered to subjects immediately following TBI can adversely impact long-term recovery (Statler et al., 2006). Furthermore, TBI may produce neurological or behavioral deficits that can be selectively impacted by ketamine. Therefore, this series of behavioral investigations tested the following hypotheses: 1) that mice exposed to mild TBI induced by a single blast overpressure exposure would exhibit greater sensitivity to a number of behavioral effects of ketamine (at doses of 10, 20 and 30 mg/kg administered intraperitoneally (i.p.)) compared to their sham controls and 2) that the greater sensitivity to ketamine would be retained throughout a recovery period.
METHODS
Animals–
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Uniformed Services University of the Health Sciences and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 84 male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) age eight weeks were housed four per cage and maintained in a humidity-controlled room with food and water ad libitum, on a 12 h light dark cycle (lights on at 06:00). Detailed methods are provided in the supplementary materials.
Experimental Design –
Cages were randomly assigned to the four experimental groups using an online random number generator; (https://www.graphpad.com/quickcalcs/randomize1/. Group 1 – sham saline, group 2 – sham ketamine, group 3 – mbTBI saline and group 4 – mbTBI ketamine with n=12 per group. On each behavioral test day, mice were treated in accordance with their group assignment. Ketamine’s initially impairing activity was screened in the context of TBI using three behavioral endpoints; gait, prepulse inhibition and step-down inhibitory avoidance. These behavioral endpoints were evaluated 15 minutes following ketamine administration on day 7, day 14 and day 15 post injury respectively. The doses of ketamine used in these assays were the threshold dose that induced impairment in healthy uninjured animals, i.e., gait 10 mg/kg, prepulse inhibition 20 mg/kg, and step down avoidance 30 mg/kg administered i.p. The human dose equivalent is approximately 0.6, 1.6 and 2.4 mg/kg. Subjects receiving ketamine for the indication of major depressive disorder are typically administered 0.5 – 1 mg/kg intravenously over a 40 minute period (Fava et al., 2020; Iqbal and Mathew, 2020). It was hypothesized that injury animals would exhibit a more pronounced effect of the drug relative to their healthy sham controls at these threshold doses. To determine whether the longevity of increased sensitivity to the impairing effects of ketamine was retained in animals, mice were again administered ketamine on day 35 and day 42 at the doses outlined above and screened on gait and prepulse inhibition respectively. Mice were also screened on day 43 for behavioral performance in the step down inhibitory avoidance but were not treated with ketamine on that day. Screening the beneficial behavioral effects of ketamine 10 mg/kg occurred at one month post injury using the forced swim test and 3 months post injury with the sucrose preference test. This dose is known to reverse the behavioral effects of stress in mice (Autry et al., 2011; Zanos et al., 2018). These assays were screened only once. Experimenters were blinded to both injury and treatment groups. All animals survived for the duration of the experimental period. A schematic of the behavioral testing procedure conducted post ABS or Sham exposure is provided in Figure 1a.
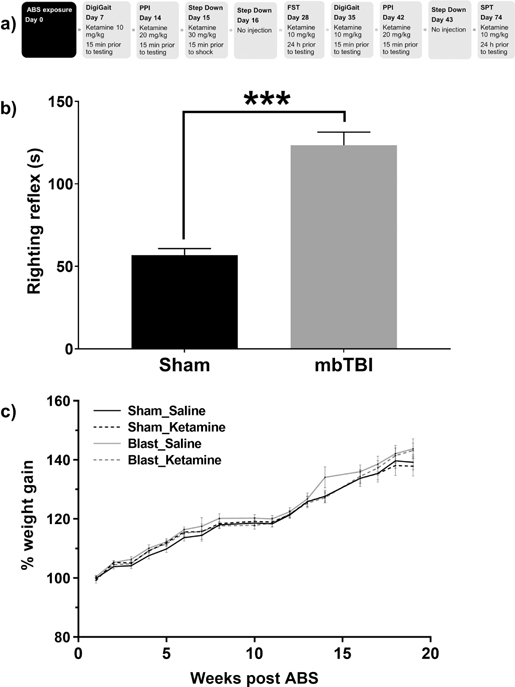
Figure 1 – ABS induced alterations in righting reflex and body weight gain.
The schematic in a outlines the study design, indicating the day post ABS or Sham exposure at which the behavioral assays were conducted. All mice were anesthetized prior to the placement in the ABS apparatus. Confirmation of the mbTBI phenotype was indicated by an increased righting reflex latency (s) in b. Mice exposed to the ABS condition designated mbTBI exhibited increased righting reflex latencies relative to Sham animals (*** denotes a p < 0.001). Weekly % weight gain throughout the experimental period is represented in c. ABS – advanced blast simulator, PPI – prepulse inhibition, FST – forced swim test, SPT – sucrose preference test, mbTBI – mild traumatic brain injury.
Mild blast traumatic brain Injury (mbTBI) –
Mice given a single exposure to the Advanced Blast Stimulator (ABS; ORA Inc., Fredericksburg, VA) were first anesthetized with 2.5% isoflurane and then received a short duration shock wave (<10 msec, mean peak pressure 19.9 psi), as previously described (Jaiswal et al., 2019; Russell et al., 2018). Sham animals that were anesthetized with 2.5% isoflurane, placed in the ABS test chamber, and were not exposed to the shock wave served as contemporary controls for TBI. All mice were immediately returned to their home cage, placed upon a warmer (38°C), and monitored until they regained consciousness. The duration of righting reflex suppression was evaluated during this time (Hamm, 2001; Russell et al., 2018; Tucker et al., 2016) as the time it took for animals to return to an upright position (forepaws touching the ground) and recorded as the time to right. This parameter was used as a correlate of duration of unconsciousness due to the anesthetic and blast exposure. Behavioral testing was initiated one week after the blast exposure.
Drugs –
R, S-ketamine hydrochloride (Mylan Pharmaceuticals, Henry Schein, NY, USA) was dissolved in sterile 0.9% saline (Quality Biological, MD, USA). Doses were derived from pilot data (not published) on assays that determined the appropriate impairing (measured within 15 min of injection), or beneficial effect (assayed 24 h post administration) at the lowest dose possible. Mice were administered either ketamine or saline via intraperitoneal injection at an injection volume of 10 ml/kg.
Gait analysis –
Motor function and coordination following Sham or mbTBI exposure was evaluated on day 7 and day 35 using the DigiGait system (Mouse Specifics Inc.), following the previously published method (Kwok et al., 2020). Ventral Plane Imaging Technology generated digital paw prints and dynamic gait signals. Following a 5 min training session, mice were administered ketamine (10 mg/kg) or saline and retested within 10 min of treatment at a treadmill speed of 9 cm/s. An average of 12 ± 4 steps were recorded for each mouse. Post drug test scores were used for all groups. Alterations in dynamic stability were evaluated across several parameters.
Prepulse Inhibition (PPI) –
The acoustic startle reflex was evaluated in three sound-attenuated chambers (SR-LAB-Startle Response System, San Diego instruments, San Diego, CA). Testing was performed on day 14 and day 42 post ABS or Sham exposure immediately following ketamine (20 mg/kg) or saline treatment. Each startle session consisted of a 10-min acclimation period in the chamber, during which a 65 dB background noise was presented. A two block PPI test session evaluated the ability of 20-ms white noise bursts at 69, 73, 77, 81, and 85 dB to attenuate the startle response to a 40 ms 115 dB white noise pulse 100 ms after the prepulse. PPI was determined using the standard metric for analysis , where sb is the animals baseline startle response to the acoustic stimulus alone and sp is startle when preceded by a prepulse.
Passive Avoidance –
The step-down inhibitory avoidance task was based on a previous publication (Sakaguchi et al., 2006) with modifications and conducted on day 15 and 16 following exposure to the ABS or Sham conditions. The apparatus consisted of a transparent Plexiglas chamber plastic platform (mouse hut platform, Bio-Serv, Flemington, NJ) located on an electrified grid floor (Bio-Signal Group, Brooklyn, NY). During training, an electric shock (0.5 mA) was delivered for 2 s approximately 10 s following “step down”. Mice were then injected with ketamine (30 mg/kg) or saline. Retention tests were performed 1 h, 24 h and 43 days after treatment, during which the latency to “step down” onto the grid floor was measured. An upper cut-off time of 180 s was set for the trial.
Forced Swim Test (FST) –
Antidepressant compounds reliably decrease immobility scores of mice (Lucki et al., 2001) and ketamine has been shown to reduced immobility up to 24 h after administration (Browne et al., 2018). Individual mice were gently placed in a cylinder (22 cm diameter) of water (25°C ± 1°C) for a period of 6 min. Immobility was defined as the absence of movement, except those required to maintain the mouse’s head above water. Mice were tested 24 h following the last of three saline or ketamine (10 mg/kg) injections, spaced every 48 h, on day 28 post ABS. This injection regimen is more clinically relevant.
Sucrose Preference Test (SPT) –
Adapted from a previously published protocol, (Jacobson et al., 2020) mice were given two overnight adaptation sessions to a two-bottle choice paradigm. One bottle contained a 1% sucrose solution (dissolved in water; Catalog # S5–500, Thermo Fisher Scientific, MD, USA), and the other was filled with water. Mice were administered ketamine (10 mg/kg) and tested for sucrose preference overnight on day 74 following ABS exposure.
Statistical analysis –
Graph Pad Prism 9 for Windows (Graph Pad Prism, La Jolla, CA) was used for all analyses. Righting reflex was evaluated with a Mann Whitney test for non-parametric data. Weight gain was analyzed with a repeated measures ANOVA with Dunnett’s multiple comparisons to compare the effects of ABS and treatment over time. For all other variables two-way ANOVA for ABS x treatment interactions were conducted, with Bonferroni multiple comparisons used where appropriate. Based on power calculations, the inclusion of 12 mice per group was required to achieve statistical significance with a power of 0.8, with alpha set at 0.05 to detect type I errors. Data points were excluded from analysis if greater than two standard deviations from the mean. Two outliers were excluded from the FST analysis, one in the sham saline and one in the sham mbTBI group. Three outliers were excluded from the SPT analysis.
RESULTS
Righting reflex and weight gain –
The immediate physiological impact of the blast wave exposure was evaluated by measuring the duration of the loss of the righting reflex caused by the ABS procedure and anesthesia. Relative to the sham group (median of 54.5 s), the median time for ABS exposed mice to right themselves was 107.5 s (U = 1, p = 0.001, sum of ranks, sham = 79, mbTBI = 221), Figure 1b, confirming a concussive blast phenotype in the ABS exposed mice.
All mice continue to gain weight over time after blast exposure, F (16, 48) = 17.23, p = 0.001, Figure 1c. Mice in the mbTBI-Saline group exhibited higher weight gain relative to Sham-Saline controls during week 13 through 16 (p < 0.001).
Gait Analysis –
Mice were tested on day 7 after ABS exposure for the effects of ketamine on gait structure. The results on a comprehensive set of movement test parameters are reported in Table 1. The ABS produced significant changes in 8 of the 21 items. Acute exposure to 10 mg/kg ketamine altered 16 of the gait parameters and there was a significant interaction between ABS and ketamine on 4 items.
Table 1 –
Gait Analysis Day 7
| Main effect of | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Sham Saline | Sham Ketamine | ABS Saline | ABS Ketamine | ABS | Ketamine | ||
| Stride length | Forepaw | 3.47 ± 0.09 | 3.86 ± 0.06 | 3.55 ± 0.06 | 4.18 ± 0.15 | n.s. | F(1,94) = 26.18, p=0.001 | n.s. |
| Hindpaw | 3.69 ± 0.07 | 3.88 ± 0.07 | 3.50 ± 0.06 | 4.06 ± 0.07 | n.s. | F(1,94) = 21.61, p=0.001 | F(1,94) = 5.04, p=0.027 | |
| Stride Frequency | Forepaw | 2.82 ± 0.17 | 2.38 ± 0.04 | 2.59 ± 0.06 | 2.27 ± 0.15 | n.s. | F(1,94) = 17.6, p=0.001 | n.s. |
| Hindpaw | 2.54±0.05 | 2.38 ± 0.06 | 2.66 ± 0.07 | 2.27 ± 0.04 | n.s. | F(1,94) = 21.26, p=0.001 | n.s. | |
| Stride Duration | Forepaw | 0.38 ± 0.01 | 0.43 ± 0.01 | 0.39 ± 0.01 | 0.46 ± 0.02 | n.s. | F(1,94) = 26.31, p=0.001 | n.s. |
| Hindpaw | 0.41 ± 0.01 | 0.43 ± 0.01 | 0.39 ± 0.01 | 0.45 ± 0.01 | n.s. | F(1,94) = 21.67, p=0.001 | F(1,94) = 4.941, p=0.028 | |
| Stance Duration | Forepaw | 0.27 ± 0.01 | 0.29 ± 0.01 | 0.29 ± 0.01 | 0.35 ± 0.02 | F(1,94) = 10.25, p=0.019 | F(1,94) = 21.6, p=0.001 | n.s. |
| Hindpaw | 0.32 ± 0.01 | 0.33 ± 0.01 | 0.29 ± 0.01 | 0.35 ± 0.01 | n.s. | F(1,94) = 18.62, p=0.001 | F(1,94) = 4.122, p=0.045 | |
| Propel Duration | Forepaw | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.01 | n.s. | F(1,94) = 15.15, p=0.002 | n.s. |
| Hindpaw | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 | n.s. | n.s. | n.s. | |
| Brake Duration | Forepaw | 0.12 ± 0.01 | 0.12 ± 0.004 | 0.13 ± 0.01 | 0.15 ± 0.01 | F(1,94) = 7.50, p=0.007 | F(1,94) = 6.08, p=0.015 | n.s. |
| Hindpaw | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.004 | 0.09 ± 0.01 | n.s. | F(1,94) = 22.45, p=0.001 | n.s. | |
| Swing Duration | Forepaw | 0.12 ± 0.004 | 0.13 ± 0.004 | 0.10 ± 0.003 | 0.12 ± 0.004 | F (1,94) =8.01, p=0.057 | F (1,94) = 12.81, p=0.005 | n.s. |
| Hindpaw | 0.09 ± 0.002 | 0.09 ± 0.001 | 0.09 ± 0.002 | 0.10 ± 0.004 | n.s. | F (1,94) = 7.264, p=0.008 | n.s. | |
| Stance/Swing | Forepaw | 2.43 ± 0.09 | 2.40 ± 0.08 | 2.9 ± 0.15 | 3.1 ± 0.15 | F(1,94) = 19.46, p=0.001 | n.s. | n.s. |
| Hindpaw | 3.47 ± 0.09 | 3.47 ± 0.09 | 3.31 ± 0.10 | 3.48 ± 0.12 | n.s. | n.s. | n.s. | |
| MAX dA/dT (cm^2/s) | Forepaw | 20.7 ± 0.88 | 24.3 ± 0.97 | 24.5 ± 1.10 | 29.7 ± 1.80 | F(1,94) = 12.41, p=0.001 | F(1,94) = 14, p=0.001 | n.s. |
| Hindpaw | 53.5 ± 2.16 | 59.11 ± 2.23 | 51.35 ± 2.31 | 51.68 ± 2.39 | F (1,94) = 4.445, p=0.036 | n.s | n.s | |
| Paw Placement Position | 0.59 ± 0.04 | 0.45 ± 0.06 | 0.37 ± 0.05 | 0.23 ± 0.07 | F (1,94) = 10, p=0.002 | F (1,94) = 7.964, p=0.006 | n.s. | |
| Overlap Distance | 2.52 ± 0.14 | 1.67 ± 0.16 | 1.5 ± 0.14 | 0.97 ± 0.16 | F (1,94) = 36.05, p=0.001 | F (1,94) = 31.22, p=0.001 | n.s. | |
| Gait Symmetry | 1.10 ± 0.04 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.99 ± 0.02 | F (1,94) = 5.396, p=0.032 | n.s. | F (1,94) = 4.753, p=0.022 | |
Analysis showed the impact of ABS was particularly strong on the forepaws, as significant changes in increased forepaw stance duration, increased forepaw brake duration, reduced forepaw swing duration, and increased forepaw stance/swing ratio. In addition, scores for forepaw and hindpaw acceleration, paw placement position, overlap and symmetry were reduced in mice exposed to ABS.
The acute administration of ketamine also produced a significant impact on movement structure, including increased stride length, stride duration, stance duration and reduced stride frequency. Reduced scores on gross measures such as paw placement position and overlap distance may reflect overall dystaxia. Finally, there was a significant interaction between ABS and ketamine on 4 movement measures. For stride length, stride duration and stance duration were reduced by ABS, but animals exposed to the ABS procedure exhibited greater stride length and duration or both stride and stance, which were significantly longer after ketamine than those of saline treated controls. For gait symmetry reduced by ABS and ketamine individually, values were not reduced further in mice that received both treatments together.
Repeated testing of mice on Day 35 post ABS exposure (Table 2), showed general recovery of most symptoms of movement impairment for both ABS and ketamine. Reduced scores for overlap distance and gait symmetry remained for ABS. Also, ketamine increased propel duration and brake duration, but did not change other movement symptoms.
Table 2 –
Gait Analysis Day 35
| Main effect of | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Sham Saline | Sham Ketamine | ABS Saline | ABS Ketamine | ABS | Ketamine | ||
| Stride length | Forepaw | 3.66 ± 0.11 | 3.38 ± 0.11 | 3.30 ± 0.16 | 3.67 ± 0.12 | n.s. | n.s | n.s. |
| Hindpaw | 3.6 ± 0.13 | 3.48 ± 0.10 | 3.6 ± 0.10 | 3.41 ± 0.12 | n.s. | n.s. | n.s. | |
| Stride Frequency | Forepaw | 2.52 ± 0.08 | 2.7 ± 0.09 | 2.75 ± 0.12 | 2.94 ± 0.19 | n.s. | n.s | n.s. |
| Hindpaw | 2.7 ± 0.19 | 2.7 ± 0.08 | 2.58 ± 0.07 | 2.73 ± 0.1 | n.s. | n.s | n.s. | |
| Stride Duration | Forepaw | 0.38 ± 0.01 | 0.43 ± 0.01 | 0.39 ± 0.01 | 0.46 ± 0.02 | n.s. | n.s | n.s. |
| Hindpaw | 0.41 ± 0.01 | 0.43 ± 0.01 | 0.39 ± 0.01 | 0.45 ± 0.01 | n.s. | n.s | n.s | |
| Stance Duration | Forepaw | 0.27 ± 0.01 | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.35 ± 0.02 | n.s. | n.s. | n.s. |
| Hindpaw | 0.32 ± 0.01 | 0.33 ± 0.01 | 0.30 ± 0.01 | 0.35 ± 0.01 | n.s. | n.s | n.s. | |
| Propel Duration | Forepaw | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.01 | n.s. | F(1,92) = 5.39, p=0.022 | n.s. |
| Hindpaw | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 | n.s. | F(1,92) = 4.72, p=0.033 | n.s. | |
| Brake Duration | Forepaw | 0.12 ± 0.01 | 0.12 ± 0.004 | 0.13 ± 0.01 | 0.15 ± 0.01 | n.s. | n.s | n.s. |
| Hindpaw | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.004 | 0.09 ± 0.01 | n.s. | F(1,92) = 6.02, p=0.016 | n.s. | |
| Swing Duration | Forepaw | 0.12 ± 0.004 | 0.13 ± 0.004 | 0.10 ± 0.003 | 0.12 ± 0.004 | n.s. | n.s. | n.s. |
| Hindpaw | 0.09 ± 0.002 | 0.09 ± 0.001 | 0.09 ± 0.002 | 0.10 ± 0.004 | n.s. | n.s | n.s. | |
| Stance/Swing | Forepaw | 2.43 ± 0.09 | 2.40 ± 0.08 | 2.9 ± 0.15 | 3.1 ± 0.15 | n.s. | n.s. | n.s. |
| Hindpaw | 3.47 ± 0.09 | 3.47 ± 0.09 | 3.31 ± 0.10 | 3.48 ± 0.12 | n.s. | n.s. | n.s. | |
| MAX dA/dT (cm^2/s) | Forepaw | 20.7 ± 0.88 | 24.9 ± 0.97 | 24.2 ± 1.10 | 29.7 ± 1.80 | n.s | n.s | n.s. |
| Hindpaw | 55.5 ± 2.16 | 59.11 ± 2.23 | 51.35 ± 2.31 | 59.67 ± 2.39 | n.s | n.s | n.s | |
| Paw Placement Position | 0.49 ± 0.56 | 0.43 ± 0.08 | 0.43 ± 0.06 | 0.33 ± 0.07 | n.s | n.s. | n.s. | |
| Overlap Distance | 1.64 ± 0.56 | 1.60 ± 0.19 | 1.3 ± 0.3 | 1.05 ± 0.2 | F (1,92) = 4.14, p=0.045 | n.s. | n.s. | |
| Gait Symmetry | 0.97 ± 0.03 | 0.99 ± 0.01 | 1.07 ± 0.05 | 1.19 ± 0.15 | F (1,92) = 5.53, p=0.029 | n.s. | n.s. | |
Passive avoidance –
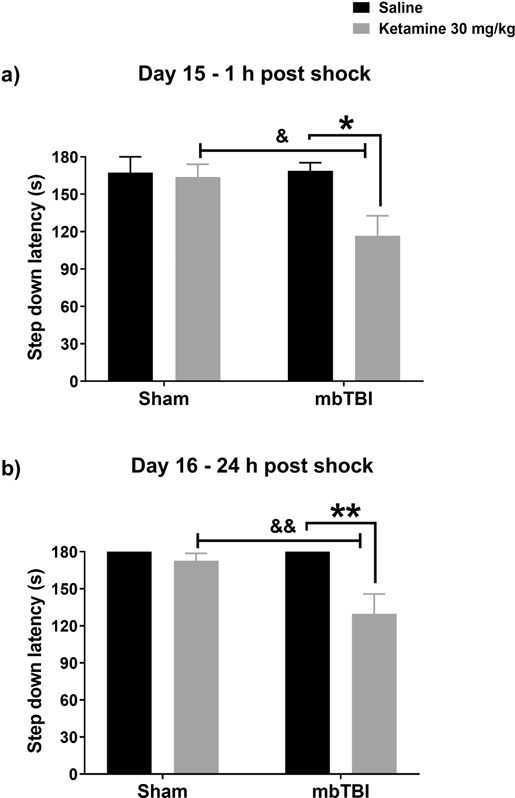
During training sessions on day 15 post injury, an effect of mbTBI on baseline performance was evident (main effect of ABS F (1, 44) = 5.795, p = 0.021, data not shown). Mice in the mbTBI group stepped off the platform faster on average (71.13 s) than Sham controls (99.92 s). The first recall test performed 1 h post shock indicated that ketamine impaired recall of the shock exposure following mbTBI (ABS x ketamine interaction, F (1, 43) = 4.079, p = 0.049, Figure 2a). Ketamine treated mbTBI mice, but not ketamine treated Sham animals, stepped down from the platform (p<0.05). The amnesic effects of ketamine were retained in mbTBI exposed mice at 24 h post treatment (ABS x treatment interaction F (1, 42), = 5.974, p = 0.019, Figure 2b), where step down latencies remained lower relative to saline treated mbTBI controls (p<0.01). When animals were retested on day 43 post injury, a significant main effect of ABS exposure was evident F (1, 44) = 6.478 = 6.478, p = 0.015 (data not shown).
Figure 2 – Increased sensitivity to the amnesic effects of ketamine in mbTBI mice.
Ketamine 30 mg/kg treatment in the mbTBI group impaired recall of a previous exposure to an aversive stimulus (shock). Relative to saline mbTBI and ketamine treated Sham controls 1 h (p<0.05) a, and at 24 h post shock (p<0.01) b.
PPI –
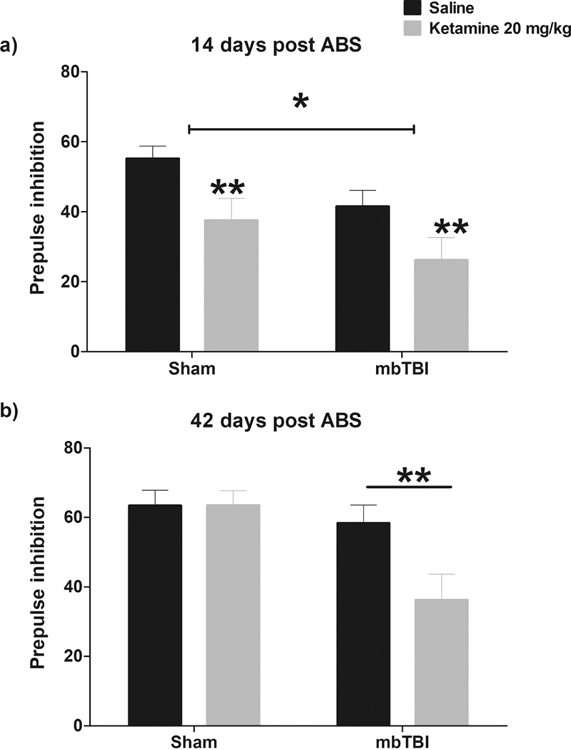
There were no differences in baseline acoustic startle response between groups (Supplementary Figure 1). For analysis, the average PPI in response to a 77-, 81- and 85-dB pulse was evaluated. On Day 14, PPI was reduced by mbTBI (main effect of ABS F (1, 43) = 4.372, p = 0.025). Nevertheless, ketamine administration reduced PPI to a comparable extent in Sham animals and mbTBI animals (main effect of ketamine F (1, 43) = 9.432, p = 0.004, Figure 3a). However, when tested on day 42, the impairing effects of ketamine on PPI were produced in mbTBI animals only (ABS x treatment interaction F (1, 44) = 4.143, p = 0.048, Figure 3b.
Figure 3 – Impaired sensorimotor gaiting in mbTBI and ketamine treated mice.
Decreased PPI was apparent in mbTBI animals on day 14 relative to Sham animals (a; p<0.05). Both Sham and mbTBI animals exhibited reductions in PPI following administration of ketamine 20 mg/kg (p<0.01) at this timepoint. In contrast, on day 42 post injury b, the ability of ketamine to reduce PPI was only apparent in the mbTBI group (p<0.01).
FST –
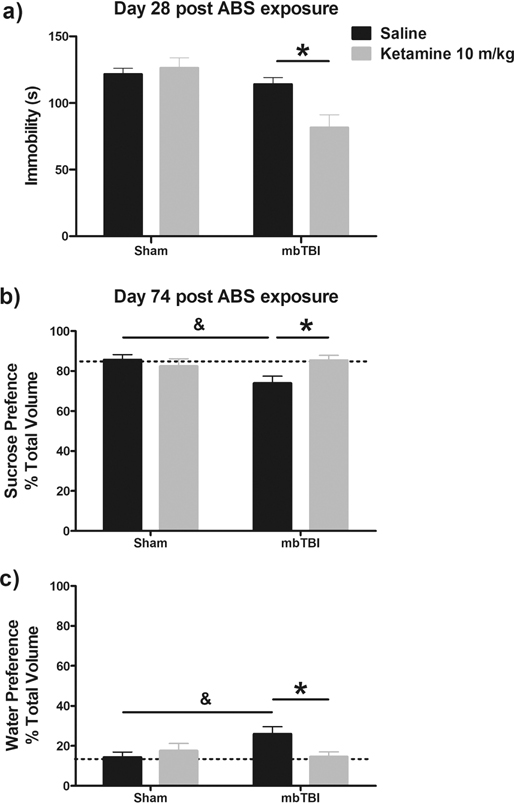
When tested on Day 28, ketamine reduced immobility scores only in mbTBI exposed animals relative to saline treated mice (p < 0.001), but not Sham animal, producing a significant ABS x ketamine treatment interaction, F (1, 42) = 6.782, p = 0.0127, Figure 4a.
Figure 4 – The beneficial effects of ketamine are retained in mbTBI mice.
Performance in the forced swim test, a, was evaluated on day 28 post ABS exposure. Ketamine 10 mg/kg reduced immobility scores of mbTBI mice relative to their saline treated controls (p<0.05). On Day 74 post ABS exposure, mbTBI was associated with a marked reduction in sucrose consumption b and increase in water intake c relative to Sham controls (p<0.05). This deficit was reversed following ketamine 10mg/kg administration (p<0.05).
SPT –
mbTBI animals, tested 74 days post injury, showed greater consumption of water (ABS x treatment interaction, F (1, 41) = 5.62, p = 0.023, Figure 4b) relative to sucrose (ABS x treatment interaction, F (1, 41) = 5.62, p = 0.023, Figure 4c). The reduced sucrose preference in mbTBI animals was then normalized by ketamine administration when tested 24 h after injection.
DISCUSSION
The goal of this study was to evaluate whether mice showed altered responsiveness to the behavioral effects of ketamine when tested at varying times after exposure to a single mbTBI. When tested acutely (15 minutes after injection), ketamine showed a series of impairing effects on gait/motor coordination, blunted sensorimotor gating and induced amnesia that were more pronounced in mice that had been exposed to mbTBI. Despite these negative consequences, when tested 24 h after injection ketamine was able to selectively modulate behavior of mbTBI animals in the (FST) and reverse behavioral deficits in the SPT, which has translational relevance as a measure of anhedonia. This interesting pattern of findings support the continued investigation of ketamine and ketamine like therapeutics for the treatment of neurobehavioral deficits that emerge in the time following blast injury.
Gait impairment measures in mice translate well to evidence of neurological symptoms, as 50% of patients with a TBI exhibit increased stance duration years following injury (Niechwiej-Szwedo et al., 2007; Williams et al., 2009). Recapitulating these effects in mice using blast overpressure, mice showed a loss of dynamic stability, exemplified by increased time spent stabilizing stance, fewer strides on average, greater maximum paw area placed on the treadmill over time and poor gait symmetry. Gait characteristics were assessed seven days following injury, at a time when gross motor deficits were no longer evident (Vu et al., 2018). In other studies, disruptions in motor function, detected by ambulatory measures such as wheel running and general home cage activity, typically resolve within 72 h of the ABS exposure (Vu et al., 2018). Few studies have explored gait characteristics following mild TBI. However, data from a study utilizing a severe injury produced by open skull controlled cortical impact (CCI) to a depth of 2 mm over the left parietal bone also reported increased forepaw stance duration relative to sham controls 7 days post injury (Sashindranath et al., 2015). Similar alterations in stance were reported in a severe CCI injury to a depth of 3 mm over the right parietal bone (Teutsch et al., 2018). As anticipated, relative to blast injury, CCI produced more impairment in hind limb measures of gait (Sashindranath et al., 2015; Teutsch et al., 2018). These data suggest that craniotomy leads to greater impairment overall. However, the consistent change in stance duration of the forepaws suggest that this gait impairment is characteristic across mild to severe TBI phenotypes in rodents. Here, direct measures of stance and stride recovered by day 35 post injury. However, persistent effects of mbTBI were evident for overlap distance and gait symmetry, highlighting prolonged gait impairment on sensitive measures post injury.
When tested after ketamine, the DigiGait data indicated that the mbTBI induced shuffling gait was exacerbated. To our knowledge this is the first report to detail the effects of ketamine on TBI induced alterations using this treadmill apparatus, although the dystaxic effects of acute ketamine on gait has been previously reported in healthy adult mice (Vecchia et al., 2018). In ABS exposed mice, the effect of ketamine was more pronounced and produced more symptoms. For example, hind paw dynamic stability was impaired by extending both the stride and stance duration. Combined with the ABS impact on forepaw measures, ketamine produced greater overall motor impairment in the context of mbTBI. Translation of these findings to patients with a history of mbTBI would suggest they be monitored closely for the acute effects of ketamine on dynamic stability when administered ketamine for treatment. Just as advised for elderly patients, patients with mbTBI receiving ketamine treatments may require a longer period of time to recover from more intense dystaxic effects after its administration.
Blast exposure may impair central auditory processing in human subjects, which causes poor sensorimotor gating and impaired selective attention (Papesh et al., 2019). In the Papesh study, self-reported measures of auditory and neurobehavioral status from both non-blast and blast-exposed participants were evaluated in addition to a paired-click sensory gating assay. The study established that a diagnosis of TBI was associated with deficits in sensory gating at the level of the cortex and a diagnosis of post-traumatic stress disorder (PTSD) was associated with poor habituation to startle (Papesh et al., 2019). Moreover, individuals exposed to impact acceleration injury also exhibit impaired startle (Blouin et al., 2007; Homayounpour et al., 2021). These data are of significant importance given the patient population of interest. It is also known that ketamine impairs prepulse inhibition in rats (Ma and Leung, 2007; Suzuki et al., 2019) and mice (Chang et al., 2019) in a dose dependent manner. The data presented in this manuscript document no difference in baseline startle of animals but a robust reduction in PPI when measured two weeks following ABS exposure. This reduction was amplified immediately after ketamine treatment. Although the impact of the blast itself on PPI diminished by day 42, these subjects remained more responsive to ketamine even when challenged at this late time point post injury. In rats, a mild fluid percussion injury, alone, and in combination with immobilization/shock stress decreased startle and PPI four days after injury (Xing et al., 2013). This decrease in startle was also reported in rats 21 and 28 days post fluid percussion injury (peak wave pressure of approximately 19 PSI) (Pang et al., 2015; Servatius et al., 2016; Sinha et al., 2017). Moreover, this blunting of startle responsivity is apparent in both male and female rats (Avcu et al., 2019). Overall, models of mTBI seem to recapitulate the profile of impaired sensorimotor gating exhibited by patients with a diagnosis of mild TBI. As ketamine is increasingly prescribed off-label to treat symptoms of PTSD (Hartberg et al., 2018), greater attention should be paid to patient history for comorbid mTBI, as the impairing effects on startle may lead to poor compliance/drop out in studies overall.
Post traumatic amnesia following a mild TBI is a common problem reported in the clinical literature. Predisposing risk factors include anticonvulsant medications, alcohol use and age (Fotakopoulos et al., 2018; Meares et al., 2015). In this study, animals that experienced blast overpressure, stepped down faster on average than Sham controls at baseline, suggesting less behavioral inhibition in a novel and potential stressful environment. Both groups demonstrated clear recall of the shock and remained on the platform for the duration of the assay. However, only mbTBI mice exhibited the amnesic effects of ketamine at the threshold impairing dose used. To our knowledge this is the first report of enhanced sensitivity to ketamine’s amnesic effects following mbTBI. This finding is in agreement with an earlier report which demonstrated increased sensitivity to the amnesic effect of the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 in rats exposed to fluid percussion injury which persisted for at least 24 h (Hamm et al., 1994). Further research is warranted in determining the underlying mediator of this enhanced sensitivity to glutamatergic agents following mild TBI.
Clinical studies emphasize the emergence of apathy and depression following traumatic brain injury (Green et al., 2021). Findings from the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study suggest that anhedonia was one of the key predictors of poor Satisfaction with Life Scale scores during a 12-month following up post injury (Agtarap et al., 2021). In this current dataset, deficits in performance on behavioral tests related to negative affect and anhedonia in mice were evaluated in two different behavioral tests assessed at one (FST) and three months (SPT) post injury. A robust reduction in preference for sucrose in the SPT was produced by mbTBI. Although, behavioral parameters in the FST were not modulated by mbTBI, the effects on SPT are indicative of disturbed performance on affective tests, although other interpretations remain to be ruled out. In contrast to the unchanged basal immobility scores in the FST at day 28 post injury, a previous study which employed open skull controlled cortical impact, reported elevated immobility scores 20 days after the initial mild (1.5 mm depth) moderate (2 mm depth) and severe (3 mm depth) injury (Washington et al., 2012). Notably, at time points closer to the initial injury no change in FST immobility scores or sucrose preference were evident in animals following a mild CCI (Tucker et al., 2017), suggesting that these behavioral deficits may emerge later in recovery. It is therefore likely, that if FST was evaluated at later time points, such as with the SPT, elevated immobility scores may have been detected in the mbTBI group. However, fluid percussion injury does not appear to augment immobility scores in the FST in rats even 133 days following injury (Blouin et al., 2007; Lapinlampi et al., 2020). These differential effects of injury type and injury severity on tests of anhedonia and affective state need to be further defined. Moreover, these studies suggest that pharmacotherapeutic interventions related to depression should preferentially be evaluated for the treatment of TBI induced neurobehavioral deficits at later times post injury, starting at least 20 days post injury. The behavioral activity of ketamine in rodent tests relevant to antidepressant effects is best measured 24 h after injection, long after ketamine is eliminated from the body and the impairing effects of ketamine are no longer evident (Browne and Lucki, 2013). In the context of mbTBI, ketamine selectively reduced immobility in mbTBI animals in the FST and normalized mbTBI induced deficits in SPT. These result are significant as they demonstrate the prolonged impact of mbTBI on measures of anhedonia and the ability of ketamine to normalize aberrant behaviors following mbTBI. The clinical significance of these data encourages the evaluation of ketamine for the treatment of neurobehavioral deficits induced following TBI.
At present, few clinical studies that have interrogated the impact of ketamine on neurobehavioral and psychiatric conditions or segmented patient responses to ketamine according to a history of traumatic brain injury. Recent clinical evidence in a small cohort of combat-injured service members treated with ketamine did not detect exaggerated ketamine-related side effects based on TBI status (Kane et al., 2020). However, a more diverse range of patients are required to confirm these observations. Only one other randomized clinical trial evaluated the utility of ketamine in patients with head and eye injuries and did not differentiate between the two groups on pain endpoints (Tran et al., 2014). In terms of the side effect profile, more agitation was reported in ketamine treated subjects relative to morphine treatment [9.5% difference (95% CI 4% to 16%) (Tran et al., 2014). Retrospective assessment of PTSD in burned service members determined that racemic ketamine administered intraoperatively following trauma may mitigate subsequent development of symptoms (McGhee et al., 2008). This is important given the intimate association between TBI and PTSD (Vasterling et al., 2018; Wisco et al., 2014; Zaninotto et al., 2016). A similar study in trauma patients, indicated that ketamine administered during the peritrauma period did not potentiate symptoms of PTSD at later time points (Highland et al., 2020; McGhee et al., 2014; Mion et al., 2017). In a randomized control trial, low-dose ketamine was shown to alleviate symptoms of PTSD within hours of infusion (Feder et al., 2014). The rapid resolution of PTSD symptoms in adults and children following a single dose of ketamine were reported to persist for over one week before the re-emergence of symptoms (D'Andrea and Andrew Sewell, 2013; Donoghue et al., 2015). These data, along with the unique capability of low-dose racemic ketamine to alleviate suicidal ideation in patients with mood disorders (DiazGranados et al., 2010; Ionescu et al., 2016), encourage the continued investigation of this novel therapeutic for stress related disorders following TBI. Nevertheless, the stratification of patients according to their history of traumatic brain injury could be an important predictor of side effects or therapeutic effects in future clinical trials.
In interpreting the data some limitations of the experimental design should be considered. Mice assigned to the two ketamine treatment groups received three i.p. injections of ketamine per week for three weeks, followed by a two week break, then one injection per week for two weeks followed by a final dosing one month later. It is possible that the repeated dosing of ketamine could potentially impact behavior, producing a sensitization or tolerance to its behavioral effects. Ketamine, administered by an intravenous, intramuscular, or intraperitoneal route produces a similar pharmacokinetic profile. Plasma and brain levels of ketamine peak within the first 5 minutes, the elimination half-life is relatively short (2–4h) (Naidoo et al., 2019; Zanos et al., 2018) but can be extended upon repeated administration (Zanos et al., 2018). Indeed, in subjects with major depressive disorder twice and thrice dosing of s-ketamine extends the efficacy of treatment for up to 15 days following the final treatment (Singh 2015). Typically, a single injection of ketamine i.p. induces alterations in behavioral assays that last from 22 h to 1 week post injection (Browne and Lucki, 2013). We utilized a clinically relevant thrice weekly injection paradigm of ketamine prior to testing in the FST in this study. Using that paradigm, the duration of ketamine’s activity on behavioral measures in the FST are extended from 24 h to 1 week follow the final injection (unpublished data). These longer lasting effects of subanesthetic doses of ketamine emerge after the elimination of ketamine from the body and are associated with neuroplastic changes induced by a glutamate surge (Aleksandrova and Phillips, 2021). However it should be noted that the extension of the duration of action of ketamine’s beneficial effects does not necessarily mean an increased duration of action of the NMDA receptor mediated side effect profile of ketamine. Sedation, dissociation, motoric impairment and amnesia are only observed when ketamine is present a physiologically relevant doses. It is also possible that the increased sensitivity to ketamine’s impairing side effect profile is due to exacerbation of TBI associated pathology. The impact of ketamine of mbTBI pathology is untested but should be considered in future studies. Based on recent published data in the literature, it appears that subanesthetic doses of ketamine would not produce pathological changes when administered alone. Indeed, ketamine administered twice weekly did not induce alterations in vacuolization, necrosis (Olney lesions), microglia activity (Iba1 labelling), and astrogliosis (GRAP reactivity) (Morris et al., 2021), suggesting that the increased sensitivity of mbTBI mice to ketamine is not due to exacerbation of TBI pathology. However, the increased sensitivity to ketamine may be explained by an intrinsic NMDA driven hyperexcitability of specific brain regions following TBI (Mayeux et al., 2017; Verley et al., 2018). This hypothesis should be tested in future studies.
The duality of ketamine’s effects in an mbTBI background on measures of side effects and affective response, urge balance when considering the use of ketamine clinically in patients with TBI. Recent advances in psychiatric medicine provide a compelling rationale to test the beneficial effects of ketamine on treatment-resistant depression and PTSD after TBI. However, the results of motor, sensory and cognitive performance suggest that there may also be increased responsiveness to the impairing effects of ketamine after TBI requiring a more cautious approach to treatment.
Supplementary Material
HIGHLIGHTS.
Mild blast traumatic brain injury (mbTBI) enhances responses to ketamine.
Ketamine increased mbTBI induced impairments in dynamic gait stability.
Ketamine further reduced prepulse inhibition after mbTBI.
Selective ketamine-induced memory impairment was apparent in mbTBI animals.
Ketamine reversed long term mbTBI deficits in sucrose consumption.
ACKNOWLEDGEMENTS
Behavioral testing and analysis were performed in the Preclinical Mouse Core and the Rat Behavior Core at the Uniformed Services University. We would like to thank Dr. Amanda Fu and Dr. Yeonho Kim for their assistance in performing the ABS procedure.
FUNDING
The authors were supported by the United States Public Health Service (USPHS) grant R01 MH105623 and Transforming Technology for the Warfighter Program grant number HU00012020014, and the Center for Neuroscience and Regenerative Medicine (TJW), which were administered by the Henry M Jackson foundation for the Advancement of Military Medicine. The funding sponsors were not involved in preparation or submission of the article for publication.
ABBREVIATIONS
- ABS
advanced blast simulator
- mbTBI
mild blast induced traumatic brain injury
- PPI
prepulse inhibition
- FST
forced swim test
- SPT
sucrose preference test.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DISCLAIMER
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University, or the Department of Defense.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agtarap SD, Campbell-Sills L, Jain S, Sun X, Dikmen S, Levin H, McCrea MA, Mukherjee P, Nelson LD, Temkin N, Yuh EL, Giacino JT, Manley GT, Stein MB, Investigators T-T, 2021. Satisfaction with Life after Mild Traumatic Brain Injury: A TRACK-TBI Study. J Neurotrauma 38, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova LR, Phillips AG, 2021. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 42, 929–942. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM, 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avcu P, Sinha S, Pang KCH, Servatius RJ, 2019. Reduced avoidance coping in male, but not in female rats, after mild traumatic brain injury: Implications for depression. Behav Brain Res 373, 112064. [DOI] [PubMed] [Google Scholar]
- Blouin JS, Siegmund GP, Timothy Inglis J, 2007. Interaction between acoustic startle and habituated neck postural responses in seated subjects. J Appl Physiol (1985) 102, 1574–1586. [DOI] [PubMed] [Google Scholar]
- Browne CA, Falcon E, Robinson SA, Berton O, Lucki I, 2018. Reversal of Stress-Induced Social Interaction Deficits by Buprenorphine. Int J Neuropsychopharmacol 21, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Lucki I, 2013. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AP, Abbas M, Alunday RL, Qeadan F, Shuttleworth CW, 2018. Spreading depolarization in acute brain injury inhibited by ketamine: a prospective, randomized, multiple crossover trial. J Neurosurg, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Ren Q, Dong C, Fujita Y, Hashimoto K, 2019. Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav 181, 53–59. [DOI] [PubMed] [Google Scholar]
- Chang LC, Raty SR, Ortiz J, Bailard NS, Mathew SJ, 2013. The emerging use of ketamine for anesthesia and sedation in traumatic brain injuries. CNS Neurosci Ther 19, 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SP, Bhatia A, Buvanendran A, Schwenk ES, Wasan AD, Hurley RW, Viscusi ER, Narouze S, Davis FN, Ritchie EC, Lubenow TR, Hooten WM, 2018. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 43, 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea D, Andrew Sewell R, 2013. Transient resolution of treatment-resistant posttraumatic stress disorder following ketamine infusion. Biol Psychiatry 74, e13–14. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr., 2010. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71, 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue AC, Roback MG, Cullen KR, 2015. Remission From Behavioral Dysregulation in a Child With PTSD After Receiving Procedural Ketamine. Pediatrics 136, e694–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI, 2020. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 25, 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS, 2014. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71, 681–688. [DOI] [PubMed] [Google Scholar]
- Fotakopoulos G, Makris D, Tsianaka E, Kotlia P, Karakitsios P, Gatos C, Tzannis A, Fountas K, 2018. The value of the identification of predisposing factors for post-traumatic amnesia in management of mild traumatic brain injury. Brain Inj 32, 563–568. [DOI] [PubMed] [Google Scholar]
- Green SL, Gignac GE, Watson PA, Brosnan N, Becerra R, Pestell C, Weinborn M, 2021. Apathy and Depression as Predictors of Activities of Daily Living Following Stroke and Traumatic Brain Injuries in Adults: A Meta-Analysis. Neuropsychol Rev. [DOI] [PubMed] [Google Scholar]
- Halbauer JD, Ashford JW, Zeitzer JM, Adamson MM, Lew HL, Yesavage JA, 2009. Neuropsychiatric diagnosis and management of chronic sequelae of war-related mild to moderate traumatic brain injury. J Rehabil Res Dev 46, 757–796. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, 2001. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma 18, 1207–1216. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, 1994. Traumatic brain injury enhances the amnesic effect of an NMDA antagonist in rats. J Neurosurg 81, 267–271. [DOI] [PubMed] [Google Scholar]
- Hartberg J, Garrett-Walcott S, De Gioannis A, 2018. Impact of oral ketamine augmentation on hospital admissions in treatment-resistant depression and PTSD: a retrospective study. Psychopharmacology (Berl) 235, 393–398. [DOI] [PubMed] [Google Scholar]
- Highland KB, Soumoff AA, Spinks EA, Kemezis PA, Buckenmaier CC 3rd, 2020. Ketamine Administration During Hospitalization Is Not Associated With Posttraumatic Stress Disorder Outcomes in Military Combat Casualties: A Matched Cohort Study. Anesthesia and analgesia 130, 402–408. [DOI] [PubMed] [Google Scholar]
- Homayounpour M, Gomez NG, Vasavada AN, Merryweather AS, 2021. Cervical Muscle Activation Due to an Applied Force in Response to Different Types of Acoustic Warnings. Ann Biomed Eng 49, 2260–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, Nyer M, Cassano P, Mischoulon D, Alpert JE, Brown EN, Nock MK, Fava M, Cusin C, 2016. Rapid and Sustained Reductions in Current Suicidal Ideation Following Repeated Doses of Intravenous Ketamine: Secondary Analysis of an Open-Label Study. J Clin Psychiatry 77, e719–725. [DOI] [PubMed] [Google Scholar]
- Iqbal SZ, Mathew SJ, 2020. Ketamine for depression clinical issues. Adv Pharmacol 89, 131–162. [DOI] [PubMed] [Google Scholar]
- Jacobson ML, Wulf HA, Browne CA, Lucki I, 2020. The kappa opioid receptor antagonist aticaprant reverses behavioral effects from unpredictable chronic mild stress in male mice. Psychopharmacology (Berl) 237, 3715–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Knutsen AK, Wilson CM, Fu AH, Tucker LB, Kim Y, Bittner KC, Whiting MD, McCabe JT, Dardzinski BJ, 2019. Mild traumatic brain injury induced by primary blast overpressure produces dynamic regional changes in [(18)F]FDG uptake. Brain research 1723, 146400. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Scott JC, Biester R, Brownlow JA, Harpaz-Rotem I, Gur RC, 2017. Comparison of blast-exposed OEF/OIF veterans with and without a history of TBI symptoms on a brief computerized neuropsychological battery. Appl Neuropsychol Adult 24, 92–97. [DOI] [PubMed] [Google Scholar]
- Kane AV, Giordano NA, Tran J, Kent ML, Highland KB, 2020. Association between traumatic brain injuries and ketamine infusion side effects following combat injury. BMJ Mil Health. [DOI] [PubMed] [Google Scholar]
- Kwok A, Rosas S, Bateman TA, Livingston E, Smith TL, Moore J, Zawieja DC, Hampton T, Mao XW, Delp MD, Willey JS, 2020. Altered rodent gait characteristics after ~35 days in orbit aboard the International Space Station. Life Sci Space Res (Amst) 24, 9–17. [DOI] [PubMed] [Google Scholar]
- Lapinlampi N, Andrade P, Paananen T, Hamalainen E, Ekolle Ndode-Ekane X, Puhakka N, Pitkanen A, 2020. Postinjury weight rather than cognitive or behavioral impairment predicts development of posttraumatic epilepsy after lateral fluid-percussion injury in rats. Epilepsia 61, 2035–2052. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ, 2001. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 155, 315–322. [DOI] [PubMed] [Google Scholar]
- Ma J, Leung LS, 2007. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology (Berl) 191, 961–974. [DOI] [PubMed] [Google Scholar]
- Mayeux J, Katz P, Edwards S, Middleton JW, Molina PE, 2017. Inhibition of Endocannabinoid Degradation Improves Outcomes from Mild Traumatic Brain Injury: A Mechanistic Role for Synaptic Hyperexcitability. J Neurotrauma 34, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH, 2008. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma 64, S195–198; Discussion S197–198. [DOI] [PubMed] [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Slater TM, Petz LN, Fowler M, 2014. The intraoperative administration of ketamine to burned U.S. service members does not increase the incidence of posttraumatic stress disorder. Mil Med 179, 41–46. [DOI] [PubMed] [Google Scholar]
- Meares S, Shores EA, Smyth T, Batchelor J, Murphy M, Vukasovic M, 2015. Identifying posttraumatic amnesia in individuals with a Glasgow Coma Scale of 15 after mild traumatic brain injury. Arch Phys Med Rehabil 96, 956–959. [DOI] [PubMed] [Google Scholar]
- Mion G, Le Masson J, Granier C, Hoffmann C, 2017. A retrospective study of ketamine administration and the development of acute or post-traumatic stress disorder in 274 war-wounded soldiers. Anaesthesia 72, 1476–1483. [DOI] [PubMed] [Google Scholar]
- Morris PJ, Burke RD, Sharma AK, Lynch DC, Lemke-Boutcher LE, Mathew S, Elayan I, Rao DB, Gould TD, Zarate CA Jr., Zanos P, Moaddel R, Thomas CJ, 2021. A comparison of the pharmacokinetics and NMDAR antagonism-associated neurotoxicity of ketamine, (2R,6R)-hydroxynorketamine and MK-801. Neurotoxicol Teratol 87, 106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V, Mdanda S, Ntshangase S, Naicker T, Kruger HG, Govender T, Naidoo P, Baijnath S, 2019. Brain penetration of ketamine: Intranasal delivery VS parenteral routes of administraion. Journal of psychiatric research 112, 7–11. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Inness EL, Howe JA, Jaglal S, McIlroy WE, Verrier MC, 2007. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture 25, 70–77. [DOI] [PubMed] [Google Scholar]
- Pacheco JM, Hines-Lanham A, Stratton C, Mehos CJ, McCurdy KE, Pinkowski NJ, Zhang H, Shuttleworth CW, Morton RA, 2019. Spreading Depolarizations Occur in Mild Traumatic Brain Injuries and Are Associated with Postinjury Behavior. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KC, Sinha S, Avcu P, Roland JJ, Nadpara N, Pfister B, Long M, Santhakumar V, Servatius RJ, 2015. Long-lasting suppression of acoustic startle response after mild traumatic brain injury. J Neurotrauma 32, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papesh MA, Elliott JE, Callahan ML, Storzbach D, Lim MM, Gallun FJ, 2019. Blast Exposure Impairs Sensory Gating: Evidence from Measures of Acoustic Startle and Auditory Event-Related Potentials. J Neurotrauma 36, 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AL, Richardson MR, Bauman BM, Hernandez IM, Saperstein S, Handa RJ, Wu TJ, 2018. Differential Responses of the HPA Axis to Mild Blast Traumatic Brain Injury in Male and Female Mice. Endocrinology 159, 2363–2375. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, Koseki M, Wakamatsu M, Matsumura E, 2006. Effects of systemic administration of beta-casomorphin-5 on learning and memory in mice. Eur J Pharmacol 530, 81–87. [DOI] [PubMed] [Google Scholar]
- Sashindranath M, Daglas M, Medcalf RL, 2015. Evaluation of gait impairment in mice subjected to craniotomy and traumatic brain injury. Behav Brain Res 286, 33–38. [DOI] [PubMed] [Google Scholar]
- Scheenen ME, Spikman JM, de Koning ME, van der Horn HJ, Roks G, Hageman G, van der Naalt J, 2016. Patients "At Risk" of Suffering from Persistent Complaints after Mild Traumatic Brain Injury: The Role of Coping, Mood Disorders, and Post-Traumatic Stress. J Neurotrauma. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Marx CE, Sinha S, Avcu P, Kilts JD, Naylor JC, Pang KC, 2016. Brain and Serum Androsterone Is Elevated in Response to Stress in Rats with Mild Traumatic Brain Injury. Front Neurosci 10, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SP, Avcu P, Spiegler KM, Komaravolu S, Kim K, Cominski T, Servatius RJ, Pang KCH, 2017. Startle suppression after mild traumatic brain injury is associated with an increase in proinflammatory cytokines, reactive gliosis and neuronal loss in the caudal pontine reticular nucleus. Brain Behav Immun 61, 353–364. [DOI] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Dixon CE, Clark RS, Jenkins L, Kochanek PM, 2006. Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J Neurotrauma 23, 97–108. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Murakami K, Tajima Y, Hara H, Kunugi A, Kimura H, 2019. TAK-137, an AMPA receptor potentiator with little agonistic effect, produces antidepressant-like effect without causing psychotomimetic effects in rats. Pharmacol Biochem Behav 183, 80–86. [DOI] [PubMed] [Google Scholar]
- Teutsch P, Jones CE, Kaiser ME, Avalon Gardner N, Lim MM, 2018. Gait and Conditioned Fear Impairments in a Mouse Model of Comorbid TBI and PTSD. Behav Neurol 2018, 6037015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KP, Nguyen Q, Truong XN, Le V, Le VP, Mai N, Husum H, Losvik OK, 2014. A comparison of ketamine and morphine analgesia in prehospital trauma care: a cluster randomized clinical trial in rural Quang Tri province, Vietnam. Prehosp Emerg Care 18, 257–264. [DOI] [PubMed] [Google Scholar]
- Tucker LB, Burke JF, Fu AH, McCabe JT, 2017. Neuropsychiatric Symptom Modeling in Male and Female C57BL/6J Mice after Experimental Traumatic Brain Injury. J Neurotrauma 34, 890–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT, 2016. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. J Neurotrauma 33, 880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Aslan M, Lee LO, Proctor SP, Ko J, Jacob S, Concato J, 2018. Longitudinal Associations among Posttraumatic Stress Disorder Symptoms, Traumatic Brain Injury, and Neurocognitive Functioning in Army Soldiers Deployed to the Iraq War. J Int Neuropsychol Soc 24, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M, Sullivan KD, 2009. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clinical psychology review 29, 674–684. [DOI] [PubMed] [Google Scholar]
- Vecchia DD, Kanazawa LKS, Wendler E, de Almeida Soares Hocayen P., Bruginski E, Campos FR, Stern CAJ, Vital M, Miyoshi E, Wohr M, Schwarting RKW, Andreatini R, 2018. Effects of ketamine on vocal impairment, gait changes, and anhedonia induced by bilateral 6-OHDA infusion into the substantia nigra pars compacta in rats: Therapeutic implications for Parkinson's disease. Behav Brain Res 342, 1–10. [DOI] [PubMed] [Google Scholar]
- Verley DR, Torolira D, Pulido B, Gutman B, Bragin A, Mayer A, Harris NG, 2018. Remote Changes in Cortical Excitability after Experimental Traumatic Brain Injury and Functional Reorganization. J Neurotrauma 35, 2448–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu PA, Tucker LB, Liu J, McNamara EH, Tran T, Fu AH, Kim Y, McCabe JT, 2018. Transient disruption of mouse home cage activities and assessment of orexin immunoreactivity following concussive- or blast-induced brain injury. Brain research 1700, 138–151. [DOI] [PubMed] [Google Scholar]
- Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP, 2012. The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J Neurotrauma 29, 2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, Morris ME, Schache A, McCrory PR, 2009. Incidence of gait abnormalities after traumatic brain injury. Arch Phys Med Rehabil 90, 587–593. [DOI] [PubMed] [Google Scholar]
- Wisco BE, Marx BP, Holowka DW, Vasterling JJ, Han SC, Chen MS, Gradus JL, Nock MK, Rosen RC, Keane TM, 2014. Traumatic brain injury, PTSD, and current suicidal ideation among Iraq and Afghanistan U.S. veterans. J Trauma Stress 27, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G, Barry ES, Benford B, Grunberg NE, Li H, Watson WD, Sharma P, 2013. Impact of repeated stress on traumatic brain injury-induced mitochondrial electron transport chain expression and behavioral responses in rats. Front Neurol 4, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto AL, Vicentini JE, Fregni F, Rodrigues PA, Botelho C, de Lucia MC, Paiva WS, 2016. Updates and Current Perspectives of Psychiatric Assessments after Traumatic Brain Injury: A Systematic Review. Front Psychiatry 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD, 2018. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 70, 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Zeiler FA, Teitelbaum J, West M, Gillman LM, 2014. The ketamine effect on ICP in traumatic brain injury. Neurocrit Care 21, 163–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.