Abstract
Background
Stenotic femoral intercondylar notch is considered as a risk factor for anterior cruciate ligament (ACL) injury and three-dimensional notch volume is used as a marker for the injury. The primary purpose of this study was to assess the difference in notch volume between the ACL-injured and uninjured in men and women combined or stratified by sex. The secondary purpose was to assess the difference in notch volume between the ACL-intact men and women.
Methods
A search of PubMed/Medline, Scopus, Google Scholar, and Cochrane databases from inception to December 9, 2020, was conducted without restrictions using the following terms: ACL, notch, volume, notch volume, femoral notch volume, and intercondylar notch volume. Studies that compared the ACL-injured with uninjured controls were included. Independent extraction of articles by two authors using predefined data fields including study quality indicators was done. All pooled analyses were based on the inverse-variance weighted random effects model and mean difference was chosen as the effect measure.
Results
Nine studies (1,169 knees) qualified for overall analysis (both sexes combined) and significant heterogeneity was observed, which disappeared after pooling studies with age-sex matched controls and those without. Notch volume in the ACL-injured was 0.75 cm3 (95% confidence interval [CI], 0.53–0.96 cm3), which was smaller than that in the age- and sex-matched controls. Six studies qualified for analysis in men. Notch volume in the ACL-injured men was smaller, especially when non-contact ACL injury was considered (1.40 cm3; 95% CI, 1.08–1.73 cm3). Five studies qualified for analysis in women and ACL-injured women had smaller notch volume irrespective of the mechanism of injury (0.38 cm3; 95% CI, 0.18–0.59 cm3). Notch volume of the uninjured men was larger than that of the uninjured women (1.86 cm3; 95% CI, 1.54–2.18 cm3).
Conclusions
ACL-injured adults have smaller notch volume than the age- and sex-matched controls. Non-contact ACL-injured males have smaller notch volume compared to ACL-intact males. ACL-injured females have smaller notch volume irrespective of the nature of injury. Men have higher notch volume than women. The quality of evidence is very low to low.
Keywords: Anterior cruciate ligament, Femur, Risk factors, Notch volume, Stenotic femoral intercondylar notch
Anterior cruciate ligament (ACL) injuries are one of the most common sports-related injuries and research efforts are directed towards identification of risk factors of ACL injury.1,2,3) The rationale behind this search is that once an at-risk knee is identified, preventive strategies can be devised and instituted more effectively. Various risk factors of ACL injury are described and are categorized as intrinsic and extrinsic factors.4) Risk factors inherent to the individual are called intrinsic factors and include anatomical, sex-related, neuromuscular, hormonal, and genetic influences.4) Sex is considered to be an independent risk factor and women are at a higher risk (> 3–4 times) of non-contact ACL injuries.5) Among the various anatomical factors, innate knee morphometry—in particular, intercondylar notch geometry—is considered an important predisposing factor for ACL injury.4,6,7,8)
Stenotic femoral intercondylar notch is considered as a risk factor and various two-dimensional (2D) notch parameters have been evaluated on radiographs and magnetic resonance imaging (MRI), including notch width (NW), notch width index (NWI), notch angle (NA), notch depth (ND), and notch shape index (NSI).2,6) Considerable diversity exists in the measurements and no single parameter has been established as a marker of stenotic notch.9,10,11,12,13,14,15,16,17) In addition, these 2D notch measurements are not only plane-specific (coronal/axial/sagittal) but also location-specific in a given plane. Recent meta-analyses on these 2D parameters suggest inconsistent association with ACL injury. A meta-analysis by Andrade et al.18) concluded smaller NW and NWI would be associated with ACL tears. However, they reported very high heterogeneity in their analyses (80% and 93% for NW and NWI, respectively). On closer analysis, the included studies differed in various aspects, most notably on the plane and location of measurements. Li et al.19) found NW to be smaller in the ACL-injured in both the axial and coronal planes but NWI was smaller in the coronal plane only. High heterogeneity was noted in the meta-analysis regarding NW in the axial plane and almost all meta-analyses pertaining to NWI. Li et al.20) found NW to be smaller in both men and women, whereas NWI was significantly smaller only in men. Significant heterogeneity was apparent in almost all the meta-analyses including those concerning NW. Presence of such high heterogeneity raises concerns about inconsistency of the results.
As 2D notch parameters can be plane- and location-specific, it is probably unreasonable to expect a single unifying 2D parameter that defines morphology of a complex three-dimensional (3D) space. Intercondylar notch volume (notch volume) being a 3D parameter was proposed to be a better marker for stenotic notch.21) Smaller notch volume has been associated with ACL injury in some studies.22) Some authors found smaller notch volume to be an independent risk factor of non-contact ACL injury.23) On the other hand, some studies have found contradictory results.24) Significant sex difference has also been suggested among various notch parameters including notch volume,6,15,23,25) and some authors26) have suggested separate analysis of male and female data due to this sex difference. While some studies have analyzed notch volume separately in men and women, others have failed to do so.24) Furthermore, some studies have included only non-contact ACL injury while others have not been restricted on the mechanism of injury.22)
To the authors' best knowledge, no systematic review or meta-analysis has been conducted yet to assess the association of notch volume with ACL injury. The primary purpose of the current study was to assess the difference in notch volume between the ACL-injured and uninjured when men and women are analyzed together or when each sex is analyzed separately. The secondary purpose was to assess the difference in notch volume between ACL-intact men and ACL-intact women. We hypothesized that smaller intercondylar notch volume would be associated with ACL injury irrespective of sex and that men have larger intercondylar notch volume than women.
METHODS
The study was conducted in compliance with preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines,27,28) and a thorough search of Medline (PubMed), Scopus, Google Scholar, and Cochrane databases was done from inception to December 9, 2020, to retrieve relevant studies that evaluated femoral intercondylar notch volume. Search string used in PubMed was as follows: (((((anterior cruciate ligament) AND (notch)) AND (volume)) OR (femoral notch volume)) OR (femoral intercondylar notch volume)) OR (intercondylar notch volume). No restrictions were placed. Similar search strings were used for all the databases as per the permissible syntax. Two authors (VJ and QA) independently assessed each study in two rounds. First round consisted of screening focused on titles and abstracts while the second round analyzed full texts for eligibility. References given in the included articles were also scrutinized to include additional studies. Any disagreement regarding qualification of study was resolved by discussion at the third round.
Criteria for inclusion were as follows: (1) studies measuring femoral intercondylar notch volume using MRI/computed tomography (CT), (2) comparative study with a control group, (3) exposure of interest being femoral intercondylar notch volume, (4) outcome of interest being ACL injury, and (5) relevant data being available for evaluation. Exclusion of the study was done if it (1) did not include the control group, (2) was a review, letter, or meeting abstract, (3) was animal study, cadaveric study, or studied graft failure after reconstruction, or (4) included pediatric population.
Data Extraction
Data extraction was done systematically by the two aforementioned authors. Following information were recorded from each study: first author's name, year of publication, country of origin, sample size of both groups, sex details, study design, inclusion/exclusion criteria for the comparison groups, and all data relevant to the objectives. Mean difference (MD) was selected as the principal summary measure. Relevant data for calculation of effect size were either directly taken from the study (if available) or were back-calculated using methods described in Cochrane handbook (using p-value of t-test, t-test statistic, confidence interval [CI], sample size, etc.).29) Subgroups were combined when deemed necessary and if data provided in an article did not allow for calculation of effect measure, the study was discarded from the meta-analysis.
Assessment of Risk of Bias
Both the aforementioned authors independently assessed the methodological quality and susceptibility to bias using Newcastle-Ottawa Scale (NOS).30) Disagreement was resolved by discussion. Considering the presence of sparse literature, perfect blinding of articles for analysis was not possible; however, journal titles, author names, and origin were blinded from the reviewers at this stage. Reliability of assessment was measured using Cohen's Kappa statistic on IBM SPSS ver. 26.0 (IBM Corp., Armonk, NY, USA).
Statistical Analysis
The data were recorded and managed using Revman 5.3 software, Cochrane collaboration, Oxford, England. Inverse-variance weighted meta-analysis on random effects model was performed and reported. Heterogeneity was evaluated using Q, τ2 statistic, and I2 statistic and when present, it was investigated through subgroup analysis using characteristics found important during full text review. A priori consideration was given to this analysis. It was determined at the time of full text review that the studies differed in some clinical aspects and needed exploration. Methods used to calculate notch volume, restriction to non-contact ACL injury, use of injured knee or contralateral healthy knee for measurement, and study methodology were identified as important clinical differences. To assess publication bias, Trim and Fill method and Egger's test were used when appropriate. Sensitivity analysis was done by excluding single study from analysis. Statistical analysis was performed by Revman 5.3. Open source Prometa 3 software was used for those analyses that were not supported by Revman 5.3 (viz Egger's test, sensitivity plots). Statistical significance was considered at p < 0.05. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) recommendations were followed to determine quality of evidence.31) Effect size was reported as MD (95% CI) in cm3.
RESULTS
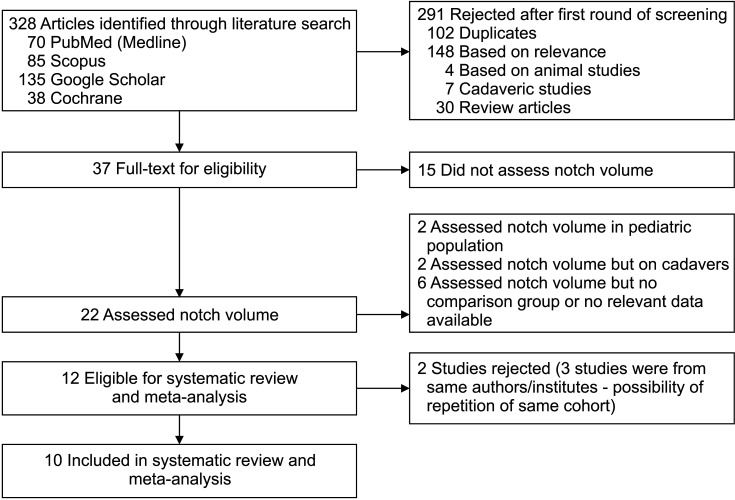
The literature search identified 328 articles and a detailed screening process (Fig. 1) yielded 12 studies14,21,22,23,24,32,33,34,35,36,37,38) that qualified for the study. Three studies were from the same authors/institutions and had overlapping duration of the study.21,23,38) Possibility of the same cohort of patients being repeated could not be ruled out, hence we decided to include only 1 study23) out of the 3 studies. We decided to include the study23) that had the same primary objective as the current study (to compare the intercondylar notch volume between the ACL-injured and uninjured). In addition, it had a larger sample size than the other 2 studies21,23) and provided sex-related data. In total, 10 studies were finally included in the analysis.14,22,23,24,32,33,34,35,36,37)
Fig. 1. Flow diagram of detailed screening process during study.
All included studies were case-control in design and 622,23,24,33,36,37) of those contained sex-related data. One study was exclusively limited to men.33) With regards to moderator characteristics that were decided a priori, 5 studies included only non-contact ACL injuries,14,23,33,34,36) while the others22,24,32,35,37) were unrestricted/unspecified. The most common method of measurement (6 studies)22,23,24,35,36,37) was on axial MRI sections as per Charlton et al.25) One study33) modified the method by including a slice interval, while 3 studies14,32,34) used different methods. Five studies had matching of controls,14,22,23,33,36) while the other 524,32,34,35,37) had unmatched selection of controls. Contralateral healthy knees were used as a surrogate for measurements of the injured knees in 3 studies14,35,36) (primarily because of interest in the pre-injury status of ACL volume), while the rest (7 studies)22,23,24,32,33,34,37) used the injured knees.
Risk of bias assessment as per NOS score is shown in Table 1. Two authors (VJ and QA) independently assessed the studies for risk of bias and the inter-rater reliability by Cohen's kappa statistic was 0.883 (p < 0.0001). Association with ACL injury was assessed stepwise by first comparing overall (men and women data combined) ACL-injured cases with controls. This was followed by sex-controlled comparison and inter-sex comparison of uninjured controls.
Table 1. Study Characteristics.
| Study (year) | Study type/sample size (n)/age (yr) | Comparability of controls/NOS score | Other study characteristics |
|---|---|---|---|
| Zhang et al. (2019)23) (assessment) | Retrospective, case control | Matched controls (age, sex) | Method by Charlton et al.25) |
| Evidence: level III | Used injured knee | ||
| Age: 29.9 ± 6.6 | NOS: 7 (3/1/3) | Only non-contact injuries | |
| Iriuchishima et al. (2021)32) | Prospective, case control | Controls unmatched | Different method of measurement - 3D CT |
| I: 47, C: 41 | Evidence: level III | Used injured knee | |
| Median age (I: 26, C: 27) | NOS: 5 (2/0/3) | Nature of injury unspecified | |
| Jha and Pandit (2021)33) | Retrospective, case control I: 80, C: 80 | Matched controls (age, sex, height) | Modified the method by Charlton et al.25) : utilized slice interval in addition to slice thickness for calculation |
| Age: NA | Evidence: level III | Used injured knee | |
| Study limited to males only | NOS: 8 (3/2/3) | Only non-contact injuries | |
| van Eck et al. (2011)24)(comparison) | Retrospective, case control | Controls unmatched | Method by Charlton et al.25) |
| Evidence: level III | Used injured knee | ||
| Age: 33.3 ± 14.3 | NOS: 5 (2/0/3) | Nature of injury unspecified | |
| Wratten et al. (2015)22) | Retrospective, case control | Matched controls (age, sex) | Method by Charlton et al.25) |
| I: 90, C: 90 | Evidence: level III | Used injured knee | |
| Age: 31.8 ± 11.3 | NOS: 7 (3/1/3) | Nature of injury unspecified | |
| Oshima et al. (2020)35) | Prospective, case control | Controls unmatched | Method by Charlton et al.25) |
| I: 19, C: 18 | Evidence: level III (controls were healthy adults) | Used contra-lateral healthy knee | |
| Age: 29.9 ± 10.5 | NOS: 5 (3/0/2) | Nature of injury unspecified | |
| Whitney et al. (2014)36) | Prospective, case control | Matched controls (age, sex) | Method by Charlton et al.25) |
| I: 88, C: 88 | Evidence: level III | Used contra-lateral healthy knee | |
| Age: NA | NOS: 9 (4/2/3) | Only non-contact injuries | |
| Simon et al. (2010)14) | Prospective, case control | Matched control (age, sex, weight, height) | Method unspecified |
| I: 27, C: 27 | Evidence: level III | Used contra-lateral healthy knee | |
| Age: NA | NOS: 6 (2/2/2) | Only non-contact injuries | |
| Taneja et al. (2018)34) | Prospective, case control | Unmatched control | Different method of measurement |
| I: 50, C: 50 | Evidence: level III | Used injured knee | |
| Age: 36.8 ± 9.3 | NOS: 7 (4/0/3) | Only non-contact injuries | |
| Kim et al. (2013)37) | Retrospective, case control | Unmatched controls | Method by Charlton et al.25) |
| I: 72, C: 80 | Evidence: level III | Used injured knee | |
| Age: 40.91 ± 11.25 | NOS: 6 (3/0/3) | Nature of injury unspecified |
Values are presented as mean ± standard deviation or number.
NOS: Newcastle-Ottawa Scale, I: sample size of anterior cruciate ligament-injured group, C: sample size of control group, 3D CT: three-dimensional computed tomography, NA: not available.
ACL-Injured versus Controls (Overall Data Including Both Sexes)
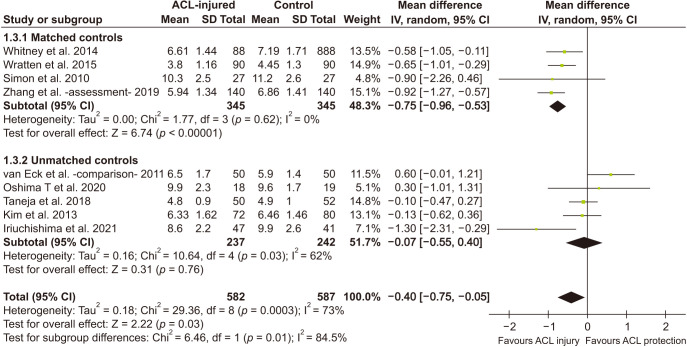
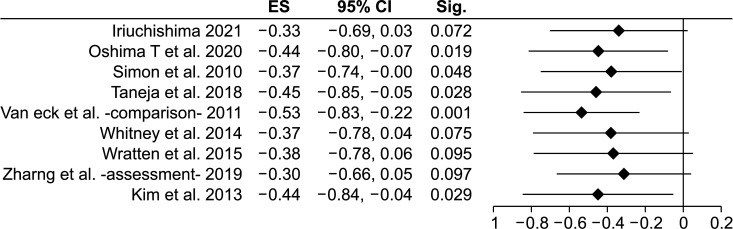
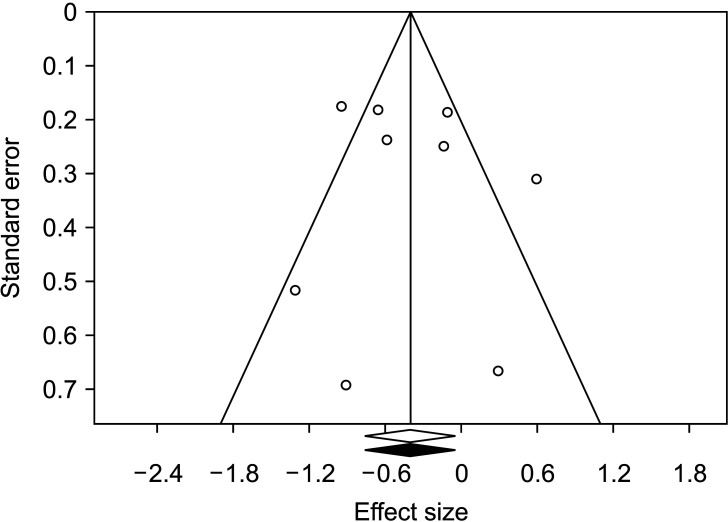
Nine studies14,22,23,24,32,34,35,36,37) qualified for this analysis that compared 582 injured knees with 587 controls. Pooled results showed significant difference between cases and controls (MD, −0.40 cm3; 95% CI, −0.75 to −0.05; p = 0.002) (Table 2). The negative value of MD points towards the ACL-injured group having smaller notch volumes compared to controls. Significant p-value denotes that effect estimate is different from null effect (zero MD). Substantial heterogeneity was noted (p = 0.0003, I2 = 73%, τ2 = 0.18 and a subgroup analysis was done based on pre-decided factors. Results of the analysis are detailed in Table 2. Subgroup analysis based on injury mechanism, method of measurement of notch volume, or use of the injured/contralateral knee for measurement did not explain heterogeneity adequately. Heterogeneity was best explained when the studies were grouped according to comparability of controls. Four studies14,22,23,36) had age and sex matching of controls, while 524,32,34,35,37) had unmatched controls. The difference between the two subgroups was statistically significant (p = 0.01) (Table 2). Pooled effect size of studies with matched controls was found to be −0.75 cm3 (95% CI, −0.96 to–0.53 without any heterogeneity (I2 = 0%, τ2 = 0.0). Fig. 2 depicts a forest plot of overall analysis with both sexes evaluated together. Sensitivity analysis by exclusion of a single study (Fig. 3) showed that pooled MD ranged from −0.53 cm3 (95% CI, −0.83 to −0.22; p = 0.001) to −0.30 cm3 (95% CI, −0.66 to 0.05; p = 0.097) with heterogeneity I2 ranging from 59% to 76%. Sensitivity analysis by segregating the prospective and retrospective studies showed no significant differences between the two subgroups (p = 0.729). The prospective group showed similar pooled MD (–0.45; 95% CI, −0.90 to −0.001; p = 0.0491; I2 = 47.64%; τ2 = 0.11), while the pooled effect size of the retrospective subgroup was nonsignificant (–0.32; 95% CI, −0.90 to 0.26; p = 0.278). Trim and fill analysis showed insignificant publication bias (Fig. 4) and the estimated effect size was the same as the observed effect size. Egger's linear regression test also revealed no publication bias (intercept 0.72, t = 0.43, p = 0.684).
Table 2. Overall Comparison: Male and Female Combined Analysis (ACL Injured vs. Control).
| Comparison* | Total knees (case vs. control) | Mean difference (95% CI, cm3) | Test for overall effect Z (p-value) | Subgroup difference | Comment |
|---|---|---|---|---|---|
| ACL injured vs. controls (male and female combined)14,22-24,32,34-37) | 1,169 (582 vs. 587) | –0.40 (–0.75 to –0.05) | Z = 2.22 (p = 0.03) | NA | Significant heterogeneity requires exploration. |
| Q = 29.36, df = 8 (p = 0.0003), I2 = 73%, τ2 = 0.18 95% prediction interval (–1.002 to 0.202) |
|||||
| Restricted to non-contact injury (4 studies)14,23,34,36 | 612 (305 vs. 307) | –0.57 (–1.02 to –0.11) | Z = 2.43 (p = 0.02) | Q = 0.75, df = 1 (p = 0.38), I2 = 0% | Restriction to non-contact ACL injury does not affect pooled effect size. |
| Q = 10.35, df = 3 (p = 0.02), I2 = 71%, τ2 = 0.14 | |||||
| Unrestricted (5 studies)22,24,32,35,37) | 557 (277 vs. 280) | –0.24 (–0.83 to 0.35) | Z = 0.78 (p = 0.43) | ||
| Q = 16.97, df = 4 (p = 0.002), I2 = 76%, τ2 = 0.31 | |||||
| Using injured knee (6 studies)22-24,32,34,37) | 902 (449 vs. 453) | –0.38 (–0.83 to 0.06) | Z = 1.68 (p = 0.09) | Q = 0.20, df = 1 (p = 0.66), I2 = 0% | Use of injured knee or uninjured contralateral knee does not affect pooled effect size. |
| Q = 27.35, df = 5 (p < 0.0001), I2 = 82%, τ2 = 0.24 | |||||
| Healthy knee as surrogate (3 studies)14,35,36) | 267 (133 vs. 134) | –0.52 (–0.94 to –0.10) | Z = 2.44 (p = 0.01) | ||
| Q = 1.87, df = 2 (p = 0.39), I2 = 0%, τ2 = 0.00 | |||||
| Standard method to measure (6 studies)22-24,35-37) | 925 (458 vs. 467) | –0.33 (–0.76 to 0.10) | Z = 1.49 (p = 0.14) | Q = 0.41, df = 1 (p = 0.52), I2 = 0% | Method of measurement of volume does not affect pooled effect size. |
| Q = 22.49, df = 5 (p = 0.0004), I2 = 78%, τ2 = 0.21 | |||||
| Other methods (3 studies) 14,32,34) | 244 (124 vs. 120) | –0.64 (–1.49 to 0.21) | Z = 1.48 (p = 0.14) | ||
| Q = 5.60, df = 2 (p = 0.06), I2 = 64%, τ2 = 0.36 | |||||
| Matched controls (4 studies)14,22,23,36) | 690 (345 vs. 345) | –0.75 (–0.96 to –0.53) | Z = 6.74 (p < 0.00001) | Q = 6.46, df = 1 (p = 0.01), I2 = 84.5% | ACL injured knees had smaller notch volume compared to matched controls. |
| Q = 1.77, df = 3 (p = 0.62), I2 = 0%, τ2 = 0.00 | |||||
| Unmatched controls (5 studies)24,32,34,35,37) | 479 (237 vs. 242) | –0.07 (–0.55 to 0.40) | Z = 0.31 (p = 0.76) | ||
| Q = 10.64, df = 4 (p = 0.03), I2 = 62%, τ2 = 0.16 | |||||
ACL: anterior cruciate ligament, CI: confidence interval, Q: Cochrane's Q statistic (χ2), df: degrees of freedom, τ2: tau2, NA: not available.
*All analyses used inverse-variance weighted random effects model. Unit of effect measure: mean difference in cm3.
Statistically significant, p < 0.05.
Fig. 2. Forest plot of overall analysis of the anterior cruciate ligament (ACL)-injured vs. control (both sexes considered together). Means in cm3. SD: standard deviation, IV: inverse variance weighted, Random: random effects analysis, CI: confidence interval, Chi2: Cochrane's Q, df: degrees of freedom; since effect size is mean difference, null effect is zero.
Fig. 3. Sensitivity analysis by exclusion of a single study (overall analysis of the anterior cruciate ligament-injured vs. control; both sexes considered together). ES: effect size (mean difference in cm3), CI: confidence interval; since effect size is mean difference, null effect is zero.
Fig. 4. Trim and fill analysis for publication bias. Visual inspection does not reveal any serious publication bias. Shaded effect size is estimated effect size, which is identical to the observed effect size.
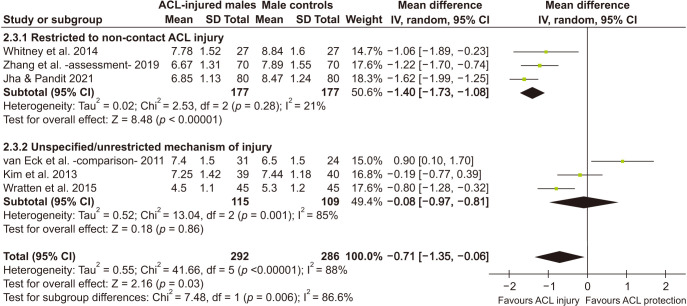
Male ACL-Injured versus Male Controls
Six studies22,23,24,33,36,37) qualified for this meta-analysis, of which 5 used the same method of measurement. One study utilized the similar method with one modification: used slice interval in addition to slice thickness in the calculation.33) Male ACL-injured patients had significantly smaller notch volume compared to controls (pooled MD = −0.71 cm3; 95% CI, −1.35 to −0.06; p = 0.03) but significant heterogeneity was present (I2 = 88%, τ2 = 0.55, p < 0.00001) (Table 3). Segregation of studies based on mechanism of injury explained the heterogeneity. Studies with restriction to non-contact injury showed negligible heterogeneity (I2 = 0%, τ2 = 0.02, p = 0.28 and significant difference was present between the two subgroups (p = 0.006). Other moderators failed to explain the heterogeneity. Fig. 5 shows a forest plot of this analysis. On sensitivity analysis with exclusion of any single study, pooled MD ranged from −0.50 cm3 (95% CI, −1.16 to 0.15 to −1.00 cm3 (95% CI, −1.50 to −0.50). The effect size altered maximally on exclusion of the study with maximal risk of bias.24)
Table 3. Comparison of ACL-Injured vs. Control: Males Only.
| Comparison* | Sample (knee) | Mean difference (95% CI) | Significance Z (p-value) | Remark | |
|---|---|---|---|---|---|
| Males (all studies)22-24,33,36,37) | 578 (292 vs. 286) | –0.71 (–1.35 to –0.06) | z = 2.16 (p = 0.03) | Significant heterogeneity needs exploration. | |
| Q = 41.66, df = 5 (p < 0.00001), I2 = 88%, τ2 = 0.55 | |||||
| Males: non-contact (3 studies)23,33,36) | 354 (177 vs. 177) | –1.40 (–1.73 to –1.08) | Z = 8.48 (p < 0.00001) | Subgroup difference Q = 7.48, df = 1 (p = 0.006), I2 = 86.6% | Non-contact ACL-injured males had smaller notch volumes. |
| Q = 2.53, df = 2 (p = 0.28), I2 = 21%, τ2 = 0.02 | |||||
| Males: unrestricted (3 studies)22,24,37) | 224 (115 vs. 109) | –0.08 (–0.97 to 0.81) | Z = 0.18 (p = 0.86) | Subgroup analysis based on mechanism of injury explains heterogeneity. | |
| Q = 13.04, df = 2 (p = 0.001), I2 = 85 %, τ2 = 0.52 | |||||
| Males: injured knee (5 studies)22-24,33,37) | 524 (265 vs. 259) | –0.64 (–1.37 to 0.09) | Z = 1.71 (p = 0.09) | Subgroup difference Q = 0.55, df = 1 (p = 0.46), I2 = 0% | Use of injured or healthy contralateral knee for measurement did not differ significantly. |
| Q = 41.62, df = 4 (p < 0.0001), I2 = 90%, τ2 = 0.62 | |||||
| Males: healthy knee (1 study)36) | 54 (27 vs. 27) | –1.06 (–1.89 to –0.23) | Z = 2.50 (p = 0.01) | ||
| NA | |||||
| Studies using standard method to measure (5 studies)22-24,36,37) | 418 (212 vs. 206) | –0.50 (–1.16 to 0.15) | Z = 1.51 (p = 0.13) | Subgroup difference Q = 8.46, df = 1 (p = 0.004), I2 = 88.2% | Method of measurement does not explain heterogeneity adequately. |
| Q = 23.73, df = 4 (p < 0.0001), I2 = 83%, τ2 = 0.45 | |||||
| Other methods33) | 160 (80 vs. 80) | –1.62 (–1.99 to –1.25) | Z = 8.64 (p < 0.00001) | ||
| NA | |||||
| Matched controls (4 studies)22,23,33,36) | 444 (222 vs. 222) | –1.21 (–1.61 to –0.81) | Z= 5.95 (p < 0.00001) | Subgroup difference Q = 6.93, df = 1 (p = 0.008), I2 = 85.6% | Because notch volume is unlikely to vary with age in adults, this association may be spurious. |
| Q = 7.52, df = 3 (p = 0.06), I2 = 60%, τ2 = 0.10 | |||||
| Unmatched controls (2 studies)24,37) | 134 (70 vs. 64) | 0.32 (–0.75 to 1.38) | Z = 0.59 (p = 0.56) | ||
| Q = 4.70, df = 1 (p = 0.03), I2 = 79%, τ2 = 0.47 | |||||
ACL: anterior cruciate ligament, CI: confidence interval, Q: Cochrane's Q statistic (χ2), df: degrees of freedom, τ2: tau2, NA: not available.
*All analyses used inverse-variance weighted random effects model. Unit of Effect measure: mean difference in cm3.
Statistically significant, p < 0.05.
Fig. 5. Forest plot (male anterior cruciate ligament [ACL]-injured vs. male control). Means in cm3. SD: standard deviation, IV: inverse variance weighted, Random: random effects analysis, CI: confidence interval, Chi2: Cochrane's Q, df: degrees of freedom; since effect size is mean difference, null effect is zero.
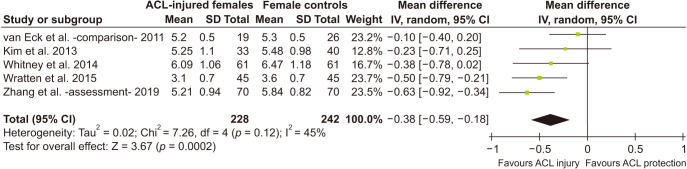
Female ACL-Injured versus Female Controls
When only females were considered, 5 studies22,23,24,36,37) qualified and all five used the same method of measurement. Table 4 and Fig. 6 show results of this analysis: females with ACL injury had smaller notch volume than controls (pooled MD, −0.38 cm3; 95% CI, −0.59 to −0.18; p = 0.0002) without any heterogeneity (I2 = 45%, τ2 = 0.02, p = 0.12).
Table 4. Comparison of ACL-Injured vs. Controls: Females Only.
| Comparison* | Sample (knee) | Mean difference (95% CI) | Significance Z (p-value) | Remark | |
|---|---|---|---|---|---|
| Female ACL-injured vs. controls (all studies)22-24,36,37) | 470 (228 vs. 242) | –0.38 (–0.59 to –0.18) | Z = 3.67 (p = 0.0002) | ACL-injured females had smaller notch volumes irrespective of nature of injury or injured/healthy knee used for measurement. | |
| Q = 7.26, df = 4 (p = 0.12), I2 = 45%, τ2 = 0.02 | |||||
| Females: non-contact injury (2 studies)23,36) | 262 (131 vs. 131) | –0.54 (–0.78 to –0.31) | Z = 4.51 (p < 0.00001) | Subgroup difference Q = 1.98, df = 1 (p = 0.16), I2 = 49.5% | Effect size increases in non-contact injury group. No significant difference between subgroups based on mechanism of injury |
| Females: unrestricted (3 studies)22,24,37) | 208 (97 vs. 111) | –0.29 (–0.55 to –0.02) | Z = 2.10 (p = 0.04) | ||
| Q = 3.67, df = 2 (p = 0.16), I2 = 45%, τ2 = 0.03 | |||||
| Females: injured knees (4 studies)22-24,37) | 348 (167 vs. 181) | –0.38 (–0.64 to –0.13) | Z = 2.92 (p = 0.003) | Subgroup difference Q = 0.00, df = 1 (p = 1.00), I2 = 0% | No significant difference between subgroups based whether injured or healthy contralateral knee was used |
| Females: healthy contralateral knee (1 study)36) | 122 (61 vs. 61) | –0.38 (–0.78 to 0.02) | Z = 1.87 (p = 0.06) | ||
| NA | |||||
ACL: anterior cruciate ligament, CI: confidence interval, Q: Cochrane's Q statistic (χ2), df: degrees of freedom, τ2: tau2, NA: not available.
*All analyses used inverse-variance weighted random effects model. Unit of Effect measure: mean difference in cm3.
Statistically significant, p < 0.05.
Fig. 6. Forest Plot (female anterior cruciate ligament [ACL]-injured vs. female control). Means in cm3. SD: standard deviation, IV: inverse variance weighted, Random: random effects analysis, CI: confidence interval, Chi2: Cochrane's Q, df: degrees of freedom; since effect size is mean difference, null effect is zero.
This difference increased further when only non-contact ACL injuries23,36) were considered (pooled MD, −0.54; 95% CI, −0.78 to −0.31; p < 0.00001; I2 = 0%; τ2 = 0.00); however, this subgroup showed no significant difference with the subgroup that did not restrict22,24,37) to non-contact ACL injuries. No subgroup difference was seen based on whether studies used injured22,23,24,37) or healthy contralateral knees36) for measurement. Since all studies used the same standard method of measurement, this moderator was rendered irrelevant. Sensitivity analysis showed nonsignificant effect on the pooled effect size. The maximal effect was seen after exclusion of the study with the highest risk of bias,24) but the effect size was still significantly different from the null effect (pooled MD, −0.49; 95% CI,–0.66 to −0.32; p < 0.00001; I2 = 0%; τ2 = 0.00).
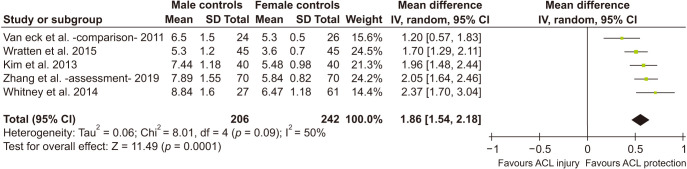
Differences between Male and Female Notch Volume
Using the same 5 studies,22,23,24,36,37) pooled data from male and female control groups were compared and significant difference between the two groups was noted. Notch volume of males was larger by 1.86 cm3 (95% CI, 1.54 to 2.18 as shown in Fig. 7. While the method of measurement was the same in all, other moderators were rendered irrelevant as this was a comparison of healthy controls.
Fig. 7. Forest plot (male controls vs. female control). Means in cm3. SD: standard deviation, IV: inverse variance weighted, Random: random effects analysis, CI: confidence interval, Chi2: Cochrane's Q, df: degrees of freedom; since effect size is mean difference, null effect is zero.
Due to the inadequate number of studies, formal assessment of publication bias in male and female analysis was not possible owing to limitations of tests.39) However, during quality of evidence assessment, some publication bias was suspected and accounted for. Prediction interval of the pooled effect at outcome level was not done due to the inadequate number of studies.40)
DISCUSSION
The most important finding of this study is that ACL-injured patients (both men and women combined) have smaller notch volumes than age- and sex-matched controls. When only males are considered, similar relationship exists, which amplifies with non-contact nature of ACL injury. ACL-injured women have significantly smaller notch volume irrespective of the nature of injury. In addition, ACL-intact men have higher notch volume than ACL-intact women. This is in accordance with our hypothesis that ACL-injured patients would have smaller notch volume than ACL-intact controls and that men would have larger notch volumes than women. Summary of findings for important outcomes is tabulated in Table 5 along with GRADE quality of evidence.
Table 5. GRADE Summary of Findings Table for Primary Outcomes.
| Outcome | Number of knees (study) | Assumed risk§ (cm3) | Corresponding risk | Quality of evidence¶ |
|---|---|---|---|---|
| ACL-injured vs. control (age, sex matched) | 690† (4 case control) | 4.45–11.20 | Notch volume in the ACL-injured is 0.75 cm3 lesser than in age and sex matched controls (0.53–0.96 cm3 lesser).∥ | Low** |
| Male ACL-injured vs. control* | 578† (6 case control) | 5.30–8.84 | Notch volume in ACL-injured males is 0.71 cm3 smaller than in controls (0.06–1.35 cm3 smaller).∥ | Very low††,‡‡ |
| Males with non-contact ACL injury vs. control | 354‡ (3 case control) | 7.89–8.84 | Notch volume in males with non-contact ACL injury is 1.40 cm3 smaller than in controls (1.08–1.73 cm3 smaller).∥ | Very low‡‡,§§ |
| Female ACL-injured vs. female control | 470† (5 case control) | 3.60–6.47 | Notch volume in ACL-injured females is 0.38 cm3 smaller than in controls (0.18–0.59 cm3 smaller).∥ | Low**,‡‡ |
| Male control vs. female control | 448‡ (5 case control) | 3.6–6.47 (Female control) | Notch volume in control males is 1.86 cm3 more than in female controls (1.54–2.18 cm3 larger).∥ | Very Low∥∥,‡‡ |
Notch volume measured on magnetic resonance imaging was compared between ACL-injured and uninjured population. Population: adult population, exposure: notch volume, comparator: adult population without ACL injury, outcome: ACL injury, studies: case control.
GRADE: Grading of Recommendations Assessment, Development, and Evaluation, ACL: anterior cruciate ligament, OIS: optimal information size.
*Concern for inconsistency by explaining heterogeneity (by exclusion of study with high risk of bias) was eliminated; however, authors decided to retain all studies and downgrade for inconsistency. †OIS criterion met. ‡OIS criterion not met. OIS calculated using α (0.05), β (0.20), minimal detectable difference in notch volume as 0.380 cm3. This value was chosen in the absence of an established minimal important difference for notch volume. The value was chosen arbitrarily based on the fact that 0.380 cm3 was the smallest pooled effect size in the above outcomes. Pooled standard deviations from all the included studies were used and the mean of those standard deviations was considered for calculation of OIS. §Calculated by considering means of notch volumes among control groups of included studies. ∥Minimal important difference for notch volume is unknown. ¶Quality of evidence starts as low quality as included studies are all observational. **Some concern regarding publication bias cannot be ruled out, but considered nonserious by authors. However, quality of evidence may be rated as “Very low” if one considers serious bias. ††Concern for inconsistency. ‡‡Concern for suspicion of some publication bias. §§Concern for imprecision. ∥∥Concern for indirectness.
Two theories may explain this relationship. First, a smaller notch volume accommodates a smaller, thinner, and vulnerable ACL, which could get injured at lower loads.21,25,41) Second, the theory suggests easier impingement of the ACL on the inner wall of the lateral femoral condyle during flexion, especially under tibial plateau rotation shear stress.21,42)
The literature regarding notch volume has two major limitations. First, there is a paucity of literature due to tedious and repetitive measurements required on multiple slices for estimation of notch volume. Second, there is considerable diversity in the existing literature. The diversity in literature is exemplified by differential analysis in studies. As with other 2D notch parameters, notch volume has also been found to have significant inter-sex variations.22,23,25) In addition, because female sex itself constitutes a separate risk factor5) for ACL injury, sex can act as a major confounder. For adequate internal validity, this confounder must be dealt with either by study design or appropriate statistical analysis. The literature has been somewhat lacking in this regard. We considered this as a major source of heterogeneity and therefore undertook separate sex analysis. Innate knee morphological risk factors such as notch volume should be evaluated in the setting of a non-contact ACL injury.43) Contact injuries can occur even in the absence of anatomical risk factors and may act as an effect modifier. Not restricting the cases to “non-contact ACL injuries” by some studies may contribute to statistical heterogeneity and thus was considered as another important moderator in the study. Differences in method of measurement contribute to variance in effect size and hence was hypothesized as a moderator. Charlton et al.25) in 2002 described a method of measurement on axial sections of MRI and this has been subsequently used by most authors. Pooled analysis of reliability of this method is hampered by a paucity of extractable data in the articles; however, interobserver reliability is considered high with coefficients ranging from 0.88 to 0.99.23,25,44) Some studies have used different methods of measurement including modification of the abovementioned method by including slice interval in the formulas, special reformatted volumetric sequences, and CT scans, whereas some did not specify the method of measurement.14,32,33,34) There is no data regarding superiority or accuracy of any particular method. Some studies have used the injured knee for notch measurements while others have used healthy contralateral knee as surrogates. It has been shown that the contralateral ACL volume and NWI can be used as surrogates for the injured side45,46) but the same has not been established for notch volume.
Sex-specific stratified analysis and explanation of heterogeneity by subgroup analysis based on pre-decided moderators contribute to the robustness of the present study. In the overall analysis (with both sexes combined), the significant heterogeneity in results was explained only by matching of controls. Compared to matched controls, ACL-injured patients have a smaller notch volume. This indirectly suggests that men and women have large difference in their notch volumes. Inter-sex comparison of controls also supports this result. Compared to ACL-intact women, ACL-intact men were found to have larger notch volumes. When only men were analyzed, significant heterogeneity was noted (with all the studies included). Further exploration of heterogeneity suggests that smaller notch volume is associated with non-contact ACL-injured men. Partial explanation of heterogeneity was also possible based on study methodology (matching of controls). Those with age-matched controls differed significantly with those with unmatched controls. The matched group, however, retained moderate heterogeneity (I2 = 60%). Furthermore, since notch volume is unlikely to differ with age, this association could be spurious.47) The method of measurement and use of injured knee/healthy contralateral knee did not adequately explain the heterogeneity (neither in overall analysis nor in men-only analysis).
Women with ACL injury were found to have significantly smaller notch volume and showed minimal heterogeneity (even when all studies were included). This suggests that notch volume in ACL-injured women is smaller irrespective of the nature of injury. This difference in notch volume amplified further when only non-contact ACL-injured patients were considered. Smaller notches and smaller/thinner ACL may lead to higher rates of injury in female athletes compared to male counterparts.23,48)
Markers of notch stenosis have been evaluated in other meta-analyses, including the very recent ones.19,20) The present study differs from these meta-analyses in two aspects. First, the abovementioned meta-analyses have considered 2D notch parameters such as NW and NWI. The present study considers a 3D parameter instead. Second, the present study anticipated statistical heterogeneity, and a conscious effort to explain the heterogeneity has been made based on very specific moderators that were decided a priori. The successful explanation of heterogeneity in the present study may also be partly because a 3D notch parameter such as notch volume may be a better marker for notch stenosis compared to the multiple, often differently measured 2D parameters.
Notch volume has been correlated with patient characteristics. Three studies23,24,35) found moderate to high positive correlations with height and weight (stronger for height), while 1 study22) did not find any correlation. The studies differed on many aspects including restriction to non-contact mechanism, use of healthy contralateral knee, and study methodology. Pooled analysis of this correlation was not attempted. Differences in height and weight between the two sexes may contribute to the difference in notch volume. Charlton et al.25) adjusted for height and weight and the difference of notch volume observed between the two sexes was rendered insignificant, suggesting that women had smaller notch volumes because of smaller height and weight. Only one study in our analysis matched controls according to height and weight, in addition to age and sex. On a multivariate regression model, neither sex nor height/weight were found to be significantly contributing to variance in notch volume (with ACL and posterior cruciate ligament volumes as factors).35) On the contrary, as a predictor for ACL injury, men had a higher odds ratio in a multivariate model.33) Meta-regression analysis using height, weight, and sex as covariates would help in this decision, but one would need at least 30 studies for such an analysis.
GRADE summary of findings49) for important outcomes are tabulated in Table 5. Being a meta-analysis of observational studies, the quality of evidence starts as low and as a result all the evidence generated here are categorized as very low to low. One of the major reasons for downgrading was inability to meet optimal information size (OIS) criteria and/or suspected publication bias. OIS was calculated using GRADE guidelines.50) A minimum notch volume difference to be detected was set arbitrarily at 0.38 cm3 (based on the lowest pooled effect size in all four meta-analyses) with a standard α (0.05) and β (0.20). A point to be stressed here is that the minimal important difference for notch volume has not been established and therefore this assumption had to be arbitrary.
Clinical implications of these results are many. Firstly, they provide a rationale for further research focusing on the ability to predict ACL injuries (determining appropriate cutoff/critical value of notch volume). Secondly, they underscore the need to screen athletes early to detect those at risk and institute customized training modules (e.g. neuromuscular control and strength training). Thirdly, indications for notchplasty can be explored based on notch volume in order to prevent repeat injury of reconstructed ACL graft and prevention of contralateral injury. Apart from ACL injury, treatment of mucoid degeneration of the ACL also may require objective identification of a stenotic notch. Notchplasty has been recommended in selective cases of mucoid degeneration of the ACL (those with a stenotic notch)51) and usefulness of notch volume may also be explored in such cases.
Inability to perform formal assessment of publication bias of sex-related outcomes is a major limitation of the study, which stems from scant literature. Broadening of inclusion criteria would neither have helped with the number of studies, nor would it have done justice to the objectives. Possible early reporting bias cannot be ruled out in this setting. Another obvious limitation is that the analysis is based on level III case-control studies, but this is the best available evidence currently. Retaining only prospective studies would leave a single study for sex-based analyses. In the overall analyses, subgroup analysis by segregating prospective and retrospective studies does not explain the heterogeneity, nor does it have adequate OIS, thus creating concerns regarding imprecision as well as inconsistency. Therefore, the authors decided to retain all the studies for evidence generation.
To conclude, ACL-injured adults have smaller notch volume than age- and sex-matched controls. Non-contact ACL-injured men have smaller notch volume compared to ACL-intact men. Women, however, have smaller notch volumes in ACL-injured patients irrespective of the nature of injury. Men have higher notch volume compared to women. The quality of evidence is very low to low.
ACKNOWLEDGEMENTS
Dr. Pooja Thakur, MS, contributed significantly in statistical analysis and interpretation for this study.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Moses B, Orchard J, Orchard J. Systematic review: annual incidence of ACL injury and surgery in various populations. Res Sports Med. 2012;20(3-4):157–179. doi: 10.1080/15438627.2012.680633. [DOI] [PubMed] [Google Scholar]
- 2.Souryal TO, Freeman TR. Intercondylar notch size and anterior cruciate ligament injuries in athletes: a prospective study. Am J Sports Med. 1993;21(4):535–539. doi: 10.1177/036354659302100410. [DOI] [PubMed] [Google Scholar]
- 3.Sood M, Kulshrestha V, Sachdeva J, Ghai A, Sud A, Singh S. Poor functional outcome in patients with voluntary knee instability after anterior cruciate ligament reconstruction. Clin Orthop Surg. 2020;12(3):312–317. doi: 10.4055/cios19143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith HC, Vacek P, Johnson RJ, et al. Risk factors for anterior cruciate ligament injury: a review of the literature - part 1: neuromuscular and anatomic risk. Sports Health. 2012;4(1):69–78. doi: 10.1177/1941738111428281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petushek EJ, Sugimoto D, Stoolmiller M, Smith G, Myer GD. Evidence-based best-practice guidelines for preventing anterior cruciate ligament injuries in young female athletes: a systematic review and meta-analysis. Am J Sports Med. 2019;47(7):1744–1753. doi: 10.1177/0363546518782460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer S, Meredith SJ, Wilson KW, et al. Knee morphological risk factors for anterior cruciate ligament injury: a systematic review. J Bone Joint Surg Am. 2020;102(8):703–718. doi: 10.2106/JBJS.19.00535. [DOI] [PubMed] [Google Scholar]
- 7.Wang YL, Yang T, Zeng C, et al. Association between tibial plateau slopes and anterior cruciate ligament injury: a meta-analysis. Arthroscopy. 2017;33(6):1248–1259.e4. doi: 10.1016/j.arthro.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Jaan T, Lopez-Alcorocho JM, Rodriguez-Inigo E, Castellan F, Hernandez JC, Guillen-Garcia P. The importance of the intercondylar notch in anterior cruciate ligament tears. Orthop J Sports Med. 2015;3(8):2325967115597882. doi: 10.1177/2325967115597882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen L, Jin ZG, Dong QR, Li LB. Anatomical risk factors of anterior cruciate ligament injury. Chin Med J (Engl) 2018;131(24):2960–2967. doi: 10.4103/0366-6999.247207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouras T, Fennema P, Burke S, Bosman H. Stenotic intercondylar notch type is correlated with anterior cruciate ligament injury in female patients using magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1252–1257. doi: 10.1007/s00167-017-4625-4. [DOI] [PubMed] [Google Scholar]
- 12.van Diek FM, Wolf MR, Murawski CD, van Eck CF, Fu FH. Knee morphology and risk factors for developing an anterior cruciate ligament rupture: an MRI comparison between ACL-ruptured and non-injured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):987–994. doi: 10.1007/s00167-013-2588-7. [DOI] [PubMed] [Google Scholar]
- 13.Vrooijink SH, Wolters F, Van Eck CF, Fu FH. Measurements of knee morphometrics using MRI and arthroscopy: a comparative study between ACL-injured and non-injured subjects. Knee Surg Sports Traumatol Arthrosc. 2011;19 Suppl 1:S12–S16. doi: 10.1007/s00167-011-1502-4. [DOI] [PubMed] [Google Scholar]
- 14.Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43(9):1702–1707. doi: 10.1016/j.jbiomech.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JS, Nam DC, Kim DH, Kim HK, Hwang SC. Measurement of knee morphometrics using MRI: a comparative study between ACL-injured and non-injured knees. Knee Surg Relat Res. 2012;24(3):180–185. doi: 10.5792/ksrr.2012.24.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoteya K, Kato Y, Motojima S, et al. Association between intercondylar notch narrowing and bilateral anterior cruciate ligament injuries in athletes. Arch Orthop Trauma Surg. 2011;131(3):371–376. doi: 10.1007/s00402-010-1254-5. [DOI] [PubMed] [Google Scholar]
- 17.Stein V, Li L, Guermazi A, et al. The relation of femoral notch stenosis to ACL tears in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2010;18(2):192–199. doi: 10.1016/j.joca.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade R, Vasta S, Sevivas N, et al. Notch morphology is a risk factor for ACL injury: a systematic review and meta-analysis. J ISAKOS. 2016;1:70–81. [Google Scholar]
- 19.Li H, Zeng C, Wang Y, et al. Association between magnetic resonance imaging-measured intercondylar notch dimensions and anterior cruciate ligament injury: a meta-analysis. Arthroscopy. 2018;34(3):889–900. doi: 10.1016/j.arthro.2017.08.299. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Li C, Li L, Wang P. Correlation between notch width index assessed via magnetic resonance imaging and risk of anterior cruciate ligament injury: an updated meta-analysis. Surg Radiol Anat. 2020;42(10):1209–1217. doi: 10.1007/s00276-020-02496-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Zhang X, Fang Z, et al. The correlation between common 2D femoral notch parameters and 3D notch volume: a retrospective MRI study. BMC Musculoskelet Disord. 2019;20(1):146. doi: 10.1186/s12891-019-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wratten CJ, Tetsworth K, Hohmann E. Three-dimensional femoral notch volume in anterior cruciate ligament-deficient versus anterior cruciate ligament-intact patients: a matched case-control study with inter-gender comparison. Arthroscopy. 2015;31(6):1117–1122. doi: 10.1016/j.arthro.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Xie G, Fang Z, Zhang X, Huangfu X, Zhao J. Assessment of relationship between three dimensional femoral notch volume and anterior cruciate ligament injury in Chinese Han adults: a retrospective MRI study. Int Orthop. 2019;43(5):1231–1237. doi: 10.1007/s00264-018-4068-7. [DOI] [PubMed] [Google Scholar]
- 24.van Eck CF, Kopf S, van Dijk CN, Fu FH, Tashman S. Comparison of 3-dimensional notch volume between subjects with and subjects without anterior cruciate ligament rupture. Arthroscopy. 2011;27(9):1235–1241. doi: 10.1016/j.arthro.2011.03.085. [DOI] [PubMed] [Google Scholar]
- 25.Charlton WP, St John TA, Ciccotti MG, Harrison N, Schweitzer M. Differences in femoral notch anatomy between men and women: a magnetic resonance imaging study. Am J Sports Med. 2002;30(3):329–333. doi: 10.1177/03635465020300030501. [DOI] [PubMed] [Google Scholar]
- 26.Alentorn-Geli E, Pelfort X, Mingo F, et al. An evaluation of the association between radiographic intercondylar notch narrowing and anterior cruciate ligament injury in men: the notch angle is a better parameter than notch width. Arthroscopy. 2015;31(10):2004–2013. doi: 10.1016/j.arthro.2015.04.088. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 [Internet] London: Cochrane Training; 2019. [cited 2021 Apr 30]. Available from: www.training.cochrane.org/handbook . [Google Scholar]
- 30.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] Ottawa, ON: Ottawa Hospital Research Institute; 2000. [cited 2021 Apr 30]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . [Google Scholar]
- 31.Schunemann H, Brozek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach [Internet] London: Cochrane Training; 2013. [cited 2021 Apr 30]. Available from: https://training.cochrane.org/resource/grade-handbook . [Google Scholar]
- 32.Iriuchishima T, Goto B, Fu FH. Truncated-pyramid shape simulation for the measurement of femoral intercondylar notch volume can detect the volume difference between ACL-injured and intact subjects. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1709–1713. doi: 10.1007/s00167-020-06204-0. [DOI] [PubMed] [Google Scholar]
- 33.Jha V, Pandit A. Notch volume measured on magnetic resonance imaging is better than 2-dimensional notch parameters for predicting noncontact anterior cruciate ligament injury in males. Arthroscopy. 2021;37(5):1534–1543.e1. doi: 10.1016/j.arthro.2020.11.050. [DOI] [PubMed] [Google Scholar]
- 34.Taneja AK, Miranda FC, Demange MK, et al. Evaluation of posterior cruciate ligament and intercondylar notch in subjects with anterior cruciate ligament tear: a comparative flexed-knee 3D magnetic resonance imaging study. Arthroscopy. 2018;34(2):557–565. doi: 10.1016/j.arthro.2017.08.296. [DOI] [PubMed] [Google Scholar]
- 35.Oshima T, Putnis S, Grasso S, Parker DA. The space available for the anterior cruciate ligament in the intercondylar notch is less in patients with ACL injury. Knee Surg Sports Traumatol Arthrosc. 2020;28(7):2105–2115. doi: 10.1007/s00167-020-05921-w. [DOI] [PubMed] [Google Scholar]
- 36.Whitney DC, Sturnick DR, Vacek PM, et al. Relationship between the risk of suffering a first-time noncontact ACL injury and geometry of the femoral notch and ACL: a prospective cohort study with a nested case-control analysis. Am J Sports Med. 2014;42(8):1796–1805. doi: 10.1177/0363546514534182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HK, Moon DK, Gwark JY, Nam DC, Kim DH, Hwang SC. Correlation of notch configuration between subjects with and subjects without anterior cruciate ligament injury. J Korean Orthop Assoc. 2013;48(6):457–463. [Google Scholar]
- 38.Zhang C, Xie G, Dong S, et al. A novel morphological classification for the femoral notch based on MRI: a simple and effective assessment method for the femoral notch. Skeletal Radiol. 2020;49(1):75–83. doi: 10.1007/s00256-019-03255-4. [DOI] [PubMed] [Google Scholar]
- 39.Page MJ, Higgins JP, Sterne JA. In: Cochrane handbook for systematic reviews of interventions version 6.0. Higgins JP, Thomas J, Chandler J, et al., editors. London: Cochrane Training; 2019. Assessing risk of bias due to missing results in a synthesis. [Google Scholar]
- 40.Deeks JJ, Higgins JP, Altman DG. In: Cochrane handbook for systematic reviews of interventions version 6.0. Higgins JP, Thomas J, Chandler J, et al., editors. London: Cochrane Training; 2019. Analysing data and undertaking meta-analyses. [Google Scholar]
- 41.Chandrashekar N, Slauterbeck J, Hashemi J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. Am J Sports Med. 2005;33(10):1492–1498. doi: 10.1177/0363546504274149. [DOI] [PubMed] [Google Scholar]
- 42.Thein R, Spitzer E, Doyle J, et al. The ACL Graft has different cross-sectional dimensions compared with the native ACL: implications for graft impingement. Am J Sports Med. 2016;44(8):2097–2105. doi: 10.1177/0363546516645531. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer CE, Beattie PF, Sacko RS, Hand A. Risk factors associated with non-contact anterior cruciate ligament injury: a systematic review. Int J Sports Phys Ther. 2018;13(4):575–587. [PMC free article] [PubMed] [Google Scholar]
- 44.Zbrojkiewicz D, Scholes C, Zhong E, Holt M, Bell C. Anatomical variability of intercondylar fossa geometry in patients diagnosed with primary anterior cruciate ligament rupture. Clin Anat. 2020;33(4):610–618. doi: 10.1002/ca.23465. [DOI] [PubMed] [Google Scholar]
- 45.Jamison ST, Flanigan DC, Nagaraja HN, Chaudhari AM. Side-to-side differences in anterior cruciate ligament volume in healthy control subjects. J Biomech. 2010;43(3):576–578. doi: 10.1016/j.jbiomech.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teitz CC, Lind BK, Sacks BM. Symmetry of the femoral notch width index. Am J Sports Med. 1997;25(5):687–690. doi: 10.1177/036354659702500517. [DOI] [PubMed] [Google Scholar]
- 47.Tuca M, Hayter C, Potter H, Marx R, Green DW. Anterior cruciate ligament and intercondylar notch growth plateaus prior to cessation of longitudinal growth: an MRI observational study. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):780–787. doi: 10.1007/s00167-016-4021-5. [DOI] [PubMed] [Google Scholar]
- 48.Montalvo AM, Schneider DK, Webster KE, et al. Anterior cruciate ligament injury risk in sport: a systematic review and meta-analysis of injury incidence by sex and sport classification. J Athl Train. 2019;54(5):472–482. doi: 10.4085/1062-6050-407-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence: imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Ventura D, Nunez JH, Joshi-Jubert N, Castellet E, Minguell J. Outcome of arthroscopic treatment of mucoid degeneration of the anterior cruciate ligament. Clin Orthop Surg. 2018;10(3):307–314. doi: 10.4055/cios.2018.10.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]