Abstract
Background
Periosteum plays a significant role in bone formation and regeneration by storing progenitor cells, and also acts as a source of local growth factors and a scaffold for recruiting cells and other growth factors. Recently, tissue-engineered periosteum has been studied extensively and shown to be important for osteogenesis and chondrogenesis. Using biomimetic methods for artificial periosteum synthesis, membranous tissues with similar function and structure to native periosteum are produced that significantly improve the efficacy of bone grafting and scaffold engineering, and can serve as direct replacements for native periosteum. Many problems involving bone defects can be solved by preparation of idealized periosteum from materials with different properties using various techniques.
Methods
This review summarizes the significance of periosteum for osteogenesis and chondrogenesis from the aspects of periosteum tissue structure, osteogenesis performance, clinical application, and development of periosteum tissue engineering. The advantages and disadvantages of different tissue engineering methods are also summarized.
Results
The fast-developing field of periosteum tissue engineering is aimed toward synthesis of bionic periosteum that can ensure or accelerate the repair of bone defects. Artificial periosteum materials can be similar to natural periosteum in both structure and function, and have good therapeutic potential. Induction of periosteum tissue regeneration and bone regeneration by biomimetic periosteum is the ideal process for bone repair.
Conclusions
Periosteum is essential for bone formation and regeneration, and it is indispensable in bone repair. Achieving personalized structure and composition in the construction of tissue engineering periosteum is in accordance with the design concept of both universality and emphasis on individual differences and ensures the combination of commonness and individuality, which are expected to meet the clinical needs of bone repair more effectively.
The translational potential of this article
To better understand the role of periosteum in bone repair, clarify the present research situation of periosteum and tissue engineering periosteum, and determine the development and optimization direction of tissue engineering periosteum in the future. It is hoped that periosteum tissue engineering will play a greater role in meeting the clinical needs of bone repair in the future, and makes it possible to achieve optimization of bone tissue therapy.
Keywords: Biomaterials, Bone defect healing, Bone repair, Osteogenesis, Periosteum, Tissue-engineered periosteum
Abbreviations: AF Antheraea pernyi fibroin, AMSCs adipose mesenchymal stem cells; BMP bone morphogenetic proteins, BMP-2 bone morphogenetic protein-2; BMSCs bone marrow stromal cell, CaPs calcium phosphate nanoparticles, COL I collagen I; co-PUPCL a mixed fiber formed by PCL and polyurethane, DEX dexamethasone; DOP dopamine, DSCs dental pulp stem cells; ECM extracellular matrix, GBR guided bone regeneration; GelMA methacrylate gelatin, HA hydroxyapatite; HAM human amniotic membrane, HCP human cultured periosteum; ICA Icariin, IGF-1 insulin-like growth factor-1; MBGNs mesoporous bioglass nanoparticles, MOX moxifloxacin hydrochloride; MSCs mesenchymal stem cells, n-HA nano-hydroxyapatite; OCN osteocalcin, OSX osterix; PCL polycaprolactone, PDCs periosteum derived cells; PDGF-BB platelet-derived growth factor-BB, PDO periosteal distraction osteogenesis; PEEK polyetheretherketone, PLA polylactic acid; PLLA l-lactic acid, PRP platelet-rich plasma; PU degradable polyurethane fibers without nano-hydroxyapatite, PUHA degradable polyurethane fibers with nano-hydroxyapatite; PVA polyvinyl alcohol, rhBMP-2 recombinant human bone morphogenetic protein-2; rMSCs rat mesenchymal stem cells, Runx2 Runt-related transcription factor 2; SEM scanning electron microscope, SF silk fibroin; SiNPs Silica nanoparticles, SIS small intestinal submucosa; s-PEEK sulfonated PEEK, SSCs skeletal stem cells; SSP synthetic scaffold periosteum, TCP tricalcium phosphate; TGF-β transforming growth factor-β, VEGF vascular endothelial growth factors
1. Introduction
Periosteum is a connective tissue envelope that covers the surface of bone. It consists of an inner layer and an outer layer containing various cells with osteogenic potential and abundant capillaries. The complex and multifunctional structure provides a niche for pluripotent cells and molecular factors that modulate cell behaviour, allowing periosteum to act as a repository. At the stage of fracture healing, osteoprogenitor cells in periosteum differentiate into cells with osteogenic potential that significantly promote fracture recovery. All components of periosteum are important factors for bone development and regeneration. Moreover, periosteum shows advanced and outstanding material properties, and its mechanical strength, chemical properties, and biological state are the characteristics that determine its material properties changes. However, autologous periosteum transplantation is limited by the finite source and the patient's health, and it is easy to cause deep tissue infection and chronic pain at the collection site. Allogeneic periosteum transplantation also has some disadvantages, such as immune rejection and easy pathogen transmission. To solve these problems, technology for efficient artificial periosteum construction by tissue engineering methods based on the characteristics of periosteum has emerged [1,2]. At present, the fast-developing field of periosteum tissue engineering is aimed toward synthesis of bionic periosteum that can ensure or accelerate the repair of bone defects. Artificial periosteum materials can be similar to natural periosteum in both structure and function, and have good therapeutic potential. Induction of periosteum tissue regeneration and bone regeneration by biomimetic periosteum is the ideal process for bone repair. With advances in tissue-engineered periosteum, research on bionic periosteum is becoming increasingly mature and reliable. Related research on tissue-engineered periosteum not only directs the development of bone tissue engineering, but also makes it possible to achieve optimization of bone tissue therapy [3].
2. Histological structure of periosteum
Periosteum is a connective tissue rich in microvascular components that covers the bone surface and is closely connected to the bone cortex by Sharpey fibres. Some researchers divide periosteum into two layers traditionally: outer fibrous layer with abundant fibrocytes and inner layer with significant osteogenic potential [4]. Meanwhile, other researchers divide periosteum into three layers from the outside-in on functional and anatomical grounds: outer fibrous layer, undifferentiated layer, and inner cambium layer [5,6] (Fig. 1). The importance of the undifferentiated layer is that it acts as a ‘shock absorber’, transferring pressure and tension above the physiological range to the osteogenic layer and initiating the surface remodeling seen in situ [7].
Fig. 1.
Histological hierarchy of the periosteum.
The outer fibrous layer can be divided into two parts: superficial layer and deep layer. The superficial layer, composed of collagen matrix and a small amount of elastic fibres, has weak elasticity and relatively few cells, but a rich neural network [6]. The superficial layer matrix has the highest degree of vascularization within periosteum tissue, and is the main source of blood supply for the bone and skeletal muscle. The deep layer, so-called fibrous elastic layer because of its many elastic fibres, is also highly collagenous and has few cells like the superficial layer, but its blood vessels are poorly developed. Periosteal tendon adhesion often ends in this fibroelastic substratum [8]. The outer fibrous layer has a fixed role of providing elasticity and flexibility, and allows resistance of pressure and tension.
The undifferentiated layer is a relatively transparent zone mainly occupied by capillaries and amorphous extracellular matrix (ECM). In this layer, fibroblasts and collagen fibres are abundant. As polymorphic cells of mesenchymal origin, pericytes partially surround the capillaries [6]. This layer provides progenitor cells for the outer fibrous layer and inner cambium layer and plays an essential role in regulating, supporting, and buffering the reconstruction of bone tissue [[9], [10], [11]].
The inner cambium layer is highly cellular and contains a variety of bone cells, including mesenchymal progenitor cells, differentiated osteogenic progenitor cells, osteoblasts, and fibroblasts, in a sparse collagenous matrix. The osteoblasts make contact with the bone cortical surface and are surrounded by small dense cells resembling fibroblasts within a fairly extensive peripheral vascular and sympathetic network. Because of the nature of the structure, there are many endothelial pericytes, whose osteogenic potential has been demonstrated in numerous studies, that can be used as a reliable auxiliary source of progenitor cells [12,13]. The composition of periosteum tissue changes with age, and the changes are most pronounced in this layer [14,15]. The numbers of osteoprogenitor cells and fibroblasts decrease with age [16], and adult periosteum is just a thin layer of tissue surrounding the bone structure [17]. The change is most pronounced in the inner cambium layer. Although periosteum changes during development, it is significant for bone remodelling and bone repair. For mechanical reasons, direct periosteum stimulation therapy may yield better anti-fracture efficacy than drugs targeting endosteal and trabecular cell populations [15].
Periosteal circulation is essential for the process of bone vascularization. Abundant blood supply provides not only sufficient nutrients for periosteum growth, but also necessary nutrients and cells for bone repair [18,19]. There are four main sources of periosteum blood supply. The first is the intrinsic periosteal system spread over the periosteum fibrous layer. The second is the musculoperiosteal system formed by connections between the muscle circulation and periosteal vessels at the muscle source. The third is the fascioperiosteal system consisting of limb artery branches at the fascial level between the muscles that supply the periosteum. The fourth is periosteocortical anastomoses that can attach periosteum and form a capillary network with blood vessels inside Haversian canals, passing into the bone marrow capillary network inside to achieve nutrition and moistening [5,19].
The nerve fibres in periosteum are predominantly unmyelinated nerve fibres, and their free nerve endings may be associated with perception of pain. Peptogenic nerves containing substance P are highly expressed after periosteum injury, resulting in sensitivity and persistent pain. Periosteum also has nerve fibres containing vasoactive intestinal peptides that regulate the capillary sphincter to control vascular tension and blood flow, but the specific mechanism remains to be clarified [20].
3. Function of periosteum
Periosteum is extremely important in the process of osteogenesis, it can not only provide needed substances and cells in the osteogenesis stage [21,22], but also play a role of bone repair under the stimulation of biological regulatory factors [[23], [24], [25]].At the same time, periosteum can also promote osteogenesis under mechanical stimulation [26].
3.1. Periosteum provides nutrients and cells with differentiation potential for the osteogenesis stage
Periosteum has an abundant capillary system that can provide oxygen, minerals, and other materials needed to rebuild bone tissue, while its vascular network is the guarantor of bone vascularization [21]. Periosteum also contains various cells with osteogenic potential, and after its stimulation by physical, chemical, or biological factors, mesenchymal stem cells (MSCs) rapidly differentiate into cells such as osteoprogenitor cells, osteoblasts, and chondroblasts. Factors like low oxygen concentration, low intensity pulse, donor age, and bone morphogenetic protein (BMP) concentrations all affect the osteogenic differentiation process of MSCs [22]. Meanwhile, the differentiation process for osteoblasts is mainly affected by genetic factors [27], hormone levels, and cell regulatory factors. Osteoblasts express receptors that regulate osteogenic factors such as parathyroid hormone, prostaglandin, and epinephrine, and the related factors can regulate the differentiation degree of osteoblasts and their precursors by binding to the receptors surrounding osteoblasts, thus participating in the repair and reconstruction of bone tissue.
3.2. Periosteum participates in bone repair under stimulation by osteogenic factors
By stimulating and regulating MSCs, osteoblasts, and chondroblasts in periosteum, osteogenic growth factors affect the expression levels of genes such as RANKL and SOST [28], and participate in the repair and reconstruction of bone tissue to varying degrees, thereby promoting osteogenesis [29].
BMPs comprise a group of highly conserved soluble bone matrix glycoproteins with similar structures. Studies have shown that BMPs can upregulate the expression of the osteogenic lineage genes, osterix (OSX) and osteocalcin (OCN) significantly, and also induce Runt-related transcription factor 2 (Runx2) expression in mesenchymal progenitors in a Smad-dependent manner [30]. Proper amounts of BMPs not only accelerate osteogenesis at bone defect sites [31], improve the success rate of bone non-union healing [32], and promote the generation of blood vessels at bone repair sites [31,33], but also reduce the occurrence of complications such as infections and pain associated with fracture [32].
Insulin-like growth factor (IGF)-1 significantly affects the expression of OSX, and promotes the expression of osteoblast marker genes. IGF-1 is a critically important regulator of bone mechanosensitivity, the osteocyte-derived IGF-1 is an important mediator of the osteogenic response to loading. Also it acts as an autocrine effector to rapidly upregulate cyclooxygenase-2 and Wnt10b expression, and at the same time to suppress SOST expression. The IGF-1 and prostaglandin E2 have a synergistic effect on bone surface osteoblasts to activate corresponding pathways in turn [34]. IGF-1 is involved in the normal metabolism of cartilage and the formation and repair of chondrocytes after cartilage injury. It is indispensable for bone repair and reconstruction, as shown by a study that employed the skull of IGF-1 gene knockout mice as a bone defect model and found a 14% reduction in the healed tissue thickness compared with control mice [35].
Transforming growth factor (TGF)-β, which promotes the proliferation and differentiation of osteoblasts in periosteum, mainly exists in platelets and bone tissue. TGF-β2 functions as a morphogenetic inducer for mesenchymal progenitors and macrophages such as osteal macrophages, osteoclasts or chondroclasts,TGF-β2 and RANKL may carry out a synergistic effect on osteoclast precursors. Metalloproteinases, related to inflammation, are also one of potential downstream genes of TGF-β signaling [36].It not only participates in the process of bone resorption by osteoclasts and the formation of new cartilage, but also induces endochondral angiogenesis, promotes vascular activity, and accelerates osteogenesis efficiency [37,38]. The role of TGF-β in modulating angiogenesis is associated with distinct TGF-b type Ι receptor/Smad pathways [37].
3.3. Periosteum promotes osteogenesis under mechanical stimulation
Normally, before the stress is applied, periosteum is in a state of tension, which acts as an elastic membrane to inhibit the growth of cartilage and is maintained by the elastic properties of fibrous periosteum. When the bone bends, the periosteum tends to contract. Because the connection between the periosteum and the bone is maintained, the tension is transmitted to the bone surface. It has been proved that changes in bones during bending is not only the result of internal strain of bones, but also the result of transformation of bones wrapped in soft tissues. The relative movement of bone causes the tension of periosteum, leading to the formation of new bone. In order to remodel the bone, the mechanical stress exerted on the periosteum will eventually be transformed into a cellular response. The periosteum changes with stress, and osteoblasts rearrange along the direction of tension decomposition, which may be influenced by the force passing through the mid and outer layers of periosteum. Delayed reaction and obvious structural changes in the mid zone further indicate that this layer can play a buffering role, so the undifferentiated layer plays an important regulatory role in bone remodeling [7,39]. At the same time, under the action of mechanical stimulation, periosteum can also induce the gene expression changes of Wnt signaling pathway, BMP signaling pathway and various cytokines, and jointly promote the differentiation and osteogenesis of osteoblasts [26,40,41].
4. Periosteum-mediated forms of osteogenesis
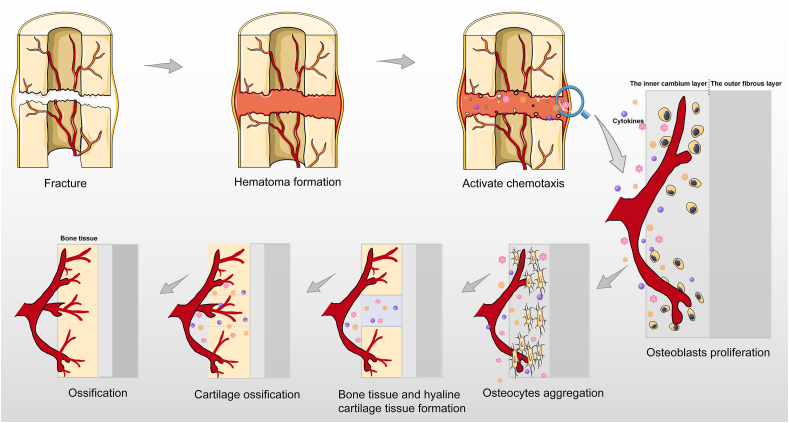
After fracture occurrence, a hematoma is formed, and chemotaxis is activated by the release of molecules such as growth factors, cytokines, and interleukins. The periosteum cambium cells proliferate and differentiate to produce different effects on different parts of the blood supply, new bone forms distal to the fracture site with sufficient blood supply, and cartilage forms at the fracture site with insufficient blood supply. Subsequently, new angiogenesis is observed, thereby promoting blood supply, cartilage absorption, and bone formation through endochondral osteogenesis(Fig. 2).
Fig. 2.
Diagram of bone and blood vessel formation after fracture.
4.1. Two main types of periosteum osteogenesis: intramembranous ossification and entochondrostosis
Intramembranous ossification is relatively simple. When bone tissue is damaged, mesenchyme differentiates into an embryonic connective tissue membrane and osteogenesis occurs within this membrane. When periosteum is in contact with the hematoma at a fracture site, MSCs in periosteum differentiate into osteoprogenitor cells that subsequently form osteoblasts. Osteoblasts secrete and embed osteoid, and the osteoid matrix is calcified to form bone tissue(Fig. 3).
Fig. 3.
Two main types of periosteum osteogenesis: intramembranous ossification and entochondrostosis.
Endochondral bone repair is one of the important forms of bone repair and the main form of osteogenesis after bionic periosteum implantation [42]. During this period, periosteum plays a major role. After fracture occurrence, the hematoma develops gradually and is stabilized by the surrounding soft tissue, followed shortly thereafter by stabilization through the reconstructed periosteum fibrous layer. In the area with favourable blood supply around the fracture, the inner cambium layer cells proliferate and differentiate, forming a bone collar by membranous ossification. Near the fracture, the inner cambium layer of periosteum generates a large number of chondrocytes, followed by recurrence of foetal bone formation through an endochondral ossification process. Finally, the cartilage is replaced with bone tissue [43](Fig. 3).
The undifferentiated progenitor cells retained on the bone surface eventually form periosteum by the intramembranous ossification pathway, while surrounding perichondrium is mainly formed by the endochondral osteogenesis pathway. Thereafter, perichondrium can be transformed into periosteum by an appropriate developmental regulation mechanism [44]. A previous study showed that, compared with bone marrow stem cells (BMSCs), periosteum-derived cells (PDCs) show better bone regeneration potential, clone growth, and differentiation ability [45].
4.2. The ECM of periosteum cooperates with osteogenesis
The ECM of periosteum is important for the process of osteogenesis by providing microstructures and appropriate biochemical signals for cell differentiation and maturation. In addition, the ECM effectively mediates acellular mineralization during bone formation, and promotes regeneration in situ and heterotopic ossification of bone defects [46].
Periosteum fibres are important components of the periosteum ECM, as the gaps between collagen fibrils in decellularized natural periosteum ECM scaffolds allow enrichment of calcium, phosphate, and carbonate ions, further inducing formation of apatite. The collagen matrix also stabilizes the precursor crystals, thereby controlling the physical characteristics of growing apatite particles [46]. The Sharpey fibres in periosteum are one of the main elements for ECM regulation because they modify the “quality” of the bone matrix structure they occupy. Above all, the fibres provide an unabridged scaffold for skeletal self-repair [47]. Many growth factors are also present in the ECM, such as BMP-2, IGF-1, and TGF-β, and these factors have an irreplaceable role in bone repair [32,35,37].
4.3. Periosteal vascular remodelling promotes osteogenesis
The abundant blood supply of periosteum not only meets its own metabolic needs, but also nourishes the surrounding bone tissue through vascular branches. After bone tissue damage occurs, periosteal vessels appear at the corresponding site. The new blood vessels quickly connect with one another to establish a new microcirculation that provides a blood transport basis for the formation of new bone. As mentioned above, periosteum is highly vascular. The pericytes in the postcapillary venules in periosteum can proliferate and differentiate into osteoblasts and become a supplementary source of osteoblasts during periosteum osteogenesis [48].
5. Application of periosteum osteogenesis in related fields
5.1. Periosteum-induced osteogenesis
Periosteum-induced osteogenesis is involved in the repair of small local bone defects through self-proliferation of periosteum at the broken ends of the fracture. In a study to determine the smallest canine mandible defect that would not show spontaneous healing in nature, presence of periosteum was associated with fast healing in small mandible defects and difficulty in healing for defects >50 mm in diameter, while removal of periosteum was associated with failure to heal for defects >15 mm in diameter [49]. Periosteum-induced osteogenesis has the characteristics of reduced trauma and simple operation, but the method is less osteogenic and slower than other methods such as periosteal distraction osteogenesis and periosteum transplantation.
5.2. Periosteal distraction osteogenesis
Periosteal distraction osteogenesis takes advantage of periosteum osteogenesis by gradually expanding the periosteum to artificially create space between the bone surface and the periosteum, leading to new bone generation without the need for corticotomy [50]. When traction force is applied to the bone fracture, undifferentiated MSCs in periosteum differentiate into highly active osteoblasts, and the newly formed bone tissue repairs the bone defect after a series of reactions such as calcification and remodelling. Similarly, when tension is located on periosteum at both ends of the bone defect, it promotes expression of osteoblasts, and gradual retraction of periosteum leads to formation of a gap below the periosteum that becomes invaded by the surrounding soft tissue, thereby stimulating the periosteum again and accelerating osteoblast secretion and new bone formation [51].
5.3. Bone formation by periosteum transplantation
Periosteum grafts include free periosteum grafts and vascularized periosteum grafts. The former grafts are less osteogenic than the latter grafts because of the influence of the regional blood supply. For example, Ritsila et al. [52] successfully repaired congenital maxillary defects in a rabbit model with free periosteum grafts and then performed spinal fusion in the same rabbit model, and found that, compared with traditional spinal fusion, spinal fusion with free periosteum grafts reduced the incidence of postoperative complications. Meanwhile, Ritsila et al. [53,54] used vascularized periosteum grafts to repair rabbit tibial bone defects, and found that more bone was generated in the vascularized periosteum transplantation group. The method of osteogenesis by periosteum transplantation expands the scope of periosteum transplantation, increases the selection of the donor area, improves local blood circulation at the wound surface, and plays the role of a biomembrane to prevent the surrounding soft tissue from entering the bone defect area, which is conducive to bone healing.
5.4. Clinical application of periosteum
Implantation of periosteum to repair bone defects and accelerate bone formation has had limited success in clinical trials. Yang et al. [55] used autogenous periosteum transplantation to repair large cartilage defects in hip and knee joints, and observed long-term efficacy. Of the 52 patients evaluated, 37 had normal joint function and good radiographic performance during a complete follow-up of >10 years, confirming the role of autogenous periosteum in promoting the repair of articular cartilage. Other researchers used vascularized periosteal flap intramedullary transplantation to repair femoral neck fractures in young and middle-aged patients. During follow-up of 47 patients, fracture healing was found without femoral head necrosis, and it was concluded that a vascularized periosteal flap could reconstruct the blood supply at the injury site, play the role of periosteum osteogenesis, and promote fracture healing [56]. At present, there are few reports on the clinical use of periosteum, and comprehensive understanding of its clinical effects is lacking. However, natural periosteum transplantation and repair methods are improving. For example, stimulation by various growth factors can improve the osteogenic performance of periosteum.
5.5. Periosteum tissue engineering
Tissue-engineered periosteum is widely studied at present. The procedure involves culture and augmentation of seed cells in vitro, followed by inoculation of the expanded seed cells onto a scaffold material once a sufficient number of cells has been acquired. The scaffold material complexed with growth factors is implanted into the bone defect. As the scaffold material gradually becomes degraded and absorbed by the body, the seed cells gradually form new bone tissue with normal physiological structure and function, and finally achieve the purpose of bone defect repair. At present, tissue-engineered periosteum is usually transplanted into the recipient by tamping. However, insufficient local nutrient supply and accumulation of metabolites in the recipient can lead to the death of a large number of the seed cells. The scaffold may also become compressed and deformed, thereby failing to play its roles of support and protection. Therefore, the focus of tissue-engineered periosteum technology is gradually shifting toward artificial bionic periosteum, to improve the macroscopic and microscopic structures of periosteum as well as its vascularization.
Different types tissue-engineered periostea have their own characteristics. First, the ossification of periosteum varies from species to species. For example, during in vitro experiments, cells derived from rabbit periosteum and human periosteum have different osteogenic potential. Human periosteum cells are relatively normal at the late stage of osteogenesis, while rabbit periosteum cells initiate an osteoclast program [57]. Second, the osteogenic potential of periosteum varies in relation to different cell source sites within the same body. Studies have shown that different parts of the body have different types and powers of osteogenesis. For example, tibial periosteum can form new bone through two mechanisms, intramembranous ossification and entochondrostosis, while skull periosteum can only form new bone through intramembranous ossification. In addition, the osteogenic ability of tibial periosteum is far stronger than that of skull periosteum [12,13]. Third, because the amounts of cambium layer cells can vary, the osteogenic capacity of periosteum can differ among different regions within the same bone [58].
6. Composition and application of tissue-engineered periosteum
Various active components of periosteum can directly or indirectly participate in the process of physiological osteogenesis through specific mechanisms. To accelerate the process of bone repair after bone injury, components of tissue engineering, including cells, scaffolds, and bioactive factors, have been applied individually or in combination. For different cases, complete fresh periosteum or composite membranes constructed according to the requirements of different components are available. The application of periosteum structure in tissue engineering is becoming increasingly diversified.
6.1. Cells
At present, there are two ways to implant cells into the body. One is to connect the cells directly to a scaffold, and the other is to form a cell sheet in vitro to improve the adhesion rate, and then use the sheet directly or attach it to a scaffold [59]. The cells applied for tissue engineering of periosteum include PDCs [60], skeletal stem cells [61], BMSCs [62], adipose mesenchymal stem cells (AMSCs), and dental pulp stem cells [63] (Fig. 4). PDCs have the strongest osteogenic differentiation potential, while BMSCs have relatively weak ability for osteogenesis. Although the osteogenic capacity of AMSCs is also relatively weak, these cells have been widely used because of their favourable characteristics such as easy isolation, relative abundance, rapid expansion, and multipotency [64,65]. Meanwhile, addition of endotheliocytes can promote the formation of microvessels during periosteum-mediated bone repair [66], and vascular pericytes have the same potential in tissue engineering.
Fig. 4.
The composition and classification of tissue engineering periosteum.AMSCs adipose mesenchymal stem cells, BMSCs bone marrow stromal cells, DSCs dental pulp stem cells, GelMA methacrylate gelatin, PCL polycaprolactone, PDCs periosteum derived cells, PDGF-BB platelet-derived growth factor-BB, PLLA l-lactic acid, SSP synthetic scaffold periosteum, VEGF vascular endothelial growth factors.
Many reports have demonstrated that the differentiation potential of the same cell type can vary depending on differences in species, preconditioning, and origin within the same organism [13,[67], [68], [69]].
6.2. Scaffolds
For tissue engineering of periosteum, scaffolds provide a three-dimensional structure for cell adhesion and a suitable microenvironment to support cell function and facilitate interactions between cells. Scaffolds are divided into endogenous and exogenous scaffolds.
Endogenous scaffolds are the ideal biological scaffolds because of their high biocompatibility and lack of immunogenicity. Periosteum ECM, the most common endogenous scaffold, is the remnant of periosteum after all of the cellular components are removed, leaving just the ECM microstructure and bioactive factors.
Initially, exogenous scaffolds were only employed to simulate the spatial structure of the ECM, and included tricalcium phosphate (TCP) [70], polylactic acid, polycaprolactone (PCL), and chitosan [66] (Fig. 4). Subsequently, drugs, key factors, BMP-2, and vascular endothelial growth factor (VEGF) were attached to the scaffolds or attached to capsules, particles, and other structures to achieve the effect of controlled release, to provide suitable conditions for new bone formation and make the cells more osteogenic [71,72]. However, abnormalities in the physiological levels of bioactive factors can produce serious side effects [73], such as inflammation and immune responses [74], excessive osteogenesis, and ectopic osteogenesis [75].
6.3. Vasoactive component
Vascular periosteal flaps can accelerate bone healing in the host after allogeneic bone transplantation, reducing the risk of non-union. However, due to the lack of donors as well as the issue of postoperative infection in allograft bone, the application of vascularized periosteal flaps is problematic. An endogenous-exogenous composite bionic periosteum has wide application prospects for triggering periosteum and bone regeneration. Taking advantage of collagen self-assembly and micro-sol electrospinning technologies, Wu et al. [76] created a hierarchical micro/nanofibrous bionic periosteum with sustained release of VEGF. Serving as an exogenous vascularized fibrous layer of periosteum, it can induce the endogenous cambium layer in vivo, leading to complete regeneration of periosteum and bone tissue. VEGF encapsulated in a core shell structure composed of hyaluronan and l-lactic acid (hyaluronan-PLLA) was confirmed to be released in a sustained manner in the fibrous layer and bone defect areas for angiogenesis. Using a polydopamine-assisted technique, Li et al. [33] constructed a functionalized periosteum consisting of an electrospun scaffold grafted with a leptin receptor antibody and BMP-2-loaded hollow MnO2 nanoparticles, and observed growth of osteogenic conjugated capillaries into the bone repair site. Upon further evaluation of the osteogenic microenvironment, they found different PDGF-BB expression levels in the scaffold compared with other groups and similar results for type H capillaries, finally coming to the conclusion that osteoblasts induced by the transplanted periosteum promoted preosteoclast secretion of PDGF-BB that guided growth of H-type capillaries to the osteogenic microenvironment(Fig. 4).
There is increasing evidence that the strategy for using organic combinations of different types of bionic periosteum with angiogenesis-promoting activity may provide a solution to the clinical problem of insufficient donor grafts.
7. Classification of bionic periosteum
Autologous periosteum transplantation is limited by the restricted tissue source and fitness of the patient, as well as the possibility of deep tissue infection and chronic pain at the collection site [77]. In allogeneic periosteum transplantation, problems such as immune rejection and pathogen transmission cannot be completely avoided [78]. To solve these problems as much as possible, development of efficient bionic periosteum is constantly evolving. With the aim of achieving bionic periosteum creation, artificial periosteum materials obtained by periosteum tissue engineering technology resemble the natural periosteum in structure and function, and have wide therapeutic potential. Artificial periostea can be divided into three categories: cell-sheet artificial periosteum, acellular scaffold artificial periosteum, and synthetic scaffold artificial periosteum.
7.1. Cell-sheet artificial periosteum
The cell-sheet technology was developed earlier than other technologies and its preparation process is relatively simple. The target cells are cultured in vitro to proliferate and fuse, and various ECM components are induced to form a complete and robust tissue sheet. This technique only requires extraction of a small number of cells through a microinvasive operation that causes little damage to the body(Fig. 4).
Cells in human mandibular periosteum have been extracted and cultured to form sheet structures. Grafts with human cultured periosteum (HCP) sheets, platelet-rich plasma, and hydroxyapatite granules were incorporated into the treatment of infrabony periodontal defects, with continuous follow-up and investigation by Okuda et al. [23], who found that the depth of detection, clinical attachment, and bone filling on imaging were all significantly improved at 5 years post-surgery. The study also proved the potential of HCP sheets as an active drug delivery system that could favourably influence cellular functions and serve as a seed for ectopic bone formation near the implantation site by producing important growth factors related to periodontal regeneration. Fu et al. [78] found that new bone formation for spine fusion was facilitated by use of BMSC-loaded TCP wrapped with a PDC-loaded artificial cell sheet. They demonstrated that this multifunctional multisubstance composite membranous scaffold could not only form a periosteum-bone biomimetic graft at the defect site, but also allow PDCs and BMSCs to interact in a probable co-culture environment. Scanning electron microscope (SEM) analysis showed that PDCs grew on the artificial electrospun mesh cell sheet and had good adhesion. The electrospun mesh cell sheet provided a three-dimensional structure and an effective method of delivering PDCs for migration, adhesion, and proliferation (Table 1).
Table 1.
The research of cell-sheet and acellular scaffold artificial periosteum.
| Year | Team | Materials and technology | Achievement | Advantage | Types |
|---|---|---|---|---|---|
| 2013 | Okuda et al. [23] | HCP+ PRP+ HA | Clinical attachment and imaging bone filling were all significantly improved at five years postsurgery | HCP sheets can serve as an active drug delivery system | Cell sheet |
| 2019 |
Fu et al. [78] |
BMSCs-loaded TCP+ PDCs-loaded cell sheet |
Promoted spinal fusion and new bone formation |
The two kinds of cells interact with each other, and the 3D structure can provide cell adhesion sites |
Cell sheet+ 3D structure |
| 2018 | Ghanmi et al. [84] | Fresh acellular HAM | Promoted bone regeneration at the critical size of bone defect | A substitute for the natural periosteum | Acellular scaffold |
| 2020 | Zhao et al. [79] | Acellular scaffold of SIS+ Rabbit MSCs | Reconstructed the critical size defect of long bone, and repaired large irregular defects | Bone and blood vessels form in the defect | Acellular scaffold+ Cells |
| 2020 | He et al. [81] | Acellular sheep periosteum+ Mouse MC3T3-E1 cells | Acellular sheep periosteum had osteogenic activity and no cytotoxicity | Acellular periosteum can avoid immune response and has high biocompatibility | Acellular scaffold+ Cells |
| 2021 | Zhao et al. [80] | Acellular scaffold of SIS+ MSCs | The therapeutic effect of MSCs combined with acellular membrane was better than allogeneic bone materials | Promote bone regeneration of long bone defect | Acellular scaffold+ Cells |
BMSCs bone marrow stromal cell, HA hydroxyapatite, HAM human amniotic membrane, HCP human cultured periosteum, MSCs mesenchymal stem cells, PDCs periosteum derived cells, PRP platelet-rich plasma, SIS small intestinal submucosa, TCP tricalcium phosphate
Wrapping an MSC sheet around a bone defect or a regenerated scaffold is a simple way to achieve cell culture through attachment to an exogenous scaffold, because the adhesion of cultured cells is particularly important. In the past, enzyme digestion would destroy any growth factors and other substances produced during the process of cell culture, and the resulting cell suspension would be unable to achieve the purpose of limited and specific transplantation due to its fluidity, with a corresponding effect on the utilization of the cells. However, the cell sheet technology ensures that cell membrane surface proteins such as cytokine receptors remain undamaged and that the ECM is retained, thereby maintaining the cell-specific phenotype. Compared with cell suspension, and due to its physical properties, the local cell inoculation rate in a cell sheet is greatly improved, and an environment for subsequent growth and differentiation of the cells is established. With the development of the technology, cell sheets have gradually upgraded from a single layer to a double layer or even multiple layers, from a single cell to multiple cells, from unidirectional differentiation to multidirectional differentiation, from in vitro experiments to animal experiments, and even clinical application. Nevertheless, the cell density in a cell sheet is high, especially when multiple layers are overlapped, and there may be insufficient nutrients and oxygen in the sheet, possibly leading to necrosis of the central cells. In addition, a monolayer cell sheet is too thin for easy transfer. These issues remain to be resolved in further studies.
7.2. Acellular scaffold artificial periosteum
Based on effective removal of the immunogenic cellular components from the natural tissue, this material retains the natural internal three-dimensional scaffold structure and a large number of effective components such as structural proteins, specialized proteins, proteoglycans, and growth factors. The resulting tissue with unique internal structures and natural components cannot be perfectly replicated by current synthetic materials(Fig. 4).
Zhao et al. [79] developed a self-made tissue-engineered periosteum that plays a role for osteogenesis and angiogenesis at bone defects. Their tissue-engineered periosteum is a flexible cellular construct made by combining osteogenic-induced rabbit MSCs with an acellular scaffold of small intestinal submucosa (SIS). They used this method to successfully reconstruct critical-size defects in long bones, as well as large irregular defects in rabbit models. Next year, his team also confirmed that the effect of acellular SIS combined with MSCs in treating large long bone defects was better than that of allogeneic materials [80]. He et al. [81,82] focused on the preparation of acellular sheep periosteum ECM material, and explored the potential application of the material for guided bone regeneration. They decellularized sheep periosteum and then inoculated the acellular periosteum with mouse MC3T3-E1 cells. Subsequently, they recorded the whole cell adhesion process by SEM. In the material characterization experiment, CCK-8 assays showed that the acellular periosteum had no toxic effect on preosteoblasts, and instead exerted a positive effect on cell proliferation. Alkaline phosphatase and quantitative real-time PCR (COL I, Runx2, OCN) assays were used to detect the osteogenic induction activity of acellular periosteum. The results showed that, unlike fresh sheep periosteum, acellular sheep periosteum did not cause serious immunogenic responses through the Th1 pathway. In a nutshell, acellular sheep periosteum had good biocompatibility and could be used to guide bone regeneration. Human amniotic membrane (HAM), the innermost layer of the placenta, has strong anti-fibrosis characteristics and immune inertia, and is an easily accessible and valuable tissue [83]. Ghanmi et al. [84] transplanted fresh acellular HAM into 40 rabbits, and found that replacement of periosteum with fresh acellular HAM promoted bone regeneration in critical-size bone defects, albeit with a different additive effect to that of natural periosteum (Table 1).
The principle of acellular technology is to remove immunogenic substances effectively and retain nonimmunogenic ECM to the greatest extent. Based on the structural and biochemical characteristics of the acellular tissue, acellular ECM has specific induction activity [85]. The residual components of mammalian periosteum after decellularization are similar and include a number of growth factors such as BMPs that can induce differentiation and osteogenesis of cells. In addition, the surface morphology and internal three-dimensional pore system facilitate cell migration, proliferation, and differentiation. The main problem for use of an acellular scaffold lies in its antigenicity, which mainly comes from non-collagen proteins in both heterogeneous or allogeneic periosteum. Therefore, after processes such as decellularization, the antigenicity of residual materials must be reduced and the risk of infectious diseases must be eliminated. However, if the degree of deproteinization is insufficient, some antigenicity will be retained and possibly lead to serious immune reactions. Currently, there are no effective methods for removing non-collagen proteins without affecting collagen proteins.
7.3. Synthetic scaffold periosteum
Synthetic scaffold periosteum material (SSP) is simple to produce in vitro. It can effectively avoid immune diseases caused by acellular matrix periosteum materials and reduce the chance of carrying pathogens into the body. SSP can be divided into two categories: monolayer SSP and multilayer SSP(Fig. 4).
7.3.1. Monolayer SSP
A hydrogel is a gel with a strong hydrophilic three-dimensional network structure. Because hydrogels contain water internally and allow free transport of nutrients and metabolic wastes, they are an effective means to simulate the ECM of periosteum, thereby enhancing the control and safety of exogenous periosteum application. Methacrylate gelatine (GelMA) is a mature hydrogel material that is rich in RGD sequences and facilitates cell adhesion. The monolayer structure of GelMA covers the bone injury area and promotes the process of bone repair. However, its excellent hydrophilicity leads to difficulty in the sustained-release function [86,87]. Xin et al. [88] initially modified GelMA by amination, and then cross-linked mesoporous bioglass nanoparticles (MBGNs) with rhBMP-2 by amide bonds using the EDC/NHS reaction. Finally, the cross-linked MBGNs and rhBMP-2 were combined with GelMA by photo-cross-linking under ultraviolet irradiation to form a GelMA/MBGNs-rhBMP-2 membrane. MBGNs were able to effectively release rhBMP-2 slowly and increase its concentration locally, thus improving the safety of rhBMP-2 in vivo. Meanwhile, the combination of GelMA and MBGNs ensured local fixation of MBGNs within a short time and avoided their removal by flowing liquid. The long-term release by MBGNs further ensured that the synthetic membrane material promoted cell proliferation and osteogenic differentiation. Hoffman et al. [48] implanted MSCs into a hydrolytic degradable hydrogel to create a periosteum mimetic and transplanted it as an allograft. After 16 weeks of healing, the results compared with untreated defects clearly showed that the new periosteum mimetic led to increased vascularization (2.4 times), endochondral bone formation (2.8 times), and biomechanical strength (1.8 times). The obvious drawback was that, compared with autologous grafts, the process of endochondral osteogenesis in this method would be delayed.
It is essential to continue to explore the fusion and healing of allografts in bone injury. Electrospinning is a special form of polymer fluid electrostatic atomization process that can be a good choice for the preparation of artificial periosteum. The principle of the fabrication process is that the droplets at the needle in the electric field will change from spherical to conical, such that polymer filaments with nanometre diameter can be obtained from the conical tip. These filaments cross-link with one another to form a net with a microstructure that resembles the periosteum fibre layer, is close to the natural ECM structure, and can be conducive to the growth of cells. Gong et al. [89] combined icariin (ICA) and moxifloxacin hydrochloride (MOX) into a composite structure with PCL as the core and gelatine as the shell, and created a membrane structure with osteogenic and antibacterial effects with the support of coaxial electrospinning technology. The results showed that based on the complex core–shell structure and the varying degradation rates of the different substances, effects on drug release and concentration control could be achieved. After 1 month, it was found that the double drug-loaded membrane released almost 100% of MOX and only 20% of ICA. In addition, the structure had an obvious antibacterial effect in vitro, and the expression of osteocalcin and type-I collagen as well as the deposition of calcium were significantly enhanced. Silica nanoparticles (SiNPs) with high specific surface area and good biocompatibility have wide application prospects in bone tissue engineering because they provide better adhesion for bone cells [90]. Lu et al. [91] used electrospinning and post-treatment processes to prepare a porous poly PLLA fibrous membrane as a bone tissue engineering substrate for SiNPs, and the main feature of the membrane was its high specific surface area. To promote the adhesion strength of SiNPs on the surface of PLLA fibres, they used dopamine (DOP) to modify the surface of PLLA fibres. Coating with SiNPs significantly improved the mechanical properties and hydrophilicity of the composite membrane. Furthermore, because of the biocompatibility of SiNPs, the PLLA/DOP/SiNP composite membrane had excellent cell biocompatibility and showed more cell adhesion and proliferation. In another study, Liu et al. [92] prepared calcium phosphate nanoparticles (CaPs) by an emulsion method and combined them with GelMA by electrospinning technology to construct an organic-inorganic hybrid biomimetic of periosteum and mixed hydrogel fibres. They found that the electrospun fibres exhibited good morphological and mechanical properties, and controllable ion release was observed for >10 days. Subsequently, upon co-culture with human umbilical vascular endothelial cells and MC3T3-E1 cells, the hybridized fibres showed potential to promote angiogenesis and osteogenesis. The study fully demonstrated that the composite periosteum structure had the function of local long-term control of ion release and in situ mineralization.
Although hydrogels and electrospinning have been widely used and studied, they cannot perfectly simulate the flexibility and ductility of natural periosteum, and need to be continuously optimized. Among the large number of periosteum materials, polyetheretherketone (PEEK) has attracted much attention because its elastic modulus conforms to the strength of the natural bone cortex, but it has not been widely used due to the limitation of its bioinert surface. Zhao et al. [93] electrospun a synthetic fluorinated PEEK polymer into nanofibres, sulfonated the resulting nanofibres, and then combined them with PCL to create a novel flexible nanocomposite periosteum (s-PEEK/PCL). Compared with pure s-PEEK, they found that the s-PEEK/PCL composite membrane had stronger hydrophilicity, better ductility, significantly improved biological activity, and stronger adsorption capacity for proteins. At the same time, the composite film was successfully mineralized by a uniform thin calcium phosphate layer at a later stage, and the potential of the film to improve the osteogenic reaction was confirmed. Shi et al. [94] developed a polylactic-glycolic acid nanosheet with a directional microgroove structure, and demonstrated that the directional microgroove structure enabled the material to match the ability of natural periosteum to adjust cell arrangement and allowed the polylactic-glycolic acid sheet to attach to a tissue engineering scaffold more firmly. Based on the excellent flexibility and ductility of paraffin membrane, they combined the directional microgroove structure with a paraffin membrane to regulate the direction of cell growth and the direction of mechanical tension applied. They also coated the membrane with a layer of polydopamine, which improved the biocompatibility of the material and enhanced the ability of cells to attach and proliferate more efficiently. The results showed that AMSCs in the stretched group outperformed AMSCs in the unstretched group for osteogenesis under mechanical stretching and spatial structure induction, and confirmed the positive stimulation effect of mechanical stress on bone regeneration and osteogenic differentiation [95]. The single-layer bionic periosteum made of polyurethane-ascorbic acid-calcium peroxide containing fibers on collagen was combined with special bone substitutes. It was found that the periosteum could not only support the primary periosteal cell survival, but also significantly improve bone formation and periosteum regeneration [96]. An artificial periosteum was prepared by biomineralizing Antheraea pernyi fibroin (AF) membrane with prenucleated nanoclusters. It was found that the biomineralization process on the membrane not only provide advanced elastic modulus and tensile strength for AF membrane, but also significantly promote osteogenic differentiation of MSCs without osteogenic inducer in vitro [97]. Liu et al. [98] developed the calcium-binding peptide-loaded PCL electrospun membrane modified by the shish-kebab structure, in which the calcium-binding peptide formed by electrostatic chelation not only prolonged the release cycle of E7 peptide-BMP-2, but also promoted the biomineralization of bionic periosteum and the regeneration of vascularized bone tissue (Table 2).
Table 2.
The research of synthetic monolayer SSP.
| Year | Team | Materials and technology | Achievement | Advantage | Types |
|---|---|---|---|---|---|
| 2013 | Hoffman et al. [48] | Degradable hydrogels+ MSCs | After sixteen weeks, periosteum vascularization, endochondral ossification and enhanced biomechanical properties were observed | Recovery is faster than the untreated group; endochondral ossification is slower than that of the autograft group | Monolayer SSP+ Cells |
| 2014 | Shi et al. [94] | A polylactic-glycolic acid nanosheet with a directional microgroove structure+ Paraffin membrane+ DOP | Improved the biocompatibility of the material, cell adhesion and proliferation were enhanced, the osteogenic ability of AMSCs in stretching group was higher | Regulate the direction of cell growth and the direction of mechanical tension applied, mechanical stress has positive effect on bone regeneration and osteogenic differentiation | Monolayer SSP+ Directional microgroove structure+ Drugs |
| 2019 | Gong et al. [89] | ICA+ MOX+ PCL+ gelatin | The membrane has antibacterial action, the expression of osteocalcin, type-I collagen and calcium deposition were significantly enhanced | Drug sustained release and drug antibacterial | Monolayer SSP+ Drugs |
| 2019 | Zhao et al. [93] | Fluorinated PEEK polymer+ PCL | Composite membrane had stronger hydrophilicity, better ductility, improved biological activity and stronger adsorption capacity for protein, homogeneous bone mineralization was seen | Composite membrane improves the osteogenic response potential | Electrospinning monolayer SSP |
| 2020 | Xin et al. [88] | MBGNs+ rhBMP-2+ GelMA | The combination of GelMA and MBGNs could ensure the local fixation of MBGNs in a short time, so as to avoid being taken away by flowing liquid, made MBGNs work for a long time | Composite membrane promoted cell proliferation and osteogenic differentiation | Monolayer SSP+ Growth factors |
| 2020 | Lu et al. [91] | Porous poly PLLA fibrous membrane+ DOP+ SiNPs | Composite membrane had good mechanical properties, hydrophilicity and biocompatibility | The membrane owns high specific surface area, provide more adhesion sites and space for cell proliferation | Electrospinning monolayer SSP+ Drugs |
| 2020 | Liu et al. [92] | Caps+ GelMA | The membrane acted as a slow-release ion to promote local angiogenesis and osteogenesis | Local long-term control of ion release, enhance the function of bone mineralization in situ | Electrospinning monolayer SSP |
| 2021 | Gupta et al. [96] | Polyurethane+ ascorbic acid+ calcium peroxide containing fibers | Supported the primary periosteal cell survival, promoted periosteum regeneration | Improve bone formation and periosteum regeneration | Monolayer SSP |
| 2021 | Shuai et al. [97] | AF+ prenucleated nanoclusters | Improved the physicochemical properties of membrane and promoted the osteogenic differentiation of MSCs | Provide a promising strategy in this field | Monolayer SSP+ Nanoclusters |
| 2021 | Liu et al. [98] | Calcium-binding peptide-loaded PCL+ E7 peptide-BMP-2 | Prolonged the release cycle of protein, promoted the biomineralization and the regeneration of tissue | Drug sustained release and promote the biomineralization | Monolayer SSP+ Growth factors |
AF Antheraea pernyi fibroin, AMSCs adipose mesenchymal stem cells, BMP-2 bone morphogenetic protein-2, Caps calcium phosphate nanoparticles, DOP dopamine, GelMA methacrylate gelatin, ICA Icariin, MBGNs mesoporous bioglass nanoparticles, MOX moxifloxacin hydrochloride, MSCs mesenchymal stem cells, PCL polycaprolactone, PEEK polyetheretherketone, PLLA l-lactic acid, rhBMP-2 recombinant human bone morphogenetic protein-2, SiNPs silica nanoparticles, SSP synthetic scaffold periosteum
The mechanical properties of grafts are very important for the reconstruction of large bone defects. However, a major drawback of hydrogel and electrospinning materials is that they cannot simulate the flexibility and ductility of natural periosteum well. However, when combined with other biomaterials to form composite scaffolds that imitate the natural bone environment as much as possible, they can be adjustable in terms of morphology, structure, and biological activity, and increase the osteogenic ability of the resulting scaffolds, making them the priority candidate materials for bone tissue engineering. Moreover, the majority of the raw materials for hydrogels and electrospinning are natural polymers, and their properties vary from source to source and can also by changed by different degumming processes and manufacturing methods. In addition, although many studies have demonstrated the potential of monolayer composite scaffolds to induce osteogenic development in vivo, most of these studies were conducted in animal models and the results may not be fully generalizable to humans. More research is needed in the future to determine the safety of nanofibrous scaffolds in clinical trials.
7.3.2. Multilayer SSP
Periosteum is divided into three layers, and therefore just a single layer of scaffold has difficulty in restoring the multilayer characteristics of periosteum. To achieve the effect of natural periosteum as much as possible, multilayer artificial synthetic scaffolds have been developed.
Silk fibroin (SF), a natural polymer biomaterial, forms collagen-like fibres. Because of its good permeability, suitable mechanical strength, excellent biocompatibility, and biodegradation, it has been widely applied in bone tissue engineering [99]. SF also possesses great strength and toughness, providing sufficient stability in vivo and support for cell adhesion, growth, and differentiation of human progenitor cells at the implantation site [100]. Su et al. [101] prepared SF scaffold-deposited nanofibres with porous and biodegradable characteristics, then formed an SF scaffold by freeze-drying technology, and subsequently combined dexamethasone with polyvinyl alcohol nanofibres with the aid of electrospinning technology. The activity of alkaline phosphatase and the amount of calcium mineralization were significantly increased after 21 days of induction culture. RT-PCR assays showed that osteoblast genes were highly expressed in the early and late stages. Wang et al. [102] used PCL, collagen, and nano-hydroxyapatite as raw materials, added hexafluoroisopropanol to prepare nanofibre sheets by electrospinning technology, and finally attached BMSCs. A biomimetic periosteum was constructed by stacking the nanofibre sheets layer by layer. After transplantation into 4-mm femoral defect segments in mice, the bionic composite periosteum repaired local bone defects and reversed the biomechanical disadvantage of allogeneic bone implantation for a period of time. Implantation of the biomimetic periosteum composite reproduced the whole process of periosteal bone repair in vivo, including the following aspects: donor-dependent formation of bone and cartilage, induction of distinct CD31-high type H endothelium, reconstitution of bone marrow, and remodelling of bone allografts. Using this method, each cell layer could be modified and adjusted, and the microenvironmental characteristics of periosteum could be simulated individually. Enlightened by the natural nacre and the most commonly used guided bone regeneration (GBR) membrane (BIO-GIDE), Zhang et al. [103] prepared a novel multifunctional double-layer GBR film using chitosan, graphene oxide, and calcium silicate nanowires as raw materials by employing a mature evaporation-induced self-assembly technology combined with ice-templating technology. The resulting morphology was very specific, in that one side was a smooth pearl layer that ensured excellent mechanical properties but prevented non-osteoblast interference, while the other side was a porous layer conducive to cell adhesion. Unlike the previous BIO-GIDE, the new membrane had better mechanical properties and more functions, such as stronger bacteriostatic effect, appropriate degradation rate, and biocompatibility. Inspired by the three-layer structure and multifunction characteristics of natural periosteum, Sun et al. [104] prepared a three-layer structure rich in two kinds of fibres belonging to natural periosteum using conjugated electrospinning technology. For this structure, they used PCL fibres to construct the outermost layer, mixed fibres of PCL and polyurethane (co-PUPCL) as the middle layer, and degradable polyurethane fibres with or without nano-hydroxyapatite (PUHA or PU) as the inner layer. The tensile strength of the three-layer structure gradually decreased from the outside to the inside, while the formation of the membrane structure by the action of electrospinning ensured that each layer had good adhesion. The asymmetry of the fibre structure resulted in different degradability and hydrophilicity of the coating, and the innermost layer was proven to have osteogenic activity. Wu et al. [105] used PCL, COL I and mineralized COL I to prepare a three-layer fiber membrane by electrospinning technology. The membrane showed tensile properties in the range of natural periosteum. Experiments showed that the inner layer of the membranes supported the attachment, proliferation, ingrowth and osteogenesis of BMSCs. Laijun et al. [106] used PCL to prepare the macroporous fluffy guiding layer to simulate the periosteal fiber layer, TCP to prepare the ECM-like bioactive layer to simulate the periosteal cambium layer, and then used PCL to prepare the middle dense layer to connect the two layers. The released periosteum not only benefits the infiltration and oriented growth of fibroblasts, but also enhances the proliferation and differentiation of BMSCs, and at the same time prevents the invasion of soft tissues at the injured site. In order to simulate the structure and function of natural periosteum, Yang et al. [107] used GelMA, l-arginine-based unsaturated poly and methacrylated hydroxyapatite nanoparticles to prepare bionic periosteum, which enhanced the material mechanical strength, facilitated tissue adhesion and maintained the continuous activation of nitric oxide-cyclic guanosine monophosphate signaling pathway(Table 3).
Table 3.
The research of synthetic multilayer SSP.
| Year | Team | Materials and technology | Achievement | Advantage | Types |
|---|---|---|---|---|---|
| 2016 | Su et al. [101] | Porous and biodegradable SF scaffold-deposited nanofibers+ PVA nanofibers+ DEX | The activity of alkaline phosphatase and the expression of calcium mineralization increased significantly after co-culture, osteoblast genes were highly expressed | Multilayers mimic natural periosteum | Electrospinning multilayer SSP |
| 2018 | Wang et al. [102] | PCL+ Collagen+ Nano-hydroxyapatite+ Hexafluoroisopropanol + BMSCs | Repaired 4 mm bone defect in mice and reversed biomechanical disadvantage of allogeneic bone | Participate in the whole process of periosteum osteogenesis repair | Electrospinning multilayer SSP |
| 2019 | Zhang et al. [103] | Chitosan+ Graphene oxide + Calcium silicate nanowires | One layer was a smooth membrane that prevented interference from non-osteoblasts, the other was a porous membrane that facilitated cell adhesion | Own better mechanical properties, stronger antibacterial effect | Multilayer SSP |
| 2019 | Sun et al. [104] | PCL+ co-PUPCL+ PUHA or PU | The inner layer had obvious osteogenic activity and the multilayer structure was tensile resistant | The asymmetry of the fiber structure resulted in different degradability and hydrophilicity | Electrospinning multilayer SSP |
| 2021 | Wu et al. [105] | PCL+ COL I+ MC | Tensile properties closed to natural periosteum, and the inner layer supported BMSCs attachment, proliferation and differentiation | Mechanical performance is improved, and promotes osteogenesis layer by layer | Multilayer SSP |
| 2021 | Laijun et al. [106] | PCL+ TCP nanowire | Conducive to fibroblast infiltration growth, enhanced the proliferation and differentiation of BMSCs, prevented soft tissue invasion | The effect of promoting vascularization and bone regeneration is obvious | Multilayer SSP |
| 2021 | Yang et al. [107] | GelMA+ Arg-UPEA+ nHAMA | Enhanced the material mechanical strength, facilitated tissue adhesion and maintained the continuous activation of NO-cGMP signaling pathway | To simulate the periosteum functionally and structurally | Multilayer SSP |
Arg-UPEA l-arginine-based unsaturated poly, BMSCs bone marrow stromal cell, COL I collagen I, co-PUPCL a mixed fiber formed by PCL and polyurethane, DEX dexamethasone, GelMA methacrylate gelatin, MC mineralized COL I, nHAMA methacrylated hydroxyapatite nanoparticles, NO-cGMP nitric oxide-cyclic guanosine monophosphate, PCL polycaprolactone, PU degradable polyurethane fibers without nano-hydroxyapatite, PUHA degradable polyurethane fibers with nano-hydroxyapatite, PVA polyvinyl alcohol, SF silk fibroin, SSP synthetic scaffold periosteum, TCP tricalcium phosphate
At present, cell-sheet technology is more widely used in the repair and regeneration of myocardium and cornea. Common cell-sheet materials need to be attached to exogenous scaffolds for cell culture, but this method is easily influenced by cell adhesion ability. Acellular scaffold can perfectly replicate the internal structure and natural components of periosteum, but the acquisition of tissue raw materials is relatively complicated and there is still the possibility of immunological rejection. Although monolayer SSP can avoid the interference of immune source, its flexibility and ductility are not as good as that of natural periosteum, and it is difficult to restore the multilayer structure of periosteum. Then multilayer SSP can solve most of the above problems, it is still in the primary stage of development. Although there have been clinical trials on the use of cell-sheet bionic periosteum in the treatment of human bone defects [23], the number of reports describing clinical application of tissue-engineered periosteum is very limited. Further optimization of the macroscopic and microscopic structures of existing tissue-engineered periosteum and improvement of the performance of tissue-engineering periosteum will accelerate translation of the basic research on periosteum into clinical treatment. However, biomimetic studies on the microstructure and biological function of artificial periosteum remain in their infancy, and the material selection, design concept, and performance evaluation require further improvements. The existing problems associated with synthetic materials mainly lie in their low cell adhesion rate, difficulty in complete degradation, teratogenicity, and carcinogenicity.
8. Conclusions and perspectives
Periosteum is essential for bone formation and regeneration, and has been gradually recognized for its indispensable role in bone repair. Periosteum has a complex and orderly organization, and due to the various components and their combinations, different structures of tissue-engineered periosteum can be produced. In this review, the histological structure, osteogenic function, and tissue engineering application of periosteum are summarized, and the potential research directions in the future are discussed.
At present, studies on periosteum tissue engineering have yielded some results, and a variety of bionic artificial periostea have been developed. The key to design and construct artificial periosteum lies in selecting scaffold materials and achieve reasonable collection as well as simulating the structure and function of natural periotseum fully. Although many kinds of periosteum materials have been developed, none of these materials have completely met the ideal requirements to date. Almost all of the materials are still at the stage of in vitro tests and animal experiments. Therefore, it is imperative to determine the pros and cons of various classical materials and lay a good foundation for clinical selection. On this basis, the macroscopic and microscopic structures require further optimization, and the corresponding angiogenesis mechanisms require further clarification to enhance the performance of tissue-engineered periosteum. With the current development of stem cell biology, gene therapy and biomaterials, it is possible to prepare tissue engineering periosteum with higher functions and less disadvantages. Achieving personalized structure and composition in the construction of tissue engineering periosteum is in accordance with the design concept of both universality and emphasis on individual differences and ensures the combination of commonness and individuality, which are expected to meet the clinical needs of bone repair more effectively. At the same time, we should speed up the research results on various scaffolds toward clinical application. In the future, the development of artificial periosteum will open a new door for the clinical treatment of bone defects, and improve the therapeutic effect to a new level.
Funding
This study was supported by the Science and Technology Innovation Foundation of Dalian (2020JJ27SN070) and the open project of Key Laboratory for Micro/Nano Technology and System of Liaoning Province, Dalian University of Technology (20210101). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
ZHL and NGW provided conception and design of study, WTZ drafted the manuscript, MY and TZS provided writing and illustrationsupport, JZ, NH and YTZ helped perform the paper selection and scoring, ZHL and NGW provided writing assistance and revised and proof read the article.
Declaration of competing interest
The authors have no conflict of interest relevant to this review.
Acknowledgments
The authors thank Alison Sherwin, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
Biography

Zhonghai Li Professor, Department of Orthopaedics, First Affiliated Hospital of Dalian Medical University, Key Laboratory of Molecular Mechanism for Repair and Remodeling of Orthopaedic Diseases, Liaoning Province, the People’s Republic of China.
References
- 1.Colnot C., Zhang X., Knothe Tate M.L. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012;30(12):1869–1878. doi: 10.1002/jor.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Merchán E.C. A review of recent developments in the molecular mechanisms of bone healing. Int J Mol Sci. 2021;22(2) doi: 10.3390/ijms22020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N., Song J., Zhu G., Li X., Liu L., Shi X., et al. Periosteum tissue engineering-a review. Biomater Sci. 2016;4(11):1554–1561. doi: 10.1039/c6bm00481d. [DOI] [PubMed] [Google Scholar]
- 4.Dwek J.R. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010;39(4):319–323. doi: 10.1007/s00256-009-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin G., Antabak A., Davila S. The periosteum. Part 1: anatomy, histology and molecular biology. Injury. 2007;38(10):1115–1130. doi: 10.1016/j.injury.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Squier C.A., Ghoneim S., Kremenak C.R. Ultrastructure of the periosteum from membrane bone. J Anat. 1990;171:233–239. [PMC free article] [PubMed] [Google Scholar]
- 7.Feik S.A., Storey E., Ellender G. Stress induced periosteal changes. Br J Exp Pathol. 1987;68(6):803–813. [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb F.O. Metabolic, degenerative, and inflammatory diseases of bones and joints. Arch Intern Med. 1974;133(1):154–155. [Google Scholar]
- 9.Bianco P., Riminucci M., Gronthos S., Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cell. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 10.Toosi S., Behravan J. Osteogenesis and bone remodeling: a focus on growth factors and bioactive peptides. Biofactors. 2020;46(3):326–340. doi: 10.1002/biof.1598. [DOI] [PubMed] [Google Scholar]
- 11.Urist M.R., DeLange R.J., Finerman G.A. Bone cell differentiation and growth factors. Science. 1983;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- 12.Fujii T., Ueno T., Kagawa T., Sakata Y., Sugahara T. Comparison of bone formation ingrafted periosteum harvested from tibia and calvaria. Microsc Res Tech. 2006;69(7):580–584. doi: 10.1002/jemt.20274. [DOI] [PubMed] [Google Scholar]
- 13.Bilkay U., Tokat C., Helvaci E., Ozek C., Zekioglu O., Onat T., et al. Osteogenic capacities of tibial and cranial periosteum: a biochemical and histologic study. J Craniofac Surg. 2008;19(2):453–458. doi: 10.1097/SCS.0b013e318052fe3d. [DOI] [PubMed] [Google Scholar]
- 14.O'Driscoll S.W., Saris D.B., Ito Y., Fitzimmons J.S. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19(1):95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 15.Ferretti J.L., Frost H.M., Gasser J.A., High W.B., Jee W.S., Jerome C., et al. Perspectives on osteoporosis research: its focus and some insights from a new paradigm. Calcif Tissue Int. 1995;57(6):399–404. doi: 10.1007/BF00301939. [DOI] [PubMed] [Google Scholar]
- 16.Chartier S.R., Mitchell S.A.T., Majuta L.A., Mantyh P.W. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178–190. doi: 10.1016/j.neuroscience.2018.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen M.R., Hock J.M., Burr D.B. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35(5):1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Maia Ferreira Alencar C.H., Sampaio Silveira C.R., Cavalcante M.M., Maia Vieira C.G., Diógenes Teixeira M.J., Neto F.A., et al. Periosteum: an imaging review. Eur J Radiol Open. 2020;7:100249. doi: 10.1016/j.ejro.2020.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson A.H. The blood supply of the periosteum. J Anat. 1985;140(Pt 4):697–704. Pt 4. [PMC free article] [PubMed] [Google Scholar]
- 20.Hohmann E.L., Elde R.P., Rysavy J.A., Einzig S., Gebhard R.L. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232(4752):868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 21.Roberto-Rodrigues M., Fernandes R.M., Senos R., Scoralick A.C., Bastos A.L., Santos T.M., et al. Novel rat model of nonunion fracture with vascular deficit. Injury. 2015;46(4):649–654. doi: 10.1016/j.injury.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Li C., Wei G., Gu Q., Wen G., Qi B., Xu L., et al. Donor age and cell passage affect osteogenic ability of rat bone marrow mesenchymal stem cells. Cell Biochem Biophys. 2015;72(2):543–549. doi: 10.1007/s12013-014-0500-9. [DOI] [PubMed] [Google Scholar]
- 23.Okuda K., Kawase T., Nagata M., Yamamiya K., Nakata K., Wolff L.F., et al. Tissue-engineered cultured periosteum sheet application to treat infrabony defects: case series and 5-year results. Int J Periodontics Restorative Dent. 2013;33(3):281–287. doi: 10.11607/prd.1545. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang Z., John J.V., Liao H., Luo J., Rubery P., Mesfin A., et al. Periosteum mimetic coating on structural bone allografts via electrospray deposition enhances repair and reconstruction of segmental defects. ACS Biomater Sci Eng. 2020;6(11):6241–6252. doi: 10.1021/acsbiomaterials.0c00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyer C.H., Kjaergaard K., Ding M., Qin L. Vascular endothelial growth factor for in vivo bone formation: a systematic review. J Orthop Translat. 2020;24:46–57. doi: 10.1016/j.jot.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito R., Matsumiya T., Kon T., Narita N., Kubota K., Sakaki H., et al. Periosteum-derived cells respond to mechanical stretch and activate Wnt and BMP signaling pathways. Biomed Res. 2014;35(1):69–79. doi: 10.2220/biomedres.35.69. [DOI] [PubMed] [Google Scholar]
- 27.Hou Y., Lin W., Li Y., Sun Y., Liu Y., Chen C., et al. De-osteogenic-differentiated mesenchymal stem cells accelerate fracture healing by mir-92b. J Orthop Translat. 2021;27:25–32. doi: 10.1016/j.jot.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q., DiFeo Jacquet R., Landis W.J. Characterization of tissue-engineered human periosteum and allograft bone constructs: the potential of periosteum in bone regenerative medicine. Cells Tissues Organs. 2020;209(2–3):128–143. doi: 10.1159/000509036. [DOI] [PubMed] [Google Scholar]
- 29.Gresham R.C.H., Bahney C.S., Leach J.K. Growth factor delivery using extracellular matrix-mimicking substrates for musculoskeletal tissue engineering and repair. Bioact Mater. 2021;6(7):1945–1956. doi: 10.1016/j.bioactmat.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marupanthorn K., Tantrawatpan C., Kheolamai P., Tantikanlayaporn D., Manochantr S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int J Mol Med. 2017;39(3):654–662. doi: 10.3892/ijmm.2017.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook S.D., Patron L.P., Salkeld S.L., Rueger D.C. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85-(A Suppl 3):116–123. doi: 10.2106/00004623-200300003-00018. [DOI] [PubMed] [Google Scholar]
- 32.Hissnauer T.N., Stiel N., Babin K., Rupprecht M., Ridderbusch K., Rueger J.M., et al. Recombinant human bone morphogenetic protein-2 (rhBMP-2) for the treatment of nonunion of the femur in children and adolescents: a retrospective analysis. BioMed Res Int. 2017;2017:3046842. doi: 10.1155/2017/3046842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H., Wang H., Pan J., Li J., Zhang K., Duan W., et al. Nanoscaled bionic periosteum orchestrating the osteogenic microenvironment for sequential bone regeneration. ACS Appl Mater Interfaces. 2020;12(33):36823–36836. doi: 10.1021/acsami.0c06906. [DOI] [PubMed] [Google Scholar]
- 34.Sheng M.H., Lau K.H., Baylink D.J. Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J Bone Metab. 2014;21(1):41–54. doi: 10.11005/jbm.2014.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng M.H., Zhou X.D., Bonewald L.F., Baylink D.J., Lau K.H. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52(1):133–144. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 36.Takeyama K., Chatani M., Inohaya K., Kudo A. TGFβ-2 signaling is essential for osteoblast migration and differentiation during fracture healing in medaka fish. Bone. 2016;86:68–78. doi: 10.1016/j.bone.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Ritz M.F., Graumann U., Gutierrez B., Hausmann O. Traumatic spinal cord injury alters angiogenic factors and TGF-beta1 that may affect vascular recovery. Curr Neurovasc Res. 2010;7(4):301–310. doi: 10.2174/156720210793180756. [DOI] [PubMed] [Google Scholar]
- 38.Wen X., Li X., Tang Y., Tang J., Zhou S., Xie Y., et al. Chondrocyte FGFR3 regulates bone mass by inhibiting osteogenesis. J Biol Chem. 2016;291(48):24912–24921. doi: 10.1074/jbc.M116.730093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellender G., Feik S.A., Ramm-Anderson S.M. Periosteal changes in mechanically stressed rat caudal vertebrae. J Anat. 1989;163:83–96. [PMC free article] [PubMed] [Google Scholar]
- 40.Birkhold A.I., Razi H., Duda G.N., Weinkamer R., Checa S., Willie B.M. The periosteal bone surface is less mechano-responsive than the endocortical. Sci Rep. 2016;6:23480. doi: 10.1038/srep23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown T.D., Pedersen D.R., Gray M.L., Brand R.A., Rubin C.T. Toward an identification of mechanical parameters initiating periosteal remodeling: a combined experimental and analytic approach. J Biomech. 1990;23(9):893–905. doi: 10.1016/0021-9290(90)90354-6. [DOI] [PubMed] [Google Scholar]
- 42.Moore S.R., Heu C., Yu N.Y., Whan R.M., Knothe U.R., Milz S., et al. Translating periosteum's regenerative power: insights from quantitative analysis of tissue genesis with a periosteum substitute implant. Stem Cells Transl Med. 2016;5(12):1739–1749. doi: 10.5966/sctm.2016-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson C., Alpern E., Miclau T., Helms J.A. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87(1–2):57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 44.Roberts S.J., van Gastel N., Carmeliet G., Luyten F.P. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10–18. doi: 10.1016/j.bone.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Duchamp de Lageneste O., Julien A., Abou-Khalil R., Frangi G., Carvalho C., Cagnard N., et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9(1):773. doi: 10.1038/s41467-018-03124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin X., Zhao C., Zhu P., Chen J., Yu H., Cai Y., et al. Periosteum extracellular-matrix-mediated acellular mineralization during bone formation. Adv Healthc Mater. 2018;7(4) doi: 10.1002/adhm.201700660. [DOI] [PubMed] [Google Scholar]
- 47.Aaron J.E. Periosteal Sharpey's fibers: a novel bone matrix regulatory system? Front Endocrinol. 2012;3:98. doi: 10.3389/fendo.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman M.D., Xie C., Zhang X., Benoit D.S. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials. 2013;34(35):8887–8898. doi: 10.1016/j.biomaterials.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huh J.Y., Choi B.H., Kim B.Y., Lee S.H., Zhu S.J., Jung J.H. Critical size defect in the canine mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(3):296–301. doi: 10.1016/j.tripleo.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Zhao D., Wang Y., Han D. Periosteal distraction osteogenesis: an effective method for bone regeneration. BioMed Res Int. 2016;2016:2075317. doi: 10.1155/2016/2075317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niemeyer F., Claes L., Ignatius A., Meyers N., Simon U. Simulating lateral distraction osteogenesis. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritsilä V.A., Santavirta S., Alhopuro S., Poussa M., Jaroma H., Rubak J.M., et al. Periosteal and perichondral grafting in reconstructive surgery. Clin Orthop Relat Res. 1994;(302):259–265. [PubMed] [Google Scholar]
- 53.Ritsilä V., Alhopuro S., Rintala A. Bone formation with free periosteal grafts in reconstruction of congenital maxillary clefts. Ann Chir Gynaecol. 1976;65(5):342–344. [PubMed] [Google Scholar]
- 54.Jaroma H.J., Ritsilä V.A. Behaviour of cancellous bone graft with and without periosteal isolation in striated muscle. An experimental study. Scand J Plast ReConstr Surg Hand Surg. 1988;22(1):47–51. doi: 10.3109/02844318809097934. [DOI] [PubMed] [Google Scholar]
- 55.Yang G.Y., Lu S.B., Wang J.F. [Long-term clinical observation on the repair of large articular cartilage defects of the hip and the knee with free autogeneous periosteum] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004;18(1):8–11. [PubMed] [Google Scholar]
- 56.Li L.H., Chen X.L., Han G.H. [Repair of femoral neck fracture with vascular pedicled periosteum flap transfer in young and middle-aged] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004;18(1):15–17. [PubMed] [Google Scholar]
- 57.Eyckmans J., Luyten F.P. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Eng. 2006;12(8):2203–2213. doi: 10.1089/ten.2006.12.2203. [DOI] [PubMed] [Google Scholar]
- 58.Gallay S.H., Miura Y., Commisso C.N., Fitzsimmons J.S., O'Driscoll S.W. Relationship of donor site to chondrogenic potential of periosteum in vitro. J Orthop Res. 1994;12(4):515–525. doi: 10.1002/jor.1100120408. [DOI] [PubMed] [Google Scholar]
- 59.van Gastel N., Stegen S., Stockmans I., Moermans K., Schrooten J., Graf D., et al. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cell. 2014;32(9):2407–2418. doi: 10.1002/stem.1783. [DOI] [PubMed] [Google Scholar]
- 60.Chang H., Docheva D., Knothe U.R., Knothe Tate M.L. Arthritic periosteal tissue from joint replacement surgery: a novel, autologous source of stem cells. Stem Cells Transl Med. 2014;3(3):308–317. doi: 10.5966/sctm.2013-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan C.K.F., Gulati G.S., Sinha R., Tompkins J.V., Lopez M., Carter A.C., et al. Identification of the human skeletal stem cell. Cell. 2018;175(1):43–56. doi: 10.1016/j.cell.2018.07.029. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui L., Xiang S., Chen D., Fu R., Zhang X., Chen J., et al. A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits. J Orthop Translat. 2021;26:151–161. doi: 10.1016/j.jot.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu V., Helder M.N., Bravenboer N., Ten Bruggenkate C.M., Jin J., Klein-Nulend J., et al. Bone tissue regeneration in the oral and maxillofacial region: a review on the application of stem cells and new strategies to improve vascularization. Stem Cells Int. 2019;2019:6279721. doi: 10.1155/2019/6279721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadegh A.B., Basiri E., Oryan A., Mirshokraei P. Wrapped omentum with periosteum concurrent with adipose derived adult stem cells for bone tissue engineering in dog model. Cell Tissue Bank. 2014;15(1):127–137. doi: 10.1007/s10561-013-9383-z. [DOI] [PubMed] [Google Scholar]
- 65.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baldwin J.G., Wagner F., Martine L.C., Holzapfel B.M., Theodoropoulos C., Bas O., et al. Periosteum tissue engineering in an orthotopic in vivo platform. Biomaterials. 2017;121:193–204. doi: 10.1016/j.biomaterials.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Danalache M., Kliesch S.M., Munz M., Naros A., Reinert S., Alexander D. Quality analysis of minerals formed by jaw periosteal cells under different culture conditions. Int J Mol Sci. 2019;20(17) doi: 10.3390/ijms20174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uddströmer L. The osteogenic capacity of tubular and membranous bone periosteum. A qualitative and quantitative experimental study in growing rabbits. Scand J Plast Reconstr Surg. 1978;12(3):195–205. doi: 10.3109/02844317809012995. [DOI] [PubMed] [Google Scholar]
- 69.Hsiao H.Y., Yang C.Y., Liu J.W., Brey E.M., Cheng M.H. Periosteal osteogenic capacity depends on tissue source. Tissue Eng Part A. 2018;24(23–24):1733–1741. doi: 10.1089/ten.TEA.2018.0009. [DOI] [PubMed] [Google Scholar]
- 70.Cheng W.X., Liu Y.Z., Meng X.B., Zheng Z.T., Li L.L., Ke L.Q., et al. PLGA/β-TCP composite scaffold incorporating cucurbitacin B promotes bone regeneration by inducing angiogenesis. J Orthop Translat. 2021;31:41–51. doi: 10.1016/j.jot.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin J., Qiu S., Shi B., Xu X., Zhao Y., Gao J., et al. Controlled release of FGF-2 and BMP-2 in tissue engineered periosteum promotes bone repair in rats. Biomed Mater. 2018;13(2) doi: 10.1088/1748-605X/aa93c0. [DOI] [PubMed] [Google Scholar]
- 72.Kim H.Y., Lee J.H., Lee H.A.R., Park J.S., Woo D.K., Lee H.C., et al. Sustained release of BMP-2 from porous particles with leaf-stacked structure for bone regeneration. ACS Appl Mater Interfaces. 2018;10(25):21091–21102. doi: 10.1021/acsami.8b02141. [DOI] [PubMed] [Google Scholar]
- 73.Yang T., Tamaddon M., Jiang L., Wang J., Liu Z., Liu Z., et al. Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration. J Orthop Translat. 2021;30:112–121. doi: 10.1016/j.jot.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robin B.N., Chaput C.D., Zeitouni S., Rahm M.D., Zerris V.A., Sampson H.W. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study. Spine. 2010;35(23):E1350–E1354. doi: 10.1097/BRS.0b013e3181e85756. [DOI] [PubMed] [Google Scholar]
- 75.Weisbrod L.J., Arnold P.M., Leever J.D. Radiographic and CT evaluation of recombinant human bone morphogenetic protein-2-assisted cervical spinal interbody fusion. Clin Spine Surg. 2019;32(2):71–79. doi: 10.1097/BSD.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 76.Wu L., Gu Y., Liu L., Tang J., Mao J., Xi K., et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555. doi: 10.1016/j.biomaterials.2019.119555. [DOI] [PubMed] [Google Scholar]
- 77.Chun Y.Y., Wang J.K., Tan N.S., Chan P.P., Tan T.T., Choong C. A Periosteum-Inspired 3D Hydrogel-Bioceramic Composite for Enhanced Bone RegenerationMacromol Biosci. 2016;16(2):276–287. doi: 10.1002/mabi.201500258. [DOI] [PubMed] [Google Scholar]
- 78.Fu T.S., Wang Y.C., Chen C.H., Chang C.W., Lin T.Y., Wong C.B., et al. Engineered periosteum-bone biomimetic bone graft enhances posterolateral spine fusion in a rabbit model. Spine J. 2019;19(4):762–771. doi: 10.1016/j.spinee.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 79.Zhao L., Zhao J., Yu J.J., Zhang C. Irregular bone defect repair using tissue-engineered periosteum in a rabbit model. Tissue Eng Regen Med. 2020;17(5):717–727. doi: 10.1007/s13770-020-00282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao L., Zhao J., Tuo Z., Ren G. Repair of long bone defects of large size using a tissue-engineered periosteum in a rabbit model. J Mater Sci Mater Med. 2021;32(9):105. doi: 10.1007/s10856-021-06579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He J., Li Z., Yu T., Wang W., Tao M., Wang S., et al. In vitro and in vivo biocompatibility study on acellular sheep periosteum for guided bone regeneration. Biomed Mater. 2020;15(1) doi: 10.1088/1748-605X/ab597f. [DOI] [PubMed] [Google Scholar]
- 82.He J., Li Z., Yu T., Wang W., Tao M., Ma Y., et al. Preparation and evaluation of acellular sheep periostea for guided bone regeneration. J Biomed Mater Res. 2020;108(1):19–29. doi: 10.1002/jbm.a.36787. [DOI] [PubMed] [Google Scholar]
- 83.Shay E., He H., Sakurai S., Tseng S.C. Inhibition of angiogenesis by HC·HA, a complex of hyaluronan and the heavy chain of inter-α-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52(5):2669–2678. doi: 10.1167/iovs.10-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghanmi S., Trigui M., Baya W., Ellouz Z., Elfeki A., Charfi S., et al. The periosteum-like effect of fresh human amniotic membrane on bone regeneration in a rabbit critical-sized defect model. Bone. 2018;110:392–404. doi: 10.1016/j.bone.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Porzionato A., Stocco E., Barbon S., Grandi F., Macchi V., De Caro R. Tissue-engineered grafts from human decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci. 2018;19(12) doi: 10.3390/ijms19124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schönmeyr B., Clavin N., Avraham T., Longo V., Mehrara B.J. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng Part A. 2009;15(7):1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 87.Cao Y., Yang S., Zhao D., Li Y., Cheong S.S., Han D., et al. Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering. J Orthop Translat. 2020;23:89–100. doi: 10.1016/j.jot.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xin T., Mao J., Liu L., Tang J., Wu L., Yu X., et al. Programmed sustained release of recombinant human bone morphogenetic protein-2 and inorganic ion composite hydrogel as artificial periosteum. ACS Appl Mater Interfaces. 2020;12(6):6840–6851. doi: 10.1021/acsami.9b18496. [DOI] [PubMed] [Google Scholar]
- 89.Gong M., Huang C., Huang Y., Li G., Chi C., Ye J., et al. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomedicine. 2019;17:124–136. doi: 10.1016/j.nano.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Paris J.L., Lafuente-Gómez N., Cabañas M.V., Román J., Peña J., Vallet-Regí M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019;86:441–449. doi: 10.1016/j.actbio.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu Z., Wang W., Zhang J., Bártolo P., Gong H., Li J. Electrospun highly porous poly(L-lactic acid)-dopamine-SiO(2) fibrous membrane for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;117:111359. doi: 10.1016/j.msec.2020.111359. [DOI] [PubMed] [Google Scholar]
- 92.Liu W., Bi W., Sun Y., Wang L., Yu X., Cheng R., et al. Biomimetic organic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110670. doi: 10.1016/j.msec.2020.110670. [DOI] [PubMed] [Google Scholar]
- 93.Zhao F., Hu S., Wang F., Wang L. A sulfonated PEEK/PCL composite nanofibrous membrane for periosteum tissue engineering application. J Mater Sci. 2019;54(18):12012–12023. [Google Scholar]
- 94.Shi X., Fujie T., Saito A., Takeoka S., Hou Y., Shu Y., et al. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv Mater. 2014;26(20):3290–3296. doi: 10.1002/adma.201305804. [DOI] [PubMed] [Google Scholar]
- 95.Shi X., Li L., Ostrovidov S., Shu Y., Khademhosseini A., Wu H. Stretchable and micropatterned membrane for osteogenic differentation of stem cells. ACS Appl Mater Interfaces. 2014;6(15):11915–11923. doi: 10.1021/am5029236. [DOI] [PubMed] [Google Scholar]
- 96.Gupta S., Teotia A.K., Qayoom I., Shiekh P.A., Andrabi S.M., Kumar A. Periosteum-Mimicking tissue-engineered composite for treating periosteum damage in critical-sized bone defects. Biomacromolecules. 2021;22(8):3237–3250. doi: 10.1021/acs.biomac.1c00319. [DOI] [PubMed] [Google Scholar]
- 97.Shuai Y., Lu H., Lv R., Wang J., Wan Q., Mao C., et al. Biomineralization directed by prenucleated calcium and phosphorus nanoclusters improving mechanical properties and osteogenic potential of Antheraea pernyi silk fibroin-based artificial periosteum. Adv Healthc Mater. 2021;10(8) doi: 10.1002/adhm.202001695. [DOI] [PubMed] [Google Scholar]
- 98.Liu L., Shang Y., Li C., Jiao Y., Qiu Y., Wang C., et al. Hierarchical nanostructured electrospun membrane with periosteum-mimic microenvironment for enhanced bone regeneration. Adv Healthc Mater. 2021;10(21) doi: 10.1002/adhm.202101195. [DOI] [PubMed] [Google Scholar]
- 99.Omenetto F.G., Kaplan D.L. New opportunities for an ancient material. Science. 2010;329(5991):528–531. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhardwaj N., Kundu S.C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 101.Su W.T., Chiou W.L., Yu H.H., Huang T.Y. Differentiation potential of SHEDs using biomimetic periosteum containing dexamethasone. Mater Sci Eng C Mater Biol Appl. 2016;58:1036–1045. doi: 10.1016/j.msec.2015.09.077. [DOI] [PubMed] [Google Scholar]
- 102.Wang T., Zhai Y., Nuzzo M., Yang X., Yang Y., Zhang X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials. 2018;182:279–288. doi: 10.1016/j.biomaterials.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang K., Gao H., Pan X., Zhou P., Xing X., Xu R., et al. Multifunctional bilayer nanocomposite guided bone regeneration membrane. Matter. 2019;1(3):770–781. [Google Scholar]
- 104.Sun F., Chen J., Jin S., Wang J., Man Y., Li J., et al. Development of biomimetic trilayer fibrous membranes for guided bone regeneration. J Mater Chem B. 2019;7(4):665–675. doi: 10.1039/c8tb02435a. [DOI] [PubMed] [Google Scholar]
- 105.Wu J., Yao M., Zhang Y., Lin Z., Zou W., Li J., et al. Biomimetic three-layered membranes comprising (poly)-ε-caprolactone, collagen and mineralized collagen for guided bone regeneration. Regen Biomater. 2021;8(6):rbab065. doi: 10.1093/rb/rbab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laijun L., Yu Z., Chaojing L., Jifu M., Fujun W., Lu W. An enhanced periosteum structure/function dual mimicking membrane forin-siturestorations of periosteum and bone. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/abf9b0. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y., Xu T., Zhang Q., Piao Y., Bei H.P., Zhao X. Biomimetic, stiff, and adhesive periosteum with osteogenic-angiogenic coupling effect for bone regeneration. Small. 2021;17(14) doi: 10.1002/smll.202006598. [DOI] [PubMed] [Google Scholar]